It is postulated that disease relapse in patients with acute myeloid leukemia (AML) is consequent upon chemoresistance within leukemic stem/progenitor cell (LSC) populations from which bulk blasts arise1. In adults with high risk AML, allogeneic hematopoietic cell transplantation (HCT) has become a central component of the treatment algorithm to overcome this chemoresistance as it delivers maximal anti-leukemic activity through both dose intensification and by the genesis of a potent graft-versus-leukemia (GVL) effect2-4. However relapse still occurs in a significant proportion of allografted patients and now represents the major cause of treatment failure particularly with reduced intensity conditioning (RIC) regimens5. Whilst minimal residual disease (MRD) from the bulk leukemic population is known to be prognostic, more accurate predictors of relapse risk might be developed from detection of putative LSC populations pre- or post-transplant. However, to date an association between LSC and transplant outcome remains uncertain.
Xenotransplantation assays that have characterised the leukemic propagating functional properties of LSCs cannot be applied as a routine clinical assay to track LSC residual disease in AML patients. As well as monitoring MRD from bulk leukemic blasts6, multiparameter flow cytometry (MFC) assays can be used to quantitate candidate immunophenotypic hematopoietic stem /progenitor populations characterised as enriched for LSC activity7, 8. We report the first study to evaluate an immunophenotypic LSC assay as a biomarker for outcome in allografted AML patients, performed in parallel with standard MFC-MRD monitoring (using conventional leukemic-aberrant-immunophenotypes, LAIPs) and chimerism studies for pre- and post-HCT time-points in 101 adults undergoing HCT for high risk AML or myelodysplasia. Our cohort included predominantly older patients, many of whom were allografted using a reduced intensity conditioning (RIC) regimen (RIC=73; myeloablative conditioning, MAC=28). The results are thus relevant to similar patient populations in many adult transplant centres, as RIC HCT is increasingly used for older patients with AML / high risk MDS.
Immunophenotypic LSC (MFC-LSC) were monitored by quantitating the lymphoid-primed multi-potential progenitor-like (LMPP-like) stem/progenitor compartment. This assay does not require an aberrant LSC profile to be identified at presentation but instead measures an abnormally expanded immunophenotypic CD34+ population previously functionally characterised to be LSC-containing7, 9, with a detection threshold of 0.02% of total nucleated bone marrow cells (validated in9 and further validated for this study, Supplementary Figure 2B). Detection of these MFC-LSC antedated morphological relapse in a prior cohort of patients post chemotherapy9 (further example Supplementary Figure 3). Detailed methods for this study are available in Supplementary Information.
The pre-transplant demographics of all 101 patients stratified by MFC-MRD and MFC-LSC status pre- and post-HCT are summarised in Table 1. In the overall cohort there were 36 deaths, 25 relapses, 17 NRM with a median follow-up among survivors of 18 months (range 7-44 months). 28 patients had acute GVHD and 10 patients received donor lymphocyte infusions (DLI).
Table 1. Demographic and clinical characteristics of study cohort - MFC-MRO and LSC status pre and post HCT.
| Parameter | All (N =101) | pre HCT MRD (MFC-MRD) (n =66) | post HCT MRD (MFC-MRD) (n =69> | pre HCT LSC (MFC-LSC) (n =72) | post HCT LSC (MFC-LSC) (n = 92) | ||||
|---|---|---|---|---|---|---|---|---|---|
| MRD+ (n=33) n(%) | MRD− (n=33) n(%) | MRD+ (n=23) n(%) | MRD− (n=46) n(%) | LSC+ (n=15) n(%) | LSC− (o=57) n(%) | LSC+ (n=16) n(%) | LSC− (n=76) n(%) | ||
| Age at transplant, years | |||||||||
| Median | 53 | 51 | 55 | 47 | 56 | 49 | 53 | 50 | 55 |
| Range | 80-70 | 18-68 | 18-70 | 19-66 | 18-70 | 18-70 | 20-70 | 19-66 | 18-70 |
| Sex | |||||||||
| Male | 61 (60%) | 20 (61%) | 20 (61%) | 16 (70%) | 26 (57%) | 5 (33%) | 31 (54%) | 12 (75%) | 45 (59%) |
| Female | 40 (40%) | 13 (39%) | 13 (39%) | 7 (30%) | 20 (43%) | 10 (67%) | 26 (46%) | 4 (25%) | 31 (41%) |
| Disease Type, | |||||||||
| De novo AML | 74 (73%) | 25 (76%) | 24 (73%) | 18 (78%) | 34 (74%) | 10 (67%) | 43 (75%) | 11 (69%) | 55 (72%) |
| Secondary AML | 22 (22%) | 6 (18%) | 8 (24%) | 5 (22%) | 8 (17%) | 4 (27%) | 13 (23%) | 5 (31%) | 17 (22%) |
| high risk MDS | 5 (5%) | 2 (6%) | 1 (3%) | 0 (0%) | 4 (9%) | 1 (1%) | 1 (2%) | 0 (0%) | 4 (5%) |
| Cytogenetic risk group | |||||||||
| Favourable | 7(7%) | 1(3%) | 2(6%) | 0(0%) | 5(11%) | 0(0%) | 3(5%) | 0(0%) | 5(7%) |
| Intermediate | 70(69%) | 17 (52%) | 25(76%) | 13(57%) | 30(65%) | 6(40%) | 43(75%) | 10(63%) | 57(75%) |
| Adverse | 21 (21%) | 14 (42%) | 5(15%) | 10(43%) | 9(20%) | 9(60%) | 10(18%) | 6(38%) | 12(16%) |
| Unknown / missing | 3(3%) | 1(3%) | 1(3%) | 0(0%) | 2(4%) | 0(0%) | 1(2%) | 0(0%) | 2(3%) |
| FLT3 ITD mutant | 31 (31%) | 10(30%) | 15(45%) | 9(39%) | 14 (30%) | 6(40%) | 21 (37%) | 7(44%) | 23(30%) |
| FLT3 ITD wild type | 60(59%) | 20(61%) | 18(55%) | 12(52%) | 30(65%) | 9(60%) | 34(60%) | 7(44%) | 46(61%) |
| FLT3 ITD no data | 10(10%) | 3(9%) | 0(0%) | 2(9%) | 2(4%) | 0(0%) | 2(4%) | 2(13%) | 7(9%) |
| NPM1 mutant | 14(14%) | 4(12%) | 5(15%) | 3(13%) | 5(11%) | 1(7%) | 9(16%) | 1(6%) | 12(16%) |
| NPM1 wild type | 26(26%) | 12(36%) | 9(27%) | 7(30%) | 13(28%) | 5(33%) | 16(28%) | 6(38%) | 19(25%) |
| NPM1 no data | 61(60%) | 17(52%) | 19 (58%) | 13(57%) | 28(61%) | 9(60%) | 32(56%) | 9(56%) | 45(59%) |
| No. of chemotherapy courses prior to HCT | |||||||||
| 1 | 5(5%) | 4(12%) | 0(0%) | 0(0%) | 4(9%) | 1(1%) | 2(4%) | 0(0%) | 5(7%) |
| 2 | 29 (29%) | 9 (27%) | 10 (30%) | 9 (39%) | 14 (30%) | 6 (40%) | 17 (30%) | 7 (44%) | 20 (26%) |
| ≥3 | 34 (34%) | 12 (36%) | 17 (52%) | 11 (48%) | 17 (37%) | 8 (53%) | 36 (63%) | 8 (50%) | 47 (62%) |
| Missing data | 33 (33%) | 8 (24%) | 6 (18%) | 3 (13%) | 11 (24%) | 0 (0%) | 2 (4%) | 1 (6%) | 4 (5%) |
| CR status pre transplant | |||||||||
| CR1 (incfcidng CR1i) | 69 (68%) | 22 (67%) | 28(85%) | 18(78%) | 34(74%) | 10(67%) | 43(75%) | 9(56%) | 54(71%) |
| CR2 (incfcidng CR2i) | 25(25%) | 6(18%) | 5(15%) | 2(9%) | 11(24%) | 2(13%) | 13(23%) | 3(19%) | 18(24%) |
| CR3 (incfcidng CR3i | 2(2%) | 0(0%) | 0(0%) | 0(0%) | 1(2%) | 0(0%) | 0(0%) | 0(0%) | 2(3%) |
| CRi (either CRIi or CR2t) ANC < 1.000/μL and/or platelets < 100.000/μL) | 23 (23%) | 7(21%) | 2(9%) | 8(17%) | 20(20%) | 4(27%) | 12(21%) | 3(19%) | 17(22%) |
| Not in CR | 5(5%) | 5(15%) | 0(0%) | 3(13%) | 1(2%) | 3(20%) | 1(2%) | 3(19%) | 2(3%) |
| Conditioning Regimen | |||||||||
| Reduced Intensify (RIC) | 73 (72%) | 23 (70%) | 26 (79%) | 14 (61%) | 37 (80%) | 10 (67%) | 41 (72%) | 13 (23%) | 57 (75%) |
| Donor type related / unrelated | 24/M9 | 9/14 | 9/17 | 5/9 | 12/25 | 4/6 | 16/25 | 4/9 | 17/40 |
| Myeloablative (MAC) | 28 (28%) | 10 (30%) | 7 (21%) | 9 (39%) | 9 (20%) | 5 (33%) | 16 (28%) | 3 (19%) | 19 (25%) |
| Donor type related / unrelated | 11/17 | 3/7 | 3/4 | 2/7 | 5/4 | 2/3 | 5/11 | 0/3 | 10/9 |
| Source of stem cells | |||||||||
| Peripheral blood | 96 (95%) | 31 (94%) | 32 (97%) | 23 (100%) | 44 (96%) | 15 (100%) | 55 (96%) | 16 (100%) | 71 (93%) |
| Bone marrow | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0 |
| Cord blood | 5(5%) | 2(6%) | 1(3%) | 0(0%) | 2(4%) | 0(0%) | 2(4%) | 0(0%) | 5(66%) |
| Patient / Donor CMV status | |||||||||
| negative / negative | 36 (36%) | 9 (27%) | 15 (45%) | 5 (22%) | 16 (35%) | 2 (13%) | 24 (42%) | 3 (19%) | 28 (37%) |
| positive / positive | 33 (33%) | 10 (30%) | 11 (33%) | 9 (39%) | 15 (33%) | 6 (40%) | 19 (33%) | 7 (44%) | 24 (32%) |
| positive / negative | 23 (23%) | 10(30%) | 3 (9%) | 6 (26%) | 10(22%) | 4 (27%) | 10(18%) | 4 (25%) | 18(24%) |
| negative / positive | 9 (9%) | 4 (12%) | 4 (12%) | 3 (13%) | 5 (11%) | 3 (20%) | 4 (7%) | 2 (12%) | 6 (8%) |
| Pre HCT MFC-LSC | |||||||||
| detected | 15 (14%) | 10 (30%) | 3 (9%) | 8 (35%) | 5 (11%) | NA | NA | 6 (38%) | 8 (11%) |
| not detected | 57 (56%) | 19 (58%) | 28 (86%) | 12 (52%) | 27 (59%) | NA | NA | 8 (50%) | 42 (55%) |
| Not done | 29 (29%) | 4 (12%) | 2 (6%) | 3 (13%) | 14 (30%) | NA | NA | 2 (12%) | 26 (34%) |
| Pre HCT MFC-MRD | |||||||||
| detected | 33 | NA | NA | 14 | 14 | 10 (67%) | 19 (33%) | 10 (63%) | 21 (28%) |
| not detected | 33 | NA | NA | 6 | 19 | 3 (20%) | 28 (49%) | 4 (25%) | 24 (32%) |
| Not done | 35 | NA | NA | 3 | 13 | 2 (13%) | 10 (18%) | 2 (12%) | 31 (41%) |
| Acute GVHD (grade 2-4) | 28 (28%) | 11 (33%) | 8 (24%) | 6 (26%) | 13 (28%) | 7 (47%) | 15 (26%) | 5 (31%) | 20 (26%) |
Abbreviations: MRD, minimal residual disease (standard multiparameter flow cytometry assay /MFC-MRD); AML, acute myeloid leukemia; HCT, hematopoietic cell transplantation; ANC, absolute neutrophil count; MFC-LSC, immunophenotypic leukemic stem cell population
Pre-HCT, MFC-LSC-positivity was less frequent (21%, 15/72) compared to MFC-MRD-positivity (50%, 33/66) in assessable patients. However MFC-LSC were detected in 10% of assessable MFC-MRD negative patients and in 17% of patients who could not be analysed for MFC-MRD. Thus MFC-LSC monitoring by this assay identifies a distinct subgroup of patients including some who could not be monitored by conventional MFC-MRD.
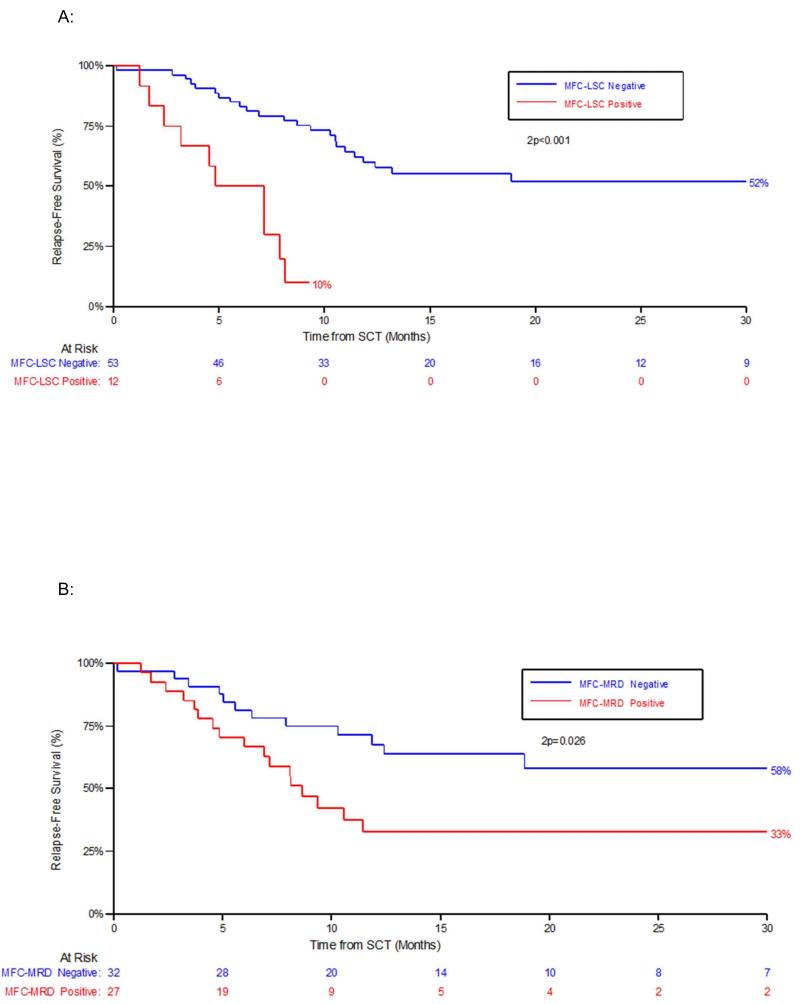
MFC-LSC-positivity within 60 days pre-HCT was highly prognostic for early disease progression with 1 year cumulative incidence of relapse (CIR) of 72% for MFC-LSC-positive vs 19% for MFC-LSC-negative patients (Hazard ratio (HR) 11.9; P<0.001), 1 year relapse-free-survival (RFS) 10% for MFC-LSC-positive vs 60% for MFC-LSC-negative patients (HR 5.84; P<0.001) (Figure 1A) and in addition appeared to be associated with a higher risk of early death with 1 year overall survival (OS) estimates of 46% for MFC-LSC-positive vs 66% for MFC-LSC-negative patients (HR 3.39; P<0.01). When analysis included patients with samples sent between 60 to 90 days pre-HCT, MFC-LSC-positivity remained prognostic for CIR, RFS and OS (Supplementary-Results Table 1).
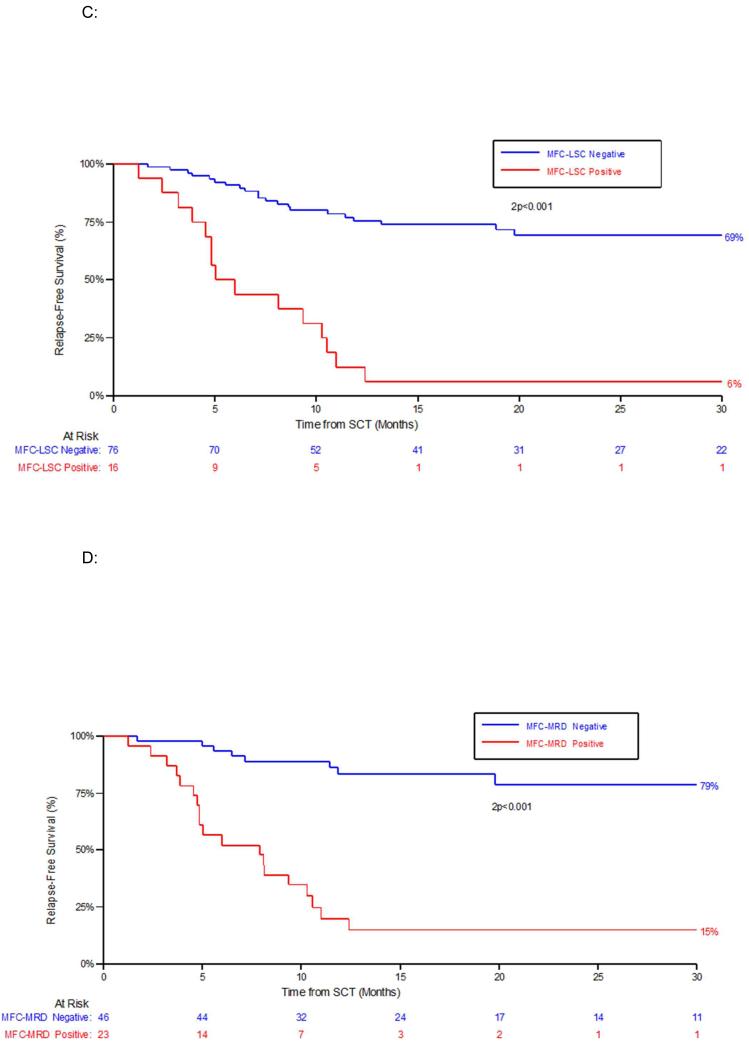
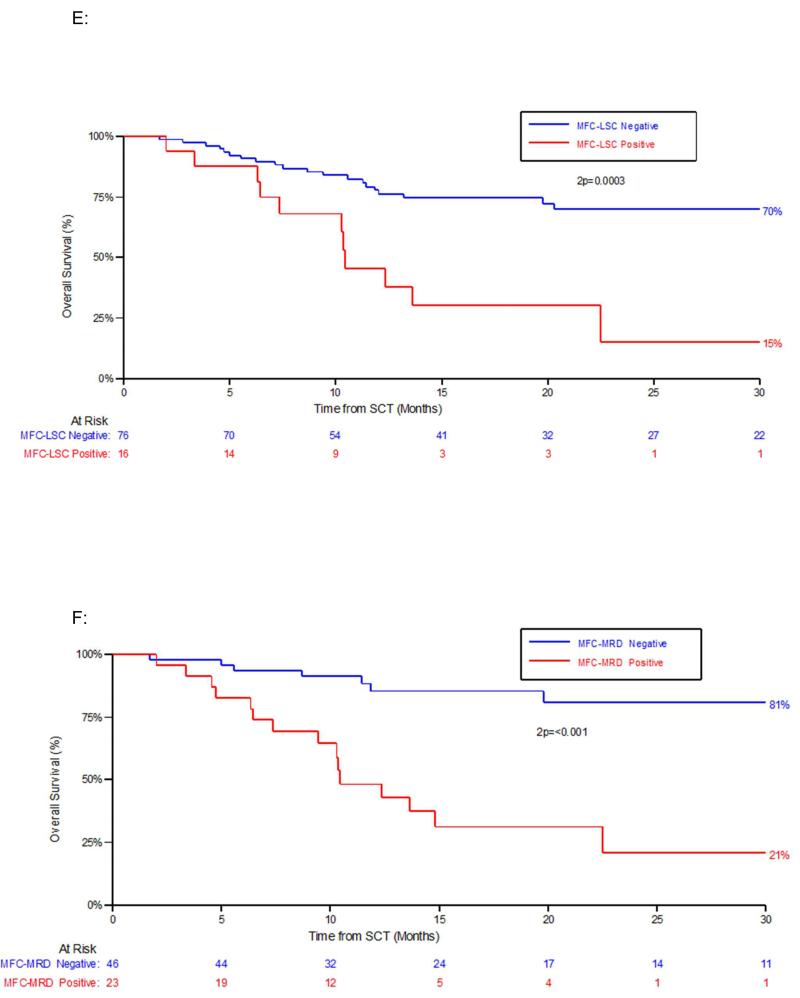
Figure 1. Outcome according to pre-HCT and post-HCT residual disease status by either immunophenotypic assay of LSC populations (MFC-LSC) or by standard flow cytometric detection (MFC-MRD).
A: Relapse free survival (RFS) by MFC-LSC status pre HCT. B: RFS by MFC-MRD status pre HCT
C: Relapse free survival (RFS) by MFC-LSC status at any time-point post HCT. D: RFS by MFC-MRD status at any time-point post HCT.
E: Overall survival (OS) by MFC-LSC status at any time-point post-HCT. F: OS by MFC-MRD status at any time-point post HCT
MFC-MRD-positivity within 60 days pre-HCT was also associated with early disease progression consistent with previous studies, but appeared less prognostic than MFC-LSC (1 year CIR, 55% for MFC-MRD-positive vs 13% for MFC-MRD-negative patients (HR 3.86; P<0.01; 1-year RFS, 33% for MFC-MRD-positive vs 66% for MFC-MRD-negative patients (HR 2.27; P=0.03) (Figure 1B, also Supplementary-Results Table 1).
To ascertain whether MFC-LSC and/or MFC-MRD were more predictive in patients undergoing RIC we excluded the small number of patients undergoing MAC from the analysis of outcome. Detectable MFC-LSC pre-HCT remained a stronger predictor of poor early outcome than MFC-MRD. All but one of RIC patients who were MFC-LSC positive pre-HCT had relapsed and/or died by 240 days from time of transplant (Supplementary Figure 4, 1 year-RFS HR, 10.07; P<0.001) and 1 year OS was 32% (versus 74% for MFC-LSC-negative patients (HR 4.95; P=0.003), (Supplementary-Results Table 1).
MFC-LSC-positivity was more frequent in the adverse risk cytogenetic subgroup [47% adverse risk vs 12% favourable/intermediate (including FLT3-mutated); P=0.003] with similar results for MFC-MRD [74% adverse risk vs 39% favourable/intermediate; P=0.01]. We therefore tested the relevance of MFC-LSC as a pre-transplant prognostic factor in a multivariate analysis that included MRD status as well as cytogenetic risk (adverse vs favourable/intermediate). Fifty-two patients (n=40 RIC, n=12 MAC) with pre-transplant bone marrow samples within 60 days of their HCT could be monitored for both MFC-MRD and MFC-LSC and had a complete data set required for multivariate analysis. Despite adjustment for MFC-MRD in this analysis, MFC-LSC status pre-transplant was an independent predictor of outcome [CIR HR, 6.62; p<0.05; RFS HR, 3.13; p<0.05] (Supplementary-Results Table 2).
Post-transplant MRD monitoring may predict subsequent relapse and therefore aid targeted interventions. This is of particular relevance to recipients of RIC allografts given their higher relapse risk but there has been little published data.
We examined the outcome of patients with MFC-LSC- or MFC-MRD-positivity detected post-HCT (whilst in morphological CR) (characteristics summarised in Supplementary-Results Table 3). The cumulative incidences of MFC-MRD- and MFC-LSC-positivity post-HCT were 33% (23/69) and 17% (16/92) respectively for those patients with sample data, with a median interval from HCT to detection of 105 days (range 38-339) for MFC-MRD and 102 days (range 38-368) for MFC-LSC.
MFC-MRD-positivity post-HCT was detected in 76% (19/25) of relapsed patients compared to 4% (3/76) of patients who had not relapsed at time of analysis (P<<0.01). MFC-LSCs were detectable prior to 60% (15/25) of relapses vs 1% (1/76) in non-relapsed patients, P<<0.01). 3/25 relapses were within 100 days, all these were patients who were positive for both MFC-LSC and MFC-MRD pre-HCT, (Supplementary-Results Table 4).
The high relapse rate of patients with MFC-MRD or MFC-LSC detected post-HCT (83% for MFC-MRD, 94% for MFC-LSC) not surprisingly resulted in a significantly poorer RFS and OS (Figure 1C-F). Median time to progression (relapse or death) of these patients from detection of post-HCT MFC-MRD and MFC-LSC was 53 days, (range 29-94 days) and 48 days (range 13-94 days) respectively. One patient was MFC-LSC-positive 2 months before MFC-MRD was detected.
Donor chimerism has been used as a surrogate marker of relapsing AML post HCT to guide post-HCT immunotherapy. In this cohort 48% (12/25) of relapses occurred in patients who maintained full donor T cell chimerism at day 90 post-HCT (D90). Twenty-nine of the 101 patients (RIC= 25, MAC=4) had progressive mixed doner chimerism (MDC, defined as <98% donor T cell chimerism) after the D90 timepoint but only 37% of these (10/29, RIC= 6 MAC=4) relapsed. Of those relapses 80% had detectable MFC-MRD/ MFC-LSC pre- and/or post-HCT. Of patients with progressive MDC but who had not relapsed at time of analysis (all RIC), none had MRD post-HCT (Supplementary-Results Tables 4, 5).
These results, although limited by small numbers, suggest that D90 chimerism or progressive MDC without evidence of post-HCT MFC-MRD / MFC-LSC is poorly predictive of early relapse, at least in RIC-HCT. This is unlikely to result from pre-emptive DLIs in this cohort as most relapses (21/25) occurred by 11 months post-transplant but only 3 patients (of which 1 was MFC-MRD-positive) received DLI within this time period.
The immunophenotypic heterogeneity of AML blasts with potential LSC activity has been defined functionally by xenotransplant models7, 10, 11. Our results provide clinical evidence for this since abnormal expansion of LMPP-like MFC-LSCs, although present in ~80% of CD34+AML at diagnosis, did not precede all relapses. However measurement of this MFC-LSC population is clinically applicable and adds valuable additional pre-transplant prognostic information to MFC-MRD, identifying a subset of patients with particularly poor early outcomes. This suggests that LSC detected by LMPP-like expansion are potentially more resistant to treatments including standard RI conditioning than LSC in other AML blast subsets such as LSC from more mature AML progenitors. It would be valuable to test whether these MFC-LSC-positive patients would benefit from further anti-leukemic therapy before HCT or more intensive conditioning.
Post-transplant, MRD was strongly associated with relapse and reduced survival with an interval between MRD positivity and relapse which may, in some patients, allow targeted manipulation of immunosuppression, pre-emptive DLI or pharmacological interventions12 when disease burden is lower. Expansion of the LSC population appears a more sensitive biomarker of relapse than standard MRD for some patients but this may vary according to the kinetics of leukemic proliferation from LSC and any GVL effect on LSC. It is as yet uncertain whether the chemoresistant properties of LSC are relevant to GVL resistance, so tracking the impact of GVL on the LMPP-like LSC compartment merits further investigation, particularly as changes in the bulk leukemic population (detected by standard MRD) such as acquired genomic abnormalities13, 14 reducing GVL may be more important to leukemia progression post-transplant. It will be interesting to evaluate whether strategies such as epigenetic manipulation of the alloreactive response using azacitidine and lenalidomide can overcome persisting LMPP-like LSCs or potential reduced immunogenicity15 of any post-transplant emerging leukemic populations in allografted AML patients.
Supplementary Material
Acknowledgements
We thank patients and clinical teams; Peter Richardson, Steve Dix and the West Midlands Regional Genetics laboratory led by Mike Griffiths and Susanna Akiki for sample processing; Authors were supported by funding from the National Institute for Health and Research, Leukaemia and Lymphoma Research, Cancer Research UK, and the CIRM Disease Team Grant DR1-01485. PV is supported by the MRC Disease Team Award
Footnotes
Conflict of Interest. The authors declare no conflict of interest
References
- 1.Dick JE. Stem cell concepts renew cancer research. Blood. 2008 Dec 15;112(13):4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR. Optimising the conditioning regimen for acute myeloid leukaemia. Best practice and research. Clinical haematology. 2009 Dec;22(4):543–550. doi: 10.1016/j.beha.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estey EH. How to manage high-risk acute myeloid leukemia. Leukemia. 2012 May;26(5):861–869. doi: 10.1038/leu.2011.317. [DOI] [PubMed] [Google Scholar]
- 4.Stelljes M, Krug U, Beelen DW, Braess J, Sauerland MC, Heinecke A, et al. Allogeneic transplantation versus chemotherapy as postremission therapy for acute myeloid leukemia:a prospective matched pairs analysis. Journal of clinical oncology. 2014 Feb 1;32(4):288–296. doi: 10.1200/JCO.2013.50.5768. [DOI] [PubMed] [Google Scholar]
- 5.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012 Feb 9;119(6):1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “Prime Time”? Blood. 2014 Jul 21; doi: 10.1182/blood-2014-05-577593. [DOI] [PubMed] [Google Scholar]
- 7.Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer cell. 2011 Jan 18;19(1):138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 8.van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S, et al. Aberrant marker expression patterns on the CD34+CD38-stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007 Aug;21(8):1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 9.Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013 Apr;27(5):1028–1036. doi: 10.1038/leu.2012.312. [DOI] [PubMed] [Google Scholar]
- 10.Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. The Journal of clinical investigation. 2011 Jan;121(1):384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(−) fraction. Blood. 2010 Mar 11;115(10):1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craddock C. Pharmacological methods to reduce disease recurrence. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:63–69. doi: 10.1182/asheducation-2013.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Stolzel F, Hackmann K, Kuithan F, Mohr B, Fussel M, Oelschlagel U, et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation. Transplantation. 2012 Apr 15;93(7):744–749. doi: 10.1097/TP.0b013e3182481113. [DOI] [PubMed] [Google Scholar]
- 14.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. The New England journal of medicine. 2009 Jul 30;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 15.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, et al. Human acute myeloid leukemia CD34+/CD38-progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer research. 2000 Aug 15;60(16):4403–4411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.