Abstract
Following the creation of an autogenous lower extremity bypass graft, the vein must undergo a series of dynamic structural changes to stabilize the arterial hemodynamic forces. These changes, commonly referred to as remodeling, include an inflammatory response, the development of a neointima, matrix turnover, and cellular proliferation and apoptosis. The sum total of these processes results in dramatic alterations in the physical and biomechanical attributes of the arterialized vein. The most clinically obvious and easily measured of these is lumen remodeling of the graft. However, though somewhat less precise, wall thickness, matrix composition, and endothelial changes can be measured in vivo within the healing vein graft. Recent translational work has demonstrated the clinical relevance of remodeling as it relates to vein graft patency and the systemic factors influencing it. By correlating histologic and molecular changes in the vein, insights into potential therapeutic strategies to prevent bypass failure and areas for future investigation are explored.
Introduction
The autogenous vein bypass remains the most effective and durable revascularization strategy for patients suffering from lower extremity ischemia despite the seemingly exponential proliferation of endovascular devices and techniques. In the United States, there are about 250,000 coronary artery and 80,000 lower extremity vein grafts implanted per year.[1] Vein grafts, in contrast to inanimate stents or prosthetic grafts, are living and evolving conduits which respond to hemodynamic stimuli and to signals from the local environment.[2] Recent randomized controlled trials inform us that 30-40% of coronary and lower extremity vein grafts occlude or develop significant stenosis within the first year following implantation.[3, 4] These figures have largely remained unchanged for the past several decades.[5] On one hand this is a cause for optimism as results remain constant despite ever more challenging and complex patients.[5] However it is discouraging to consider that 5 decades of high-powered science has not effectively changed bypass graft outcomes.
Endophlebectomy of vein graft stenosis, described first in 1965 at the University of Rochester, was used to treat a 56 year old man who developed a one centimeter stenosis in his femoro-politeal bypass 16 months after its construction.[6] Here the authors describe a white fibrous tissue which was sharply excised and repaired with a vein patch angioplasty. This all too familiar description betrays the underlying inflammatory mayhem which conspired to produce such a bland appearing lesion. We now characterize the lesion as intimal hyperplasia which is present to some extent in all vein grafts. Unlike coronary bypass grafts, duplex surveillance of lower extremity vein grafts can detect hemodynamically significant stenosis due to the vein graft's superficial location within the leg. The distribution of ultrasound-detected stenosis are diffuse in about 12% vein grafts but the majority of stenotic lesions are focal often occurring in the peri-anastomotic regions or at valve sites.[7-9]
Limitations of existing animal models
Growth factor inhibitors, transcription factors, cell cycle regulators, immunomodulators, nitric oxide donors among others have all been effective at reducing intimal hyperplasia in experimental models.[10] Yet surprisingly, very few of these have entered into phase 1 human clinical trials. The lack of translation may be due to the fact that existing animal models do not adequately represent human counterparts. They are generally constructed with short interposition grafts in high flow environments, produce minimal to moderate stenosis, and rarely develop the severe occlusive lesions seen in the human vein grafts. Most preclinical programs have relatively short endpoints, commonly 28 days, which may not be sufficient to account for the late lumen loss due to fibrous expansion.[11-14] The healing of human vein grafts are known to occur well beyond this time frame suggesting more chronic models are necessary to fully study complex mature lesions.
The remodeling of human vein bypass
While the extent and time frame of development of intimal hyperplasia in animals substantially differs from humans, one important similarity is the ability of the vein to rapidly remodel in order to stabilize hemodynamic stress.[12, 15] The idea of human vein graft remodeling is hardly novel. Szilagyi noted in the 1960s studying autopsy specimens that vein grafts had increased their diameter by as much as 50% to 75%.[16] More recently serial ultrasound studies in patient cohorts have demonstrated in vivo changes in human vein grafts.[17]
Remodeling of the vein graft can be thought of as the morphologic and geometric changes in the vein which happens through luminal dilation, reorganization of matrix and collagen, and the development of a neointima. The effects of the arterial environment on the vein have been best characterized by Dobrin and others whereby 4 pairs of deformations and counteracting stresses (circumferential, longitudinal, radial (compressive), and pulsatile) in addition to the well known shear stress. Hence exposing a vein graft to arterial pressure subjects it simultaneously to deformations and stresses in 9 different directions.[18, 19]
We hypothesized that the early geometric remodeling of the vein graft is a crucial determinant to successful long-term function of the bypass graft. To test this hypothesis, we initiated a prospective cohort study to systematically determine remodeling characteristics of lower extremity bypass grafts over the first year of implantation.[20-23] Employing high-resolution ultrasound images, luminal and wall changes were characterized from a defined region of the vein graft. We also employed pulse wave velocity (PWV) analysis to determine stiffness changes in the vein over time. PWV is the speed at which the flow pulse propagates through the conduit and is one measure of stiffness that is relatively independent of the outflow. Because it was impractical to map the entire bypass graft we used a 5 cm segment (no branches or valves) of the graft as a surrogate for the behavior of the entire graft. Using high resolution M-mode ultrasound, vein graft lumen measurements were conducted at pre-determined time points beginning in the operating room after the anastomoses were complete ,and then subsequently at 1, 3, 6, 9, and 12 months thereafter.
In these same patients, we collected demographic information, cardiovascular risk factors and tracked bypass- and limb-related outcomes. Pre-operative blood samples were obtained to measure lipids, biomarkers, and cytokines associated with inflammation and thrombosis to assess their clinical value and also to provide insights into mechanisms of vein graft failure. To ensure that these markers were not spuriously elevated, any patient with active infection, a recent procedure, or concurrent systemic illness was excluded from biomarker evaluation.
Our early findings of this study were largely descriptive in nature. We determined that the majority of the luminal and wall remodeling of the graft occurs in the first 30 days followed by relative stability. There was on average about a 25% increase in lumen change of the vein graft between the operating room and 1 month, but there was substantial variability in the luminal remodeling response.[20, 21] While the majority of the grafts increased their lumens, about one quarter decreased in size. Similarly there was on average a 35% increase in wall thickness during this same period. As expected from animal data, the initial shear stress at the time of implantation was the single biggest hemodynamic factor accounting for the variability in luminal remodeling but even so only explained about 10% of luminal remodeling. This begins to get at some of the discrepancy between animal and human data as most animal models of vein grafts employ juvenile healthy animals without severe systemic illness such as advanced diabetes mellitus, hypertension, or dyslipidemia.
Our PWV studies determined that bypass grafts developed an increase in stiffness but was un-expectantly temporally delayed from the wall thickness changes. In fact, stiffness initially decreased and then rapidly rose, reflecting re-organization of matrix proteins.[21] On average, the arterialized vein dramatically increased in stiffness by about 65% from 3 to 6 months. Considering the vein wall consists of 3 principle components, cells and proteoglycans, elastin, and collagen, only an increase in the fibrous protein collagen could account for this.[20] This observation nicely complements animal data whereby the wall thickness changes over the first 6 months were accompanied by a marked increase in collagen production.[11, 12, 24]
These early observations began to paint a picture that early luminal and wall thickness changes were followed by a period of stiffening of the graft and changes could be measured for at least 6 months following implantation. Because we encountered so much variability in lumen caliber that was not explained by the graft's inflow, outflow, or hemodynamic stress, other explanations were sought. By assessing the patient's baseline level of inflammation, determined by pre-operative measurement of high sensitivity C-reactive protein (hsCRP), it was noted that there was an inverse correlation between inflammation and the magnitude of luminal remodeling.[22] Specifically veins placed in patients who had elevated preoperative hsCRP levels (≥ 5 mg/l) dilated substantially less than those with hsCRP < 5 mg/l and were, on average, 0.5 mm smaller by the end of the first month. This was true despite having similar initial size at the time of implantation to those patients with hsCRP < 5 mg/l. Other significant demographic and clinical factors found to be associated with the early remodeling of vein grafts included the patient's race and the use of a statin at the time of operation, both of which have been shown to be associated with vein graft patency.[25, 26] Specifically, African American race was associated with less positive remodeling over the first month of implantation and vein grafts implanted in these patients never achieved the diameter of those in Caucasians.[23] Just as importantly diabetes mellitus, hypertension, and hyperlipidemia were not associated with remodeling; none of which have been shown to be associated with reduced patency of lower extremity vein grafts.
By linking bypass outcome data with serial imaging data, we next determined that early vein graft remodeling is associated with mid-term vein graft patency independent of initial vein size or other risk factors. Veins that do not enlarge or get smaller over the first postoperative month, referred to by us as poor remodelers, have a 13–fold increase risk of failure at 2 years compared to “robust remodelers”, ie. those demonstrating >25% change in lumen diameter, Figure 1.[23] To put this in perspective the use of small veins for bypass only has about a 2.5-fold increased risk of failure at one year[27] suggesting that the remodeling of the vein in the first 30 days is at least as important as vein implantation size.
Figure 1.
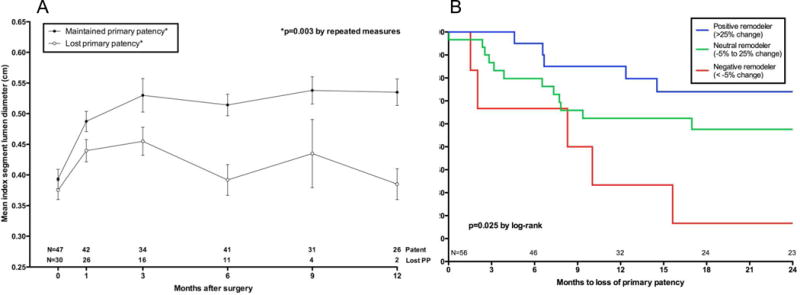
Index segment diameter based on eventual loss of primary patency. Values shown represent the mean diameter ± standard error of the mean at each interval. Although the starting diameter was at a similar size as those that remained patent, vein grafts that eventually lost primary patency failed to dilate and had a significantly smaller lumen size over time, A. Early (30 day) remodeling is associated with primary patency, P=.02. Adapted from Gasper et al.
Thus vein graft failure cannot be thought of as simply a segmental hyper-proliferative disease which develops within a static tube. But rather, intimal hyperplasia develops within a dynamic conduit, molded by hemodynamics, under the influence of systemic and regional factors. Thus inflammation,[22] race,[25] gender,[28] and genetics[29] can act globally on the entire vein graft to influence its adaptation in the arterial circulation. However, should local levels of shear stress and wall tension be impeded from reaching or reestablishing baseline conditions – due to either local environmental conditions, flow disturbances, or intrinsic vein disease - the proliferative intimal reaction would be expected to continue and stenosis to supervene.[30, 31] Therefore one explanation for segmental stenosis may be a hyper-proliferative response superimposed on a restrictive pattern of inadequate outward remodeling, Figure 2.
Figure 2.
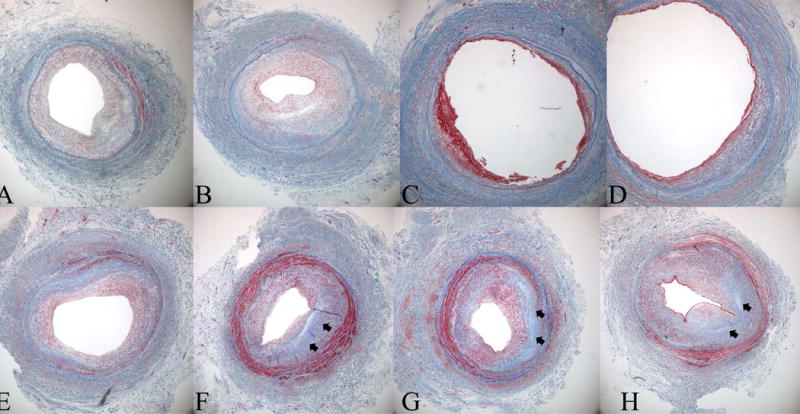
Histological evidence for negative remodeling and intimal hyperplasia as a cause of late lumen loss in a great saphenous vein bypass graft. Masson's Trichrome sections are from an 8 month old femoro-posterior tibial artery vein bypass graft that was explantated due to hemodynamically significant stenosis identified by surveillance duplex ultrasound. The repair was constructed by an interposition graft and an 8 cm piece of diseased segment was explanted, registered, and serially sectioned from A (proximal graft) to H (distal graft). Note two areas of significant stenosis, sections A and B, and sections F-H with an intervening area of relatively normal vein. While the vein was uniform size and luminal caliber at the time of original surgery the stenotic areas demonstrate loss of total vessel area indicating lumen loss is due not only to initimal hyperplasia but also negative remodeling. Note the amount of fibrous protein, blue stain (arrows), in the stenotic segments of the graft.
Given the critical importance of early vein graft remodeling, mechanistic insights may inform future local or systemic therapy to improve patency. However studies specific to lower extremity venous bypass remodeling are relatively scarce. Examining other experimental models such as arteriogenesis (collaterogenesis), arterio-venous fistula (AVF) maturation, and even varicose vein development may provide important clues to direct future research.[32-34] However, there are fundamental differences between these models and vein bypass remodeling as denoted in table 1. We believe that revisiting some of the early histological and ultrastructural studies of experimental vein grafts through the lens of recent molecular biology is informative in understanding inciting pivotal events.[35-37]
Table 1. Characteristics Human Models of Vascular Remodeling.
| Predilation diameter | Postdilation diameter | Percent remodeled | Flow after 1 month of remodeling | Pressure | Importance of EF in remodeling | |
|---|---|---|---|---|---|---|
| Collateral vessels | 30-50 μm | 600-1000 | 2000% | ??? | < arterial | ++++ |
| Vein graft | >3.0 mm | 4.5-5.0 mm | 20-30% | Medium (100-200 cc/min) | Arterial | +/- |
| AVF | > 3.0 mm | 5-7 mm | 100% | High (600 cc/min) | Low (tapers quickly) | +++ |
Histological remodeling
The injury associated with venous harvesting and implantation into the arterial environment is unlike any other known vascular injury including development of atherosclerosis, balloon angioplasty and stenting, and even creation of the AVF. It is abrupt, severe, and affects the entire length of the bypass graft. Histologic and ultrastructural consequences of this have been well described and are depicted in Figure 3.[35-41] Within 24 hours to 3 days after pressurization, vein graft endothelial cells (EC) are either focally absent or appear attenuated and elevated by sub-endothelial edema and infiltrating inflammatory cells which are present in the subendothelial space as early as 4 hours following implantation.[42, 43] Platelets, inflammatory cells and fibrin are adherent to denuded areas of endothelium where they release growth factors such as platelet derived growth factor BB (PDGF-BB), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF-1) among many others.[36, 44, 45] Areas of intact endothelial cells either subsequently slough or lose their barrier function so that they are permeable to plasma proteins, macromolecules, lipids, and growth factors. Remaining endothelial cells demonstrate vacuolation and increase in golgi and rough endoplasmic reticulum (rER), indicative of conversion to a pro-inflammatory phenotype.[36] By 3 days, bare collagen, elastin and other matrix proteins are visible with adherent platelets, red blood cells and fibrin.
Figure 3.
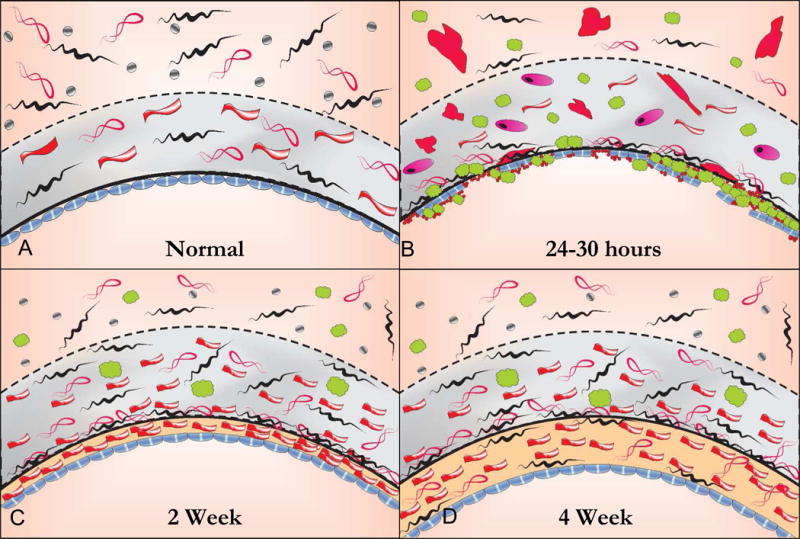
The histology of the healing autogenous vein graft. In the normal vein, the intima is lined by large flat endothelial cells that are more permeable than those in arteries. The intima is separated from the media by a fenestrated internal elastic laminae. The tunica media is thin compared to an artery with 2 or more layers of smooth muscle cells (SMC) while the adventitia is relatively thick consisting of a loose collagenous network interspersed with fibroblasts and vaso vasorum and small autonomic nerves, A. Within 24 hours following implantation the vein grafts exhibit significant endothelial cell loss and sub-endothelial edema. Inflammatory cells, platelets and fibrin are seen adherent to the surface and infiltrating underneath the attenuated endothelial cell monolayer. There is edema in the tunica media with extensive SMC necrosis or swelling and hypertrophy of the remaining SMCs with infiltration of inflammatory cells, B. By 2 to 4 weeks there is re-endothelialization of the luminal surface and a developing neointima. While the endothelium is continuous, it remains dysfunctional as evidenced by organelle hypertrophy and adhesion molecule expression. The medial edema and inflammation is reduced and there is increased collagen content. Surviving medial SMCs appear hypertrophic with increased rough endoplasmic reticulum and Golgi apparatus indicating synthetic transformation. Over time the adventitia becomes incorporated in surrounding tissue and vaso vasorum and adrenergic nerve fibers grow in from adjacent arteries and connective tissue, C. By 4 weeks there is a predominant layer of intimal thickening characterized by SMCs embedded in a matrix of collagen and ground substance. While early medial thickening is caused predominantly by edema and inflammation, fibrous transformation is responsible for late medial thickening. Areas of the medial wall are devoid of cells and entirely replaced by fibrosis. Clinically stiffening of the vein graft is likely from from the increase in fibrous protein as well as increased cross linking of extracellular matrix proteins.
Depending on the animal model, the endothelial monolayer is largely restored by 10 days to 2 weeks but it is likely that functional restoration in a 60 cm long human bypass graft takes far longer than the short interposition grafts used in animals.[36, 39, 46] While the exact time frame of human vein graft re-endothelialization is currently not known, we do know that mature (>12 months) vein grafts exhibit endothelium dependent relaxation mediated by nitric oxide (NO).[2] Evidence is emerging that the production of endothelium-derived relaxing factors, may be delayed for up to 6 months following bypass grafting - long after the critical geometric remodeling period is complete suggesting that early luminal remodeling is independent of this process.(Owens unpublished) We now believe that reconstitution of a physiologically functional endothelium represents the third and final clinical stage of vein graft remodeling following luminal and wall thickness changes and stiffening. Programs focusing on earlier restoration of a functional endothelial cell monolayer and clinical measurements re-endothelialization are likely to provide valuable data to our understanding of vein graft failure with immediate translational impact.[47]
Early after implantation, the media is marked by edema and focal hemorrhage which likely accounts for the early thickness changes which can be measured by ultrasound.[36] The increased radial (compressive) stress of pressurization and disruption from the vaso vasorum creates a zone of ischemia in the vein media. Almost immediately SMC show evidence of apoptosis or frank necrosis, as evidenced by marked vacuolated and pyknotic nuclei. Remaining SMCs, like the ECs, demonstrate severe structural changes including cellular hypertrophy, mitochondrial swelling and bleb formation, and increased rER and golgi. Inflammatory cells, particularly macrophages, gradually increase in the media and engulf the necrotic SMCs where as many as 70% of medial SMC cells are lost during this early time period.
Despite the substantial acute loss of medial SMCs, most remaining SMCs resist apoptosis and enter the cell cycle as early as 48 hours following injury.[48-50] The early injury response transcription factors c-fos and c-jun of the activator protein 1 (AP-1) complex, can be seen to be induced in SMCs subjacent to the intima where the first wave growth and serum factors from adherent platelets and inflammatory cells emerge.[51, 52] PDGF, IGF-1, and other growth factors signal increases in SMC migration and proliferation via phosphoinositol-3-kinase (PI3K)-dependent pathways by binding to receptor tyrosine kinases and G protein-coupled receptors.[53] PI3K in turn activates numerous downstream pivotal effector molecules related to cell proliferation including mammalian target of rapamycin (mTOR), p38MAPK, extracellular signal related kinases -1 and -2 (ERK 1/2) and Akt/PKB which collectively lead to neointimal hyperplasia.[54, 55] Inhibition of c-jun and PI3K have been shown to reduce vein graft stenosis in experimental models.[51, 56] Many excellent reviews address SMC proliferation and migration with respect to vascular injury and intimal hyperplasia.[55, 57-59]
The adventitia is characterized by fibroblasts within a loose connective tissue stroma with occasional vaso vasorum and vaso nervosum.[39] Adventitial fibroblasts, rich in nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, have been shown to be a source of reactive oxygen species (ROS) in blood vessels following mechanical stretch and injury.[60] In models of balloon injury and vein grafts, perivascular fibroblasts can be converted into SMC-like cells (“myofibroblasts”) that have migratory and synthetic capacity.[61-63] In vivo marker gene transfer studies show that these cells can migrate into the developing intima and contribute to intimal hyperplasia.[63] Further evidence is supported by disruption of transforming growth factor-β (TGF-β) or PDGF-BB signaling pathways which can attenuate myofibrobast migration into the neointima,reduce collagen content, and reduce constrictive remodeling following balloon angioplasty.[64, 65]
Soon after implantation, breaks in collagen fibers, thrombosis of the vaso vasorum and fragmentation of the adventitia can be seen. The vasa vasorum, initially disrupted by harvesting the vein, has been shown to return fully functional to the adventitia and outer media as early as 7 days following implantation where it participates not only in nourishing the healing vein but also inflammatory cell trafficking into and out of the vein graft.[40, 66] It has been generally assumed that the newly implanted vein graft receives oxygenation via passive diffusion from luminal arterial blood.[67] However, the vasa vasorum in veins penetrate close to the intima and possibly through to the lumen so that retrograde filling by oxygenated blood may be possible.[68, 69] The in situ bypass, originally described by Hall in 1962 [70] and more recently advanced by Shah and Leather, has several theoretical advantageous over the reversed saphenous vein graft.[71, 72] First, there is less dissection and therefore less disruption of the vaso vasorum which should reduce the ischemia reperfusion injury. Second, small arteriovenous fistula could increase the shear stress through the vein and improve outward remodeling. And third there is a reduced size disparity at the femoral and distal anastomosis. However, in practice these theoretical advantageous have not translated to increased patency. It is likely that traumatic lysis of the valve leaflets, mobilization of the proximal and distal swing segments and ligation of the numerous arteriovenous fistula offsets these advantages.
The adventitia is also a compartment housing progenitor cells which contribute to vascular repair by differentiating into a myofibroblast phenotype and possibly other cell types such as pericytes or ECs.[73, 74] Because the adventitia lies between the vessel wall and surrounding tissues, it likely contributes to vein graft remodeling by integrating diverse signals from the vessel wall and the local environment. Indeed a number of experimental programs have exploited adventitial delivery of therapeutic agents to the vein graft to take advantage of these mechanisms.[75-78]
By 3 weeks the media and adventitia demonstrate extensive fibrous replacement with collagen and a much smaller amount of elastin. Histologic studies of mature grafts demonstrate normal appearing endothelium, a thick neointima composed of abundant collagen and ground substance and a relatively thinner media.[41]
Molecular remodeling
The cellular stress and tissue damage associated with venous implantation activate the innate immune system through several different mechanisms. Necrotic cellular debris exposes modified lipids and proteins such as phosphoenthanolamine and phosphorylcholine which is recognized by C-reactive protein (CRP), a member of the pentraxin family of proteins. Pentraxins can be thought of as primitive antibodies which circulate at normally low levels and police for a limited repertoire of damage patterns commonly seen in invading microbes or damaged cell surfaces.[79] In this sense they can be thought of as the humoral arm of the innate immune system.[80] CRP can bind to these normally cryptic epitopes and activate the complement system and recruit inflammatory cells to the injured vein which exacerbates injury and necrosis. CRP may also activate local SMC to promote migration. Thus, although frequently viewed as a non-specific biomarker of inflammation, CRP may act directly as a modulator of acute vascular injury.[81-86]
The release of endogenous stress-response proteins such as heat shock protein 60 (HSP60), extracellular high mobility group box 1 (HMGB1), tenascin-C, and biglycan are some of the first mediators of immune activation.[87-91] These proteins, collectively referred to as damage associated molecular patterns, (DAMPs) are released by shear stress and matrix remodeling and are the endogenous ligands of the toll like receptors (TLRs).[92] TLRs transmit stress signals through adaptor proteins myeloid differentiation protein-88 (MyD88) or the toll or interferon (TIR) domain-containing adapter-inducing interferon (TRIF) to orchestrate the inflammatory response through transcription factors including nuclear factor kappa B (NFKB).[93, 94] TLR-4 and its endogenous ligands are found in human vein grafts demonstrating its relevance to the current discussion. In TLR-4 deficient mice, vein grafts demonstrate a reduction in outward remodeling.[95] However TLR4 deficient mice also exhibit reduced wall thickening and reduced SMC content causally implicating this pathway in the formation of intimal hyperplasia and making it difficult to separate lumen dilation and wall thickening.
To separate remodeling from intimal hyperplasia, carotid ligation models have been used. Following carotid artery ligation, flow in the contralateral carotid artery increases in a compensatory manner resulting in flow mediated vasodilation without the induction of intimal hyperplasia. In mice lacking TLR-4, there is defective flow-induced outward remodeling of the carotid artery and there is an increase in collagen content suggesting that TLR-4 is necessary for the matrix turnover required for expansile remodeling.[96] By contrast, mice deficient in the NF-KB p50 subunit demonstrate increased flow-induced outward remodeling and reduced collagen content.[97] Homodimers of p50 bind to DNA but inhibit transcription activity by other NF-κB dimers thereby acting as a brake on NF-κB.[98] Therefore p50 null mice have a more pronounced inflammatory reaction in response to a flow stimulus. [97] Other studies show that TLR-4 is integral to osteocalcin-induced myofibroblast transformation from adventitial fibroblast which involves the inflammatory mediators, protein kinase C-δ(PKC-δ) and cylco-oxygenase 2 (cox-2).[99] These studies highlight the link between innate immune activation and matrix turnover with respect to vascular remodeling.
Reactive oxygen and nitrogen species (ROS) are also integral messengers in the innate immune system and important mediators of hemodynamic remodeling in the vein. Shear stress in the great saphenous vein, normally about 0-4 dynes/cm2, abruptly rises to as high as 25-30 dynes/cm2 after implantation[21] which is more than sufficient for induction of ROS.[100] ROS can be generated from NADPH oxidases, nitric oxide synthase (NOS) isoforms including the inducible form (iNOS), xanthine oxidase, Cox-2, cytochrome P450, and the mitochondrial electron transport chain.[101] Hydrogen peroxide generated from the electron transport chain is essential for flow mediated dilation of the microcirculation and is a recognized hyperpolarizing factor.[102] ROS, particularly peroxynitrite, generated from the NADPH oxidase subunit p47phox – a cytosolic component of the NADPH oxidase complex- in response to a high flow fistula, mediates MMP gelatinase induction and outward remodeling of murine AVF.[103] NADPH oxidase and superoxide are abundant in the early vein graft wall, as they are produced not only by infiltrating neutrophils but also by SMCs and ECs.[104] In vein grafts and AVFs, increased superoxide production has been directly correlated with de-differentiated cells in the neointima as well as a reduction in free radical scavenging enzymes, super oxide dismutase (SOD) and Cu/Zn SOD activity. [105, 106] The presence of peroxynitrite, a product of superoxide and NO, suggest uncoupling of NOS isoforms and demonstrates the altered redox state which exists in the healing vein.[103, 106]
ROS are essential for activation of MMPs by cleaving their pro-domain and unmasking the active site.[100, 103, 107] MMPs consists of a family of about 20 related proteins that collectively can degrade all of the core proteins and proteoglycans in the venous wall during remodeling.[108] Because of this potential destructiveness, MMPs are tightly regulated at several levels including transcription, activation of protease proforms, secretion of stored MMPs, and through binding to their natural inhibitor, tissue inhibitor of MMP (TIMP).[109] Thrombin, plasmin, and neutrophil proteases activates MMP2 bound to membrane type 1 metalloproteinase (MT1-MMP) while the signal transducer ERK 1/2 controls MMPs in experimental vein graft models.[43, 110] In addition, shear stress results in phosphorylation of the p65 subunit of the inflammatory transcription factor NFκB which, along with AP-1, and other transcription factors stimulate MMP transcription through coordinated promoter binding.[100, 109] MMP-2 and MMP-9, referred to as gelatinases due to their ability to break down gelatin and several other collagens in particular the type IV collagen of the basement membrane, are upregulated within as little as 3 hours after venous implantation.[43, 111-113] Ultrastructural studies have documented progressive loss of type IV collagen in the early bypass graft indicating destruction of basement membrane (BM) which normally surrounds 95% of saphenous vein medial SMCs.[114, 115] The SMC BM not only represents a physical barrier to SMC migration but focal contacts between SMC and BM laminin keeps the cell in a quiescent, differentiated phenotype.[116] Therefore destruction of the BM by MMPs both liberates the SMC to migrate and results in loss of differentiation.[117, 118] Experimental inhibition of MMPs limits flow-mediated arterial enlargement and elastin degradation in rat and rabbit models[119, 120] whereas various in vivo and ex vivo techniques to increase TIMPs in the venous wall have been successfully employed to mitigate intimal hyperplasia.[121-123] These studies highlight the fact that matrix turnover, intimal hyperplasia and vascular remodeling are inextricably linked to one another.
The local renin-angiotensin-alsosterone system may be an important link between vein graft collagen production and inflammation. Aldosterone, signaling through the mineralocorticoid receptor (MR), has been shown to be an important mediator of vascular inflammation, oxidative stress, and fibrosis in both clinical and experimental settings.[124-126] Aldosterone promotes vascular fibrosis in response to injury and the MR regulates a number of profibrotic genes in SMCs including type 1 and type 3 collagens and connective tissue growth factor.[127] The mineralocorticoid receptor has been demonstrated to be upregulated in experimental vein graft models as well as in human explanted vein grafts establishing its clinical relevance.[128] More recently, antagonism of the MR with spironolactone reduced vein graft wall thickening, fibrosis, and inflammation in a mouse vein graft model.[126] In this study, spironolactone treatment reduced vein graft intima-media collagen area by 53% while reducing the number of infiltrating PMNs by 3-fold.
The cytokine tumor necrosis factor-α (TNF-α) is also involved in immune-mediated vascular remodeling in animal models. In arteriogenesis, TNF-α co-localizes with macrophages located in a perivascular cuff surrounding remodeling arteries and mice lacking either TNF-α or the p55 TNF receptor show significant reduction in collateral blood flow. By contrast, inhibitors of TNF-α attenuate collateral artery development.[129] While it was once thought that flow mediated vascular remodeling was a phenomena intrinsic to the vascular wall, recent work has demonstrated that macrophages are prerequisite for dilation or shrinkage of conduit vessels in response to flow stimuli.[130-132]
Inflammatory cell phenotype and the fate of remodeling
The homing, trafficking, and retention of inflammatory cells into the vein graft wall is unlikely to be uniform along the entire graft length but rather dependent on local flow disturbances and injury variance.[133, 134] In general however, monocytes and polymorphonuclear cells (PMN) can bind to the vein graft in high shear conditions (20-40 dynes/cm2) through the leukocyte β2-integrins, αMβ2 (MAC-1) and αLβ2 (LFA-1) receptors allowing firm adhesion to vascular cells and more than 30 proteins of the extracellular matrix including fibrinogen.[135, 136] Early induction of MMPs to breakdown BM and other ECM barriers not only permits SMC migration out toward the intima, but also facilitates PMNs and monocyte entry into the vessel wall.[43] While macrophages are essential for vessel remodeling, they also contribute to the inflammatory state of the newly implanted vein as evidenced in macrophage depletion studies in which there is both a reduction in inflammatory cytokines as well as intimal hyperplasia.[137, 138]
Monocyte chemotactic factor-1 (MCP-1) and its receptor CC-chemokine receptor-2 (CCR2) is the classic chemoattractant pathway for monocyte invasion in the vein graft. Its importance in arteriogenesis is evidenced by the fact that MCP-1 administration augments collateral artery development after femoral artery ligation in experimental models.[139] Conversely transgenic mice deficient in MCP-1 have reduced collateral artery formation.[140] MCP-1 has been shown to be induced in SMCs and ECs within 24 hours of exposure to increases in circumferential wall tension or stretch (cyclic stress) in a manner that is dependent on the inflammatory transcription factor AP-1.[141] AP-1 regulates the expression of many stress response genes including those associated with the proinflammatory phenotype of ECs and VSMC and is activated by ROS.[142] Increasing venous pressure in vivo increases SMC proliferation and MMP2 activation in an AP-1-dependent manner.[143] MCP-1 also directly participates in growth and migration of SMCs by targeting cyclins through the inflammatory transcription factor, nuclear factor of activated T cells (NFAT).[144-147] Hence, cyclic stress may induce MCP-1 production in vascular cells through AP-1-dependent mechanisms and in turn MCP-1 can induce neointimal formation through NFAT. Inhibition of either MCP-1 or CCR2 reduces vein graft intimal hyperplasia.[145, 148]
Other pathways are involved in inflammatory cell recruitment to the vein graft besides MCP-1. The CXC chemokine ligand-12 (CXCL12), also called stromal cell-derived factor-1α (SDF-1) an essential cytokine for stem cell mobilization and is involved in homing circulating cells to the vein graft. Theoretically SDF-1 and its receptor CXCR4 could be beneficial by recruiting progenitor cells to repair the healing vein graft.[149] However CXCR4+/- heterozygote mice have significantly lower CXCR4 cell surface receptor levels on bone marrow-derived mononuclear cells and are less responsive to SDF-1. Vein bypasses placed in these mice exhibit less inflammatory cell infiltrate and less neointimal formation than do wild type controls.[150] A CXCR4 small molecule antagonist, inhibits neointimal formation and smooth muscle progenitor cell mobilization after arterial injury in an apoE-/- mice model providing further circumstantial evidence of the importance of this pathway in vein graft failure.[151]
That inflammation and macrophages are involved in both inward and outward remodeling suggests that we should not be looking solely at the magnitude of inflammation but rather the nature of the inflammatory response.[131] Receptor characteristics and densities on the macrophage cell surface dictates what it “sees” and contributes to preferential trafficking and retention into blood vessels. For example longer lived resident macrophages expressing the chemokine receptor CXCR3 have been found in humans within the adventitia of abdominal aortic aneurysms and in areas of flow disturbance such as the carotid bifurcation, suggesting that they are associated with pathological states.[130] CXCR3 is the receptor for interferon (IFN)-induced protein of 10 kD (IP-10) as well as monokine induced IFN-γ (Mig). IFN-γ in turn is the classic T- cell Helper (TH1) cytokine which induces macrophage activation and production of the prototypical cytokines, TNF-α, IL-1, and IL-6. These macrophages are further classified by a low expression of the lymphocyte antigen 6c (Ly6clow) and CXCR3+, Ly6clow macrophages are known to patrol the microvasculature including adventitia microvessels.[152] In mice, CXCR3 signaling contributes both to accumulation of adventitial macrophages and is involved in negative remodeling associated with reduced flow.[130] Signaling through the CXCR3 receptor stimulates the transglutaminase crosslinking enzyme, factor XIII subunit a (XIIIa) which exerts its effects by cross-linking ECM proteins and serves as a biological glue.[130, 153, 154] The combination of fixation of the ECM by cross linking and increased collagen production clearly would prohibit luminal expansion. Might persistent flow disturbance around valve sites lead to a more pathologic inflammatory cell subset accumulation into the wall leading to focal stenosis or failure of luminal expansion?
Polarization of the initial immune response may decide the ultimate fate of the vein graft. While infiltrating monocytes contribute most conspicuously to vein graft remodeling, lymphocyte subpopulations and macrophage phenotype switching might ultimately set the stage for resolution or chronic inflammation and fibrosis. Both the professional antigen presenting cell, the dendritic cell and CD3+ T-lymphocytes have been identified in human vein graft specimens.[155, 156] Studies involving lung and liver fibrosis indicate that TH2 type dominant cytokine responses involving IL-4, IL-5, IL-13, and IL-21 are profibrotic whereas TH1 associated cytokines, dominated by IFN-γ and IL-12, may be important in the resolution of inflammation and possess anti-fibrotic properties.[157] Both IFN-γ and IL-12 treatment attenuates fibrosis in experimental pulmonary and renal models of fibrosis.[158-160]
Of note, recent work has demonstrated that the resolution of inflammation, formerly viewed as a passive decrescendo of pro-inflammatory signals, is in fact an orchestrated process driven by specific “pro-resolving mediators” (PRM). Using unbiased lipidomics in models of self-limited inflammation, it was discovered that novel lipid mediators derived from polyunsaturated fatty acids (PUFA) are generated by specific biosynthetic pathways [161-163]. There are four distinct classes of PRMs that have been recognized: the lipoxins derived from the ω-6 PUFA arachidonic acid (AA), and the resolvins, protectins, and maresins derived from the ω -3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)[164-166]. Resolvins act via specific G-protein coupled receptors (GPCRs)[167-169] to reduce expression of pro-inflammatory cytokines and adhesion molecules, increase expression of anti-inflammatory cytokines, and increase clearance of cellular debris. Emerging evidence from our group and others has demonstrated biological activity of PRMs on vascular cells [170, 171]. Lipoxins and resolvins regulate leukocyte-endothelial interactions, reduce the formation of reactive oxygen species, and regulate the production of prostacyclin and nitric oxide [172-176]. More recently we have demonstrated a broad spectrum of beneficial actions of D-series resolvins on vascular cells.[177] These include: i) inhibition of leukocyte adhesion and adhesion molecule expression; ii) inhibition of cytokine expression; iii) inhibition of VSMC proliferation and migration; iv) reduction in oxidative stress; v) and a reduction in neointima formation following balloon angioplasty in rabbit arteries. These studies suggest that endogenous resolution mechanisms may be an important element of the homeostatic process of vascular remodeling, and may offer a new therapeutic target to manipulate vascular healing.
Summary
We have described 3 measurable, temporarlly-distinct clinical stages of vein graft remodeling: luminal and wall thickness changes, changes in stiffness, and the return of endothelial function, table 2. To gain some mechanistic insights into these clinical stages we have attempted to relate them to both histologic and molecular changes associated with vein graft implantation. Of course this is an over simplification. For example, stimulating macrophages alone through the TLR4 and its 2 adaptor proteins discussed above produces 775 unique proteins including 52 cytokines![178] Regulation of this single receptor and its effectors occurs not only at the genetic and epigenetic layers but also dependent on adaptor protein interplay which can be either synergic or redundant. As demonstrated in figure 2, intimal hyperplasia and constrictive remodeling often occur together to reduce lumen area suggesting common signaling pathways. However, we have chosen to highlight specific examples to create a temporal molecular framework of the innate immune system's role in the clinical stages vein graft remodeling.
Table 2. Three Clinical Stages of Vein Graft Healing.
| Clinical stages | Time frame | Ultrastructural correlates | Molecular correlates |
|---|---|---|---|
| Lumen dilation and increased wall thickness | 0-30 days | Endothelial denudation, medial wall edema, Cellular homing/ trafficking Apoptosis/necrosis Proliferation Matrix remodeling |
Inducers: (DAMPS, neo-antigens, ROS serum growth factors) Amplifiers: (TNF-α, IL-1) Receptors: TLRs, NLRs, IL-1R, and TNF-αR 1,-2 Transducers: (AP-1, NFκB, NFAT) |
| Stiffness increase in Young's elastic modulus. | 3-6 months | Myofibrobast transformation, Collagen deposition |
Fibrosis (TGF-β, CTGF, SMAD 1/2, TGFR1, TGFR2) Cross linking ECM transglutaminases (factor XIIIa) |
| Endothelial recovery, vein graft exhibits flow mediated vasodilation | > 6 months | Complete endothelial coverage, barrier function, and return of vasoreactivity | EDRF: eNOS, NO, prostacyclin, EDHF |
Innate immune inducers: DAMPS damage associated molecular patterns, ROS reactive oxygen species. Amplifiers: TNF tumor necrosis factor, IL-1 interleukin-1. Receptors: TLR toll like receptor, NLR nod like receptor. Transducers: AP-1 activated protein-1, NFκB nuclear factor kappa B, NFAT nuclear factor of activated T cells. Fibrosis: TGF-β transforming growth factor-β, CTGF connective tissue growth factor, SMAD mothers against decapentaplegic homologue, TGFR transforming growth factor-β receptor. EDRF endothelium derived relaxing factors, eNOS endothelium nitric oxide synthase, NO nitric oxide, EDHF endothelium derived hyperpolarizing factor
While it is clear that inflammation is crucial for remodeling of the new vein graft we must be specific when characterizing inflammation. It is paradoxical that diabetes mellitus and renal failure, two diseases hallmarked by systemic inflammation and oxidative stress, have not been shown to directly affect vein graft remodeling or patency.[179, 180] It is possible that an intense tissue-level inflammatory response to implantation that quickly subsides is most conducive to vascular wall remodeling as denoted in figure 4. However should vein graft wall inflammation fail to resolve then the stage is set for pathologic remodeling and fibrosis and vein graft stenosis. Likewise, time and again, hemodynamic stress has been shown in animal models to influence the development of intimal hyperplasia.[18, 19, 181] High shear conditions skew the cytokine repertoire to a Th2 type response with lower inflammatory and higher anti-inflammatory cytokines compared with low shear stress.[182] However, blood flow and shear stress are largely un-modifiable factors dictated by the inflow and outflow conditions and vein diameter. Vascular surgeons know that vein grafts remain patent and function successfully in conditions of extremely low flow, such as the pedal bypass.[179] Even more extreme is the bypass to an isolated popliteal artery segment, initially described by Mannick, which provides testimony to the functionality of vein grafts whose outflow to the leg is solely through popliteal artery collateral circulation.[183] Hemodynamic stresses are the blunt forces of global remodeling but it is the nature of the inflammatory response that finely sculpts vein graft geometry.
Figure 4.
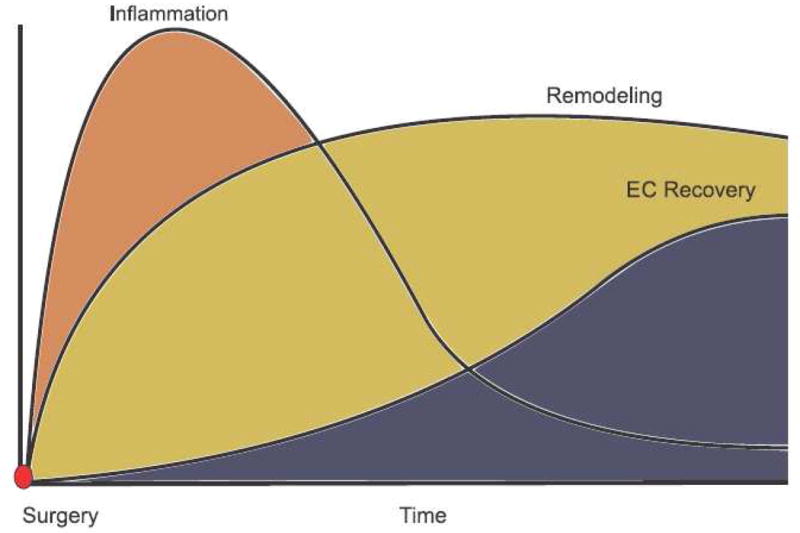
Hypothetical sequence of vein graft healing. Inflammation peaks early following implantation and then subsides. The critical period of vein graft luminal remodeling is largely complete by the first 30 days. Functional endothelial recovery is temporally delayed by several months. Mature vein grafts exhibit an endothelial layer overlying a stable neointima. Endothelium-dependent relaxation in mature grafts is mediated by nitric oxide.
The autogenous vein bypass graft remains the gold standard revascularization method for the ischemic limb. Newly implanted vein grafts undergo dramatic structural changes in response to the new high flow, high pressure environment. These changes, commonly referred to as remodeling, include a pronounced inflammatory response accompanied by the development of a neointima and significant changes in matrix composition. Similar to how maturation of arm veins predicts the performance of an arteriovenous fistula, recent translational work has demonstrated that remodeling of the vein graft is important for subsequent patency of the lower extremity bypass graft.
Footnotes
Author conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhasin M, Huang Z, Pradhan-Nabzdyk L, Malek JY, LoGerfo PJ, Contreras M, et al. Temporal network based analysis of cell specific vein graft transcriptome defines key pathways and hub genes in implantation injury. PLoS One. 2012;7(6):e39123. doi: 10.1371/journal.pone.0039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009;50(5):1063–70. doi: 10.1016/j.jvs.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. Jama. 2005;294(19):2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 5.Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg. 2001;233(3):445–52. doi: 10.1097/00000658-200103000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslau RC, Deweese JA. Successful Endophlebectomy of Autogenous Venous Bypass Graft. Ann Surg. 1965;162:251–4. doi: 10.1097/00000658-196508000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills JL, Fujitani RM, Taylor SM. The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg. 1993;17(1):195–204. doi: 10.1067/mva.1993.42796. discussion 204-6. [DOI] [PubMed] [Google Scholar]
- 8.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 9.Bandyk DF, Schmitt DD, Seabrook GR, Adams MB, Towne JB. Monitoring functional patency of in situ saphenous vein bypasses: the impact of a surveillance protocol and elective revision. J Vasc Surg. 1989;9(2):286–96. [PubMed] [Google Scholar]
- 10.Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J. 2010;74(8):1501–12. doi: 10.1253/circj.cj-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong AP, Nili N, Jackson ZS, Qiang B, Leong-Poi H, Jaffe R, et al. Expansive remodeling in venous bypass grafts: novel implications for vein graft disease. Atherosclerosis. 2008;196(2):580–9. doi: 10.1016/j.atherosclerosis.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987;5(1):126–36. [PubMed] [Google Scholar]
- 13.Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009;297(4):H1200–7. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, et al. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293(1):H482–8. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kalra M, Miller VM. Early remodeling of saphenous vein grafts: proliferation, migration and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J Vasc Res. 2000;37(6):576–84. doi: 10.1159/000054091. [DOI] [PubMed] [Google Scholar]
- 16.Szilagyi DE, Smith RF, Elliott JP. Venous Autografts In Femoropopliteal Arterioplasty. Observations In The Treatment Of Occlusive Disease. Arch Surg. 1964;89:113–25. doi: 10.1001/archsurg.1964.01320010115011. [DOI] [PubMed] [Google Scholar]
- 17.Fillinger MF, Cronenwett JL, Besso S, Walsh DB, Zwolak RM. Vein adaptation to the hemodynamic environment of infrainguinal grafts. J Vasc Surg. 1994;19(6):970–8. doi: 10.1016/s0741-5214(94)70208-x. discussion 978-9. [DOI] [PubMed] [Google Scholar]
- 18.Dobrin PB, Littooy FN, Golan J, Blakeman B, Fareed J. Mechanical and histologic changes in canine vein grafts. J Surg Res. 1988;44(3):259–65. doi: 10.1016/0022-4804(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 19.Dobrin PB, Littooy FN, Endean ED. Mechanical factors predisposing to intimal hyperplasia and medial thickening in autogenous vein grafts. Surgery. 1989;105(3):393–400. [PubMed] [Google Scholar]
- 20.Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, et al. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. J Vasc Surg. 2004;39(3):547–55. doi: 10.1016/j.jvs.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 21.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, et al. Early biomechanical changes in lower extremity vein grafts--distinct temporal phases of remodeling and wall stiffness. J Vasc Surg. 2006;44(4):740–6. doi: 10.1016/j.jvs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, et al. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg. 2008;47(6):1235–42. doi: 10.1016/j.jvs.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasper WJ, Owens CD, Kim JM, Hills N, Belkin M, Creager MA, et al. Early (30-day) vein remodeling is predictive of midterm graft patency after lower extremity bypass. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCabe M, Cunningham GJ, Wyatt AP, Rothnie NG, Taylor GW. A histological and histochemical examination of autogenous vein grafts. Br J Surg. 1967;54(2):147–55. doi: 10.1002/bjs.1800540216. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, et al. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119(1):123–30. doi: 10.1161/CIRCULATIONAHA.108.810341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39(6):1178–85. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46(6):1180–90. doi: 10.1016/j.jvs.2007.08.033. discussion 1190. [DOI] [PubMed] [Google Scholar]
- 28.Hiramoto JS, Owens CD, Kim JM, Boscardin J, Belkin M, Creager MA, et al. Sex-based differences in the inflammatory profile of peripheral artery disease and the association with primary patency of lower extremity vein bypass grafts. J Vasc Surg. 2012;56(2):387–95. doi: 10.1016/j.jvs.2012.01.059. discussion 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conte MS, Owens CD, Belkin M, Creager MA, Edwards KL, Gasper WJ, et al. A single nucleotide polymorphism in the p27(Kip1) gene is associated with primary patency of lower extremity vein bypass grafts. J Vasc Surg. 2013;57(5):1179–1185 e2. doi: 10.1016/j.jvs.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheanvechai C, Effler DB, Hooper JR, Eschenbruch EM, Sheldon WC, Sones FM, Jr, et al. The structural study of the saphenous vein. Ann Thorac Surg. 1975;20(6):636–45. doi: 10.1016/s0003-4975(10)65755-4. [DOI] [PubMed] [Google Scholar]
- 31.Vesti BR, Primozich J, Bergelin RO, Strandness E., Jr Follow-up of valves in saphenous vein bypass grafts with duplex ultrasonography. J Vasc Surg. 2001;33(2):369–74. doi: 10.1067/mva.2001.111744. [DOI] [PubMed] [Google Scholar]
- 32.Dixon BS. Why don't fistulas mature? Kidney Int. 2006;70(8):1413–22. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 33.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95(5):449–58. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 34.Nath KA, Kanakiriya SK, Grande JP, Croatt AJ, Katusic ZS. Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. Am J Pathol. 2003;162(6):2079–90. doi: 10.1016/S0002-9440(10)64339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 36.Brody WR, Angeli WW, Kosek JC. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972;66(1):111–30. [PMC free article] [PubMed] [Google Scholar]
- 37.Stark VK, Warner TF, Hoch JR. An ultrastructural study of progressive intimal hyperplasia in rat vein grafts. J Vasc Surg. 1997;26(1):94–103. doi: 10.1016/s0741-5214(97)70152-6. [DOI] [PubMed] [Google Scholar]
- 38.Spaet TH, Stemerman MB, Veith FJ, Lejnieks I. Intimal injury and regrowth in the rabbit aorta; medial smooth muscle cells as a source of neointima. Circ Res. 1975;36(1):58–70. doi: 10.1161/01.res.36.1.58. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs JC, Mitchener JS, 3rd, Hagen PO. Postoperative changes in autologous vein grafts. Ann Surg. 1978;188(1):1–15. doi: 10.1097/00000658-197807000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boerboom LE, Olinger GN, Liu TZ, Rodriguez ER, Ferrans VJ, Kissebah AH. Histologic, morphometric, and biochemical evolution of vein bypass grafts in a nonhuman primate model. I. Sequential changes within the first three months. J Thorac Cardiovasc Surg. 1990;99(1):97–106. [PubMed] [Google Scholar]
- 41.Dilley RJ, McGeachie JK, Prendergast FJ. A review of the histologic changes in vein-to-artery grafts, with particular reference to intimal hyperplasia. Arch Surg. 1988;123(6):691–6. doi: 10.1001/archsurg.1988.01400300033004. [DOI] [PubMed] [Google Scholar]
- 42.Berguer R, Higgins RF, Reddy DJ. Intimal hyperplasia. An experimental study. Arch Surg. 1980;115(3):332–5. doi: 10.1001/archsurg.1980.01380030078019. [DOI] [PubMed] [Google Scholar]
- 43.Sharony R, Pintucci G, Saunders PC, Grossi EA, Baumann FG, Galloway AC, et al. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and -2. Am J Physiol Heart Circ Physiol. 2006;290(4):H1651–9. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27(8):1744–51. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 45.Francis SE, Hunter S, Holt CM, Gadsdon PA, Rogers S, Duff GW, et al. Release of platelet-derived growth factor activity from pig venous arterial grafts. J Thorac Cardiovasc Surg. 1994;108(3):540–8. [PubMed] [Google Scholar]
- 46.Ehsan A, Mann MJ, Dell'Acqua G, Tamura K, Braun-Dullaeus R, Dzau VJ. Endothelial healing in vein grafts: proliferative burst unimpaired by genetic therapy of neointimal disease. Circulation. 2002;105(14):1686–92. doi: 10.1161/01.cir.0000013775.02396.93. [DOI] [PubMed] [Google Scholar]
- 47.Li FD, Sexton KW, Hocking KM, Osgood MJ, Eagle S, Cheung-Flynn J, et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. J Surg Res. 2013;180(1):e55–62. doi: 10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49(3):327–33. [PubMed] [Google Scholar]
- 49.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56(1):139–45. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 50.Rosen EM, Goldberg ID, Myrick KV, Levenson SE. Radiation survival of vascular smooth muscle cells as a function of age. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;48(1):71–9. doi: 10.1080/09553008514551081. [DOI] [PubMed] [Google Scholar]
- 51.Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits, and intimal hyperplasia in human saphenous veins. J Biol Chem. 2010;285(6):4038–48. doi: 10.1074/jbc.M109.078345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Miano JM, Vlasic N, Tota RR, Stemerman MB. Localization of Fos and Jun proteins in rat aortic smooth muscle cells after vascular injury. Am J Pathol. 1993;142(3):715–24. [PMC free article] [PubMed] [Google Scholar]
- 53.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292(1):C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 54.Saunders PC, Pintucci G, Bizekis CS, Sharony R, Hyman KM, Saponara F, et al. Vein graft arterialization causes differential activation of mitogen-activated protein kinases. J Thorac Cardiovasc Surg. 2004;127(5):1276–84. doi: 10.1016/j.jtcvs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 56.Hata JA, Petrofski JA, Schroder JN, Williams ML, Timberlake SH, Pippen A, et al. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg. 2005;129(6):1405–13. doi: 10.1016/j.jtcvs.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 57.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 58.Kumar MS, Owens GK. Combinatorial control of smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2003;23(5):737–47. doi: 10.1161/01.ATV.0000065197.07635.BA. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96(3):280–91. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 60.Rey FE, Pagano PJ. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol. 2002;22(12):1962–71. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94(7):1655–64. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 62.Shi Y, O'Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95(12):2684–93. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 63.Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59(1):212–21. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 64.Mallawaarachchi CM, Weissberg PL, Siow RC. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25(7):1383–7. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 65.Mallawaarachchi CM, Weissberg PL, Siow RC. Antagonism of platelet-derived growth factor by perivascular gene transfer attenuates adventitial cell migration after vascular injury: new tricks for old dogs? FASEB J. 2006;20(10):1686–8. doi: 10.1096/fj.05-5435fje. [DOI] [PubMed] [Google Scholar]
- 66.Wyatt AP, Rothnie NG, Taylor GW. The Vascularization of Vein-Grafts. Br J Surg. 1964;51:378–81. doi: 10.1002/bjs.1800510522. [DOI] [PubMed] [Google Scholar]
- 67.Dashwood MR, Anand R, Loesch A, Souza DS. Hypothesis: a potential role for the vasa vasorum in the maintenance of vein graft patency. Angiology. 2004;55(4):385–95. doi: 10.1177/000331970405500405. [DOI] [PubMed] [Google Scholar]
- 68.Ohta O, Kusaba A. Development of vasa vasorum in the arterially implanted autovein bypass graft and its anastomosis in the dog. Int Angiol. 1997;16(3):197–203. [PubMed] [Google Scholar]
- 69.Crotty TP. The path of retrograde flow from the lumen of the lateral saphenous vein of the dog to its vasa vasorum. Microvasc Res. 1989;37(1):119–22. doi: 10.1016/0026-2862(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 70.Hall KV. The great saphenous vein used in situ as an arterial shunt after extirpation of the vein valves. A preliminary report. Surgery. 1962;51:492–5. [PubMed] [Google Scholar]
- 71.Leather RP, Shah DM, Chang BB, Kaufman JL. Resurrection of the in situ saphenous vein bypass. 1000 cases later. Ann Surg. 1988;208(4):435–42. doi: 10.1097/00000658-198810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah DM, Darling RC, 3rd, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long-term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann Surg. 1995;222(4):438–46. doi: 10.1097/00000658-199510000-00003. discussion 446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105(27):9349–54. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113(9):1258–65. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng QH, Irvine S, Tagalakis AD, McAnulty RJ, McEwan JR, Hart SL. Inhibition of neointimal hyperplasia in a rabbit vein graft model following non-viral transfection with human iNOS cDNA. Gene Ther. 2013 doi: 10.1038/gt.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48(6):1566–74. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 77.Huang WC, Newby GB, Lewis AL, Stratford PW, Rogers CA, Newby AC, et al. Periadventitial human stem cell treatment reduces vein graft intimal thickening in pig vein-into-artery interposition grafts. J Surg Res. 2013;183(1):33–9. doi: 10.1016/j.jss.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 78.Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. Sustained local drug delivery from a novel polymeric ring to inhibit intimal hyperplasia. J Biomed Mater Res A. 2010;93(2):656–65. doi: 10.1002/jbm.a.32307. [DOI] [PubMed] [Google Scholar]
- 79.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440(7088):1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 80.Agrawal A, Singh PP, Bottazzi B, Garlanda C, Mantovani A. Pattern recognition by pentraxins. Adv Exp Med Biol. 2009;653:98–116. doi: 10.1007/978-1-4419-0901-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho KJ, Owens CD, Longo T, Sui XX, Ifantides C, Conte MS. C-reactive protein and vein graft disease: evidence for a direct effect on smooth muscle cell phenotype via modulation of PDGF receptor-beta. Am J Physiol Heart Circ Physiol. 2008;295(3):H1132–H1140. doi: 10.1152/ajpheart.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190(12):1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Torzewski J, Torzewski M, Bowyer DE, Frohlich M, Koenig W, Waltenberger J, et al. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18(9):1386–92. doi: 10.1161/01.atv.18.9.1386. [DOI] [PubMed] [Google Scholar]
- 84.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094–9. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 85.Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab. 2004;24(11):1214–8. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- 86.Jabs WJ, Theissing E, Nitschke M, Bechtel JF, Duchrow M, Mohamed S, et al. Local generation of C-reactive protein in diseased coronary artery venous bypass grafts and normal vascular tissue. Circulation. 2003;108(12):1428–31. doi: 10.1161/01.CIR.0000092184.43176.91. [DOI] [PubMed] [Google Scholar]
- 87.Hochleitner BW, Hochleitner EO, Obrist P, Eberl T, Amberger A, Xu Q, et al. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2000;20(3):617–23. doi: 10.1161/01.atv.20.3.617. [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Chen L, Ding J, Rong H, Dong W, Li X. High mobility group box-1 induces migration of vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway activation. Mol Biol Rep. 2012;39(3):3361–7. doi: 10.1007/s11033-011-1106-6. [DOI] [PubMed] [Google Scholar]
- 89.Wallner K, Li C, Fishbein MC, Shah PK, Sharifi BG. Arterialization of human vein grafts is associated with tenascin-C expression. J Am Coll Cardiol. 1999;34(3):871–5. doi: 10.1016/s0735-1097(99)00272-7. [DOI] [PubMed] [Google Scholar]
- 90.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 91.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–33. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect. 2006;8(7):1859–65. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 93.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 94.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 95.Karper JC, de Vries MR, van den Brand BT, Hoefer IE, Fischer JW, Jukema JW, et al. Toll-like receptor 4 is involved in human and mouse vein graft remodeling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler Thromb Vasc Biol. 2011;31(5):1033–40. doi: 10.1161/ATVBAHA.111.223271. [DOI] [PubMed] [Google Scholar]
- 96.Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, et al. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109(3):393–8. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 97.van Keulen JK, Timmers L, van Kuijk LP, Retnam L, Hoefer IE, Pasterkamp G, et al. The Nuclear Factor-kappa B p50 subunit is involved in flow-induced outward arterial remodeling. Atherosclerosis. 2009;202(2):424–30. doi: 10.1016/j.atherosclerosis.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 98.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, et al. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102(9):1645–52. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuen CY, Wong SL, Lau CW, Tsang SY, Xu A, Zhu Z, et al. From skeleton to cytoskeleton: osteocalcin transforms vascular fibroblasts to myofibroblasts via angiotensin II and Toll-like receptor 4. Circ Res. 2012;111(3):e55–66. doi: 10.1161/CIRCRESAHA.112.271361. [DOI] [PubMed] [Google Scholar]
- 100.Castier Y, Ramkhelawon B, Riou S, Tedgui A, Lehoux S. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal. 2009;11(7):1641–9. doi: 10.1089/ars.2008.2393. [DOI] [PubMed] [Google Scholar]
- 101.Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal. 2006;8(7–8):1121–9. doi: 10.1089/ars.2006.8.1121. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93(6):573–80. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 103.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97(6):533–40. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 104.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 105.West N, Guzik T, Black E, Channon K. Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia: role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2001;21(2):189–94. doi: 10.1161/01.atv.21.2.189. [DOI] [PubMed] [Google Scholar]
- 106.Tsapenko MV, d'Uscio LV, Grande JP, Croatt AJ, Hernandez MC, Ackerman AW, et al. Increased production of superoxide anion contributes to dysfunction of the arteriovenous fistula. Am J Physiol Renal Physiol. 2012;303(12):F1601–7. doi: 10.1152/ajprenal.00449.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98(11):2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 109.Chase AJ, Newby AC. Regulation of matrix metalloproteinase (matrixin) genes in blood vessels: a multi-step recruitment model for pathological remodelling. J Vasc Res. 2003;40(4):329–43. doi: 10.1159/000072697. [DOI] [PubMed] [Google Scholar]
- 110.Monea S, Lehti K, Keski-Oja J, Mignatti P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol. 2002;192(2):160–70. doi: 10.1002/jcp.10126. [DOI] [PubMed] [Google Scholar]
- 111.Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res. 2006;134(2):327–34. doi: 10.1016/j.jss.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 112.George SJ, Zaltsman AB, Newby AC. Surgical preparative injury and neointima formation increase MMP-9 expression and MMP-2 activation in human saphenous vein. Cardiovasc Res. 1997;33(2):447–59. doi: 10.1016/s0008-6363(96)00211-8. [DOI] [PubMed] [Google Scholar]
- 113.Misra S, Fu AA, Misra KD, Glockner JF, Mukhopadyay D. Evolution of shear stress, protein expression, and vessel area in an animal model of arterial dilatation in hemodialysis grafts. J Vasc Interv Radiol. 2010;21(1):108–15. doi: 10.1016/j.jvir.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fulton GJ, Channon KM, Davies MG, Annex BH, Hagen PO. Alterations in collagen subtype III and IV protein in experimental venous bypass grafting. Coron Artery Dis. 1998;9(4):191–7. doi: 10.1097/00019501-199809040-00004. [DOI] [PubMed] [Google Scholar]
- 115.Aguilera CM, George SJ, Johnson JL, Newby AC. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res. 2003;58(3):679–88. doi: 10.1016/s0008-6363(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 116.Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45(6):837–46. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- 117.Campbell GR, Campbell JH. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985;42(2):139–62. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- 118.Yahalom J, Eldor A, Fuks Z, Vlodavsky I. Degradation of sulfated proteoglycans in the subendothelial extracellular matrix by human platelet heparitinase. J Clin Invest. 1984;74(5):1842–9. doi: 10.1172/JCI111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20(12):E120–6. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- 120.Karwowski JK, Markezich A, Whitson J, Abbruzzese TA, Zarins CK, Dalman RL. Dose-dependent limitation of arterial enlargement by the matrix metalloproteinase inhibitor RS-113,456. J Surg Res. 1999;87(1):122–9. doi: 10.1006/jsre.1999.5707. [DOI] [PubMed] [Google Scholar]
- 121.Eefting D, de Vries MR, Grimbergen JM, Karper JC, van Bockel JH, Quax PH. In vivo suppression of vein graft disease by nonviral, electroporation-mediated, gene transfer of tissue inhibitor of metalloproteinase-1 linked to the amino terminal fragment of urokinase (TIMP-1.ATF), a cell-surface directed matrix metalloproteinase inhibitor. J Vasc Surg. 2010;51(2):429–37. doi: 10.1016/j.jvs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 122.George SJ, Wan S, Hu J, MacDonald R, Johnson JL, Baker AH. Sustained reduction of vein graft neointima formation by ex vivo TIMP-3 gene therapy. Circulation. 2011;124(11 Suppl):S135–42. doi: 10.1161/CIRCULATIONAHA.110.012732. [DOI] [PubMed] [Google Scholar]
- 123.Puhakka HL, Turunen P, Gruchala M, Bursill C, Heikura T, Vajanto I, et al. Effects of vaccinia virus anti-inflammatory protein 35K and TIMP-1 gene transfers on vein graft stenosis in rabbits. In Vivo. 2005;19(3):515–21. [PubMed] [Google Scholar]
- 124.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 125.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 126.Ehsan A, McGraw AP, Aronovitz MJ, Galayda C, Conte MS, Karas RH, et al. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg. 2013;145(6):1642–9. 1649 e1. doi: 10.1016/j.jtcvs.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31(8):1871–80. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bafford R, Sui XX, Park M, Miyahara T, Newfell BG, Jaffe IZ, et al. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Physiol Heart Circ Physiol. 2011;301(1):H41–7. doi: 10.1152/ajpheart.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grundmann S, Hoefer I, Ulusans S, van Royen N, Schirmer SH, Ozaki CK, et al. Anti-tumor necrosis factor-{alpha} therapies attenuate adaptive arteriogenesis in the rabbit. Am J Physiol Heart Circ Physiol. 2005;289(4):H1497–505. doi: 10.1152/ajpheart.00959.2004. [DOI] [PubMed] [Google Scholar]
- 130.Zhou J, Tang PC, Qin L, Gayed PM, Li W, Skokos EA, et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207(9):1951–66. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, Vanbavel E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc Res. 2008;78(2):341–8. doi: 10.1093/cvr/cvn050. [DOI] [PubMed] [Google Scholar]
- 132.Tang PC, Qin L, Zielonka J, Zhou J, Matte-Martone C, Bergaya S, et al. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205(13):3159–71. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eslami MH, Gangadharan SP, Belkin M, Donaldson MC, Whittemore AD, Conte MS. Monocyte adhesion to human vein grafts: a marker for occult intraoperative injury? J Vasc Surg. 2001;34(5):923–9. doi: 10.1067/mva.2001.118590. [DOI] [PubMed] [Google Scholar]
- 134.Sakaguchi T, Asai T, Belov D, Okada M, Pinsky DJ, Schmidt AM, et al. Influence of ischemic injury on vein graft remodeling: role of cyclic adenosine monophosphate second messenger pathway in enhanced vein graft preservation. J Thorac Cardiovasc Surg. 2005;129(1):129–37. doi: 10.1016/j.jtcvs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 135.Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP, et al. Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1) J Biol Chem. 2004;279(43):44897–906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 136.Reinhardt PH, Kubes P. Differential leukocyte recruitment from whole blood via endothelial adhesion molecules under shear conditions. Blood. 1998;92(12):4691–9. [PubMed] [Google Scholar]
- 137.Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126(2):428–37. [PubMed] [Google Scholar]
- 138.Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg. 2004;39(4):878–88. doi: 10.1016/j.jvs.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 139.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80(6):829–37. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 140.Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, et al. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9(4):287–92. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 141.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103(5):477–84. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 142.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81(1):1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 143.Feldner A, Otto H, Rewerk S, Hecker M, Korff T. Experimental hypertension triggers varicosis-like maladaptive venous remodeling through activator protein-1. FASEB J. 2011;25(10):3613–21. doi: 10.1096/fj.11-185975. [DOI] [PubMed] [Google Scholar]
- 144.Singh NK, Kundumani-Sridharan V, Kumar S, Verma SK, Kotla S, Mukai H, et al. Protein kinase N1 is a novel substrate of NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling. J Biol Chem. 2012;287(43):36291–304. doi: 10.1074/jbc.M112.361220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, et al. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26(9):2063–9. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 146.Karpurapu M, Wang D, Singh NK, Li Q, Rao GN. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. J Biol Chem. 2008;283(39):26577–90. doi: 10.1074/jbc.M800423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Halterman JA, Kwon HM, Zargham R, Bortz PD, Wamhoff BR. Nuclear factor of activated T cells 5 regulates vascular smooth muscle cell phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31(10):2287–96. doi: 10.1161/ATVBAHA.111.232165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eefting D, Bot I, de Vries MR, Schepers A, van Bockel JH, Van Berkel TJ, et al. Local lentiviral short hairpin RNA silencing of CCR2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein E3-Leiden mice. J Vasc Surg. 2009;50(1):152–60. doi: 10.1016/j.jvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 149.Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49(2):502–10. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang L, Brian L, Freedman NJ. Vein graft neointimal hyperplasia is exacerbated by CXCR4 signaling in vein graft-extrinsic cells. J Vasc Surg. 2012;56(5):1390–7. doi: 10.1016/j.jvs.2012.03.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Karshovska E, Zagorac D, Zernecke A, Weber C, Schober A. A small molecule CXCR4 antagonist inhibits neointima formation and smooth muscle progenitor cell mobilization after arterial injury. J Thromb Haemost. 2008;6(10):1812–5. doi: 10.1111/j.1538-7836.2008.03086.x. [DOI] [PubMed] [Google Scholar]
- 152.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-Dependent Ly6C(low) Monocytes Monitor Endothelial Cells and Orchestrate Their Disposal. Cell. 2013;153(2):362–75. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91(3):931–72. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 154.Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45(4):271–8. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- 155.Cherian SM, Bobryshev YV, Inder SJ, Wang AY, Lord RS, Farnsworth AE. Dendritic cells in aortocoronary saphenous vein bypass grafts. Heart Lung Circ. 2000;9(1):39–42. doi: 10.1046/j.1444-2892.2000.009001039.x. [DOI] [PubMed] [Google Scholar]
- 156.Inder SJ, Bobryshev YV, Cherian SM, Lord RS, Masuda K, Yutani C. Accumulation of lymphocytes, dendritic cells, and granulocytes in the aortic wall affected by Takayasu's disease. Angiology. 2000;51(7):565–79. doi: 10.1177/000331970005100705. [DOI] [PubMed] [Google Scholar]
- 157.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21(5):791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- 159.Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L92–7. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 160.Oldroyd SD, Thomas GL, Gabbiani G, El Nahas AM. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int. 1999;56(6):2116–27. doi: 10.1046/j.1523-1755.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- 161.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–55. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 162.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 163.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107(10):1170–84. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 166.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11(6):629–47. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Im DS. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog Lipid Res. 2012;51(3):232–7. doi: 10.1016/j.plipres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 169.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107(4):1660–5. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22(10):3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177(4):2116–23. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Lett. 1989;245(1–2):167–72. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- 173.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200(1):69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97(1):88–98. [PubMed] [Google Scholar]
- 175.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 Limits Polymorphonuclear Leukocytes Recruitment to Inflammatory Loci: Receptor-Dependent Actions. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27(6):2220–32. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Meissner F, Scheltema RA, Mollenkopf HJ, Mann M. Direct proteomic quantification of the secretome of activated immune cells. Science. 2013;340(6131):475–8. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- 179.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37(2):307–15. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 180.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 181.Jiang Z, Yu P, Tao M, Ifantides C, Ozaki CK, Berceli SA. Interplay of CCR2 signaling and local shear force determines vein graft neointimal hyperplasia in vivo. FEBS Lett. 2009;583(21):3536–40. doi: 10.1016/j.febslet.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ozaki CK. Cytokines and the early vein graft: strategies to enhance durability. J Vasc Surg. 2007;45(Suppl A):A92–8. doi: 10.1016/j.jvs.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Davis RC, Davies WT, Mannick JA. Bypass vein grafts in patients with distal popliteal artery occlusion. Am J Surg. 1975;129(4):421–5. doi: 10.1016/0002-9610(75)90187-7. [DOI] [PubMed] [Google Scholar]


