Abstract
Background
Specific antibody deficiency (SAD) involves a deficient response to a polysaccharide vaccine in the setting of normal IgG levels and chronic infections. Patients with chronic rhinosinusitis (CRS) are often evaluated for SAD. There is limited data describing patients with CRS and SAD.
Objective
To better characterize the role of SAD in CRS.
Methods
We reviewed electronic records of adults with CRS who were evaluated for immunodeficiency with quantitative immunoglobulin levels and pre- and post-antibody titers to a pneumococcal polysaccharide vaccine (PPV).
Results
14 pneumococcal serotypes were determined in 239 subjects from 2002–2009. 64 subjects had adequate protective titers of 1.3 μg/mL or higher in 7 or more serotypes out of the 14 serotypes checked. 56 (23%) had less than 7 protective titers post-PPV and were diagnosed with SAD. 119 subjects had an adequate response to the vaccine with 7 or more serotypes being higher than 1.3 μg/mL (>50% response) and were characterized as “responders”. Subjects with SAD received more antibiotic courses relative to responders in the two years following immunization (3.19 ± 2.64 vs. 2.19 ± 2.24, p<0.05). Ten of 56 subjects (17.9%) with SAD received immunoglobulin (Ig) replacement therapy. Subjects receiving Ig had fewer numbers of protective pneumococcal titers post PPV and had more pneumonia (40.0%) versus subjects with SAD not receiving Ig (10.9%).
Conclusions
Of 239 CRS patients with normal IgG levels evaluated for immunodeficiency, 56 (23.4%) had SAD. A majority of patients with SAD may not need Ig replacement however, a subset of patients with SAD benefit from Ig replacement.
Keywords: Chronic rhinosinusitis, specific antibody deficiency, immunoglobulin replacement therapy, pneumococcal antibody concentration, primary immunodeficiency
INTRODUCTION
Chronic rhinosinusitis (CRS) is a disease that affects over 30 million Americans and is associated with significant morbidity and a high healthcare burden.1 CRS is defined as sinonasal symptoms lasting more than 12 weeks in association with objective evidence of sinus inflammation on computed tomography (CT) scan and/or endoscopy.2,3 Patients with CRS may present with or without nasal polyps (CRSwNP or CRSsNP, respectively). CRS is a significant comorbidity for patients with asthma; in those patients, CRS exacerbations often lead to asthma exacerbations.4,5,6
Risk factors associated with CRS include atopy, structural abnormalities and immune dysfunction. Thus, CRS patients who are refractory to medical therapy, or those who present with recurrent sinus infections, warrant evaluation for immunodeficiency. Antibody deficiencies, such as specific antibody deficiency, are common among the primary immunodeficiencies (PID) and may predispose certain individuals to recurrent sinopulmonary infections.
Specific antibody deficiency (SAD) is defined as an impaired antibody response to immunization with polysaccharide antigens and normal quantitative immunoglobulin levels in the presence of recurrent or chronic respiratory infections.7 Antibiotics may be used to manage recurrent or chronic infections; however, therapies for SAD, in particular immunoglobulin (Ig) replacement therapy, have not been well described.
The use of polysaccharide vaccines to assess the functional competency of humoral immunity is widely used in clinical practice. However, there is a dearth of studies assessing vaccine nonresponders in otherwise healthy individuals and those with recurrent or chronic sinopulmonary infections. The range of anti-pneumococcal antibody titers in healthy adults has been demonstrated by evaluating normal levels of protective titers.8,9,10 In a recent study using a multiplexed bead-based assay, patients with SAD were unable to achieve antibody titer levels at least as great as the fifth percentile of healthy patients.8 The prevalence of all PID in the United States population has been estimated as 1 in every 1,200 persons.11 SAD constitutes the most common disease phenotype (23.1%) seen in pediatric patients with PIDs.12 In children with frequent respiratory infections, the prevalence of SAD is 5–15%.12,13,14,15,16
Despite the use of polysaccharide vaccines as a diagnostic challenge system to assess for SAD in patients with CRS, there are only a limited number of studies of this group of patients. Previous studies have reported SAD in as few as 11.6 % and as many as 67% of adult patients with CRS who failed medical therapy and underwent functional endoscopic sinus surgery (FESS).16,17 To better characterize the role of SAD in CRS, we examined the clinical characteristics, comorbidities, and therapies of patients with CRS and SAD in a tertiary care setting.
METHODS
The present study used a retrospective electronic database chart review of all patients 18 years of age or older at a tertiary care allergy-immunology clinic in Chicago, Illinois. This review was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine. Subjects met inclusion criteria if they had a visit at the allergy-immunology clinic at Northwestern Medical Faculty Foundation (NMFF) between 2002 and 2009, received an ICD-9 diagnosis of CRS, and underwent evaluation for immunodeficiency by assessment of quantitative serum immunoglobulin levels and pneumococcal antibody titers pre- and post-immunization with a pneumococcal polysaccharide vaccine (PPV). The treating physician made the decision to evaluate the individual patient with CRS for immunodeficiency. All subjects had normal IgG levels (>550 mg/dL). Subjects were included if they had a sinus CT scan documenting chronic rhinosinusitis and met criteria for CRS as defined by nationally-recognized consensus statements.2,18
Presence of nasal polyposis was determined by rhinoscopy or surgical pathology specimens, and subjects were subsequently classified as CRSsNP or CRSwNP. Atopy was determined in all subjects via skin testing to pollens, dust-mites, pets, molds, and cockroach using Hollister-Stier Canada (Toronto, ON) extracts. Asthma diagnosis and severity was determined in accordance with the 2007 NAEPP Expert Panel Report 3 guidelines using %FEV1 values.19
Subjects were excluded if they had common variable immunodeficiency (CVID), allergic bronchopulmonary aspergillosis (ABPA), Churg-Strauss syndrome, primary ciliary dyskinesia, cystic fibrosis, malignancy, IgA deficiency, or any acquired immunodeficiencies (e.g. HIV/AIDS or secondary to medications such as chemotherapy).
Evaluation of specific antibody deficiency
Patient immune responses were classified on the basis of number of protective pneumococcal antibody titers according to recent vaccine parameters.20 Serum IgG antibody titers to 14 common pneumococcal serotypes (1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, 23F) were measured for each subject (Specialty Laboratories Inc., Valencia, CA). To avoid overdiagnosis, an adequate response was defined as protective antibody titers (≥1.3 μg/mL) against at least seven of 14 pneumococcal serotypes.7,20 Subjects with adequate pre-immunization titers (≥ 1.3 μg/mL in seven or more out of 14 pneumococcal serotypes) were categorized as “normal baseline”. Subjects with less than seven out of 14 pre-immunization titers greater than or equal to 1.3 μg/mL were categorized as “low baseline,” immunized with Pneumovax® (23-valent unconjugated pneumococcal polysaccharide vaccine, Merck), and had their titers rechecked 6 weeks later. Individuals with seven or more serotypes greater than or equal to1.3 μg/mL after PPV immunization were characterized as “responders”.7,21 Nonresponders were subjects with less than seven out of 14 titers greater than or equal to 1.3 μg/mL post-immunization; these subjects were subsequently diagnosed with SAD.7
Statistical analysis
Evaluation of subjects’ characteristics, anti-pneumococcal antibody titers, and serum immunoglobulin levels was performed via ANOVA with post-hoc Tukey analysis. Analogous Chi-square tests were used to compare percentages for categorical variables. Data are expressed as mean with standard deviation. A p-value less than 0.05 was considered statistically significant; such results have been marked with an asterisk in the table in which they appear.
RESULTS
Population Characteristics
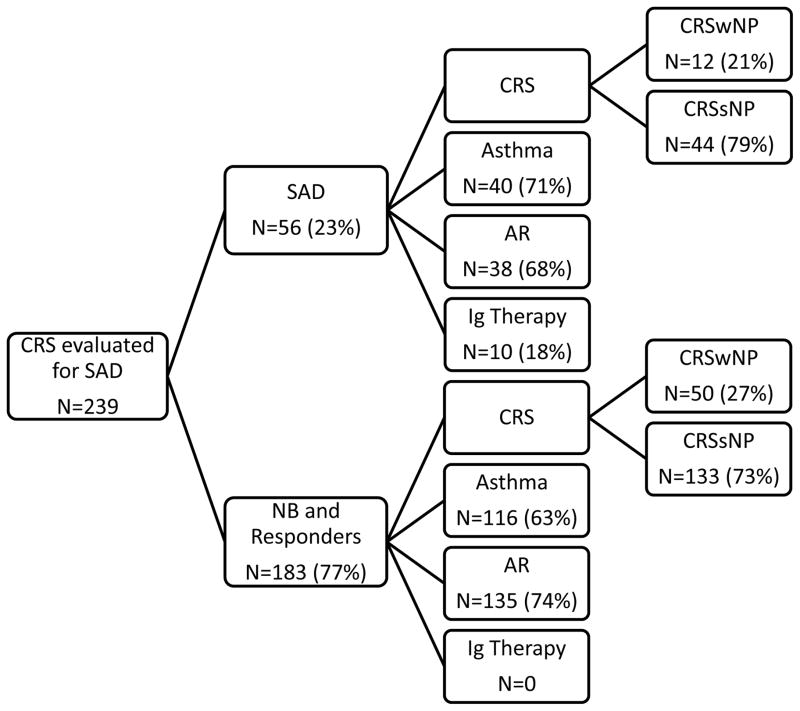
The retrospective review identified 528 patients with an ICD-9 code for CRS who had an abnormal sinus CT scan. Of these 239 (45%) were evaluated for the presence of SAD because of persistent sinonasal disease despite medical and/ or surgical therapy and had normal quantitative immunoglobulins. The decision to evaluate for immunodeficiency was up to the treating allergist. Numbers of pre-immunization pneumococcal antibody titers were protective (levels greater than or equal to 1.3 μg/mL in at least seven of 14 serotypes checked) at baseline in 64 (26.8%) subjects (normal baseline). Numbers of post-immunization pneumococcal antibody titers were protective in 119 (49.8%) subjects (responders). Fifty six (23.4%) subjects were classified as nonresponders and had less than 50% of the pneumococcal serotypes greater than or equal to 1.3 μg/mL after PPV immunization (Figure 1). Of the nonresponders, nine had received the vaccine but had had their pre-immunization titers checked elsewhere. Nonresponders were subsequently diagnosed with SAD on the basis of an inadequate response to a polysaccharide vaccine. There was no difference in age, sex, or race among subjects with SAD, responders, and normal baseline subjects (Table I).
FIG 1.
Comorbidities and immunoglobulin treatment in patients with CRS and SAD and CRS patients without SAD.
NB, normal baseline; SAD, specific antibody deficiency; CRS, chronic rhinosinusitis, CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; AR, allergic rhinitis, Ig, immunoglobulin
Table I.
Patient Demographics
| Nonresponders (N=56) | Responders (N=119) | Normal Baseline (N=64) | |
|---|---|---|---|
| Age, years (mean ± SD) | 47.96 ± 11.40 | 47.66 ± 13.57 | 50.78 ± 13.94 |
| Female sex | 38 (67.9%) | 81 (68.1%) | 47 (73.4%) |
| Race | |||
| Caucasian | 40 (71.4%) | 87 (73.1%) | 51 (79.7%) |
| African American | 4 (7.1%) | 5 (4.2%) | 4 (6.2%) |
| Hispanic | 1 (1.8%) | 6 (5.0%) | 3 (4.7%) |
| Asian | 0 | 1 (0.8%) | 1 (1.6%) |
| Other/Unknown | 11 (19.6%) | 20 (16.8%) | 5 (7.8%) |
Number of Protective Titers
Subjects with SAD had the lowest number of protective titers pre-immunization, followed by responders, and then normal baseline subjects (1.51 ± 1.49 vs. 2.53 ± 1.69 vs. 9.70 ± 2.09; p<0.05). Post-immunization, subjects with SAD had lower numbers of protective titers than responders (4.18 ± 1.79 vs. 10.11 ± 2.19; p<0.05; Figure 2). Subjects with SAD receiving Ig replacement therapy had significantly fewer numbers of protective titers post PPV compared to the subjects with SAD who did not receive Ig therapy (Table IV). All subjects had IgG levels greater than 550 mg/dL. Although within the normal range, subjects with SAD had lower levels of total serum IgG as compared to normal baseline subjects (918.70 ± 264.48 mg/dL vs. 1035.03 ± 292.21 mg/dL; p<0.05).
FIG 2.
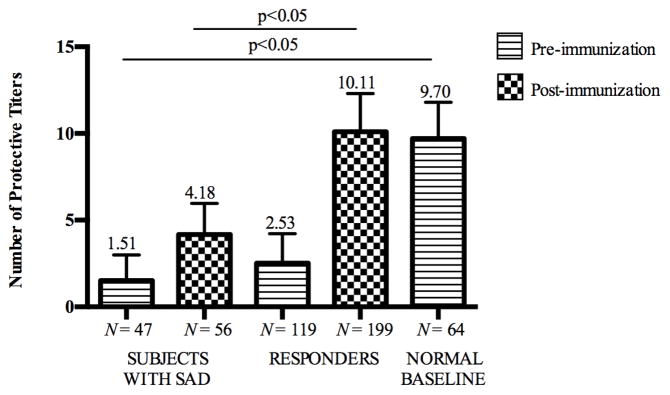
Numbers of protective pre- and post-post polysaccharide vaccine immunization titers were lower in subjects with SAD compared to responders and subjects with normal baseline.
Table IV.
Clinical Characteristics of Subjects with SAD by Ig Replacement Therapy
| Received Ig (N=10) | No Ig Received (N=46) | P-value | |
|---|---|---|---|
| Age, years (mean ± SD) | 53.10 ± 11.36 | 46.85 ± 11.22 | 0.12 |
| Female Sex | 7 (70.0%) | 31 (67.4%) | 0.60 |
| Caucasian | 4 (40.0%) | 36 (78.3%) | <0.05 |
| African American | 2 (20.0%) | 2 (4.3%) | <0.05 |
| Hispanic | 1 (10.0%) | 0 | <0.05 |
| Other/Unknown | 3 (30.0%) | 8 (17.4%) | <0.05 |
| # of pre-PPV titers >1.3μg/mL | 1.10 ± 1.37 | 1.62 ± 1.52 | 0.33 |
| # of post-PPV titers >1.3μg/mL | 2.70 ± 2.06 | 4.50 ± 1.57 | <0.05 |
| IgG, mg/dL (mean ± SD) | 860.10 ± 301.47 | 932.02 ± 257.32 | 0.44 |
| IgA, mg/dL (mean ± SD) | 117.80 ± 47.11 | 191.09 ± 85.95 | <0.05 |
| Allergic Rhinitis | 5 (50.0%) | 33 (71.7%) | 0.17 |
| Asthma | 9 (90.0%) | 31 (67.4%) | 0.15 |
| Imaging abnormalities | 6/8† (75.0%) | 17/29† (58.6%) | 0.34 |
| History of pneumonia | 4 (40.0%) | 5 (10.9%) | <0.05 |
| FESS | 5 (50.0%) | 25 (54.3%) | 0.54 |
| Antibiotics (mean ± SD) δ | 3.70 ± 2.31 | 3.07 ± 2.72 | 0.50 |
Denominators represent total number of individuals in each group that underwent an imaging modality (either chest CT or chest X-ray).
Courses of antibiotics received in the two years following immunization.
PPV, polysaccharide pneumococcal vaccine; Ig, immunoglobulin; FESS, functional endoscopic sinus surgery
Numbers of Protective Post-Immunization Titers by CRS Type and Asthma Status
A subgroup analysis was performed based on the presence or absence of asthma. Within the CRSsNP group, the numbers of protective titers post-immunization were significantly lower in the CRSsNP patients with asthma as compared to the CRSsNP patients without asthma (7.41 ± 3.70 vs. 9.30 ± 3.23; p<0.05;). When further categorized by severity based on %FEV1, the moderate-to-severe CRSsNP asthmatics had significantly lower numbers of protective titers post-immunization relative to non-asthmatics and mild asthmatics (6.76 ± 3.68 vs. 9.14 ± 3.30 vs. 7.90 ± 3.41; p<0.05; Figure 3). Within the CRSwNP group, there was no significant difference in the numbers of protective post-immunization titers based on the presence of asthma.
FIG 3.
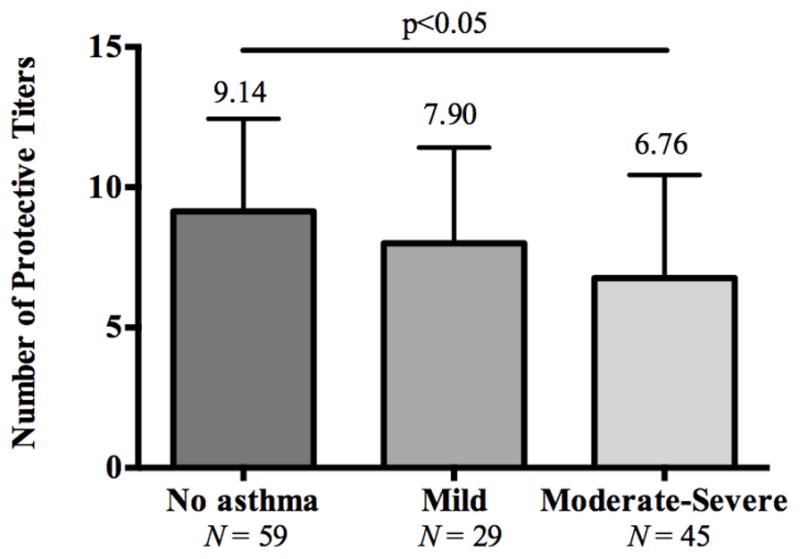
Numbers of protective titers post immunization with a polysaccharide vaccine were lower in moderate-severe asthmatics compared to subjects with mild asthma.
Radiographic Abnormalities
Radiographic lung abnormalities were studied by chest X-ray and/or CT scan, if available. Not all patients had chest imaging, as lung architecture is not routinely assessed in subjects with CRS. A chest radiologist verified abnormal findings on imaging and they included atelectasis, bronchiectasis, pleural effusions, nodules, consolidations, fibrosis, and linear infiltrates. There was no difference among the groups (Table II).
Table II.
Lung Pathology by Responder Status
| SAD (N=56) | Responders (N=119) | Normal Baseline (N=64) | |
|---|---|---|---|
| Imaging abnormalities | 23/37† (62.2%) | 31/80† (38.8%) | 25/49† (51.0%) |
| History of Pneumonia | 9 (16.1%) | 17 (14.3%) | 12 (18.8%) |
Denominators represent total number of individuals in each group that underwent at least one imaging modality (either chest CT, chest X-ray, or both).
SAD, specific antibody deficiency
All subjects had an abnormal sinus CT scan however, there was no difference in severity in subjects with SAD compared to those with normal baseline or responders (data not reported).
Therapies
Subjects with SAD tended to have fewer FESS procedures (30, 53.6%) relative to responders (83, 69.7%) and normal baseline subjects (45, 70.3%; p=0.08). Ten of the subjects with SAD (17.9%) were receiving immunoglobulin (Ig) replacement therapy. No other subjects, either in the responders or normal baseline groups, were receiving Ig. Subjects with SAD received more antibiotics courses for sinopulmonary infections relative to responders in the two years following immunization with PPV (3.19 ± 2.64 vs. 2.19 ± 2.24; p<0.05; Table III). This information was collected from telephone records, clinic visit documentations, and from the medication section of the electronic medical records. Within the SAD group, patients with asthma received more courses of antibiotics than patients without asthma (3.82 ± 2.60 vs. 1.43 ± 1.87; p<0.05).
Table III.
Therapies by Responder Status
| SAD (N=56) | Responders (N=119) | |
|---|---|---|
| Functional endoscopic sinus surgery (FESS) | 30 (53.6%) | 83 (69.7%) |
| Immunoglobulin replacement therapy* | 10 (17.9%) | 0 |
| Antibiotics courses (mean ± SD)*, δ | 3.19 ± 2.64 | 2.19 ± 2.24 |
| - In patients with asthma* | 3.82 ± 2.60 | 2.89 ± 2.38 |
| - In patients without asthma* | 1.43 ± 1.87 | 1.28 ± 1.67 |
Significant difference at the p<0.05 level.
Courses of antibiotics received in the two years following immunization.
SAD, specific antibody deficiency
Characteristics of CRS Subjects with SAD Receiving Ig Replacement Therapy
The clinical characteristics of subjects with SAD receiving Ig replacement therapy were compared to those with SAD not receiving Ig therapy (Table IV). Of note, four of 10 patients with SAD receiving Ig (40.0%) had a history of clinically documented pneumonia versus five of the 46 patients with SAD not receiving Ig (10.9%; p<0.05). Subjects with SAD receiving Ig replacement therapy had significantly fewer numbers of protective titers post PPV compared to the subjects with SAD who did not receive Ig therapy (Table IV). Global assessment by the treating physician demonstrated improvement in all subjects on Ig relative to their medical condition before starting Ig (Table V). Prior to receiving Ig, chart review documented frequent infections. After initiation of Ig replacement, seven of the ten (70.0%) had fewer sinopulmonary infections. Three of ten continued to have sinus infections, albeit fewer than before receiving Ig.
Table V.
Global Assessment of Patients with SAD Receiving Immunoglobulin Replacement
| Age | Pertinent Past Medical History | Global Assessment by Treating Physician |
|---|---|---|
| 55 |
|
Improved greatly with fewer sinus infections. |
| 37 |
|
Improved greatly. Reduced frequency of sinus infections and asthma exacerbations. Less prednisone and fewer acute physician visits. |
| 64 |
|
Improved greatly. Reduced severity and frequency of sinus infections. |
| 49 |
|
Improved greatly. Reduced severity and frequency of sinus infections. |
| 66 |
|
Improved greatly: Reduced severity and frequency of sinus infections. Fewer antibiotics courses. |
| 43 |
|
Improved but continues to have sinus infections about three times a year requiring treatment with antibiotics. |
| 59 |
|
Improved greatly. |
| 37 |
|
Improved but continues to have sinus infections. |
| 52 |
|
Improved but sinus disease remains hard to manage; however fewer antibiotics courses. |
| 67 |
|
Improved greatly with fewer sinus infections. |
Asthma severity was graded according to the National Heart, Lung, and Blood Institute’s 2007 NAEPP Expert Panel Report 3 guidelines using %FEV1 values19
Functional endoscopic sinus surgery.
DISCUSSION
This study was conducted to characterize patients with CRS and SAD. Guidelines for the treatment of specific antibody deficiency (SAD) are unclear, and patients with SAD present a therapeutic challenge. In this study of 239 adult patients with CRS with normal immunoglobulin levels who underwent evaluation for immunodeficiency, 56 (23.4%) met the criteria for SAD. A previous study found SAD in 67% of CRS patients; however, this study used more liberal criteria to define SAD.17 The definition of SAD and what constitutes an adequate number of protective serotypes after a polysaccharide vaccine is based on consensus documents and is controversial. This study considered an adequate response as protective titers (≥1.3 μg/mL) in seven out of 14 (50%) of pneumococcal serotypes after PPV immunization so as to not overdiagnose SAD.
Lung imaging abnormalities on either chest x-ray or CT scan tended to be more common in patients with SAD versus responders or normal baseline patients; however this was not significant. No significant difference was found in pneumonia prevalence among the patient groups; however, it is worth noting that the normal baseline group in this study included patients with CRS, not healthy controls. Not all patients underwent chest radiography and this was a limitation of the study. These findings also suggest that most patients with CRS and SAD don’t have worse lower airway disease compared to CRS patients without SAD. Within the SAD group, there was a trend toward a history of more pneumonia in patients receiving immunoglobulin (Ig) replacement therapy (40.0%) compared to those who did not receive any Ig (10.9%). In addition, those with SAD were more likely to receive antibiotics in the two years following immunization with pneumococcal vaccine relative to those who had an adequate response to the vaccine. This suggests that CRS patients with SAD may have a higher propensity for infectious exacerbations than the patients with CRS who have an adequate response to PPV. It is also possible that the pneumococcal vaccine, which was given for diagnostic purposes, has a therapeutic benefit in some patients.20 A limitation of this retrospective study is the fact that the subjects assessed in this study were managed by different physicians in a group practice and there were no uniform criteria for antibiotic prescriptions and potentially treating physicians had a lower threshold for prescribing antibiotics to those patients who carried a diagnosis of SAD.
Asthmatics with SAD received significantly more courses of antibiotics relative to patients with SAD who did not have asthma (Table III). Further study is needed to determine the role of asthma in these patients. The nature of the relationship between asthma and bacterial infection is a topic of great interest within the field. Airway microbiotic patterns have been found to be altered in those with asthma, and the composition of the flora may be associated with bronchial hyperreactivity in these patients.22 Specifically, asthmatics may face increased colonization with S. pneumoniae,23 and patients with asthma are at increased risk for invasive pneumococcal infections as compared to those without asthma.21,24,25,26,27 As a result, it is currently recommended that adults (19–64 years) with asthma receive immunization with the 23-valent pneumococcal polysaccharide vaccine.20,28
Interestingly, in this study asthmatics with CRSsNP had a less robust response to the pneumococcal vaccine as compared to the CRSsNP patients without asthma; this trend was not seen in the CRSwNP subjects. The lowest response to vaccine was seen in the moderate-to-severe asthmatics in the CRSsNP group. This suggests that CRSsNP asthmatics may have an impaired mucosal response to S. pneumoniae exposure as well as an impaired systemic polysaccharide antibody response as compared to their non-asthmatic counterparts (Figure 3).
Despite receiving more antibiotics than those without SAD, only 10 (18%) of the patients with SAD received Ig. Ig is costly and has potential side effects.29,30,31 In one retrospective study of 75 patients with SAD, Cheng et al. found that 30 (40%) had received gamma-globulin replacement. These patients developed significantly fewer infections after Ig as compared to before.32 In the present study, only a minority of subjects with SAD received Ig. In our study, we did not prospectively follow those with SAD who did not receive Ig long term. However, the fact that they do not differ significantly from those without SAD in terms of radiographic abnormalities and only received slightly more courses of antibiotics suggests that these patients fare well without Ig.
The subgroup of subjects with SAD receiving Ig had a higher prevalence of pneumonia prior to Ig replacement relative to the subgroup not receiving Ig. The subjects with SAD receiving Ig also tended to be older, with lower numbers of protective titers post-immunization, lower levels of IgA and more likely to have asthma compared to subjects with SAD not receiving Ig (Table IV). In this study, patients with SAD who received Ig tended to have more radiographic lung abnormalities relative to patients with SAD who did not receive Ig. These findings suggest that it is important to identify patients with SAD as some of them tend to have more severe disease. However, this retrospective study is not designed to address the question of who should receive Ig replacement.
The treatment of SAD patients has been driven on the basis of their clinical characteristics and severity of infections. It appears that most patients with SAD appear to fare well without the use of Ig, and very few may actually need such therapy for management of their immunodeficiency. In general, this study suggests that patients with CRS and SAD who develop pneumonia and lower airway lung damage may be the optimal group for Ig, as they are likely the most severely affected. Pneumococcal vaccine responses, or lack thereof, may also be classified on the basis of severity (mild, moderate, or severe) or phenotype (presence of defects in long-term “memory” in the form of plasma cells derived from memory B-cells), and some have suggested that only patients with the severe and/or memory phenotypes of SAD should be given Ig.33,34 The current study supports these recommendations by showing that those receiving Ig therapy had significantly fewer numbers of protective pneumococcal serotypes post PPV compared to the patients with CRS and SAD not receiving Ig therapy.
In this retrospective study of 239 adult CRS patients evaluated for immunodeficiency, 23.4% were diagnosed with SAD. Patients with SAD received slightly more courses of antibiotics than those without SAD, and only 17.9% of patients with SAD received Ig. The study has a number of methodological limitations. First, potential biases were present in this study since the patients were treated by multiple physicians in the practice and there were no uniform criteria for starting Ig replacement or antibiotics for respiratory exacerbations. Second, patients in this study were adults managed at an academic center and the study did not include children so results cannot be generalized to all patient groups. Third, study criteria included only patients who were referred to an allergy-immunology clinic for a work-up of primary immunodeficiency. Fourth, not all patients underwent chest radiography and this is a major limitation of this retrospective study. Future studies conducted in a prospective manner with a larger sample of patients controlling for these factors, as well as prospective population-based studies of vaccine responders and nonresponders in healthy individuals, may help clarify which patients with SAD should be considered for immunoglobulin replacement therapy.
What is already known about this topic?
Guidelines for the treatment of SAD using antibiotics or immunoglobulin replacement are unclear. Current therapies include antibiotics and immunoglobulin replacement therapy.
What does this article add to our knowledge?
23% of patients with CRS and normal IgG levels who were evaluated for immunodeficiency had SAD. Subjects with CRS and SAD received significantly more antibiotic courses compared to the subjects with CRS without SAD. Subjects with SAD receiving Ig replacement therapy had significantly fewer numbers of protective titers post pneumococcal vaccine and had more lower airway disease compared to the subjects with SAD who did not receive Ig therapy.
How does this study impact current practice management guidelines?
Consider immunodeficiency evaluation in patients with difficult to treat CRS. This study suggests recommending immunoglobulin replacement therapy only to a carefully selected group of patients with SAD; those patients with SAD who also have pneumonia may be suitable candidates for immunoglobulin replacement therapy.
Acknowledgments
Declaration of all sources of funding: The Ernest S. Bazley Grant to Northwestern University and Northwestern Memorial Hospital and the Division of Allergy-Immunology at Northwestern University, Feinberg School of Medicine.
Abbreviations used
- CRS
Chronic rhinosinusitis
- CRSsNP
Chronic rhinosinusitis without nasal polyps
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- CT
Computed tomography
- dL
Deciliter
- FEV1
Forced expiratory volume in the first second of expiration
- ICD-9
International Classification of Diseases, Ninth Revision
- Ig
Immunoglobulin
- mg
Milligram
- μg
Microgram
- mL
Milliliter
- PID
Primary immunodeficiencies
- PPV
Pneumococcal polysaccharide vaccine
- S. pneumoniae
Streptococcus pneumoniae
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;193:3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists Rhinol. 2012;50(1):1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 4.Slavin RG, Spector SL, Bernstein IL, Kaliner MA, Kennedy DW, Virant FS, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunl. 2005;116(6 Suppl):S13–47. doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 5.Oehling AK. Bacterial infection as an important triggering factor in bronchial asthma. J Investig Allergol Clin Immunol. 1999;9(1):6–13. [PubMed] [Google Scholar]
- 6.Lotvall J, Ekerljung L, Lundback B. Multi-symptom asthma is closely related to nasal blockage, rhinorrhea and symptoms of chronic rhinosinusitis-evidence from the West Sweden Asthma Study. Respir Research. 2010;11:163. doi: 10.1186/1465-9921-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 8.Borgers H, Moens L, Picard C, Jeurissen A, Raes M, Sauer K, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134(2):198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Musher DM, Luchi MJ, Watson DA, Hamilton R, Baughn RE. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Inf Dis. 1990;161(4):728–35. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 10.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Inf Dis. 2010;201(4):516–24. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 11.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27(5):497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- 12.Javier FC, 3rd, Moore CM, Sorensen RU. Distribution of primary immunodeficiency diseases diagnosed in a pediatric tertiary hospital. Ann Allergy Asthma Immunol. 2000;84(1):25–30. doi: 10.1016/S1081-1206(10)62736-6. [DOI] [PubMed] [Google Scholar]
- 13.Epstein MM, Gruskay F. Selective deficiency in pneumococcal antibody response in children with recurrent infections. Ann Allergy Asthma Immunol. 1995;75(2):125–31. [PubMed] [Google Scholar]
- 14.Hidalgo H, Moore C, Leiva LE, Sorensen RU. Preimmunization and postimmunization pneumococcal antibody titers in children with recurrent infections. Ann Allergy Asthma Immunol. 1996;76(4):341–6. doi: 10.1016/S1081-1206(10)60035-X. [DOI] [PubMed] [Google Scholar]
- 15.Boyle RJ, Le C, Balloch A, Tang ML. The clinical syndrome of specific antibody deficiency in children. Clin Exp Immunol. 2006;146(3):486–92. doi: 10.1111/j.1365-2249.2006.03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr TF, Koterba AP, Chandra R, Grammer LC, Conley DB, Harris KE, et al. Characterization of specific antibody deficiency in adults with medically refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):241–4. doi: 10.2500/ajra.2011.25.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alqudah M, Graham SM, Ballas ZK. High prevalence of humoral immunodeficiency patients with refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(6):409–12. doi: 10.2500/ajra.2010.24.3532. [DOI] [PubMed] [Google Scholar]
- 18.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 19.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 20.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 Suppl):S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Landesman SH, Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Inf Dis. 1981;3 (Suppl):S184–97. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- 22.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PloS one. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jounio U, Juvonen R, Bloigu A, Silvennoinen-Kassinen S, Kaijalainen T, Kauma H, et al. Pneumococcal carriage is more common in asthmatic than in non-asthmatic young men. Clin Respir J. 2010;4(4):222–9. doi: 10.1111/j.1752-699X.2009.00179.x. [DOI] [PubMed] [Google Scholar]
- 24.Juhn YJ, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Clin Allergy Immunol. 2008;122(4):719–23. doi: 10.1016/j.jaci.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung JA, Kita H, Dhillon R, Jacobson RM, Nahm MH, Park M, et al. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad Med. 2010;122(5):116–24. doi: 10.3810/pgm.2010.09.2208. [DOI] [PubMed] [Google Scholar]
- 26.Jung JA, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, et al. Increased risk of serious pneumococcal disease in patients with atopic conditions other than asthma. J Allergy Clin Immunol. 2010;125(1):217–21. doi: 10.1016/j.jaci.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352(20):2082–90. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease C, Prevention, Advisory Committee on Immunization P. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morbidity and mortality weekly report. 2010 Sep 3;59(34):1102–6. [PubMed] [Google Scholar]
- 29.Wasserman RL, Sorensen RU. Evaluating children with respiratory tract infections: the role of immunization with bacterial polysaccharide vaccine. Ped Inf Dis J. 1999;18(2):157–63. doi: 10.1097/00006454-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 30.May A, Zielen S, von Ilberg C, Weber A. Immunoglobulin deficiency and determination of pneumococcal antibody titers in patients with therapy-refractory recurrent rhinosinusitis. Eur Arch Otorhinolaryngol. 1999;256(9):445–9. doi: 10.1007/s004050050186. [DOI] [PubMed] [Google Scholar]
- 31.Jefferis R, Kumararatne DS. Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol. 1990;81(3):357–67. doi: 10.1111/j.1365-2249.1990.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng YK, Decker PA, O’Byrne MM, Weiler CR. Clinical and laboratory characteristics of 75 patients with specific polysaccharide antibody deficiency syndrome. Ann Allergy Asthma Immunol. 2006;97(3):306–11. doi: 10.1016/S1081-1206(10)60794-6. [DOI] [PubMed] [Google Scholar]
- 33.Fried AJ, Bonilla FA. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev. 2009;22(3):396–414. doi: 10.1128/CMR.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orange JS, Ochs HD, Cunningham-Rundles C. Prioritization of Evidence-Based Indications for Intravenous Immunoglobulin. J Clin Immunol. 2013;33(6):1033–36. doi: 10.1007/s10875-013-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]