Abstract
Studies using surrogate estimates show high prevalence of insulin resistance in hepatitis C infection. This study prospectively evaluated the correlation between surrogate and directly measured estimates of insulin resistance and the impact of obesity and ethnicity on this relationship. Eighty-six nondiabetic, noncirrhotic patients with hepatitis C virus (age = 48 ± 7 years, 74% male, 44% white, 22% African American, 26% Latino, 70% genotype 1) were categorized into normal-weight (body mass index [BMI] < 25, n = 30), overweight (BMI = 25–29.9, n = 38), and obese (BMI ≥ 30, n = 18). Insulin-mediated glucose uptake was measured by steady-state plasma glucose (SSPG) concentration during a 240-minute insulin suppression test. Surrogate estimates included: fasting glucose and insulin, glucose/insulin, homeostasis model assessment (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), insulin (I-AUC) and glucose (G-AUC) area under the curve during oral glucose tolerance test, and the Belfiore and Stumvoll indexes. All surrogate estimates correlated with SSPG, but the magnitude of correlation varied (r = 0.30–0.64). The correlation coefficients were highest in the obese. I-AUC had the highest correlation among all ethnic and weight groups (r = 0.57–0.77). HOMA-IR accounted for only 15% of variability in SSPG in the normal weight group. The common HOMA-IR cutoff of ≤ 3 to define insulin resistance had high misclassification rates especially in the - group independent of ethnicity. HOMA-IR > 4 had the lowest misclassification rate (75% sensitivity, 88% specificity). Repeat HOMA-IR measurements had higher within-person variation in the obese (standard deviation = 0.77 higher than normal-weight, 95% confidence interval = 0.25–1.30, P = 0.005).
Conclusion
Because of limitations of surrogate estimates, caution should be used in interpreting data evaluating insulin resistance especially in nonobese, nondiabetic patients with HCV.
Epidemiologic studies support an association between chronic hepatitis C virus (HCV) infection and type 2 diabetes mellitus.1–3 The mechanism by which HCV may induce diabetes is thought to be related to insulin resistance and potential defects in insulin signaling pathways.4,5 Studies to date have shown a higher prevalence of insulin resistance in HCV infection compared to hepatitis B virus infection and other causes of liver disease.6 Insulin resistance and diabetes in the setting of HCV infection is of great importance due to its association with increased rates of fibrosis and faster progression of liver disease7,8 as well as potentially lower response to antiviral HCV therapy.9–11
To date, all human studies except for one that evaluated insulin resistance in the setting of HCV have used surrogate estimates of insulin resistance rather than direct measurements of insulin-mediated glucose uptake (IMGU).9–12 The most commonly used surrogate estimate in the HCV population is the homeostasis model assessment of insulin resistance (HOMA-IR) that is calculated from a single measurement of fasting insulin and glucose.13 Although these estimates are convenient in large-scale epidemiologic studies,14 they have significant limitations that affect their reliability; for example, HOMA-IR coefficient of variation can exceed 30%13 and can be affected by degrees of obesity and by ethnicity.14–17 In addition, the HOMA-IR cutoff values used to define insulin resistance in the HCV literature vary significantly, ranging from 1.5 to >3.6,18 These cutoff values are either arbitrarily defined9,11,19 or derived from non-HCV populations which are often heterogeneous without a comprehensive description of the population, have small sample size, include diabetics, and have limited criteria for excluding presence of liver disease.10,18,20 Furthermore, even within the HCV population, there is a large variation in the reported mean HOMA-IR values ranging from 1 to >3.10,21,22
Reliability of surrogate estimates is essential in interpreting the reported conclusions in the literature such as prevalence of insulin resistance and its impact on the natural history and treatment of HCV. Moreover, identification of a reliable surrogate estimate of insulin resistance is needed as direct measurements can be impractical and costly when evaluating large populations. The gold standards for direct physiologic measurement of insulin resistance are the validated hyperinsulinemic euglycemic clamp test and insulin suppression test.23 Despite the known limitations of the surrogate estimates in other populations, no study has evaluated the reliability and accuracy of these markers in comparison to the direct measurement of insulin resistance in the HCV population.
In this study, we evaluated the correlation between the directly measured resistance to insulin mediated glucose uptake during insulin suppression test and surrogate estimates of insulin resistance in a large, ethnically diverse, nondiabetic HCV population. In addition, we evaluated the impact of obesity and ethnicity on the relationship between the direct measurement and the surrogate estimates. As HOMA-IR is the most commonly used estimate of insulin resistance in the HCV population, we addressed misclassification rates using different HOMA-IR cutoff values as well as the within-person variation of HOMA-IR estimates from repeat measurements.
Patients and Methods
Study Subjects
Nondiabetic HCV-infected subjects between ages 18–60 with detectable hepatitis C viral load (HCV RNA) were recruited from the San Francisco General Hospital (SFGH) at the University of California San Francisco (UCSF) and other UCSF-affiliated clinics. The American Diabetes Association criteria of fasting glucose ≥7.0 mmol/L was used to defined diabetes.24 Subjects with hepatitis B virus or human immunodeficiency virus coinfection or other causes of liver disease, presence of clinical, histologic, or known diagnosis of cirrhosis or evidence of decompensated liver disease, prior treatment for HCV, steroid or anabolic drug therapy, or those with medical conditions that impaired their ability to participate in the study were excluded. Each subject provided written informed consent prior to enrollment. This study was approved by the UCSF Internal Review Board, the UCSF Committee on Human Research and SFGH Data Governance Committee.
Study Procedures
Subjects underwent a medical interview, physical examination including anthropometric measurements, and fasting laboratory evaluation at the screening visit. Seventy-six (88%) subjects underwent a liver biopsy and 89% of those had stage ≤2 fibrosis. Subjects were admitted to the UCSF Clinical and Translational Science Institute-Clinical Research Center (CRC) at SFGH for study tests. A 75-g oral glucose tolerance test (OGTT) was performed at the CRC after an overnight 12-hour fast. Venous blood samples of plasma glucose and insulin were collected at 0, 30, 60, 120, and 180 minutes after an oral ingestion of 75-g glucose load. After another overnight 12-hour fast, each subject underwent the modified insulin-suppression test.23,25 The infusion study consisted of two 120-minute periods. During both periods, octreotide was infused at a rate of 0.27 μg m−2 minute−1 to suppress endogenous insulin secretion. Insulin and glucose were infused at rates of 6 mU m−2 minute−1 and 50 mg m−2 minute−1, respectively during the low-dose period to simulate basal conditions and at rates of 32 mU m−2 minute−1 and 267 mg m−2 minute−1, respectively, during the high-dose period in order to achieve physiologic hyperinsulinemia. Blood was drawn for plasma glucose and insulin measurement at 0, 90, 100, 110, 120, 210, 220, 230, and 240 minutes. The four values obtained from 210–240 minutes were averaged to represent the steady-state plasma glucose (SSPG) and the steady-state plasma insulin concentrations (SSPI). Because SSPI concentrations are similar in all patients given identical infusion rates of insulin, the SSPG concentration is a direct measure of the ability of insulin to mediate the disposal of infused glucose load. Higher SSPG concentrations therefore represent higher degrees of insulin resistance.
Laboratory Evaluation
Plasma glucose concentrations were measured by glucose oxidase method (with the YSI 2300 STAT-Plus Analyzer, Yellow Springs, OH). Plasma insulin was measured by using a single antibody radioimmunoassay without cross reactivity with human proinsulin (Millipore, Billerica, MA). The intra and interassay CVs for plasma insulin measurements were averaged at 3.2% and 3.9%, respectively.
Surrogate Estimates
The following surrogate estimates of insulin resistance were assessed (Table 1): fasting insulin and glucose, HOMA-IR, insulin sensitivity check index (QUICKI), fasting glucose/insulin ratio, total integrated glucose (G-AUC) and insulin (I-AUC) responses during OGTT, Belfiore’s insulin sensitivity index for glycemia, and Stumvoll index. The Matsuda index was not calculated, because this measure incorporates a nonstandard 90-minute time point in OGTT.26
Table 1.
Surrogate Estimates of Insulin Resistance
| Fasting glucose (mmol/L) |
| Fasting insulin (pmol/L) |
| HOMA-IR = fasting plasma insulin (μU/mL) · fasting plasma glucose (mmol/L)/22.5 (Matthews et al.13) |
| QUICKI = 1/[log(insulin0) + log(glucose0)] with insulin expressed in μU/mL and glucose in mg/dL (Katz et al.37) |
| Glucose/insulin ratio = fasting glucose (mg/dL)/insulin (μU/mL) |
| G-AUC = area under the curve of glucose during OGTT using trapezoidal method |
| I-AUC = area under the curve of insulin during OGTT using trapezoidal method |
| Belfiore index = 2/[({(0.5 · fasting PG) + OGTT 1-hour PG + (0.5 · OGTT 2- hour PG)}/11.36) · ({(0.5 · fasting PI) + OGTT 1-hour PI + (0.5 · OGTT 2- hour PI)}/638)+1] with plasma glucose (PG) expressed in mmol/L and plasma insulin (PI) expressed in pmol/L (Belfiore et al.38) |
| Stumvoll index = 0.222 – (0.00333 · BMI) – (0.0000779 · Ins 120 minutes) – (0.000422 · age) with insulin expressed in pmol/L (Stumvoll et al.39) |
It is important to note that there is a spectrum of insulin sensitivity in the population and that there are no single absolute cutoff values to define insulin resistance versus sensitivity. However, insulin resistance was operationally defined as the upper tertile of SSPG (SSPG > 10 mmol/L) in the healthy nondiabetic population 27 that has been shown prospectively to significantly increase risk of developing clinical syndromes associated with insulin resistance.28,29 In addition, we also added a second definition of insulin resistance as SSPG > 8.3 mmol/L that represents the upper tertile of SSPG in our HCV study population which is the largest HCV population with direct measurements of insulin mediated glucose uptake to date.
Statistical Analysis
Baseline characteristics of subjects were summarized using mean ± SD, median (range), and frequencies. Kruskal-Wallis test for continuous variables and chi-squared tests (or Fisher’s exact test when appropriate) for dichotomous variables were used to compare baseline characteristics between BMI and ethnicity categories. Subjects were divided into three BMI categories: normal weight (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Pearson correlation coefficients were calculated between SSPG and the surrogate estimates of insulin resistance. Sensitivity, specificity, and misclassification rates of HOMA-IR in predicting insulin resistance were determined using both definitions of SSPG > 10 mmol/L and SSPG > 8.3 mmol/L. Multiple logistic regression was used to evaluate BMI categories and ethnicity as predictors of false positive rates of HOMA-IR > 3 for predicting insulin resistance. The within-person standard deviation of three repeated HOMA-IR measurements for each person was calculated and then analyzed by linear regression with BMI and ethnicity categories as predictors. P values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Eighty-nine HCV-infected subjects were enrolled in the study. Three subjects with a 2-hour plasma glucose level greater than 11.1 mmol/L during the OGTT were subsequently excluded from the study. The baseline characteristics of subjects stratified by BMI category are summarized in Table 2. There were more males in the overweight group. In general, insulin resistance as determined by surrogate estimates and SSPG increased with degrees of obesity.
Table 2.
Baseline Characteristics of Study Subjects by BMI Category
| Characteristic | Normal Weight (n = 30) | Overweight (n = 38) | Obese (n = 18) | P Value |
|---|---|---|---|---|
| Age (mean ± SD), years | 47.4 ± 6.3 | 48.1 ± 6.7 | 48.0 ± 8.1 | 0.63 |
| BMI (mean ± SD), kg/m2 | 22.7 ± 1.8 | 27.2 ± 1.3 | 33.5 ± 3.1 | 0.0001 |
| Male sex (%) | 63 | 89 | 61 | 0.01 |
| Ethnicity (%) | 0.37 | |||
| White | 57 | 34 | 44 | |
| African American | 10 | 26 | 33 | |
| Latino | 27 | 29 | 17 | |
| Other | 6 | 11 | 6 | |
| Family history of diabetes (%) | 40 | 46 | 47 | 0.82 |
| Histologic fibrosis score* (%) | 0.56 | |||
| Stage 0 and 1 | 57 | 60 | 44 | |
| Stage 2 and 3 | 43 | 40 | 56 | |
| Genotype (%) | 0.22 | |||
| 1 | 67 | 79 | 52 | |
| 2 | 23 | 8 | 24 | |
| 3 | 10 | 13 | 24 | |
| Log10 HCV RNA (mean ± SD) IU/mL | 5.7 ± 0.8 | 5.9 ± 0.5 | 5.8 ± 0.8 | 0.91 |
| Fasting glucose (mean ± SD) mmol/L | 5.0 ± 0.3 | 5.2 ± 0.6 | 5.2 ± 0.7 | 0.27 |
| Fasting insulin (mean ± SD) pmol/L | 87.9 ± 38.7 | 110.5 ± 50.4 | 144.6 ± 88.9 | 0.03 |
| SSPG (mean ± SD) mmol/L | 6.7 ± 4.1 | 7.3 ± 3.7 | 9.1 ± 4.6 | 0.10 |
| Fasting glucose/insulin | 9.1 ± 6.2 | 7.4 ± 4.8 | 6.0 ± 3.2 | 0.10 |
| HOMA-IR (mean ± SD) | 2.8 ± 1.3 | 3.7 ± 1.9 | 4.9 ± 3.1 | 0.03 |
| QUICKI (mean ± SD) | 0.33 ± 0.03 | 0.32 ± 0.02 | 0.31 ± 0.03 | 0.03 |
| G-AUC (mean ± SD) mmol · L−1 · 3 h−1 | 19.7 ± 2.7 | 20.6 ± 3.4 | 21.8 ± 3.3 | 0.38 |
| I-AUC (mean ± SD) pmol · L−1 · 3 h−1 | 1108.9 ± 579.9 | 1225.6 ± 507.2 | 2161.4 ± 1684.8 | 0.04 |
| Belfiore index (mean ± SD) | 0.89 ± 0.34 | 0.81 ± 0.25 | 0.62 ± 0.34 | 0.03 |
| Stumvoll index (mean ± SD) | 0.10 ± 0.02 | 0.08 ± 0.03 | 0.06 ± 0.02 | 0.0001 |
By Metavir scoring system (Bedossa and Poynard40).
When examining the entire study population, the correlation coefficients between SSPG and all surrogate estimates of insulin resistance were statistically significant but the magnitude of the correlation varied with the fasting glucose having the lowest correlation (r = 0.30, 95% confidence interval [CI] 0.09 to 0.48, R2 = 0.09) and I-AUC (r = 0.64, 95% CI = 0.49 to 0.76, R2 = 0.41) and Belfiore index r = −0.63, 95% CI −0.75 to −0.48, R2 = 0.41) having the highest correlation. Fasting insulin had a similar correlation coefficient as HOMA-IR and QUICKI estimates (r = 0.54 versus 0.57 and −0.52, respectively).
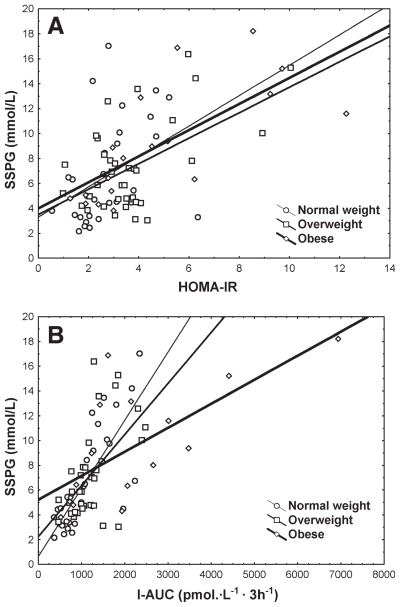
In Table 3, the correlation coefficients between SSPG and surrogate estimates are summarized by BMI categories. Overall, the highest correlations occurred in the obese group. In the overweight and normal weight groups, only certain estimates had significant correlation coefficients and the surrogate estimates based on OGTT (I-AUC, Belfiore index, Stumvoll index) had the highest correlation across both weight groups. In addition, the correlation between SSPG and certain estimates increased with increasing degrees of obesity. For example, HOMA-IR had a low correlation with SSPG in the normal weight group at 0.39 but this correlation increased to 0.72 in the obese group. Of all of the estimates, I-AUC had the highest correlation with the SSPG in the normal and overweight groups in addition to having a high correlation coefficient in the obese group. The scatter plots of SSPG versus HOMA-IR and I-AUC in different weight categories are shown in Fig. 1. Because of potential differences in mechanisms of insulin resistance with genotype 3, a separate analysis excluding genotype 3 was performed and showed similar results (Supporting Table 1). With respect to ethnicity (Table 4), the magnitude of correlation varied across ethnicities with I-AUC and Belfiore index having the highest correlation with SSPG among whites, African Americans, and Latinos. There were not enough patients with other ethnicities to allow meaningful analysis. The scatter plots of SSPG versus HOMA-IR and I-AUC in different ethnic categories are shown in Fig. 2.
Table 3.
Pearson Correlation Coefficients Between SSPG and Surrogate Measures of Insulin Resistance by BMI Categories
| Normal Weight (n = 30)
|
Overweight (n = 38)
|
Obese (n = 18)
|
||||
|---|---|---|---|---|---|---|
| r (95% CI) | R2 | r (95% CI) | R2 | r (95% CI) | R2 | |
| Fasting glucose | 0.01 (−0.36 to 0.37) | 0.00 | 0.18 (−0.15 to 0.48) | 0.03 | 0.71 (0.37 to 0.89)** | 0.51 |
| Fasting insulin | 0.41 (0.06 to 0.67)* | 0.17 | 0.53 (0.24 to 0.73)** | 0.28 | 0.61 (0.20 to 0.84)** | 0.37 |
| Glucose/insulin | −0.34 (−0.62 to 0.02) | 0.11 | −0.23 (−0.52 to 0.11) | 0.05 | −0.55 (−0.81 to −0.11)* | 0.30 |
| HOMA-IR | 0.39 (0.04 to 0.66)* | 0.15 | 0.54 (0.25 to 0.74)** | 0.29 | 0.72 (0.38 to 0.89)** | 0.52 |
| QUICKI | −0.40 (−0.66 to −0.04)* | 0.16 | −0.40 (−0.64 to −0.08)* | 0.16 | −0.76 (−0.91 to −0.45)** | 0.58 |
| G-AUC | 0.48 (0.14 to 0.71)** | 0.23 | 0.24 (−0.10 to 0.53) | 0.06 | 0.75 (0.41 to 0.91)** | 0.57 |
| I-AUC | 0.77 (0.57 to 0.89)** | 0.60 | 0.57 (0.28 to 0.76)** | 0.32 | 0.69 (0.30 to 0.89)** | 0.48 |
| Belfiore index | −0.66 (−0.83 to −0.40)** | 0.44 | −0.53 (−0.73 to −0.24)** | 0.28 | −0.61 (−0.85 to −0.16)* | 0.37 |
| Stumvoll index | −0.73 (−0.87 to −0.51)** | 0.54 | −0.44 (−0.67 to −0.14)** | 0.20 | −0.61 (−0.87 to −0.08)* | 0.36 |
P < 0.05,
P < 0.01.
Fig. 1.
Relationship between SSPG and (A) HOMA-IR and (B) I-AUC by BMI category.
Table 4.
Pearson Correlation Coefficients Between SSPG and Surrogate Measures of Insulin Resistance by Ethnicity
| White (n = 38)
|
African American (n = 19)
|
Latino (n = 22)
|
||||
|---|---|---|---|---|---|---|
| r (95% CI) | R2 | r (95% CI) | R2 | r (95% CI) | R2 | |
| Fasting glucose | 0.20 (−0.13 to 0.49) | 0.04 | 0.38 (−0.09 to 0.71) | 0.14 | 0.09 (−0.35 to 0.49) | 0.007 |
| Fasting insulin | 0.64 (0.40 to 0.80)** | 0.41 | 0.44 (−0.02 to 0.74) | 0.19 | 0.57 (0.18 to 0.80)** | 0.32 |
| Glucose/insulin | −0.36 (−0.61 to −0.04)* | 0.13 | −0.43 (−0.74 to 0.03) | 0.19 | −0.50 (−0.77 to −0.08)* | 0.25 |
| HOMA-IR | 0.69 (0.47 to 0.83)** | 0.47 | 0.51 (0.07 to 0.78)* | 0.26 | 0.53 (0.12 to 0.78)* | 0.28 |
| QUICKI | −0.54 (−0.74 to −0.27)** | 0.30 | −0.51 (−0.78 to −0.07)* | 0.26 | −0.57 (−0.81 to −0.19)** | 0.33 |
| G-AUC | 0.41 (0.09 to 0.64)* | 0.16 | 0.61 (0.21 to 0.83)** | 0.37 | 0.26 (−0.19 to 0.62) | 0.07 |
| I-AUC | 0.66 (0.43 to 0.81)** | 0.44 | 0.63 (0.22 to 0.85)** | 0.40 | 0.68 (0.35 to 0.86)** | 0.46 |
| Belfiore index | −0.65 (−0.81 to −0.41)** | 0.42 | −0.59 (−0.82 to −0.19)** | 0.35 | −0.74 (−0.89 to −0.44)** | 0.54 |
| Stumvoll index | −0.35 (−0.61 to −0.03)* | 0.12 | −0.50 (−0.79 to −0.03)* | 0.25 | −0.66 (−0.85 to −0.32)** | 0.44 |
P < 0.05,
P < 0.01.
Fig. 2.
Relationship between SSPG and (A) HOMA-IR and (B) I-AUC by ethnicity.
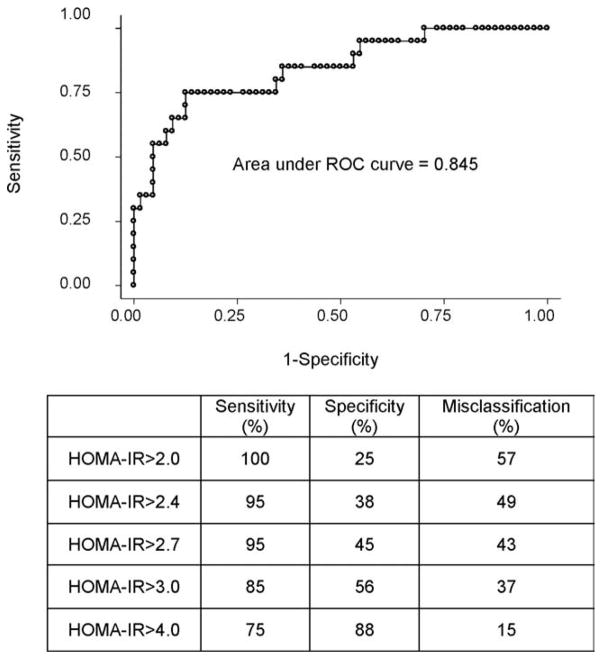
Because HOMA-IR is the most commonly used surrogate estimate in the HCV population, we evaluated the misclassification rates using the most frequently used HOMA-IR cutoff values cited in the HCV literature. The ROC curve for HOMA-IR using SSPG > 10 mmol/L to define true insulin resistance as well as the sensitivity, specificity, and misclassification rates for different HOMA-IR cutoff values is summarized in Fig. 3. For example, HOMA-IR > 3 had a 37% misclassification rate. In multiple logistic regression models on those subjects without true insulin resistance by SSPG, the odds of a false positive for insulin resistance using HOMA-IR > 3 was 3.7 times higher in the overweight group (95% CI: 1.0 to 13.4, P = 0.04) compared to normal weight group when controlling for ethnicity. However, the false positive rates were not substantially different in the obese group compared to the normal weight group. HOMA-IR > 4 has the lowest misclassification rate (15%) in identifying patients with insulin resistance. Furthermore, when SSPG > 8.3 mmol/L (the upper tertile of SSPG in this HCV population) was used, HOMA-IR > 4 once again best identified patients with insulin resistance resulting in sensitivity of 64%, specificity of 91%, and misclassification rate of 18%.
Fig. 3.
Receiver operator characteristic (ROC) curve and sensitivity, specificity, and misclassification rate of HOMA-IR in identifying subjects with insulin resistance. ROC curve for HOMA-IR performance is shown, where SSPG > 10 mmol/L is used to define insulin resistance. The area under the curve is 0.845. The table at the bottom of this figure outlines the sensitivity, specificity, and the misclassification rates of HOMA-IR. The HOMA-IR > 4 has the lowest misclassification rate.
To better understand within-person HOMA-IR variability, we compared three HOMA-IR values obtained on three separate days and usually consecutive days (ranging from 3–66 days). After controlling for time elapsed between the first and last HOMA-IR measurement, the obese subjects had larger within-person standard deviations for HOMA-IR that averaged 0.77 units (95% CI 0.19 to 1.57, P = 0.01) higher than normal weight subjects when controlled for ethnicity. Latinos had higher within-person SD for HOMA-IR that averaged 0.48 units (95% CI −0.01 to 1.06, P = 0.051) higher than whites when controlled for BMI category.
Discussion
To the best of our knowledge, this is the first published study to evaluate the reliability and limitations of a comprehensive set of surrogate estimates in comparison to direct measurement of insulin resistance in the HCV population while accounting for obesity as well as ethnicity. In addition, this study is the first to characterize the misclassification rates of different HOMA-IR cutoff values to define insulin resistance in HCV. In our study insulin mediated glucose uptake (SSPG) was directly measured by insulin suppression test. Both insulin suppression test and the euglycemic clamp test measure glucose disposal rates during steady-state physiologic hyperinsulinemia and are highly correlated (r > 0.9).23
The magnitude of correlation between surrogate estimates and direct measurement of insulin resistance varied and the highest correlation coefficients were observed with I-AUC and Belfiore index. These correlation coefficients were similar to other large nondiabetic populations.15 Studies with higher reported correlations were limited by small numbers of nondiabetic subjects and less rigorous BMI category definitions.13,30 Fasting insulin was as predictive of insulin resistance as HOMA-IR and QUICKI because the magnitude of variability in insulin is significantly higher than glucose as observed from the measurement standard deviations and thus it has a greater contribution to the calculation of these estimates. This is compatible with the fact that physiologically insulin resistance leads to hyperinsulinemia that maintains glucose homeostasis.31,32
Our study clearly showed that the reliability of surrogate estimates varies significantly with degrees of obesity in the HCV population similar to that observed in the healthy population.15 A study by Kim et al. evaluated the impact of obesity on relationship between SSPG and fasting glucose, fasting insulin, HOMA-IR, QUICKI, and I-AUC in the nondiabetic healthy population.15 They showed that lowest correlations were seen in the normal weight group, the highest in the obese group, and I-AUC was the most useful estimate in all weight groups (r = 0.69 to 0.72). In the HIV nondiabetic population, I-AUC also had the highest correlation coefficient with SSPG at 0.78 compared to other surrogate estimates.33 Similarly, in this study we showed that highest correlations between the surrogate estimates and SSPG occurred in the obese HCV patients and that I-AUC had the highest correlation across normal and overweight groups in addition to having a high correlation in the obese group (r > 0.6).
Insulin resistance is impacted by ethnicity. African Americans and Latinos have higher degrees of insulin resistance than whites.14,17,34 It has been shown that estimated indices of insulin resistance derived from OGTT are less likely to detect differences among ethnic groups than the directly measured indices.16 Chiu et al. compared Matsuda index and Stumvoll index to hyperglycemic clamp in 105 glucose tolerant subjects in four ethnic groups: Asian, Caucasian, Mexican-Americans, and African Americans. They concluded that there were significant ethnic differences in directly measured insulin sensitivity and that Asians were most insulin resistant and Caucasians were most insulin sensitive. Although the estimated indices correlated with directly measured indices (ranging from r = 0.30 to 0.52), the estimated indices did not accurately reflect the variation observed by the measured indices among different ethnic groups.16 In our study, we also found a significant correlation between estimated indices derived from OGTT and direct measures of insulin resistance, but these correlations varied between different ethnic groups. For example, whereas the correlation between Stumvoll index and SSPG was −0.35 among whites, this correlation increased to −0.66 among Latinos. I-AUC however, correlated consistently with SSPG among all ethnic groups.
In this study we have highlighted limitations of HOMA-IR in defining insulin resistance. First, HOMA-IR had a particularly low correlation (r = 0.39) with SSPG in the normal weight group and accounted for only 15% of variability in SSPG in this group. Second, the most commonly cited HOMA-IR cutoff values for insulin resistance have high misclassification rates and even at HOMA-IR > 3, over one-third of patients were misclassified. In addition, degrees of obesity impacted the rate of false positivity of HOMA-IR cutoff values. Overweight subjects were particularly likely to be misclassified having a nearly four times higher odds of false positivity for insulin resistance compared to the normal weight group independent of ethnicity. Obesity has been associated with increased insulin secretion,35 decreased insulin clearance, 36 and increased insulin resistance.31 Therefore, dynamic interplay between these physiologic mechanisms as the degree of adiposity increases may impact the relationship between direct measurement of insulin mediated glucose uptake during the steady-state physiologic hyperinsulinemia and the surrogate estimates that are based on plasma insulin and glucose concentrations. Finally, this study is the first to show that HOMA-IR > 4 is the optimal value in arbitrarily defining insulin resistance.
Our study is unique in that we evaluated the within-person standard deviation of HOMA-IR with repeated measurements and evaluated whether ethnicity or BMI were independently predictive of higher within-person standard deviations of HOMA-IR. We showed that obesity was associated with a statistically significant higher within-person standard deviation of HOMA-IR by 0.77 points when controlled for ethnicity. This may be due to the fact that obese individuals had a higher variation in the fasting insulin levels likely secondary to higher degrees of insulin resistance than other weight groups.31 Interestingly, Latinos also had a higher within-person standard deviation of HOMA-IR. Therefore, there may be greater inaccuracies in HOMA-IR measurements in obese individuals and potentially in Latinos.
In summary, our results highlight the impact of degrees of obesity and ethnicity on the relationship between surrogate estimates and direct measurements of insulin resistance in nondiabetic HCV-infected persons. I-AUC appears to best correlate with insulin resistance across all weight and ethnic groups. There is a high rate of false positivity of HOMA-IR when using the commonly reported cutoffs cited in the literature that may in turn overestimate prevalence of insulin resistance in the HCV population. In addition, HOMA-IR has higher within-person variation on repeated measurements in obese patients, which should be taken into account when evaluating changes in HOMA-IR over time. Considering the relatively low correlation of certain estimates with direct measurements of insulin resistance, caution should be used in interpreting the data evaluating insulin resistance in HCV-infected persons using surrogate estimates especially in the overweight and normal weight groups.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant numbers R01 DK074673 (to M.K.), UL1 RR024131 (NIH/NCRR UCSF-CTSI), P30 DK 026743 (UCSF Liver Center), UCSF Dean’s Office Medical Student Research Program (to K.L.), and the American Diabetes Foundation grant number 1-08-CR-30 (to M.K.).
Abbreviations
- G-AUC
glucose area under the curve during oral glucose tolerance test
- HOMA-IR
homeostasis model assessment of insulin resistance
- I-AUC
insulin area under the curve during oral glucose tolerance test
- IMGU
insulin-mediated glucose uptake
- OGTT
oral glucose tolerance test
- QUICKI
quantitative insulin sensitivity check index
- SFGH
San Francisco General Hospital
- SSPG
steady-state plasma glucose concentration
- SSPI
steady-state plasma insulin concentration
- UCSF
University of California San Francisco
Footnotes
Potential conflict of interest: Nothing to report.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or American Diabetes Association.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 2.Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349–355. doi: 10.1002/lt.20092. [DOI] [PubMed] [Google Scholar]
- 3.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 4.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384–1392. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault M-P, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol. 2005;100:1509–1515. doi: 10.1111/j.1572-0241.2005.41403.x. [DOI] [PubMed] [Google Scholar]
- 8.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Grasso A, Malfatti F, Leo P, Martines H, Fabris P, Toscanini F, et al. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984–990. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Cammà C, Di Marco V, Alessi N, Cabibi D, Caldarella R, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136–1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care. 2004;27:1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]
- 16.Chiu KC, Chuang LM, Yoon C. Comparison of measured and estimated indices of insulin sensitivity and beta cell function: impact of ethnicity on insulin sensitivity and beta cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab. 2001;86:1620–1625. doi: 10.1210/jcem.86.4.7432. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 18.Duseja A, Dhiman RK, Chawla Y, Thumburu KK, Kumar A, Das A, et al. Insulin resistance is common in patients with predominantly genotype 3 chronic hepatitis C. Dig Dis Sci. 2009;54:1778–1782. doi: 10.1007/s10620-009-0844-y. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C-S, Liu C-J, Liu C-H, Wang C-C, Chen C-L, Lai M-Y, et al. High hepatitis C viral load is associated with insulin resistance in patients with chronic hepatitis C. Liver Int. 2008;28:271–277. doi: 10.1111/j.1478-3231.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 20.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 21.Vanni E, Abate ML, Gentilcore E, Hickman I, Gambino R, Cassader M, et al. Sites and mechanisms of insulin resistance in nonobese, non-diabetic patients with chronic hepatitis C. Hepatology. 2009;50:697–706. doi: 10.1002/hep.23031. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 24.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Deng A, Gonzales O, Aillaud M, Yee G, Lamendola C, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51:2303–2308. doi: 10.1007/s00125-008-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 27.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23:171–175. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 29.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 32.Reaven GM, Moore J, Greenfield M. Quantification of insulin secretion and in vivo insulin action in nonobese and moderately obese individuals with normal glucose tolerance. Diabetes. 1983;32:600–604. doi: 10.2337/diab.32.7.600. [DOI] [PubMed] [Google Scholar]
- 33.Chu JW, Abbasi F, Beatty GW, Khalili M, Koch J, Rosen A, et al. Methods for quantifying insulin resistance in human immunodeficiency virus-positive patients. Metab Clin Exp. 2003;52:858–861. doi: 10.1016/s0026-0495(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 34.Aguirre MA, Jones CN, Pei D, Villa ML, Reaven GM. Ethnic differences in insulin resistance and its consequences in older Mexican Ameri- HEPATOLOGY, Vol. 52, No. 1, 2010 LAM ET AL. 45 can and non-Hispanic white women. J Gerontol A Biol Sci Med Sci. 1997;52:M56–M60. doi: 10.1093/gerona/52a.1.m56. [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–E508. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- 37.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 38.Belfiore F, Iannello S, Volpicelli G. Insulin sensitivity indices calculated from basal and OGTT-induced insulin, glucose, and FFA levels. Mol Genet Metab. 1998;63:134–141. doi: 10.1006/mgme.1997.2658. [DOI] [PubMed] [Google Scholar]
- 39.Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001;24:796–797. doi: 10.2337/diacare.24.4.796. [DOI] [PubMed] [Google Scholar]
- 40.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.