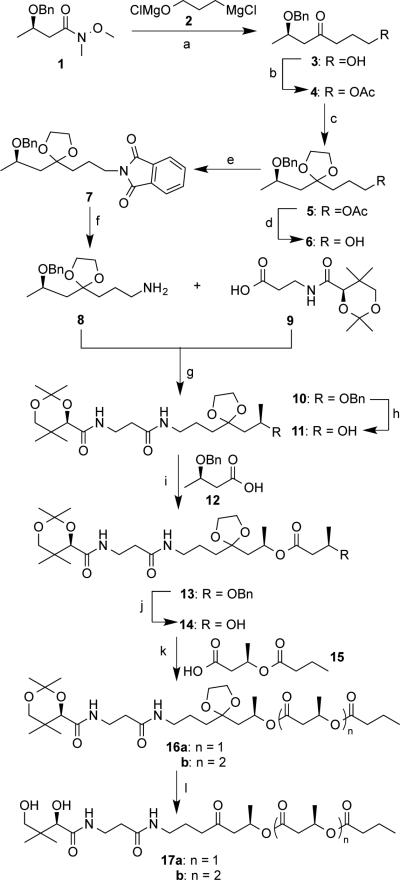
Scheme 3.
Chemical synthesis of precursors to enzymatic reactions: a) 2 (1.1 equiv.), THF, 0 °C, 50 min, then aq. NH4Cl, 86%; b) Ac2O (2.0 equiv.), pyridine (3.0 equiv.), 4-(dimethylamino)pyridine (DMAP, 0.05 equiv.), CH2Cl2, 12 hrs, 97%; c) ethylene glycol (10 equiv.), CH(OEt)3 ( 4.0 equiv.), camphorsulfonic acid (CSA, 0.05 equiv.), 55 °C, 8 hrs, 65%; d) 2 M NaOH, 4 hrs, 70%; e) Ph3P (1.1 equiv.), phthalimide (1.1 equiv.), diisopropyl diazene-1,2-dicarboxylate (DIAD, 1.1 equiv.), THF, 0°C, 12 hrs, 90%; f) N2H4•H2O (3.0 equiv.), EtOH, reflux, 3 hrs, 90%; g) 9 (1.1 equiv.), Et3N (2.5 equiv.), N-ethyl- N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.5 equiv.), hydroxybenzotriazole (HOBT, 1.5 equiv.), CH2Cl2, 12 hrs, 60%; h) H2 (1 atm), 10% Pd-C (0.15 equiv.), 5 hrs, 74%; i) 12 (3.0 equiv.), 2,4,6-trichlorobenzoyl chloride (TCBC, 3.0 equiv.), Et3N (3.5 equiv.), DMAP (3.5 equiv.), CH2Cl2, 17 hrs, 99%; j) same as (h), 89%; k) n = 1: n-butyryl chloride (3.0 equiv.), DMAP (3.4 equiv.), 0 °C to r.t., 24 hrs, 43%; n = 2: 15 (1.6 equiv.), (COCl)2 (3.2 equiv.), N, N′-dimethylformamide (DMF, one drop), CH2Cl2, 2hrs, 83%; l) 1M HCl, MeCN, 2.5 hrs, 96% for n = 1, 83% for n = 2.