Abstract
People living with HIV (PLWH) report elevated levels of posttraumatic stress disorder symptoms (PTSS) and associated comorbidities. The present study tested the efficacy of prolonged exposure (PE) at reducing PTSS, depression, negative posttraumatic cognitions, and substance use in PLWH. Participants were randomly assigned to receive PE (n = 40) or to a weekly monitoring control group (n = 25). Assessments occurred at baseline, post-intervention and 3-months post-treatment. Following the 3-month assessment, controls were offered the intervention. All PE recipients (whether originally from the PE or control group) completed a 6-month assessment. Intent-to-treat mixed model repeated measures ANOVAs were conducted through 3-months post-treatment; within group analyses were conducted through 6-months. PE recipients reported fewer PTSS and negative posttraumatic cognitions and were more likely to achieve good end-state functioning; gains were maintained at 6-months. No between-group differences emerged for substance use. Overall, results support the efficacy of PE in PLWH.
Keywords: people living with HIV, prolonged exposure, posttraumatic stress disorder, depression, intervention
Introduction
With the publication of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)[1], diagnosis with a life-threatening or terminal disease was first recognized as a traumatic event that could lead to the development of posttraumatic stress disorder (PTSD). Since then, research has established that PLWH report some of the highest rates of PTSD observed for any medical trauma/diagnosis population (e.g., cancer patients, myocardial infarction, cardiac surgery, stroke, childbirth, miscarriage, abortion, etc.)[2]. Between 30–61% of PLWH develop HIV-related PTSD at some point in their lives from either a diagnosis of HIV, or from other issues related to the disease [3–6]. In addition, PLWH report disproportionately high levels of nonHIV-related traumatic experiences (i.e., childhood sexual and physical abuse, sexual assaults, parental neglect [7–9]), and rates of PTSD in general (from either HIV- or nonHIV-related events) ranging from 14–61% [3, 4, 10–13]. These percentages are significantly higher than reported rates of PTSD in the general population (7–10%) [14, 15].
To receive a diagnosis of PTSD, an individual must react with intense fear, helplessness, or horror after exposure to a traumatic event that involved actual or threatened death to oneself or others. Additionally, PTSD involves a host of symptoms generally categorized in the following three clusters: reexperiencing, avoidance and numbing, and hyperarousal [1]. Individuals who suffer from PTSD also display heightened risk of comorbid disorders (e.g., depression, anxiety) [14, 16, 17], suicidal ideation [17], impaired psychosocial functioning [18, 19], physical health complications [20, 21], and poor quality of life [21]. In addition, individuals with PTSD often have negative posttraumatic cognitions, such as negative thoughts about the self and the world, and self blame [22, 23].
PTSD may be particularly detrimental to PLWH; research has suggested that the course of HIV infection is susceptible to influence by psychosocial factors including PTSD [24]. It has been suggested that the presence of traumatic stress and depression may explain some of the variability in HIV disease course [24 – 29]. Both PTSD and depression have been associated with lower CD4/CD8 cell count ratios [12, 30], and HIV-related PTSD specifically has been associated with greater reporting of physical symptoms [31]).
PTSD may also indirectly impact disease course in PLWH through several mechanisms. PTSD symptoms of avoidance may lead PLWH to not adhere to medication regimens or to miss more doctors’ appointments, as both of these events serve as reminders of their diagnosis [32, 33]. Avoidance symptoms may also prevent individuals from disclosing their HIV status to significant others (i.e., friends or sexual partners), which may have serious implications for the spread of the disease [33].
PTSD rarely occurs in a “pure” form and is often comorbid with depression; rates of PTSD/depression comorbidity range from 35–50% [14, 29, 34–36]. Depression has been associated with faster HIV disease progression [14, 29, 37 – 39] and three times the risk of nonadherence to medical treatment regimens in a variety of medically ill populations [39]. Though it is difficult to isolate the unique effects of PTSD and depression on disease progression and adherence due to the high rates of comorbidity, results from a recent cross-sectional study revealed that depressive symptoms (as compared to PTSD symptoms and comorbid symptomology) had the strongest relationship to CD4 cell counts and medication adherence [41]. Similarly, Boarts and colleagues [37] found that both PTSD and depression were associated with low medication adherence, but that depression, and not PTSD, predicted lower CD4 cell counts and higher viral load.
Substance use is also commonly comorbid with PTSD. PTSD rates range from 30–50% in substance abusers [42], and comorbidity rates of substance abuse/dependence in PTSD are high (up to 43%)[14, 43 46]. Further, HIV is associated with increased risk for substance use disorders, as alcohol and substance use disorders are between 2.5–7.5 times more prevalent in PLWH than in the general population [7]. Substance use alone is independently associated with faster disease progression, less than optimal medication adherence, failure of HAART medication regimens, and immune suppression [30, 47, 48], and comorbid PTSD/substance use is associated with a greater decline in immune function and health status [11, 49, 50]. Taken together, PTSD, depression, and substance use independently and collectively confer a detrimental effect on HIV disease course, adherence to medication regimens, and negative health behaviors [30. 51].
Given the high incidence of post-traumatic distress and the negative consequences of PTSD and comorbid disorders, PLWH are in need of effective mental health interventions [9, 24, 52, 53]. Despite the demonstrated efficacy of psychotherapeutic interventions for depression [54 – 56], the majority of mental health needs, specifically regarding PTSD, are unmet in PLWH [9]. In a sample of female HIV patients seeking medical care, Martinez and colleagues [4] estimated that 78% of women with partial PTSD and 59% of women with full PTSD were not receiving treatment for their disorder. Additionally, Israelski and colleagues [57] reported that 43% of patients recruited from a primary care services system who met criteria for either depression, PTSD, or acute stress disorder were not being treated.
Prolonged Exposure therapy
Prolonged exposure (PE) therapy, a form of cognitive-behavioral therapy, is considered the most appropriate first-line PTSD treatment [58 – 60]. To date, PE is the only empirically supported evidence-based treatment available for individuals with PTSD [61]. Research has demonstrated the efficacy of PE for the treatment of PTSD and comorbid symptoms across several controlled studies with varied trauma samples (for a review, see Powers and colleagues [62]). Recent research has suggested that the treatment of PTSS in substance-using individuals may consequently result in decreases in substance use, and PE may be beneficial for those who are substance dependent [63]. Further, in a recent state-of-the-science review, Nemeroff and colleagues [64] reported that the Substance Abuse and Mental Health Services Administration recommends PE for national dissemination, as it represents a model program for PTSD treatment. The superior efficacy of PE (as compared to cognitive restructuring and the combination of cognitive restructuring and PE) at producing improvements in good end-state functioning was also cited [64].
PE involves repeated imaginal exposure to the traumatic event (trauma reliving) and repeated in-vivo exposure to situations encountered in daily life that may be avoided due to the traumatic memory or PTSS [65]. Through habituation, the traumatic memory becomes incorporated into normal cognitive schemas which then prevent generalization of the traumatic memory to safe situations [66]. PE also involves systematic repeated confrontation with traumatic memories that serve to disconfirm negative posttraumatic cognitions [22]. PE therapy has been found to be just as effective at reducing negative posttraumatic cognitions, depression, and general anxiety as PE combined with cognitive restructuring in a sample of adult female assault victims [22]. Despite the high rates of PTSD in PLWH, and the demonstrated efficacy of PE therapy in individuals with PTSD, no study to date has examined the efficacy of PE for PTSD in a sample of PLWH.
The present study aimed to test the efficacy of PE therapy at reducing PTSD symptoms in male and female PLWH in a small randomized controlled trial design. Further, given the lack of evidence concerning efficacious treatments for PTSD stemming from diagnosis/living with HIV, we assessed the efficacy of PE at decreasing PTSD symptoms both from HIV- and nonHIV-related traumas. We hypothesized that HIV- and nonHIV-related PTSS, depression symptoms, negative posttraumatic cognitions, and substance use would decrease over time for PE recipients. It was also hypothesized that participants receiving PE therapy would report significantly fewer PTSD symptoms regarding both HIV and nonHIV-related trauma compared to the control group. Further, we hypothesized that the PE group would report fewer depression symptoms, fewer negative posttraumatic cognitions, and lower levels of substance use than the control group. Additionally, we hypothesized that a higher percentage of PE versus control participants would meet criteria for good end-state functioning.
Method
Participants
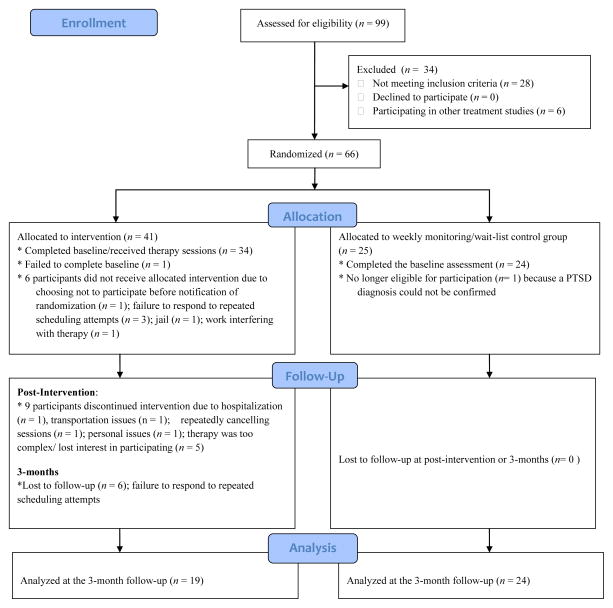
PLWH were recruited between 2005 and 2008 from two social service agencies near Cleveland, OH. Eligible participants were fluent in English, met criteria for a likely diagnosis of PTSD as assessed through the self-report PTSD Diagnostic Scale (PDS [67]) and were currently taking antiretroviral medications for HIV. Exclusionary criteria included diagnosis of a psychotic disorder, current or previous diagnosis of schizophrenia, and current suicidal ideation. Of 99 PLWH screened for the study, 34 did not meet eligibility requirements (see Figure 1 for a flow diagram of participant progress). Thus, 65 (24 females and 41 males) eligible participants completed the baseline questionnaires. The sample at baseline consisted of 29 (44.6%) African Americans, 25 (38.5%) Caucasians, 4 (6.1%) Hispanics, and 7 (10.8%) individuals who identified with more than one race. The mean age was 46 years (Range: 31–61). Participants had been living with a diagnosis of HIV for approximately 13 years (Range: 13 months to 26 years) and were a low income sample, with 85% (n = 55) of the participants earning below 20,000 dollars annually.
Figure 1.
Flow diagram of participant progress throughout the study.
Thirty-four participants were randomly assigned to the PE treatment group, while 24 were assigned to the weekly monitoring control group. At the post-intervention assessment, 23 participants were retained in the PE group (32% drop-out rate) and 24 participants were retained in the control group (0% drop-out rate). At the 3-month follow-up 19 PE recipients were retained as were all the control participants.
After completion of the 3-month assessment, participants in the control group were given the opportunity to participate in the intervention; nineteen chose to receive the intervention. All participants receiving PE (whether they had originally been in the control group or not) were assessed at 6-months post-intervention. Seventeen of the original PE participants were retained throughout 6-months (2 participants lost to follow-up between 3- and 6-months) and 13 (68.4% retention rate) of the original nineteen control participants who opted to receive the intervention were retained throughout the 6-month follow-up. Therefore, we were able to analyze data on 30 individuals (18 males and 12 females) through the 6-month follow-up.
At the post-intervention follow-up, a greater number of participants in the PE group dropped out of the study than participants in the control group (χ2 (1, n = 65) = 11.38, P < .001). Participants who dropped out of the study reported lower levels of PTSS in reference to the nonHIV-related trauma than participants who were retained at this time point (F(1,62) = 5.49, P = .02). There were no significant differences in any other demographic or study variables between participants who dropped out and those who were retained at any time point. Further, drop-out rates did not differ between participants whose most distressing trauma was HIV-related compared to nonHIV-related.
Study groups
Prolonged Exposure
Individual PE therapy sessions were conducted in the social service agency by one of two trained clinical psychology post-doctoral fellows. The treatment followed standard PE protocol [65] and consisted of 10 sessions (lasting between 90–120 minutes each) conducted twice per week for 5 weeks. The therapy was individually tailored to each participant (their most distressing trauma) and focused on HIV-related trauma for some, and nonHIV-related trauma for others.
Weekly monitoring/wait list control group
Participants assigned to the weekly monitoring/wait list control group continued with their standard visits to the social service agency, but were also contacted by their case manager once a week for the duration of the PE therapy to ensure that they were not experiencing any symptom exacerbation.
Procedure
The study protocol was approved by the human subjects review boards of Kent State University and Summa Health System. Potential participants were initially approached about the study by their case manager at the social service agency. Interested participants completed the PDS [67] to determine eligibility for the study. Participants who likely met criteria for PTSD then met with a Ph.D.-level clinical psychology student who described the study in detail to them. Interested participants provided written informed consent. The Structured Clinical Interview with Psychotic Screen for the DSM-IV (SCID [68]) was then administered to ensure the presence of diagnostic levels of PTSD, including endorsing Criterion A, associated functional impairment, and the absence of any psychotic disorder or other exclusionary criteria.
The baseline assessment occurred within one week of this initial screening procedure. All eligible participants were administered the PTSD Symptom Scale-Interview (PSS-I) with regards to both HIV-related PTSD and the most severe nonHIV-related trauma. They then completed self-report baseline questionnaires including the Center for Epidemiological Studies – Depression Scale (CES-D), the Posttraumatic Cognitions Inventory (PTCI), and a self-report scale assessing substance use over the past 30 days. Following this baseline assessment, participants were randomly assigned to the PE therapy or the weekly monitoring/wait list control group. The principal investigator (DLD) generated the allocation sequence using blocked randomization (4:3 ratio of experimental: control participants), and the graduate student conducting the assessments remained blind to group membership. Unequal numbers of participants were assigned to each group, as it was anticipated that the PE group would have a higher dropout rate. PE participants completed the self-report PSS at every other therapy session (i.e., 2, 4, 6, 8, and 10) to track symptom progression through therapy, and reported on substance use at the start of each therapy session.
All follow-up assessments were conducted at the social service agency by the same blind interviewer who conducted the baseline assessment. Participants completed the same questionnaire battery that was administered at baseline. Finally, all participants who received PE (the original PE group and the weekly monitoring/wait listed control group who elected to receive the intervention) also completed the same assessment 6-months post-intervention. Participants received $25 for each assessment and $10 for every intervention session they completed (up to $100 total with an extra $25 if they completed all of the intervention sessions).
Measures
Sociodemographics
Standard demographic questions about age, gender, race/ethnicity, number of years living with HIV, sexual orientation (rated on a scale of 1–9 where 1 = exclusively homosexual/gay, 5 = bisexual, and 9 = exclusively heterosexual/straight), and income were administered at baseline.
PTSD
Participants completed the 17-item Posttraumatic Stress Diagnostic Scale [67, 69] at screening to determine whether they met likely criteria for a PTSD diagnosis. Participants were asked to rate the frequency (0 = not at all or only one time; 3 = five or more times a week/almost always) with which they experienced each of the 17 PTSD symptoms corresponding to the criteria in the DSM-IV within the past month. A likely diagnosis of PTSD was considered present if the participant endorsed at least one reexperiencing symptom, three avoidance symptoms, and two arousal symptoms. Diagnoses were verified at screening with the SCID with Psychotic Screen for the DSM-IV [68]. The SCID is a gold standard structured interview that allows interviewers to make psychiatric diagnoses based on DSM-IV criteria.
At each assessment participants completed the PTSD Symptom Scale-Interview (PSS-I [70]). The PSS-I is a 17-item semi-structured interview designed to measure the frequency and severity of PTSD symptoms. The total score is calculated based on the sum of the individual items. Internal consistency for the PSS-I was acceptable in our sample (HIV-related trauma baseline alpha = .81; Post-intervention = .89; 3-month = .86; 6-months = .84; NonHIV-related trauma baseline alpha = .81; Post-intervention = .93; 3-month = .86; 6-months = .78).
Depression
The Center for Epidemiological Studies – Depression Scale (CESD [71]) was administered to assess participants’ level of self-reported depression symptoms within the week prior to assessment at all time points. The CES-D consists of 20 items summed to create a total score. The CES-D displayed acceptable internal consistency in our sample (baseline alpha = .71; Post-intervention = .76; 3-months = .77; 6-months = .84).
End-state functioning
End-state functioning represents a composite of psychological outcomes, and is typically computed from scores on measures of PTSD, depression, and anxiety [64, 72]; however, end-state functioning has also been computed from PTSD and depression scores alone [75]. Given relatively low rates of anxiety in PLWH, composite scores for good end state functioning were created based on reports of HIV-related PTSS, nonHIV-related PTSS, and depression. The criteria for good end-state functioning (yes or no) were calculated based on the following cut-off scores: 20 or less on the HIV-related PSS-I [59], 20 or less on the nonHIV-related PSS-I, and 16 or less on the CES-D [71].
Substance Use
A self-report substance use questionnaire was administered at each time point to assess the frequency (measured in # of days) of use of 11 different substances including alcohol, marijuana, cocaine, ecstasy, amphetamines, GHB, PCP, hallucinogens, rohypnol, ketamine, and heroin. At the baseline, 3- and 6-month follow-up assessments, frequency of substance use was assessed within the past 30 days. However, given that substance use was tracked during each PE therapy session to ensure no exacerbation of use, at the post-intervention time point (which occurred 1 week post-therapy), substance use was assessed over the past 7 days. Total frequency was obtained by summing the number of days during which substances were used.
Posttraumatic Cognitions
The self-report Posttraumatic Cognitions Inventory [23] was used to assess negative posttraumatic thoughts. Participants rated each of the 36 items based on how much they agreed or disagreed with the negative thought. This instrument yields a sum total score as well as three individual subscale scores: negative cognitions about the self, negative cognitions about the world, and self-blame. Higher scores on the PTCI indicate greater endorsement of negative posttraumatic cognitions. Internal consistency for the present sample was high (baseline alpha = .94; Post-intervention = .94; 3-month = .96; 6-month = .95).
Data Analysis
Preliminary Analyses
Preliminary analyses were conducted with Statistics Package for the Social Sciences Version 16 (SPSS 2006). An alpha level of .05 (two-tailed) was used to determine significance in all analyses. In order to test for possible control variables, chi square analyses were applied between categorical variables (group, gender, race). One-way ANOVAs were conducted between categorical and continuous variables to examine group differences in age, time living with HIV, and sexual orientation. If significant differences emerged, these variables were used as covariates. Further, to test whether randomization was successful, one-way ANOVAs were conducted to determine whether the study groups differed on any demographic or study-related variables.
Intent-to-Treat Analyses
A series of mixed-model repeated measures ANOVAs were conducted to test whether PE was efficacious at reducing HIV- and nonHIV-related PTSS, depressive symptoms, negative posttraumatic cognitions, and substance use on the randomized sample from baseline to the 3-month follow-up. For these analyses, group (PE vs. control) was the between subjects factor and time of assessment was the within subjects factor. An intent-to-treat (ITT) method was used in order to remove potential bias due to participant attrition and non-compliance with the study protocol [76]. The conservative approach of last observation carried forward (LOCF) was applied, such that missing values at each assessment were replaced by the last known value reported by the participant. Therefore, between group analyses were conducted on the 65 participants (n = 40 for the intervention group; n = 25 for the control group) randomized to the original study conditions. In the presence of a significant group x time interaction, post-hoc analyses were conducted in the form of one-way ANOVAs on the change scores between each time point.
To test whether the gains of PE therapy were maintained over the 6-month follow-up (non-randomized sample), within subjects repeated measures ANOVAs were conducted using data from the baseline, post-treatment, 3-month, and 6-month post-treatment assessments on outcomes of interest for all participants who received the PE therapy (including control group participants who later received PE). The sample size for this analysis consisted of 59 participants; the participants randomized to receive PE (n = 40) and the original weekly monitoring/wait list control participants who opted to later receive PE (n = 19). If a significant main effect of time emerged, pairwise comparisons within SPSS were conducted to determine which time points were significantly different from each other.
Secondary Completer Analyses
A series of mixed-model repeated measures ANOVAs were also conducted on participants who completed the entire study protocol. Between group analyses conducted on the randomized sample throughout 3-months consisted of 43 participants, and within group analyses throughout 6-months consisted of 30 participants. Finally, chi square analyses were conducted to determine whether groups differed in good end state functioning at the 3-and 6-month follow-up assessments.
Missing data
To include data from retained participants, missing values were imputed for less than 1% of missing data points.
Results
Preliminary Analyses
On average, participants reported experiencing 4.91 (standard deviation (SD) = 1.78) different types of prior trauma, and 34% of the participants reported that their most distressing trauma was related to their HIV diagnosis. Sixty-four of the participants reported experiencing both an HIV-and nonHIV-related trauma, and one participant only endorsed an HIV-related trauma. Baseline descriptive statistics for all study variables are presented in Table 1, and descriptive statistics for the outcomes at the follow-up assessments are displayed in Table 2. Results from chi square analyses revealed that the PE and control groups did not differ by gender or race. Results from one-way ANOVAs also revealed no significant differences between groups on age, time living with HIV, sexual orientation, HIV-related PTSS, nonHIV-related PTSS, depression, posttraumatic cognitions, and substance use (see Table 1).
Table 1.
Means and Standard Deviations from the Full Sample for Continuous Level Demographic Variables
| Baseline (n = 65)
|
||||
|---|---|---|---|---|
| Demographics | Prolonged Exposure Mean (SD) |
Wait-list/Control Mean (SD) |
F test a | Total Sample Mean (SD) |
| Age | 46 (5.8) | 48(7.0) | 1.92 | 46.37 (6.30) |
| Sexual Orientation b | 5.12 (3.5) | 5.76 (3.5) | .51 | 5.37 (3.49) |
| Months Living with HIV | 157 (68) | 163(55) | .70 | 159 (63) |
| HIV-related PTSS | 25.30 (10.26) | 26.00 (9.62) | .08 | 25.57 (9.95) |
| nonHIV-related PTSS c | 27.45 (9.52) | 30.42 (10.28) | 1.37 | 28.56 (9.84) |
| Depressive symptoms | 25.72 (10.96) | 26.00 (10.58) | .01 | 25.83 (10.73) |
| Negative Posttraumatic Cognitions | 115.66 (37.38) | 111.32 (41.09) | .19 | 113.99 (38.59) |
| Substance Use | 6.72 (11.49) | 6.76 (10.70) | .00 | 6.74 (11.11) |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms.
F test = F value from analyses examining group differences in demographic variables.
Sexual orientation = The mean score here reflects sexual orientation on a continuum from 1=9 (1= completely homosexual; 5 = bisexual; 9 = completely heterosexual).
sample size for analyses regarding nonHIV-related PTSS is 64 (1 participant failed to report a nonHIV-related trauma).
P <.05,
P <.01,
P <.001.
Table 2.
Means and Standard Deviations from the Completer Sample for all Study Outcomes
| Demographics | Post-Intervention (n = 47) | 3-month Follow-up (n = 43) | ||
|---|---|---|---|---|
|
| ||||
| PE (n = 23) Mean (SD) |
Control (n = 24) Mean (SD) |
PE (n = 19) Mean (SD) |
Control (n = 24) Mean (SD) |
|
| HIV-related PTSS | 8.30 (4.83) | 24.13 (10.66) | 7.32 (6.74) | 20.46 (8.94) |
| nonHIV-related PTSS a | 7.30 (3.98) | 28.09 (11.13) | 6.11 (5.48) | 21.26 (7.80) |
| Depressive symptoms | 16.00 (9.46) | 27.50 (11.68) | 13.53 (9.99) | 22.08 (12.55) |
| Negative Posttraumatic Cognitions | 83.70 (30. 75) | 115.08 (33.00) | 78.26 (27.83) | 100.71 (46.76) |
| Substance Use | -- | -- | 4.53 (12.46) | 7.21 (11.07) |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms.
overall sample size for analyses regarding nonHIV-related PTSS is 63 (1 control participant failed to report a nonHIV-related trauma.
Mixed Model Repeated Measures ANOVAs: ITT Analyses
After applying the LOCF method, mixed model (between and within group) repeated measures ANOVAs were conducted on HIV-related PTSS, nonHIV-related PTSS, depression, negative posttraumatic cognitions, and substance use. Inferential and descriptive statistics for all group x time interactions are displayed in Table 3. Results revealed a main effect of time for both HIV-related PTSS (Wilk’s λ = .67; F(2, 62) = 15.65, P < .001; partial η2 = .34) and nonHIV-related PTSS (Wilk’s λ = .50; F(2, 61) = 31.85, P < .001; partial η2 = .51) in that all participants demonstrated significant decreases in symptoms over time. This main effect was qualified by a significant group x time interaction indicating that PE participants experienced a greater decrease in HIV- and nonHIV-related PTSS than the controls. Post-hoc analyses revealed significant differences between baseline to post-intervention for HIV-related PTSS (F(1,63) = 8.54, P < .01) and nonHIV-related PTSS (F(1,62) = 9.98, p < .01). However, differences between baseline and 3-months were not significant.
Table 3.
Group x Time Interactions: Intent-to-Treat Mixed Model Repeated Measures ANOVAs
| Outcomes | Baseline Mean (SD) |
Post-Intervention Mean (SD) |
3-month follow-up Mean (SD) |
Wilk’s λ | F | Partial η2 |
|---|---|---|---|---|---|---|
| HIV-related PTSS (n = 65) | .86 | 4.86*** | .14 | |||
| PE | 25.30 (10.26) | 14.90 (11.09) | 14.43 (11.76) | |||
| Control | 26.00 (9.62) | 23.28 (11.26) | 19.76 (9.43) | |||
| NonHIV-related PTSS (n = 64) | .76 | 9.55*** | .24 | |||
| PE | 27.45 (9.52) | 14.60 (11.28) | 13.98 (11.96) | |||
| Control | 30.42 (10.29) | 26.96 (12.21) | 20.42 (8.83) | |||
| Depressive Symptoms (n = 65) | .92 | 2.82+ | .09 | |||
| PE | 25.72 (10.96) | 19.79 (10.46) | 18.56 (10.96) | |||
| Control | 26.55 (10.57) | 26.60 (12.29) | 21.40 (12.75) | |||
| Posttraumatic Cognitions (n = 65) | .90 | 3.28* | .10 | |||
| PE | 115.66 (37.38) | 97.25 (36.36) | 94.28 (35.46) | |||
| Control | 111.32 (41.09) | 112.56 (34.68) | 98.76 (46.80) | |||
| Substance Use (n = 65) | .99 | .12 | .00 | |||
| PE | 6.73 (11.49) | --- | 7.75 (14.05) | |||
| Control | 6.76 (10.70) | --- | 6.92 (10.93) |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms; PE = Prolonged Exposure Group; Control = Wait-list/Control Group.
P <.05,
P <.01,
P <.001.
A main effect of time was also revealed for depression symptoms, such that they decreased throughout the course of the study for all participants (Wilk’s λ = .80; F (2, 62) = 7.70, P = .001; partial η2 = .20). Results also revealed a trend towards significance for a group x time interaction, with PE participants experiencing a marginally greater decrease over time in depression symptoms. Post-hoc analyses revealed a significant reduction in depression symptoms from baseline to post-intervention (F (1,63) = 4.12, P = .047), but differences between baseline and 3-months were not significant.
Regarding posttraumatic cognitions, a significant main effect of time indicated that negative posttraumatic cognitions decreased throughout the study (Wilk’s λ = .79; F(2, 62) = 8.25, P = .001; partial η2 = .21). Further, a significant group x time interaction revealed that PE participants experienced a greater improvement in PTCI scores than control participants. Post-hoc analyses indicated significant differences from baseline to post-intervention (F (1,63) = 5.93, P = .02), but results were not significant through 3-months.
Results the outcome of substance use, a mixed model repeated measures ANOVA conducted on the baseline and the 3-month follow-up assessment revealed no main effect of time (Wilk’s λ = .10; F(1, 63) = .23, P = .63; partial η2 = .00) or group x time interaction.
Mixed Model Repeated Measures ANOVAs: Completer analysis
The following analyses were performed on the 43 participants who were retained throughout all phases of the study. Inferential and descriptive statistics for the group x time interactions are displayed in table 4. Results from mixed model repeated measures ANOVAs replicated the ITT findings regarding PTSS such that reductions in HIV-related (Wilk’s λ = .34; F(2, 40) = 38.94, P < .001; partial η2 = .66) and nonHIV-related PTSS (Wilk’s λ = .20; F(2, 39) = 77.99, P < .001; partial η2 = .80) occurred for all participants throughout the course of the study. Further, significant group x time interactions revealed that PE participants experienced a greater decrease in HIV- and nonHIV-related PTSS than control participants. Post-hoc analyses indicated significantly larger reductions in HIV- and nonHIV-related PTSS between baseline and post-intervention (F(1,45) = 37.21, P < .001; F(1,44) = 50.62, P < .001) and between baseline and 3-month follow-up PTSS scores (F(1,41) = 16.51, P < .001; F(1,40) = 19.70, P < .001) in PE participants versus controls, respectively.
Table 4.
Group x Time Interaction Results from Mixed Model Repeated Measures ANOVAs: Completer Sample
| Outcomes | Baseline Mean (SD) |
Post-Intervention Mean (SD) |
3-month follow-up Mean (SD) |
Wilk’s λ | F | Partial η2 |
|---|---|---|---|---|---|---|
| HIV-related PTSS (n = 43) | .55 | 16.35*** | .43 | |||
| PE | 26.42 (9.76) | 8.32 (5.15) | 7.32 (6.74) | |||
| Control | 26.96 (8.52) | 24.13 (10.66) | 20.46 (8.94) | |||
| NonHIV-related PTSS (n = 42) | .45 | 23.99*** | .55 | |||
| PE | 28.79 (8.87) | 7.42 (4.11) | 6.11 (5.48) | |||
| Control | 31.70 (8.34) | 28.08 (11.13) | 21.26 (7.80) | |||
| Depressive Symptoms (n = 43) | .85 | 3.44* | .15 | |||
| PE | 26.47 (11.38) | 16.05 (10.07) | 13.53 (9.99) | |||
| Control | 26.87 (9.84) | 27.50 (11.68) | 22.08 (12.55) | |||
| Posttraumatic Cognitions (n = 43) | .77 | 5.84** | .23 | |||
| PE | 117.00 (36.97) | 84.53 (33.27) | 78.26 (27.83) | |||
| Control | 113.79 (40.03) | 115.08 (33.00) | 100.71 (46.76) | |||
| Substance Use (n = 43) | .10 | .08 | .00 | |||
| PE | 3.37 (7.11) | --- | 4.53 (12.46) | |||
| Control | 7.04 (10.83) | --- | 7.21 (11.07) |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms; PE = Prolonged Exposure Group; Control = Wait-list/Control Group.
P <.05,
P <.01,
P <.001.
Results also revealed that depression symptoms were reduced throughout the duration of the study (Wilk’s λ = .69; F(2, 40) = 8.85, P = .001; partial η2 = .31), and this main effect was qualified by a significant group x time interaction revealing that PE participants demonstrated greater improvements in depression symptoms over time as compared to control participants. Post-hoc analyses indicated larger decreases in depression for PE participants than for control participants from baseline to post-intervention (F(1,45) = 7.05, P = .011). However, the change from baseline to the 3-month follow-up assessment was only marginally significant (F(1,42) = 3.11, P = .087).
Also similar to the ITT findings, results revealed a main effect for PTCI scores to improve over time (Wilk’s λ = .61; F(2, 40) = 12.83, P < .001; partial η2 = .39), and this main effect was qualified by a significant group x time interaction. Post-hoc analyses indicated greater reductions in PTCI for PE participants between baseline and post-intervention (F(1,45) = 12.16, p = .002) and baseline and 3-month follow-up (F(1,42) = 16.51, P = .02).
With regards to substance use, no exacerbation of substance use occurred during the PE sessions, and a one-way ANOVA conducted between groups on the post-intervention assessment supported the results from therapy sessions, in that no between group differences on substance use within the past week were present (F (1,42) = .09, P = .77). The PE group reported an average of 2.58 (6.65) and the control group reported an average of 2.12 (3.15) instances of substance within the past seven days. A mixed model repeated measures ANOVA conducted on baseline and the 3-month follow-up assessment confirmed the ITT findings and revealed no main effect of time (Wilk’s λ = .10; F(1, 41) = .14, P = .71; partial η2 = .00) or group x time interaction.
Within Subjects ITT Analyses – Through the 6-month Follow-up
After applying the LOCF method, within subjects repeated measures ANOVAs were conducted on 59 participants to determine whether treatment gains persisted at the 6-month follow-up. Time of assessment (baseline, post-intervention, 3- and 6-months post-treatment) was the within subjects factor. Descriptive and interferential statistics are displayed in Table 5. Results revealed significant main effects of time for the outcomes of HIV-related PTSS, nonHIV-related PTSS, depression and negative posttraumatic cognitions, such that PE participants experienced a reduction in symptoms over the course of the study. Post-hoc analyses revealed significant mean differences between baseline, post-intervention, 3- and 6-month assessments (see Table 7), implying that the effects of the intervention were maintained through 6 months after the completion of therapy. No significant main effects of time emerged for substance use.
Table 5.
Results for Intent-to-Treat Within Subjects Repeated Measures ANOVA: Main Effects of Time
| Outcomes | Baseline Mean (SD) |
Post-Intervention Mean (SD) |
3-month follow-up Mean (SD) |
6-month follow-up Mean (SD) |
Wilk’s λ | F | Partial η2 |
|---|---|---|---|---|---|---|---|
| HIV-related PTSS (n = 59) | 24.21 (9.78)a,b,c | 13.25 (10.19)a | 13.51 (10.78)b | 13.07 (11.16)c | .46 | 21.95*** | .54 |
| NonHIV-related PTSSS (n = 58) | 24.93 (9.74) a,b,c | 13.50 (10.43) a | 13.67 (11.11) b | 13.20 (10.91) c | .49 | 18.76*** | .51 |
| Depressive Symptoms (n = 59) | 24.24 (10.67) a,b,c | 18.88 (11.10) a | 18.64 (11.32) b | 19.91 (11.89) c | .76 | 5.80** | .24 |
| Negative Posttraumatic Cognitions (n = 59) | 112.78 (41.84) a,b,c | 95.85 (43.22) a | 93.95 (40.03) b | 93.14 (43.43) c | .70 | 7.90*** | .30 |
| Substance Use (n = 59) | 7.66 (11.80) a,b,c | --- | 7.00 (12.86) b | 7.14 (11.01) c | .99 | .19 | .07 |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms.
Mean(SD) = Means that share superscripts are significantly different from each other.
P <.05,
P <.01,
P <.001.
Table 7.
Post-hoc Pairwise Comparisons for Within Subjects Analyses
| Outcomes |
t Baseline to post-intervention |
t Baseline to 3-month follow-up |
t Baseline to 6-month follow-up |
|---|---|---|---|
| HIV-related PTSS: ITT | 8.04*** | 6.80*** | 7.20*** |
| NonHIV-related PTSS: ITT | 7.58*** | 6.82*** | 7.07*** |
| Depressive Symptoms: ITT | 3.84*** | 3.54** | 3.41** |
| Negative Posttraumatic Cognitions: ITT | 4.67*** | 4.38*** | 4.73*** |
| HIV-related PTSS: Completer Sample | 10.55*** | 7.01*** | 7.80*** |
| NonHIV-related PTSS: Completer Sample | 11.46*** | 8.13*** | 9.00*** |
| Depressive Symptoms: Completer Analysis | 3.88** | 3.00** | 2.73* |
| Negative Posttraumatic Cognitions: Completer Analysis | 4.75*** | 3.88** | 4.35*** |
PTSS = Posttraumatic Stress Symptoms; ITT = Intent-to-Treat Analyses
P <.05,
P <.01,
P <.001.
Within subjects Completer Analyses – Through the 6-month Follow-up
Results from within subjects repeated measures ANOVAs conducted on 30 participants replicated the ITT findings (see table 6), in that PE participants experienced significant reductions in HIV-related and nonHIV-related PTSS, depressive symptoms, and negative posttraumatic cognitions throughout 6-months post-treatment. Post-hoc pairwise comparisons again revealed significant differences between baseline and all follow-up assessments (See table 7). No significant main effects of time emerged for substance use.
Table 6.
Results for Within Subjects Repeated Measures ANOVA Completer Sample: Main Effects of Time (n = 30)
| Outcomes | Baseline Mean (SD) |
Post-Intervention Mean (SD) |
3-month follow-up Mean (SD) |
6-month follow-up Mean (SD) |
Wilk’s λ | F | Partial η2 |
|---|---|---|---|---|---|---|---|
| HIV-related PTSS | 24.27 (8.87) a,b,c | 8.43 (5.64) a | 9.57 (7.54) b | 8.80 (8.13) c | .19 | 39.65*** | .82 |
| NonHIV-related PTSS (n = 29) | 25.62 (9.11) a,b,c | 7.76 (5.07) a | 8.62 (7.86) b | 7.79 (6.57) c | .16 | 44.41*** | .84 |
| Depressive Symptoms | 23.87 (11.31) a,b,c | 16.47 (10.58) a | 15.67 (11.68) b | 18.02 (13.20) c | .62 | 5.47** | .38 |
| Negative Posttraumatic Cognitions | 107.84 (42.61) a,b,c | 83.04 (40.46) a | 81.72 (35.38) b | 79.96 (41.53) c | .54 | 7.75** | .47 |
| Substance Use | 5.07 (9.79) a,b,c | --- | 4.10 (10.55) b | 5.80 (11.15) c | .94 | .89 | .06 |
(SD) = Standard deviation; PTSS = Posttraumatic Stress Symptoms.
Mean(SD) = Means that share superscripts are significantly different from each other.
P <.05,
P <.01,
P <.001.
End-State Functioning
Results from chi square analyses revealed that 10 PE participants (52.6%) compared to 2 control participants (8.3%) met criteria for good end state functioning (χ2 (1, n = 47) = 10.43, P = .002) post-intervention. Three-months post-treatment, 12 PE participants (63.2%) compared to 6 control participants (25%) met criteria for good end-state functioning (χ2 (1, n = 43) = 6.34, P = .01). Examination of the larger sample size due to providing PE to the control group revealed that 18 (58%), 19 (63%), and 15 (50%) met good end-state functioning at the post-intervention, 3- and 6-month follow-up assessments.
Discussion
To our knowledge, the current study was the first randomized controlled trial to test the efficacy of PE therapy for PTSD in PLWH. Consistent with hypotheses, results supported the use of PE in this population; PE recipients demonstrated significant improvement in both HIV- and nonHIV-related PTSS and achieved good end-state functioning at higher rates than a weekly monitoring/wait list control group. PE was also successful at reducing negative posttraumatic cognitions and depressive symptoms, although this latter result was primarily supported in completer analyses as ITT analyses were at the trend level. Gains achieved in therapy for the outcomes of HIV- and nonHIV-related PTSS, and negative posttraumatic cognitions were maintained throughout 3-months in the completer analysis, but this pattern of results did not emerge for the ITT analyses. However, given that LOCF is a conservative approach to ITT analysis, failure to find significance with this analysis may simply reflect limited power due to a small sample size. Further, results from within subjects analyses suggest that the gains achieved from PE therapy were maintained throughout 6-months for PTSS, depression, and negative posttraumatic cognitions.
Though all group x time interaction effect sizes findings were large (partial η2 ≥ .14), the intervention accounted for the largest proportion of variance in HIV- and nonHIV-related PTSS. Additionally, the magnitude of change for both types of PTSS in the completer analyses was robust—the decline in HIV-related PTSS for PE participants was 19.58 points compared to a reduction of 6.47 for control participants. The change in nonHIV-related PTSS was equally large—a 22.68 point reduction for PE participants compared to a 9.96 point reduction for control participants. It is important to keep in mind that although the largest reduction in PTSS occurred for the PE participants in regards to their HIV-related trauma, the PE therapy itself was aimed at treating participants’ most distressing trauma. As mentioned before, only 34% of participants regarded their HIV-related trauma as being the most traumatic and thus targeted it in therapy, implying that the remaining 66% of participants targeted a nonHIV-related trauma in PE therapy. Given the observed significant reductions in both HIV- and nonHIV-related PTSS, results suggest that participants may have learned skills allowing them to transfer the gains of therapy to other traumas. PE participants also experienced significant reductions in depressive symptoms that were maintained throughout 3-months for the completers, and reductions in negative posttraumatic cognitions which were maintained throughout 6-months in both sets of analyses, supporting the efficacy of PE at decreasing a range of posttraumatic sequalae. Consistent with prior research [77, 78], substance use did not worsen over the course of treatment which lends support to the continued use of PE in PLWH who may have comorbid substance use issues.
Although the dropout rate (32%) for PE participants in the current study was within the range of dropout rates reported in exposure therapies for treating PTSD in non-medical populations (20.5–34% [79 – 81]; for recent reviews, see [82 and 83]), differential drop-out rate was an issue for the present sample. A greater proportion of PE recipients versus controls dropped out by post-intervention. Given that differential drop-out can bias the outcome estimates and their corresponding significance, the present results warrant replication and should be viewed with caution until further investigations confirm these findings. Despite this differential dropout, only 5 participants withdrew from the therapy because they either lost interest in continuing PE or thought the therapy was too much to handle at the current time. Additionally, as the present results were significant using conservative LOCF method in the ITT analyses, this suggests that differential dropout did not bias the present findings.
The high rates of post-traumatic distress observed in PLWH combined with the success of the present pilot study would underscore the importance of screening for PTSS and depression symptoms in routine visits to health care professionals [57, 84 – 86]. Further, as prior research has suggested that addressing PTSD and mental health comorbidities may be a necessary step in the process of improving adherence in PLWH [30, 87, 88], perhaps PE could provide an efficient means by which to decrease post-traumatic distress and comorbid disorders and possibly increase the efficacy of subsequent adherence and substance use interventions.
Strengths of the present study include the heterogeneity of the sample (males, females, varying sexual orientations, varying amounts of time living with HIV), as the exclusionary criteria were limited which allowed for the implementation of a practical intervention in a “real-life” setting. Several prior studies in PLWH have been conducted in limited samples of only women [4, 11, 31], or homosexual/bisexual men [3, 89]. Further, the inclusion of PLWH with various comorbidities allowed for high external validity, as it is rare for PTSD to exist as an independent psychological disorder. PTSS were also assessed via interview rather than self-reported questionnaire.
Though the present results provide preliminary evidence for the efficacy of PE in PLWH, they should be viewed with caution in the presence of some limitations. The small sample size of the present study limits power in conducting mediation or moderation analyses, and the lack of a control group at the 6-month assessment excludes the possibility for comparison of the long-term effects of therapy. Despite these limitations, results from this study provide support for the utility of PE therapy as an efficacious intervention for PLWH. The therapy was readily accepted by PLWH, with a drop-out rate comparable to other studies utilizing exposure therapy. The results expand and improve upon previous mental health interventions that have been offered to PLWH by specifically targeting PTSD with an evidence-based, first-line intervention for the disorder.
Acknowledgments
Funding for this study was provided by the National Institute of Mental Health (R34 MH 71201).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Tedstone JE, Terrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Rev. 2003;23:409–448. doi: 10.1016/s0272-7358(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 3.Kelly B, Raphael B, Judd F, Kernutt G, Burnett P, Burrows G. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry. 1998;20:345–352. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 4.Martinez A, Israelski D, Walker C, Koopman C. Posttraumatic stress disorder in women attending human immunodeficiency virus outpatient clinics. AIDS Patient Care STDs. 2002;16(6):283–291. doi: 10.1089/10872910260066714. [DOI] [PubMed] [Google Scholar]
- 5.Safren SA, Gershuny BS, Hendriksen E. Symptoms of posttraumatic stress and death anxiety in persons with HIV and medication adherence difficulties. AIDS Patient Care STDs. 2003;17:657–664. doi: 10.1089/108729103771928717. [DOI] [PubMed] [Google Scholar]
- 6.Theuninck AC, Lake N, Gibson S. HIV-related posttraumatic stress disorder: Investigating the Traumatic Events. AIDS Patient Care STDs. 2010;24(8):485–491. doi: 10.1089/apc.2009.0231. [DOI] [PubMed] [Google Scholar]
- 7.Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in Southeastern United States. J Acquir Immune Defic Syndr. 2006;42(3):298–306. doi: 10.1097/01.qai.0000219773.82055.aa. [DOI] [PubMed] [Google Scholar]
- 8.Whetten K, Reif S, Ostermann J, et al. Improving health outcomes among individuals with HIV, mental illness, and substance use disorders in the Southeast. AIDS Care. 2006;18(Suppl 1):18–26. doi: 10.1080/09540120600839330. [DOI] [PubMed] [Google Scholar]
- 9.Whetten K, Reif S, Whetten R, Murphy-McMillan LK. Trauma, mental health, distrust, and stigma among HIV-positive persons: Implications for effective care. Psychosom Med. 2008;70:531–538. doi: 10.1097/PSY.0b013e31817749dc. [DOI] [PubMed] [Google Scholar]
- 10.Gore-Felton C, Koopman C, Speigel D. The influence of traumatic stress responses on HIV risk behavior. Presentation from the 22nd Annual Scientific Conference of the Society of Behavioral Medicine; Seattle, Washington. February, 2001. [Google Scholar]
- 11.Kimerling R, Calhoun KS, Forehand R, et al. Traumatic stress in HIV-infected women. AIDS Educ Prev. 1999;11:321–330. [PubMed] [Google Scholar]
- 12.Reilly KH, Clark RA, Schmidt N, Benight CC, Kissinger P. The effects of post-traumatic stress disorder on HIV disease progression following hurricane Katrina. AIDS Care. 2009;21(10):1298–1305. doi: 10.1080/09540120902732027. [DOI] [PubMed] [Google Scholar]
- 13.Smith MY, Egert J, Winkel G, Jacobson J. The impact of PTSD on pain experience in persons with HIV/AIDS. Pain. 2002;98:9–17. doi: 10.1016/s0304-3959(01)00431-6. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–768. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 15.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 16.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 17.Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL. Comorbidity, impairment, and suicidality in subthreshold PTSD. Am J Psychiatry. 2001;158:1467–1473. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn E, Blanchard EB, Fuse T, Hickling EJ, Broderick J. Heart rate of motor vehicle accident survivors in the emergency department, peritraumatic psychological reactions, ASD, and PTSD severity: A 6 –month prospective study. J Trauma Stress. 2006;19(5):735–540. doi: 10.1002/jts.20150. [DOI] [PubMed] [Google Scholar]
- 19.Maia DB, Marmar CR, Metzler T, et al. Posttraumatic stress symptoms in an elite unit of Brazilian police officers: Prevalence and impact on psychosocial functioning and on physical and mental health. J Affect Disord. 2007;97(1):241–245. doi: 10.1016/j.jad.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Lauterbach D, Vora R, Rakow M. The relationship between posttraumatic stress disorder and self-reported health problems. Psychosom Med. 2005;67:939–947. doi: 10.1097/01.psy.0000188572.91553.a5. [DOI] [PubMed] [Google Scholar]
- 21.Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJG. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;l69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- 22.Foa EB, Rauch SAM. Cognitive changes during prolonged exposure versus prolonged exposure plus cognitive restructuring in female assault survivors with posttraumatic stress disorder. J Counsult Clin Psychol. 2004;72(5):879–884. doi: 10.1037/0022-006X.72.5.879. [DOI] [PubMed] [Google Scholar]
- 23.Foa EB, Ehlers A, Clark DM, Tolin DF, Orsillo SM. The posttraumatic cognitions inventory (PTCI): Development and validation. Psychol Assess. 1999;11(3):303–314. [Google Scholar]
- 24.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatr. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 25.Chandra PS, Desai G, Ranjan S. HIV and psychiatric disorders. Indian J Med Res. 2005;121:451–467. [PubMed] [Google Scholar]
- 26.Cohen S, Herbert TB. Health psychology: Psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psychol. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- 27.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 28.Leserman J, Jackson ED, Petitto JM, et al. Progression to AIDS: The effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Tsao JCI, Dobalian A, Moreaua C, Dobalian K. Stability of anxiety and depression in a national sample of adults with human immunodeficiency virus. J Nerv Ment Dis. 2004;192(2):111–118. doi: 10.1097/01.nmd.0000110282.61088.cc. [DOI] [PubMed] [Google Scholar]
- 30.Brief DJ, Bollinger AR, Vielhauer MJ, et al. Understanding the interface of HIV, trauma, posttraumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care. 2004;16 (Suppl1):S97–S120. doi: 10.1080/09540120412301315259. [DOI] [PubMed] [Google Scholar]
- 31.Katz S, Nevid JS. Risk factors associated with posttraumatic stress disorder symptomology in HIV-infection women. AIDS Patient Care STDs. 2005;19(2):110–120. doi: 10.1089/apc.2005.19.110. [DOI] [PubMed] [Google Scholar]
- 32.Delahanty DL, Bogart LM, Figler JL. Posttraumatic stress disorder symptoms, salivary cortisol, medication adherence, and CD4 levels in HIV-positive individuals. AIDS Care. 2004;16(2):247–260. doi: 10.1080/09540120410001641084. [DOI] [PubMed] [Google Scholar]
- 33.Radcliffe J, Landau Fleisher CL, Hawkins LA, et al. Posttraumatic stress and trauma history in adolescents and young adults with HIV. AIDS Patient Care STDs. 2007;21(7):501–508. doi: 10.1089/apc.2006.0144. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- 35.Breslau N, Kessler RC, Chilcoat SS, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 36.Keane TM, Kaloupek DG. Comorbid psychiatric disorders in PTSD: Implications for research. Ann N Y Acad Sci. 1997;821:24–34. doi: 10.1111/j.1749-6632.1997.tb48266.x. [DOI] [PubMed] [Google Scholar]
- 37.Boarts JM, Sledjeski EM, Bogart LM, Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10(3):253–261. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- 38.Kalichman SC, Sikkema KJ, DiFonzi K, Luke W, Austin J. Emotional adjustment in survivors of sexual assault living with HIV-AIDS. J Trauma Stress. 2002;15(4):289–296. doi: 10.1023/A:1016247727498. [DOI] [PubMed] [Google Scholar]
- 39.Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Perkins DO, Folds JD, Evans DL. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32(6):1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 40.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 41.Sledjeski EM, Delahanty DL, Bogart LM. Incidence and impact of posttraumatic stress disorder and comorbid depression on adherence to HAART and CD4+ counts in people living with HIV. AIDS Patient Care STDs. 2005;19(11):728–737. doi: 10.1089/apc.2005.19.728. [DOI] [PubMed] [Google Scholar]
- 42.Dansky BS, Roitzsch JC, Brady KT, Saladin ME. Posttraumatic stress disorder and substance abuse: Use of research in a clinical setting. J Trauma Stress. 1997;10:141–148. doi: 10.1023/a:1024872800683. [DOI] [PubMed] [Google Scholar]
- 43.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 44.Deering CG, Glover SG, Ready D, Edelman HC, Alarcon RD. Unique patterns of comorbidity in posttraumatic stress disorder from different sources of trauma. Compr Psychiatry. 1996;37:336–346. doi: 10.1016/s0010-440x(96)90015-2. [DOI] [PubMed] [Google Scholar]
- 45.Friedman MJ. Biological approaches to the diagnosis and treatment of post-traumatic stress disorder. J Trauma Stress. 1991;4:67–89. [Google Scholar]
- 46.Friedman MJ, Yehuda R. Post-traumatic stress disorder and comorbidity: Psychobiological approaches to differential diagnosis. In: Friedman MJ, Charney DS, editors. Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-traumatic Stress Disorder. Philadelphia, PA: Lippincott-Raven Press; 2005. [Google Scholar]
- 47.O’Cleirigh C, Hart TA, James CA. HIV and anxiety. In: Zvolensky MJ, Smits JA, editors. Anxiety in Health Behaviors and Physical Illness. New York, NY: Springer Science and Business Media, LLC; 2008. [Google Scholar]
- 48.Petry N. Alcohol use in HIV patients: what we don’t know may hurt us. Int J STD AIDS. 1999;10:561–570. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- 49.Crum RM, Galai N, Cohn S, Celentano DD, Vlahov D. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: a prospective analysis. Alcohol Clin Exp Res. 1996;20:364–371. doi: 10.1111/j.1530-0277.1996.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 50.Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol Clin Exp Res. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 51.Myers HF, Durvasula RS. Psychiatric disorders in African American men and women living with HIV/AIDS. Cultur Divers Ethnic Minor Psychol. 1999;5(3):249–262. [Google Scholar]
- 52.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the South. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 53.Sloan E, Collado-Hidalgo A, Cole S. Psychobiology of HIV infection. In: Ader R, editor. Psychoneuroimmunology. 4. Vol. 2. New York, NY: Elsevier Academic Press; 2007. [Google Scholar]
- 54.Himelhoch S, Medoff D, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: A systematic review and meta-analysis. AIDS Patient Care STDs. 2007;21(10):732–739. doi: 10.1089/apc.2007.0012. [DOI] [PubMed] [Google Scholar]
- 55.Olatunji BO, Mimiaga MJ, O’Cleirigh C, Safren SA. A review of treatment studies of depression in HIV. Top HIV Med. 2006;14(3):112–124. [PubMed] [Google Scholar]
- 56.Scott-Sheldon LAJ, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol. 2008;27(2):129–139. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Israelski DM, Prentiss DE, Lubega S, et al. Psychiatric co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007;19(2):220–225. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]
- 58.Ballenger JC, Davidson JRT, Lecrubier Y, et al. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. J Clin Psychiatry. 2000;61 (Suppl 5):60–6. [PubMed] [Google Scholar]
- 59.Foa EB, Dancu CB, Hembree EA, Jaycox LH, Meadows EA, Street GPA. Comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67:194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- 60.Foa EB, Keane T, Friedman M, Cohen J, editors. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. New York, NY: Guilford Press; 2009. [Google Scholar]
- 61.Institute of Medicine. Treatment of Posttraumatic Stress Disorder: An assessment of the evidence. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 62.Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635–41. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Henslee AM, Coffey SF. Exposure therapy for posttraumatic stress disorder in a residential substance use treatment facility. Prof Psychol: Res Pract. 2010;41(1):34–40. doi: 10.1037/a0018235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemeroff CB, Bremner D, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: A state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Foa EB, Rothbaum BO, Riggs D, Murdock T. Treatment of PTSD in rape victims: A comparison between cognitive-behavioral procedures and counseling. J Consult Clin Psychol. 1991;59:715–723. doi: 10.1037//0022-006x.59.5.715. [DOI] [PubMed] [Google Scholar]
- 66.Foa EB, Rothbaum BO. Treating the Trauma of Rape. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 67.Foa EB. Posttraumatic Stress Diagnostic Scale (PDS) manual. Minneapolis, MN: National Computer Systems; 1995. [Google Scholar]
- 68.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 69.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The posttraumatic diagnostic scale. Psychol Assess. 1997;9:445–451. [Google Scholar]
- 70.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6(4):459–473. [Google Scholar]
- 71.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1997;1(3):385–401. [Google Scholar]
- 72.Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. J Consult Clin Psychol. 1998;66(1):185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- 73.Rothbaum BO, Astin MC, Marsteller F. Prolonged exposure versus eye movement desensitization and reprocessing (EMDR) for PTSD rape victims. J Trauma Stress. 2005;18(6):607–616. doi: 10.1002/jts.20069. [DOI] [PubMed] [Google Scholar]
- 74.van Minnen A, Foa EB. The effect of imaginal exposure length on outcome of treatment for PTSD. J Trauma Stress. 2006;19(4):427–438. doi: 10.1002/jts.20146. [DOI] [PubMed] [Google Scholar]
- 75.Bryant RA, Moulds ML, Guthrie RM, Dang ST, Nixon RDV. Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder. J Consult Clin Psychol. 2003;71(4):706–712. doi: 10.1037/0022-006x.71.4.706. [DOI] [PubMed] [Google Scholar]
- 76.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med. 2010;152 doi: 10.4103/0976-500X.72352. Epub 24 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brady KT, Dansky BS, Back SE, Foa EB, Carroll KM. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: preliminary findings. J Subst Abuse Treat. 2001;21:47–54. doi: 10.1016/s0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 78.Triffleman E, Carroll K, Kellogg S. Substance dependence posttraumatic stress disorder therapy. J Subst Abuse Treat. 1999;17:3–14. doi: 10.1016/s0740-5472(98)00067-1. [DOI] [PubMed] [Google Scholar]
- 79.Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. J Consult Clin Psychol. 2005;73(5):953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- 80.van Etten ML, Taylor S. Comparative efficacy of treatments for post-traumatic stress disorder: A meta-analysis. Clin Psychol Psychother. 1998;5:126–144. [Google Scholar]
- 81.van Minnen A, Arntz A, Keijsers GPJ. Prolonged exposure in patients with chronic PTSD: predictors of treatment outcome and dropout. Behav Res Ther. 2002;40(4):439–457. doi: 10.1016/s0005-7967(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 82.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162(2):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 83.Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress. 2003;16(6):555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- 84.Basu S, Chwastiak LA, Bruce RD. Clinical management of depression and anxiety in HIV- infected adults. AIDS. 2005;19:2057–2067. doi: 10.1097/01.aids.0000182518.84407.32. [DOI] [PubMed] [Google Scholar]
- 85.Frank LR, Knox MD, Wagganer AM. HIV/AIDS and mental disorders. In: Levin BL, Becker MA, editors. A Public Health Prospective of Women’s Mental Health. New York, NY: Springer; 2010. [Google Scholar]
- 86.Mugavero M, Ostermann J, Whetten K, et al. Barriers to antiretroviral adherence: The importance of depression, abuse, and other traumatic events. AIDS Patient Care STDs. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 87.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients. Drugs. 2006;66(6):769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 88.Cook JA, Grey D, Burke-Miller J, et al. Effects of treated and untreated depressive symptoms on highly active antiretroviral therapy use in a US multi-site cohort of HIV-positive women. AIDS Care. 2006;18:93–100. doi: 10.1080/09540120500159284. [DOI] [PubMed] [Google Scholar]
- 89.Reisner SL, Mimiaga MJ, Safren SA, Mayer KH. Stressful or traumatic life events, posttraumatic stress disorder (PTSD) symptoms, and HIV sexual risk taking among men who have sex with men. AIDS Care. 2009;29(12):1481–1489. doi: 10.1080/09540120902893258. [DOI] [PubMed] [Google Scholar]