Abstract
Introduction
Endoscopic lung volume reduction (ELVR) provides a minimally invasive therapy for patients with severe lung emphysema. As its impact on right ventricular (RtV) function is undefined, we examined the extent of RtV functional changes following ELVR, as assessed by use of speckle tracking-based RtV deformation analysis.
Methods
We enrolled 32 patients with severe emphysematous COPD scheduled for bronchoscopic LVR using endobronchial valves (Zephyr, PulmonX, Inc.), comprising 16 matched clinical responders and 16 non-responders. Echocardiography was conducted one day prior to ELVR and at an eight-week postprocedural interval.
Results
Patients were predominantly of late middle-age (65.8±8.7yrs), male (62.5%) and presented advanced COPD emphysema (means FEV1 and RV: 32.6% and 239.1% of predicted, respectively). After ELVR, RtV apical longitudinal strain improved significantly in the total study cohort (-7.96±7.02% vs. -13.35±11.48%, p=0.04), whereas there were no significant changes in other parameters of RtV function such as RtV global longitudinal strain, TAPSE or pulmonary arterial systolic pressure. In responding patients, 6MWT-improvement correlated with a decrease in NT-proBNP (Pearson´s r: -0.53, p=0.03). However, clinical non-responders did not exhibit any RtV functional improvement.
Discussion
ELVR beneficially impacts RtV functional parameters. Speckle tracking-based RtV apical longitudinal strain analysis allows early determination of RtV contractile gain and identification of clinical responsiveness.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory condition that will shortly constitute the third leading cause of mortality worldwide [1]. As a result of progressive limitations to airflow, a decrease in the number of small airways and emphysematous destruction of lung parenchyma, alveolar hypoxia and respiratory insufficiency arise. Right ventricular (RtV) dysfunction in COPD is the result of multifactorial mechanisms. Alveolar hypoxemia is the major cause of pulmonary vascular disease that follows two different ways of action: whereas acute hypoxemia induces hypoxic pulmonary vasoconstriction, chronic hypoxemia causes structural changes in the pulmonary vasculature, referred to as “remodelling” of the pulmonary vascular bed [2]. However, apart from hypoxemia-induced increase in pulmonary vascular resistance that finally leads to pulmonary hypertension (PH)-mediated right heart impairment, RtV dysfunction in COPD can also be observed in the absence of PH. Hilde et al. reported impaired RtV systolic function, RtV hypertrophy and dilation in COPD patients without PH as compared to healthy controls [3]. These additional mechanisms comprise, among others, systemic inflammation, endothelial dysfunction as well as lung hyperinflation.
As the degree of RtV dysfunction and presence of RtV failure determine prognosis in COPD [4], non-invasive imaging modalities for evaluation of RtV function are imperative. Right ventricular speckle tracking and two-dimensional visualization of RtV strain permit an accurate analysis of RtV deformation capabilities and is reported to predict future right heart failure [5,6].
In consideration of the progressive and wasting nature of COPD [7], new therapeutic modalities—complementing the established medical management of inhaler and anti-inflammatory therapy—have been proposed [8]. They primarily address the advanced emphysematous lung destruction in COPD that in turn is not responsive to medical treatment. Emphysema-induced dyspnoea arises from loss of elastic retraction, causing static and dynamic hyperinflation and consequently inefficiency of respiratory muscle activity [9]. Lung volume reduction surgery was studied in the National Emphysema Treatment Trial, which demonstrated, in a subset of patients, significant improvements in pulmonary function and exercise performance [10]. The concept guiding this approach is to remove damaged, overinflated tissue to alleviate the mechanical burden on the respiratory muscles and thereby reduce breathlessness. In view of the procedure’s associated increase in short-term mortality and morbidity [11], less invasive, bronchoscopically conducted methods to achieve lung volume reduction—without the complications accompanying surgery—have been developed. Endoscopic lung volume reduction (ELVR) comprises diverse techniques, of which endobronchial valve (EBV) implantation is the most studied [12,13].
The aim of the present study was to investigate the impact that ELVR by EBV placement has on right ventricular function, as measured by echocardiography and two-dimensional strain analysis, and to correlate the results with patient clinical outcomes.
Methods
Study population
A total of fifty-six patients with heterogeneous emphysema, admitted to the University Hospital Bonn, Germany, for ELVR by EBV placement, from January 2013 to January 2014, were evaluated for study participation. Consistent with VENT study criteria [12], patients were found eligible for the study if they met the following inclusion criteria: a heterogeneous emphysema distribution, a forced expiratory volume in one second (FEV1) of 15 to 45% of predicted, a residual volume (RV) ≥150% of predicted, a total lung capacity (TLC) ≥100% of predicted and a six-minute walk test distance (6MWTD) ≥140 m. The exclusion criteria for study enrolment comprised pre-existence of coronary artery disease, dilated cardiomyopathy and advanced congestive heart failure. On the day prior to ELVR and eight weeks thenceforth, a 6MWT was performed by serial measurement of two walks and use of a “best-of-two” approach [14]. Clinical responder status was defined by a postprocedural improvement in 6MWTD ≥25 meters [15]. Thirty-eight out of fifty-six patients (67.9%) showed clinical responsiveness to ELVR, whereas eighteen patients (32.1%) were clinical non-responders and represented the failure portion to ELVR. On the basis of demographic and preprocedural lung functional parameters, sixteen clinical non-responders to ELVR were consecutively compared to sixteen responders, resulting in a final study population size of thirty-two patients.
Ethics statement
The study was approved by the medical ethics committee of Bonn and was conducted in accordance with the Declaration of Helsinki. All patients gave their written informed consent of participation.
Follow up
Clinical and laboratory examinations were conducted at baseline and at 8-week interval post-ELVR. They comprised echocardiography and two-dimensional speckle tracking, pulmonary function testing according to the ERS guidelines [16] and laboratory measurement of plasma N-terminal pro-BNP (NT-proBNP) levels. Additionally, post-procedural chest X-ray at week 8 was conducted and compared to baseline thoracic computed tomography for the occurrence of pulmonary atelectasis and diaphragmatic movement.
Procedure
We performed flexible bronchoscopy under moderate sedation with midazolam and propofol, permitting spontaneous breathing and without requirement for anaesthesiological assistance. Zephyr endobronchial valves (Pulmonx, Inc., Redwood City, Calif., USA) were placed unilaterally to isolate the target lobe. Target lobe was previously identified by computed tomography visualized severity of lobar emphysema and scintigraphically determined perfusional and ventilatory reduction. Prior to valve implantation, the Chartis Pulmonary Assessment System (Pulmonx, Inc., Redwood City, Calif., USA) was employed to assess the level of collateral ventilation (CV) [17]. Under the premise of CV-absence, ELVR by EBV was completed. All bronchoscopies were performed by the same experienced bronchoscopist (D.S.).
Transthoracic echocardiography
Transthoracic two-dimensional echocardiography and colour tissue Doppler imaging (TDI) were obtained by experienced cardiac sonographers (R.S., C.H.) using a 2.5-MHz phased-array transducer and conventional equipment (Vivid 7, General Electric Medical Health, Waukesha, Wisconsin, USA; iE 33, Philips Medical Systems, Koninklijke N.V., Hamburg, Germany). With the patient in the left lateral decubitus position, echocardiography followed a standardized protocol, beginning with the parasternal position (long and short axis view) and then in the apical four, five, two and three chamber views. The pulmonary arterial systolic pressure (PASP) was estimated from the peak tricuspid regurgitation jet velocities. Echocardiographic recordings accorded with the recommendations of the American Society of Echocardiography [18].
Two-dimensional speckle tracking echocardiography
Speckle tracking was used to assess angle-independent myocardial deformation. An apical four-chamber view of the right ventricle was transferred to medical imaging software (TomTec Imaging Systems GmbH, Unterschleissheim, Germany). Beginning and ending at the tricuspidal annulus, the RtV endocardium was traced manually in the context of offline analysis, and was tracked with software support throughout two cardiac cycles. Apical, midventricular and basal segmentation was performed visually alongside the lateral free RtV wall, permitting global and regional longitudinal strain evaluation (Fig 1). Speckle tracking analysis was performed by versed cardiologists, blinded to hemodynamic and clinical information.
Fig 1. Speckle tracking derived visualization of right ventricular strain.
(A) Upper left: In an apical four-chamber view, speckle artefacts in the right ventricle which are caused by refraction, reflection and scattering of echo beams, are tracked throughout the heart cycle. The visualization of their displacement allows deduction of the contractility of the right ventricular myocardium. Two different types of myocardial wall strains, the radial (upper right) and the longitudinal strain (bottom right), are illustrated over the cardiac cycle. (B) For the radial (upper half) and the longitudinal strain (lower half), division into myocardial regions—i.e. the apical, midventricular and basal segments—is performed. Additionally, an average value indicates global strain. Quantitative data and their correspondent graphical representation are colour-coded.
Statistics
Descriptive statistics are presented as absolute numbers and percentages, means (±SD) or medians (range), when appropriate. In the case of categorical variables, Pearson’s χ2 test was used to test for associations; the unpaired t-test was applied to compare the means of continuous parameters. Correlations between variables were examined with Pearson correlation coefficient. Non-parametric testing by use of Kruskal-Wallis one-way analysis of variance was performed for evaluation of ordinal scaled variables. A p-value below 0.05, resulting from a two-tailed test, was considered statistically significant. Receiver operating characteristic (ROC) curve analysis for predicted probability was conducted for the RtV apical longitudinal strain; the correspondent area under the curve with its 95% CI was calculated. In 10 random patients, inter- and intraobserver reproducibility of strain measurement was tested by intraclass correlation coefficient with a coefficient ≥0.8 indicating a good reliability. All statistical analyses were performed by use of SPSS Statistics 22 software (IBM, Armonk, NY, USA).
Results
The preinterventional demographic and clinical data of the 32 enrolled patients are presented in Table 1. The study population was predominantly male (62.5%, n = 20) and late middle-aged (65.8±8.7 years). The baseline pulmonary function testing evinced a mean FEV1 of 0.92 l (±0.25 l) in absolute terms and 32.6% (±8.0%) of the predicted value. The baseline residual volume accounted for 5.53 l (±1.05 l) and 239.1% (±52.4%) of predicted. The left lower lobe was targeted in a majority of patients (43.8%), followed by the right upper lobe (in 31.3%), the right lower lobe (in 18.7%) and the left upper lobe in the remaining 6.2%. The preprocedural echocardiographic parameters are summarised in Table 1.
Table 1. Patient demographic, clinical and echocardiographic characteristics at baseline, subdivided according to clinical responder status.
| All patients | Responders | Non-Responders | p-value | |||
|---|---|---|---|---|---|---|
| Subjects | 32 | 16 | 16 | |||
| Age, years | 65.8 ± 8.7 | 67.1 ± 8.7 | 64.6 ± 8.8 | 0.44 | ||
| Male gender (n,%) | 20 (62.5%) | 10 (62.5%) | 10 (62.5%) | 1.00 | ||
| Smoking status (n,%) | Current smoker | 0 (0%) | 0 (0%) | 0 (0%) | 0.64 | |
| Former smoker | 32 (100%) | 16 (100%) | 16 (100%) | |||
| Never smoker | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Pack-years | 40 (15–200) | 40 (20–80) | 35 (15–200) | 0.69 | ||
| FEV1, l | 0.92 ± 0.25 | 0.90 ± 0.23 | 0.94 ± 0.28 | 0.72 | ||
| FEV1, % predicted | 32.60 ± 7.98 | 31.71 ± 6.28 | 33.48 ± 9.51 | 0.54 | ||
| RV, l | 5.53 ± 1.05 | 5.55 ± 1.00 | 5.50 ± 1.14 | 0.90 | ||
| RV, % predicted | 239.11 ± 52.41 | 235.29 ± 50.40 | 242.93 ± 55.73 | 0.69 | ||
| RV/TLC ratio | 74.11 ± 7.04 | 74.19 ± 5.82 | 74.03 ± 8.28 | 0.95 | ||
| RV/TLC ratio, % predicted | 185.00 ± 24.36 | 183.47 ± 24.80 | 186.53 ± 24.62 | 0.73 | ||
| 6MWTD, m | 300 (140–600) | 300 (140–390) | 325 (140–600) | 0.13 | ||
| NT-proBNP, pg/ml | 162.1 ± 119.2 | 161.3 ± 123.9 | 163.0 ± 118.5 | 0.97 | ||
| Serum creatinine concentration, mg/dl | 0.95 ± 0.44 | 1.01 ± 0.56 | 0.90 ± 0.28 | 0.49 | ||
| Target lobe (n,%) | Right upper lobe | 10 (31.3%) | 4 (25.0%) | 6 (37.5%) | 0.60 | |
| Right lower lobe | 6 (18.7%) | 4 (25.0%) | 2 (12.5%) | |||
| Left upper lobe | 2 (6.2%) | 1 (6.2%) | 1 (6.2%) | |||
| Left lower lobe | 14 (43.8%) | 7 (43.8%) | 7 (43.8%) | |||
| Antiobstructive and antiinflammatory medication use (n,%) | Short-acting beta2 agonist | 20 (62.5%) | 11 (68.8%) | 9 (56.3%) | 0.47 | |
| Short-acting anticholinergic | 22 (68.8%) | 9 (56.3%) | 13 (81.3%) | 0.13 | ||
| Long-acting beta2 agonist | 32 (100%) | 16 (100%) | 16 (100%) | * | ||
| Long-acting anticholinergic | 32 (100%) | 16 (100%) | 16 (100%) | * | ||
| Inhaled glucocorticoids | 30 (93.8%) | 16 (100%) | 14 (87.5%) | 0.14 | ||
| Triple inhaler therapy | 30 (93.8%) | 16 (100%) | 14 (87.5%) | 0.14 | ||
| Systemic glucocorticoids | 6 (18.8%) | 1 (6.3%) | 5 (31.3%) | 0.07 | ||
| PDE-4 inhibitor (Roflumilast) | 16 (50.0%) | 7 (43.8%) | 9 (56.3%) | 0.48 | ||
| Home oxygen therapy (n,%) | 18 (56.3%) | 8 (50.0%) | 10 (62.5%) | 0.48 | ||
| Cardiovascular medication use (n,%) | Aspirin | 10 (31.3%) | 5 (31.3%) | 5 (31.3%) | 1.00 | |
| Clopidogrel | 1 (3.1%) | 1 (6.3%) | 0 (0%) | 0.31 | ||
| Statin | 9 (28.1%) | 3 (18.8%) | 6 (37.5%) | 0.24 | ||
| ACE inhibitor | 13 (40.6%) | 8 (50.0%) | 5 (31.3%) | 0.28 | ||
| Angiotensin II receptor blocker | 6 (18.8%) | 3 (18.8%) | 3 (18.8%) | 1.00 | ||
| Beta-blocker | 10 (31.3%) | 4 (25.0%) | 6 (37.5%) | 0.45 | ||
| Calcium channel blocker | 9 (28.1%) | 5 (31.3%) | 4 (25.0%) | 0.69 | ||
| Diuretics | 13 (40.6%) | 7 (43.8%) | 6 (37.5%) | 0.72 | ||
| Comorbidities (n,%) | Arterial hypertension | 22 (68.8%) | 12 (75.0%) | 10 (62.5%) | 0.45 | |
| Diabetes mellitus | 5 (15.6%) | 1 (6.3%) | 4 (25.0%) | 0.14 | ||
| Unipolar depression | 11 (34.4%) | 4 (25.0%) | 7 (43.8%) | 0.26 | ||
| Echocardiographic parameters | ||||||
| LVEF, % | 61.1 ± 10.9 | 61.1 ± 11.7 | 61.2 ± 10.5 | 0.97 | ||
| Diastolic LV dysfunction | No diastolic LV dysfunction | 4 (12.5%) | 1 (6.3%) | 3 (18.8%) | 0.48 | |
| Grade I | 24 (75.0%) | 13 (81.3%) | 11 (68.8%) | |||
| Grade II | 4 (12.5%) | 2 (12.5%) | 2 (12.5%) | |||
| Grade III | 0 (0%) | 0 (0%) | 0 (0%) | |||
| PASP, mmHg | 29.0 ± 14.3 | 29.4 ± 15.6 | 28.6 ± 13.3 | 0.87 | ||
| PASP (n,%) | <30mmHg | 18 (56.3%) | 12 (75.0%) | 6 (37.5%) | 0.05 | |
| 30 -<50mmHg | 11 (34.4%) | 3 (18.8%) | 8 (50.0%) | |||
| 50-<70mmHg | 2 (6.3%) | 0 (0%) | 2 (12.5%) | |||
| ≥70mmHg | 1 (3.1%) | 1 (6.3%) | 0 (0%) | |||
| TAPSE, mm | 22.0 ± 5.3 | 20.8 ± 6.1 | 23.3 ± 4.2 | 0.17 | ||
| 2D global RV-Sl, % | -11.23 ± 6.47 | -8.24 ± 6.25 | -14.21 ± 5.32 | 0.01 | ||
| 2D apical RV-Sl, % | -7.96 ± 7.02 | -5.39 ± 4.80 | -10.54 ± 8.04 | 0.05 | ||
| 2D medial RV-Sl, % | -11.46 ± 7.16 | -8.94 ± 5.43 | -13.97 ± 7.94 | 0.06 | ||
| 2D basal RV-Sl, % | -14.72 ± 10.17 | -10.48 ± 8.17 | -18.96 ± 10.44 | 0.06 | ||
Data are presented as total number and percentage (in parentheses), mean ± SD or median and range (in parentheses).
* P-values not calculable
Abbreviations:
FEV1 = forced expiratory volume in 1 second
RV = residual volume
RV/TLC = residual volume/total lung capacity
CAT = COPD Assessment Test
6MWTD = 6 minute walk test distance
NT-proBNP = N-terminal pro-BNP
LVEF = left ventricular ejection fraction
LV = left ventricular
PASP = pulmonary arterial systolic pressure
TAPSE = tricuspid annular plane systolic excursion
2D global RV-Sl, % = two dimensional right ventricular global longitudinal strain
2D apical RV-Sl, % = two dimensional right ventricular apical longitudinal strain
At eight weeks, pulmonary function exhibited an increase in FEV1 of predicted by 4.11% (95%CI: +0.99%–+7.24%; p = 0.01) and 12.6%, in absolute and relative terms respectively. The mean residual volume decreased by an absolute 12.51% (95%CI: -32.08%–+7.05%; p = 0.20) of the predicted value, corresponding to a relative decrease of 5.23 percentage points. The RV/TLC ratio, used as a descriptive marker for air trapping and airways obstruction, revealed an absolute and relative decrease of 8.56% and 4.63% of predicted, respectively (95%CI: -17.27 –+0.14%; p = 0.05). Lung functional parameters as a function of 6MWT-guided clinical responsiveness are summarised in Table 2.
Table 2. Pulmonary function and echocardiographic outcomes at eight-week follow up.
| All patients | Responders | Non-Responders | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-ELVR | Post-ELVR | p-value | Pre-ELVR | Post-ELVR | p-value | Pre-ELVR | Post-ELVR | p-value | |
| FEV1, l | 0.92 ± 0.25 | 1.03 ± 0.35 | 0.01 | 0.90 ± 0.23 | 1.07 ± 0.41 | 0.04 | 0.94 ± 0.28 | 1.00 ± 0.30 | 0.22 |
| FEV1, % predicted | 32.60 ± 7.98 | 36.71 ± 11.57 | 0.01 | 31.71 ± 6.28 | 37.02 ± 10.87 | 0.03 | 33.48 ± 9.51 | 36.40 ± 12.57 | 0.18 |
| RV, l | 5.53 ± 1.05 | 5.30 ± 1.47 | 0.30 | 5.55 ± 1.00 | 5.27 ± 1.26 | 0.35 | 5.50 ± 1.14 | 5.33 ± 1.70 | 0.61 |
| RV, % predicted | 239.11 ± 52.41 | 226.60 ± 58.38 | 0.20 | 235.29 ± 50.40 | 221.19 ± 52.71 | 0.32 | 242.93 ± 55.73 | 232.00 ± 64.83 | 0.48 |
| RV/TLC ratio | 74.11 ± 7.04 | 71.06 ± 9.76 | 0.08 | 74.19 ± 5.82 | 71.48 ± 8.62 | 0.20 | 74.03 ± 8.28 | 70.65 ± 11.05 | 0.24 |
| RV/TLC ratio, % predicted | 185.00 ± 24.36 | 176.44 ± 25.40 | 0.05 | 183.47 ± 24.80 | 175.58 ± 22.50 | 0.15 | 186.53 ± 24.62 | 177.29 ± 28.74 | 0.20 |
| CAT, points | 22.7± 5.9 | 22.8 ± 7.3 | 0.89 | 22.5 ± 5.0 | 23.3 ± 8.1 | 0.67 | 22.9 ± 6.9 | 22.4 ± 6.6 | 0.73 |
| 6MWTD, m | 302.50 ± 130.20 | 349.06 ± 127.86 | 0.02 | 267.50 ± 87.98 | 401.88 ± 74.56 | <0.001 | 337.50 ± 156.79 | 296.25 ± 149.26 | 0.02 |
| NT-proBNP, pg/ml | 162.1 ± 119.3 | 178.6 ± 235.2 | 0.70 | 161.3 ± 123.9 | 111.6 ± 93.7 | 0.15 | 163.0 ± 118.5 | 245.6 ± 309.9 | 0.29 |
| Serum creatinine concentration, mg/dl | 0.95 ± 0.44 | 0.96 ± 0.44 | 0.78 | 1.01 ± 0.56 | 1.07 ± 0.57 | 0.24 | 0.90 ± 0.28 | 0.86 ± 0.22 | 0.27 |
| Echocardiographic parameters | |||||||||
| LVEF, % | 61.1 ± 11.1 | 60.1 ± 9.2 | 0.45 | 61.5 ± 12.1 | 60.7 ± 9.4 | 0.88 | 61.2 ± 10.5 | 59.6 ± 9.2 | 0.31 |
| PASP, mmHg | 29.0 ± 14.3 | 30.2 ± 14.6 | 0.50 | 29.4 ± 15.6 | 28.9 ±16.8 | 0.44 | 28.6 ± 13.3 | 31.5 ± 12.5 | 0.40 |
| TAPSE, mm | 22.0 ± 5.3 | 21.9 ± 5.3 | 0.92 | 20.8 ± 6.1 | 20.9 ± 4.9 | 0.85 | 23.3 ± 4.2 | 22.9 ± 5.6 | 0.82 |
| 2D global RV-Sl, % | -11.23 ± 6.47 | -13.31 ± 6.53 | 0.19 | -8.24 ± 6.25 | -13.47 ± 7.61 | 0.06 | -14.21 ± 5.32 | -13.14 ± 5.49 | 0.45 |
| 2D apical RV-Sl, % | -7.96 ± 7.02 | -13.35 ± 11.48 | 0.04 | -5.39 ± 4.80 | -13.55 ± 12.48 | 0.04 | -10.54 ± 8.04 | -13.15 ± 10.79 | 0.49 |
| 2D medial RV-Sl, % | -11.46 ± 7.16 | -11.96 ± 6.92 | 0.78 | -8.94 ± 5.43 | -12.66 ± 6.63 | 0.11 | -13.97 ± 7.94 | -11.25 ± 7.35 | 0.34 |
| 2D basal RV-Sl, % | -14.72 ± 10.17 | -11.13 ± 8.97 | 0.06 | -10.48 ± 8.17 | -8.15 ± 7.58 | 0.35 | -18.96 ± 10.44 | -14.10 ± 9.48 | 0.12 |
Data are presented as mean ± SD.
Abbreviations:
FEV1 = forced expiratory volume in 1 second
RV = residual volume
RV/TLC = residual volume/total lung capacity
CAT = COPD Assessment Test
6MWTD = 6 minute walk test distance
NT-proBNP = N-terminal pro-BNP
LVEF = left ventricular ejection fraction
PASP = pulmonary arterial systolic pressure
TAPSE = tricuspid annular plane systolic excursion
2D global RV-Sl, % = two dimensional right ventricular global longitudinal strain
2D apical RV-Sl, % = two dimensional right ventricular apical longitudinal strain
The two-dimensional speckle tracking evaluation of right ventricular deformation capabilities revealed significant improvement in RtV apical longitudinal strain (-7.96±7.02% vs. -13.35±11.48%, p = 0.04), whereas global and regional medial and basal RtV longitudinal strain did not change significantly over the follow-up period (Table 2).
The 6MWTD increased an average 46.6 m (95%CI: +7.9 m—+85.3 m; p = 0.02) in the total study cohort. The clinical responders exhibited a mean 6MWTD improvement of 134.4 m (95%CI: +102.3 m—+166.5 m; p<0.001), a significant increase in RtV apical longitudinal strain (-5.39±4.80% vs. -13.55±12.48%, p = 0.04; Fig 2) and a statistical trend towards a postprocedural improvement in RtV global longitudinal strain (-8.24±6.25% vs. -13.47±7.61%, p = 0.06). In contrast, the global and regional RtV deformation properties remained unchanged in the case of the clinical non-responsiveness (Table 2).
Fig 2. Pre- to post-procedural presentation of 2D global and apical RV-Sl in responding patients.
Abbreviations: 2D global RV-Sl = two dimensional right ventricular global longitudinal strain 2D apical RV-Sl = two dimensional right ventricular apical longitudinal strain.
Intra- and interobserver reliability analysis for speckle tracking- based strain data showed a good reproducibility. For the apical, medial, basal and global right ventricular strain, the interobserver difference was 1.93±4.05, 1.84±3.73, 0.54±5.71 and 0.64±3.12 of the mean strain for both observers, respectively. The correspondent intraclass correlation coefficient of interobserver variability was 0.86 (95%CI: 0.53–0.97, p<0.01), 0.80 (95%CI: 0.27–0.95, p<0.01), 0.89 (95%CI: 0.55–0.97, p<0.01) and 0.92 (95%CI: 0.68–0.98, p = 0.001), respectively. For intraobserver repeatability, the intraclass correlation coefficients for the apical, medial, basal and global right ventricular strain accounted for 0.99 (95%CI: 0.96–0.99, p<0.001), 0.93 (95%CI: 0.73–0.98, p<0.001), 0.96 (95%CI: 0.82–0.99, p<0.001) and 0.92 (95%CI: 0.66–0.98, p = 0.001), respectively.
Pulmonary arterial systolic pressure (PASP), as a conventional right ventricular functional parameter, remained statistically unchanged pre- to post-procedurally. Biventricular end-diastolic and end-systolic measured dimensions, stroke volumes and their correspondent indices did not differ pre-procedurally between the clinically responding and non-responding group (Table 3). Post-procedurally, a significant difference in the left and right ventricular stroke volume indices (p = 0.04 and <0.01, respectively), as well as in the right ventricular end-systolic volume index (p = 0.03) was observed by comparison of both groups. In case of clinical responsiveness, pre- to post-postprocedural comparison revealed a significant gain in right ventricular stroke volume and stroke volume index (p = 0.007 and p = 0.006, respectively), while neither the non-responding subgroup, nor the total study collective per se offered significant ELVR-mediated changes.
Table 3. Volumetric data as a function of clinical responder status.
| All patients | Responders | Non-Responders | p-value | |
|---|---|---|---|---|
| Pre-ELVR | ||||
| Left ventricular end-diastolic volume, ml | 82.6 ± 33.7 | 88.3 ± 40.9 | 76.9 ± 24.4 | 0.34 |
| Left ventricular end-diastolic volume index, ml/m2 | 44.8 ± 17.2 | 48.2 ± 21.9 | 41.4 ± 10.4 | 0.27 |
| Left ventricular end-systolic volume, ml | 33.3 ± 21.7 | 35.9 ± 26.4 | 30.6 ± 16.1 | 0.50 |
| Left ventricular end-systolic volume index, ml/m2 | 17.9 ± 11.5 | 19.7 ± 14.5 | 16.3 ± 7.5 | 0.41 |
| Left ventricular stroke volume, ml | 49.3 ± 17.2 | 52.4 ± 21.2 | 46.3 ± 12.0 | 0.32 |
| Left ventricular stroke volume index, ml/m2 | 26.8 ± 8.6 | 28.6 ± 10.8 | 25.1 ± 5.6 | 0.27 |
| Right ventricular end-diastolic volume, ml | 40.2 ± 19.5 | 37.9 ± 17.7 | 42.6 ± 21.5 | 0.50 |
| Right ventricular end-diastolic volume index, ml/m2 | 21.3 ± 8.8 | 20.2 ± 7.6 | 22.5 ± 9.9 | 0.46 |
| Right ventricular end-systolic volume, ml | 20.7 ± 12.1 | 19.7 ± 12.9 | 21.6 ± 11.5 | 0.67 |
| Right ventricular end-systolic volume index, ml/m2 | 10.9 ± 5.8 | 10.6 ± 6.4 | 11.4 ± 5.4 | 0.68 |
| Right ventricular stroke volume, ml | 19.6 ± 10.7 | 18.1 ± 8.7 | 21.0 ± 12.5 | 0.45 |
| Right ventricular stroke volume index, ml/m2 | 10.3 ± 4.9 | 9.6 ± 3.6 | 11.1 ± 6.0 | 0.40 |
| Post-ELVR | ||||
| Left ventricular end-diastolic volume, ml | 81.7 ± 30.6 | 89.5 ± 33.7 | 73.8 ± 25.9 | 0.15 |
| Left ventricular end-diastolic volume index, ml/m2 | 44.4 ± 14.7 | 48.9 ± 16.8 | 39.9 ± 11.1 | 0.08 |
| Left ventricular end-systolic volume, ml | 34.3 ± 19.4 | 36.6 ± 20.9 | 32.0 ± 18.0 | 0.51 |
| Left ventricular end-systolic volume index, ml/m2 | 18.5 ± 9.5 | 20.0 ± 10.8 | 17.0 ± 8.2 | 0.39 |
| Left ventricular stroke volume, ml | 47.3 ± 16.7 | 52.9 ± 18.2 | 41.8 ± 13.4 | 0.06 |
| Left ventricular stroke volume index, ml/m2 | 25.9 ± 8.3 | 29.0 ± 9.0 | 22.9 ± 6.4 | 0.04 |
| Right ventricular end-diastolic volume, ml | 41.5 ± 15.2 | 42.4 ± 14.4 | 40.6 ± 16.4 | 0.74 |
| Right ventricular end-diastolic volume index, ml/m2 | 22.3 ± 6.6 | 22.9 ± 5.9 | 21.6 ± 7.4 | 0.59 |
| Right ventricular end-systolic volume, ml | 21.0 ± 9.4 | 17.9 ± 7.7 | 24.1 ± 10.1 | 0.06 |
| Right ventricular end-systolic volume index, ml/m2 | 11.2 ± 4.3 | 9.5 ± 3.3 | 12.8 ± 4.6 | 0.03 |
| Right ventricular stroke volume, ml | 20.5 ± 9.3 | 24.5 ± 9.5 | 16.5 ± 7.3 | 0.01 |
| Right ventricular stroke volume index, ml/m2 | 11.1 ± 4.6 | 13.4 ± 4.5 | 8.8 ± 3.3 | <0.01 |
Data are presented as mean ± SD.
Abbreviations:
ELVR = endoscopic lung volume reduction
Complete atelectasis with lobar exclusion and diaphragmatic movement occurred in 56.3% of total cohort (responding subgroup: 62.5%, non-responding subgroup: 50.0%). Overall, laboratory testing revealed a non-significant relative mean increase in NT-proBNP of 10.2% (161.1±119.2 pg/ml vs. 178.6±235.2 pg/ml). In view of the established interaction between renal function and NT-proBNP-levels, we tested the correlation between serum creatinine concentration and NT-proBNP that presently permitted exclusion of an interparametral correlation (Pearson´s r: -0.11, p = 0.56). In the clinical responder subgroup, the increase in the 6MWTD was significantly associated with a reduction of the NT-proBNP-level (Pearson´s r: -0.53, p = 0.03, Fig 3), that in turn occurred independently of RtV strain adaptations. Receiver operating characteristic (ROC) curve analysis of RtV apical longitudinal strain for predicted probability of response to ELVR evinced an area under the curve of 0.73 (95%CI: 0.55–0.91 m; p = 0.03), indicating an overall fair accuracy. A cut-off value of -7.5 showed a specificity of 81.2% and a sensitivity of 68.8% (Fig 4). Neither pulmonary function nor echocardiographic changes manifested dependency on treated lobe.
Fig 3. Changes in 6-minute walk test distance and NT-proBNP levels in clinical responders to endoscopic lung volume reduction at eight-week follow up.
Abbreviations: 6MWTD = 6 minute walk test distance, NT-proBNP = N-terminal pro-BNP.
Fig 4. Receiver operating characteristic (ROC) cure analysis of 2D apical RV-Sl for the identification of patients responding to endoscopic lung volume reduction by endobronchial valve placement.
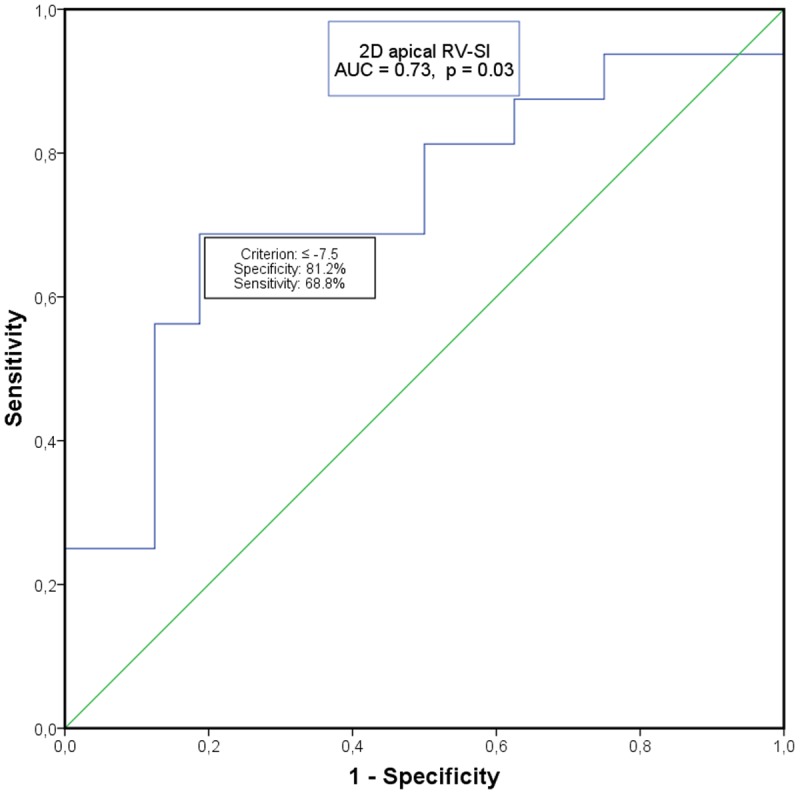
Abbreviations: 2D apical RV-Sl = two dimensional right ventricular apical longitudinal strain, AUC = Area under the curve.
Complications were limited to post-procedural occurrence and comprised early exacerbation of COPD in 9.4% of patients (n = 3), resolving under medical treatment without need for valve removal. Development of pneumothorax occurred in 4 patients (12.5%) and resolved adequately by chest tube implantation. No other kind of complications were observed.
Discussion
This study analysed the impact of ELVR by EBV placement on global and regional RtV dysfunction and identified ELVR-mediated RtV improvement. RtV apical longitudinal strain might be a valuable tool to evaluate short-term RtV functional amelioration and clinical responsiveness to ELVR.
The course of advanced emphysematous COPD is sustainably complicated by cardiac complications on the right heart side, with alterations of right ventricular function being predictive of patient´s outcome [19,20]. Numerous efforts to identify the optimal parameter to evaluate systolic and diastolic RtV function have been undertaken. Speckle tracking echocardiography was introduced by Reisner and Leitman in 2004 [21,22] and represents by now an established imaging modality. Acoustic reflections, generated by ultrasound interrogation of tissue, allow a semiautomated measurement of myocardial deformation properties. The resulting dimensionless strain quantifies global and regional ventricular contractility. In contrast to tissue Doppler imaging, speckle tracking is independent of the angle of the ultrasound beam, facilitating an easy and robust application [6]. In the field of newly diagnosed pulmonary arterial hypertension (WHO group 1), Sachdev et al. identified RtV longitudinal strain and strain rate to be predictive of future right heart failure, clinical worsening and mortality [23]. In a subsequent study, he demonstrated consistence of results by serial strain assessment even after initiation of medical PAH-treatment, emphasizing the prognostic utility of strain measurement [24].
In COPD, the mechanisms leading to RtV dysfunction are diverse. They comprise a much broader spectrum than sole pulmonary hypertension caused by hypoxic pulmonary vasoconstriction and pulmonary vascular remodelling [2]. These additional mechanisms comprehend lung hyperinflation, endothelial dysfunction and systemic inflammation, all of them able to exert a direct negative impact on the right ventricle [3]. In a COPD study population that comprised both confirmed and excluded pulmonary hypertension COPD patients, Hilde et al. showed that reduced RtV systolic function, RtV hypertrophy and dilation were even present in COPD patients without pulmonary hypertension as compared to healthy controls [3]. In this line of thought, the presently described ELVR-mediated improvement in exercise capacity—measured by 6MWT-perfomance—and RtV function would be ascribable to an improvement in lung hyperinflation that in turn has been correlated to right and left ventricular volumetric conditions. Jörgensen et al. [25] compared 13 patients with severe emphysema to matched healthy controls and objectified impairment in RtV and LV performance status in case of severe emphysema. The compromised cardiac performance was ascribed to a preload reduction, primarily caused by intrathoracic hypovolemia in consequence of pulmonary hyperinflation. Transferring these considerations to our study, we additionally measured biventricular end-diastolic and end-systolic dimensions, as well as biventricular stroke volumes. Clinical responder status was associated with a significant gain in RtV stroke volume (p<0.01) and stroke volume index (p<0.01). Simultaneously, though not significantly, RtV end-diastolic volume index increased by an absolute 2.75±7.17ml/m2 and a relative 13.6%. In case of surgically performed lung volume reduction, the response of right ventricular performance was investigated by Mineo et al. [26]. Twelve patients undergoing bilateral lung volume reduction surgery (LVRS) were assessed preoperatively and six months afterwards: they showed a significant increase in right ventricular stroke volume both at rest and during exercise.
The effect of endoscopic lung volume reduction (ELVR) on RtV function remains undetermined. ELVR by EBV placement represents an emerging, minimally invasive therapeutic modality to achieve reduction of overinflated, functionless pulmonary regions without the LVRS-associated perioperative complications [27]. Theoretically, the diminishment of emphysematous tissue is expected to ameliorate RtV performance through different mechanisms. On the one hand, the elimination of damaged areas decreases overall hyperinflation, improves rib cage and diaphragm mechanics and thereby renders more effective the work of breathing. By reducing alveolar hypoxia, hypoxic pulmonary vasoconstriction is diminished and the ventilation/perfusion ratio improves. On the other hand, a reduction in the hyperinflation-driven compression both of the vascular bed and of the ventricular relaxation, as well as an amelioration of the polycythemia-caused hyperviscosity also contributes to RtV relief.
The impact of ELVR by EBV on pulmonary hypertension in 6 patients with severe emphysematous COPD and established pulmonary hypertension was recently reported by Eberhardt et al. [28]. Right heart catheterization was performed prior to and at 90 days after ELVR. In 5 out of 6 patients hemodynamics improved with a decrease in mean pulmonary artery pressure and pulmonary capillary wedge pressure and gain in cardiac index. By contrast, our study collective comprised only a minority of PH-patients (14 patients (43.8%)) and echocardiographically-measured pulmonary arterial systolic pressure (PASP) elevation was only modest and did not change substantially post-ELVR, pointing at other mechanisms responsible for RtV functional and clinical improvement, as indicated previously.
In the presently studied COPD collective, the mean baseline RtV global longitudinal strain was -11.2±6.5% and differed considerably from the reference values in healthy subjects that have recently been described to average -27±2% [29]. This discrepancy is primarily attributable to the impairment in RtV systolic function that emphysematous COPD is associated with. In a COPD study population that comprised both confirmed and excluded pulmonary hypertension COPD subgroups [3], longitudinal strain assessment—though performed from the basal RtV free wall—was -22% and -18% in case of PH-absence and PH-presence, respectively, versus -31% in healthy controls. Moreover, strain correlated significantly with forced expiratory volume in 1sec (FEV1). In concrete terms, FEV1 was 46% of predicted in the COPD-subgroup without pulmonary hypertension and consequently considerably higher than in our study population (FEV1% predicted: 32.6%).
We presently identified RtV apical longitudinal strain to be the best correlative marker of clinical responsiveness. Its value in predicting the presence of pulmonary hypertension has been investigated by Lopez-Candales et al., who analysed longitudinal strain and velocity data in PH patients, compared them to healthy controls and ascribed the highest degree of PH predictability to alterations in apical longitudinal RtV strain [30]. Moreover, its impairment has been demonstrated repeatedly to be predictive of RtV heart failure, clinical deterioration and mortality [23,30]. Against the present background of our unobserved ELVR-mediated changes in conventional RtV functional parameters, such as PASP or TAPSE, the diagnostic advantage of strain analysis appears to be its early detection of functional adaptations post-ELVR, adaptations that might possibly precede changes in the above-mentioned traditional echocardiographic measures. Increasing evidence suggests that a post-ELVR clinical performance status enables a more feasible evaluation of procedural benefit than mere pulmonary function testing [31]. Here, we present as statistically significant a gain in FEV1 and a decrease in the RV/TLC ratio that, however, occurred independently of exercise improvement and amelioration of myocardial function, and which conditioned our decision to assess clinical responsiveness by changes in the 6MWT.
On the whole and in comparison with the responding subgroup, clinical non-responders appeared to be “fitter” at baseline in terms of age (64.6 vs. 67.1 years), 6MWTD (325 vs. 300m) and echocardiographic performance (PASP, global and regional longitudinal RtV strain). After ELVR, the global RtV-Sl strain increased in the responding subgroup by an absolute 5.2% and a relative 63.5%. However,—in case of clinical non-responsiveness—the global RtV-Sl strain even worsened by 1.07% and 7.5% in absolute and relative terms, respectively, that was additionally accompanied by a lack in PFT parameter improvement. In the light of the above, though baseline global RtV-Sl strain differed significantly between groups—a fact that seems to be indicative of major baseline RtV functional reserves in non-responders—, the opposite global strain development after ELVR illustrates the basic differing treatment response to ELVR between groups.
There are several limitations to this study that should be addressed. Given the small number of patients examined, the generalization of results is somewhat restricted. Although matching was performed to attain demographic and lung functional group homogeneity, selection bias on the basis of baseline echocardiographic parameters cannot be excluded. Due to the lack of a third subgroup comprising COPD patients without ELVR, estimation of RtV function alteration in ELVR-naive COPD is limited. To test for correlations with PASP, invasive hemodynamic data would have been required, but these presently were not obtained. Nonetheless, the accuracy of echocardiographically-determined pulmonary arterial pressures has repeatedly been demonstrated, such as by Hammerstingl and colleagues, who ascertained non-invasive, echocardiographic assessment inclusive of RtV speckle tracking analysis to be of reliable value for estimating PH, with non-invasively measured PASP correlating significantly with invasively determined mean pulmonary arterial pressure [32]. In order to additionally capture RtV functional adaptations that are driven by ELVR-mediated changes in chronic-hypoxemia caused pulmonary vascular remodelling, complementary long-term follow-up of our study cohort would be of additional value.
In conclusion, the non-invasive evaluation of speckle-tracking-based RtV longitudinal function permits an early estimation of the beneficial effects of ELVR on RtV contractility and the differentiation of clinical responder status.
Data Availability
All relevant data are within the paper.
Funding Statement
This study has been funded by Pulmonx, Inc., Redwood City, Calif., USA. None of the authors had any ownership interest in the Company. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997; 349: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 2. Weitzenblum E, Chaouat A. Cor pulmonale. Chron Respir Dis. 2009; 6: 177–185. 10.1177/1479972309104664 [DOI] [PubMed] [Google Scholar]
- 3. Hilde JM, Skjørten I, Grøtta OJ, Hansteen V, Melsom MN, Hisdal J, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013; 62: 1103–1111. 10.1016/j.jacc.2013.04.091 [DOI] [PubMed] [Google Scholar]
- 4. Burgess MI, Mogulkoc N, Bright-Thomas RJ, Bishop P, Egan JJ, Ray SG. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr. 2002; 15: 633–639. [DOI] [PubMed] [Google Scholar]
- 5. Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr. 2010; 11: 81–96. 10.1093/ejechocard/jep234 [DOI] [PubMed] [Google Scholar]
- 6. Blessberger H, Binder T. Non-invasive imaging: Two dimensional speckle tracking echocardiography: basic principles. Heart. 2010; 96: 716–722. 10.1136/hrt.2007.141002 [DOI] [PubMed] [Google Scholar]
- 7. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007; 370: 765–773. [DOI] [PubMed] [Google Scholar]
- 8. Martinez FJ, Chang A. Surgical therapy for chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2005; 26: 167–191. [DOI] [PubMed] [Google Scholar]
- 9. O'Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol. 2008; 105: 753–755. 10.1152/japplphysiol.90336.2008b [DOI] [PubMed] [Google Scholar]
- 10. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003; 348: 2059–2073. [DOI] [PubMed] [Google Scholar]
- 11. Naunheim KS, Wood DE, Krasna MJ, DeCamp MM Jr, Ginsburg ME, McKenna RJ Jr, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. 2006; 131: 43–53. [DOI] [PubMed] [Google Scholar]
- 12. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010; 363: 1233–1244. 10.1056/NEJMoa0900928 [DOI] [PubMed] [Google Scholar]
- 13. Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012; 39: 1334–1342. 10.1183/09031936.00161611 [DOI] [PubMed] [Google Scholar]
- 14. Wise RA, Connett J, Kurnow K, Grill J, Johnson L, Kanner R, et al. Selection of spirometric measurements in a clinical trial, the Lung Health Study. Am J Respir Crit Care Med. 1995; 151: 675–681. [DOI] [PubMed] [Google Scholar]
- 15. Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010; 91: 221–225. 10.1016/j.apmr.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Work Group on Standardization of Respiratory Function Tests. European Community for Coal and Steel. Official position of the European Respiratory Society. Rev Mal Respir. 1994; 11: 5–40. [PubMed] [Google Scholar]
- 17. Mantri S, Macaraeg C, Shetty S, Aljuri N, Freitag L, Herth F, et al. Technical advances: measurement of collateral flow in the lung with a dedicated endobronchial catheter system. J Bronchology Interv Pulmonol. 2009; 16: 141–144. 10.1097/LBR.0b013e3181a40a3e [DOI] [PubMed] [Google Scholar]
- 18. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. J Am Soc Echocardiogr. 2011; 24: 229–267. 10.1016/j.echo.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 19. Almagro P, Barreiro B, Ochoa de Echaguen A, Quintana S, Rodríguez Carballeira M, Heredia JL, et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006; 73: 311–317. [DOI] [PubMed] [Google Scholar]
- 20. Marti S, Muñoz X, Rios J, Morell F, Ferrer J. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J. 2006; 27: 689–696. [DOI] [PubMed] [Google Scholar]
- 21. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004; 17: 630–633. [DOI] [PubMed] [Google Scholar]
- 22. Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004; 17: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 23. Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011; 139: 1299–1309. 10.1378/chest.10-2015 [DOI] [PubMed] [Google Scholar]
- 24. Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol. 2013; 111:143–148. 10.1016/j.amjcard.2012.08.061 [DOI] [PubMed] [Google Scholar]
- 25. Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007; 131: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 26. Mineo TC, Pompeo E, Rogliani P, Dauri M, Turani F, Bollero P, et al. Effect of lung volume reduction surgery for severe emphysema on right ventricular function. Am J Respir Crit Care Med. 2002; 165: 489–494. [DOI] [PubMed] [Google Scholar]
- 27. Hopkinson NS, Toma TP, Hansell DM, Goldstraw P, Moxham J, Geddes DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med. 2005; 171: 453–460. [DOI] [PubMed] [Google Scholar]
- 28. Eberhardt R, Gerovasili V, Kontogianni K, Gompelmann D, Ehlken N, Herth FJ, et al. Endoscopic Lung Volume Reduction with Endobronchial Valves in Patients with Severe Emphysema and Established Pulmonary Hypertension. Respiration. 2015; 89: 41–48. 10.1159/000368369 [DOI] [PubMed] [Google Scholar]
- 29. Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, et al. Reference Values for Right Ventricular Strain in Patients without Cardiopulmonary Disease: A Prospective Evaluation and Meta-Analysis. Echocardiography. 2014. October 17 10.1111/echo.12806 [DOI] [PubMed] [Google Scholar]
- 30. Dambrauskaite V, Delcroix M, Claus P, Herbots L, D'hooge J, Bijnens B, et al. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr. 2007; 20: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 31. Argula RG, Strange C, Ramakrishnan V, Goldin J. Baseline regional perfusion impacts exercise response to endobronchial valve therapy in advanced pulmonary emphysema. Chest. 2013; 144: 1578–1586. 10.1378/chest.12-2826 [DOI] [PubMed] [Google Scholar]
- 32. Hammerstingl C, Schueler R, Bors L, Momcilovic D, Pabst S, Nickenig G, et al. Diagnostic value of echocardiography in the diagnosis of pulmonary hypertension. PLoS One. 2012; 7 10.1371/journal.pone.0038519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.





