Abstract
Objective
Ischemic postconditioning (stutter CPR) and sevoflurane have been shown to mitigate the effects of reperfusion injury in cardiac tissue after 15 minutes of ventricular fibrillation (VF) cardiac arrest. Poloxamer 188 (P188) has also proven beneficial to neuronal and cardiac tissue during reperfusion injury in human and animal models. We hypothesized that the use of stutter CPR, sevoflurane, and P188 combined with standard advanced life support would improve post-resuscitation cardiac and neurologic function after prolonged VF arrest.
Methods
Following 17 minutes of untreated VF, 20 pigs were randomized to Control treatment with active compression/decompression (ACD) CPR and impedance threshold device (ITD) (n=8) or Bundle therapy with stutter ACD CPR + ITD + sevoflurane + P188 (n=12). Epinephrine and post-resuscitation hypothermia were given in both groups per standard protocol. Animals that achieved return of spontaneous circulation (ROSC) were evaluated with echocardiography, biomarkers, and a blinded neurologic assessment with a cerebral performance category score.
Results
Bundle therapy improved hemodynamics during resuscitation, reduced need for epinephrine and repeated defibrillation, reduced biomarkers of cardiac injury and end-organ dysfunction, and increased left ventricular ejection fraction compared to Controls. Bundle therapy also improved rates of ROSC (100% vs. 50%), freedom from major adverse events (50% vs. 0% at 48 hours), and neurologic function (42% with mild or no neurologic deficit and 17% achieving normal function at 48 hours).
Conclusions
Bundle therapy with a combination of stutter ACD CPR, ITD, sevoflurane, and P188 improved cardiac and neurologic function after 17 minutes of untreated cardiac arrest in pigs.
Keywords: Ventricular fibrillation, cardiac arrest, postconditioning, ischemic postconditioning, stutter CPR, sevoflurane, poloxamer 188, advanced cardiovascular life support, cardiopulmonary resuscitation, left ventricular function, neurological function, survival, hemodynamics
1. INTRODUCTION
Over 350,000 people are affected by sudden cardiac death in the United States each year [1] while only 8–10% experience neurologically intact survival [2–4]. Clinical studies have demonstrated severely limited neurologic outcomes when the cardiac arrest is longer than 10 minutes [5]. Likewise, animal studies show that prolonged ventricular fibrillation (VF), lasting 12–13 minutes, prohibits neurologically intact survival with standard therapy [6–8].
Further study of global ischemia has revealed two types of injury with distinct mechanisms. The first is related to ischemic damage incurred during the period of arrest. The second is injury induced by tissue reperfusion [9–11]. In contrast to ischemic injury which occurs prior to the arrival of emergency medical services, reperfusion injury, the injury caused by reestablishing blood flow, is amenable to medical management as it occurs when therapy begins.
Recent studies in animal models of cardiac arrest provide promising evidence for improved cardiac function and neurologic recovery when therapies targeting reperfusion injury are used. Ischemic postconditioning, which utilizes structured pauses in CPR, was shown to improve left ventricular ejection fraction (LVEF) and neurologically intact survival in a porcine model of cardiac arrest including 15 minutes of untreated VF [12]. Sevoflurane has been shown to reduce release of cardiac biomarkers, reduce apoptosis, and improve LVEF; however, no difference in neurologic function was reported [13, 14]. Poloxamer 188 (P188) is a nonionic copolymer thought to adhere to gaps in the cell membrane, thereby blocking pores caused by severe cellular stress [15].
While each of these treatments is likely to provide protection against cardiac and neuronal reperfusion injury induced by cardiac arrest, we hypothesized that combining these therapies would provide a synergistic benefit even in severe injury. We therefore combined these therapies in a porcine model of cardiac arrest including 17 minutes of untreated VF. The tissue damage induced by this duration of cardiac arrest has generally been considered irreversible. We assessed hemodynamic parameters, markers of cardiac injury and function, neurologic recovery, and freedom from serious adverse events.
2. MATERIALS AND METHODS
All studies were performed with approval from the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation and the National Research Council’s Guidelines for the Care and Use of Laboratory Animals. Yorkshire farm pigs weighing 38.6 ± 0.4 kg were used.
2.1 Preparatory Phase
The anesthesia, data monitoring and recording, and surgical preparation have been described in detail previously [12]. The femoral artery and right external jugular vein were cannulated percutaneously with 8 French sheaths to provide access for continuous hemodynamic monitoring and repeated blood tests.
2.2 Hemodynamic Monitoring
Surface electrocardiographic tracings were recorded continuously. Central aortic and right atrial pressures were recorded continuously with micromanometer-tipped catheters (Millar Instruments) placed at the proximal descending thoracic aorta via the femoral artery and right atrium via the right external jugular vein, respectively. Coronary perfusion pressure (CPP) was calculated as the difference between the diastolic aortic pressure and right atrial pressure during the decompression phase of CPR. End tidal CO2 (ETCO2), blood oxygen saturation, tidal volume, and minute ventilation were continuously monitored with a respiratory monitor (CO2SMO Plus, Novametrix Medical Systems). All data were recorded with a digital recording system (BIOPAC MP150, BIOPAC Systems Inc.).
2.3 Experimental Protocol
The animals were allowed to stabilize with oxygen saturation >95% and ETCO2 between 35 and 42 mmHg for 5 minutes. Isoflurane anesthesia was stopped 5 minutes prior to VF induction with direct intracardiac current delivered via a temporary pacing wire (St. Jude Medical). Following 17 minutes of untreated VF, 20 pigs were randomized to Control or Bundle treatment resulting in 8 pigs in the Control group and 12 pigs in the Bundle group.
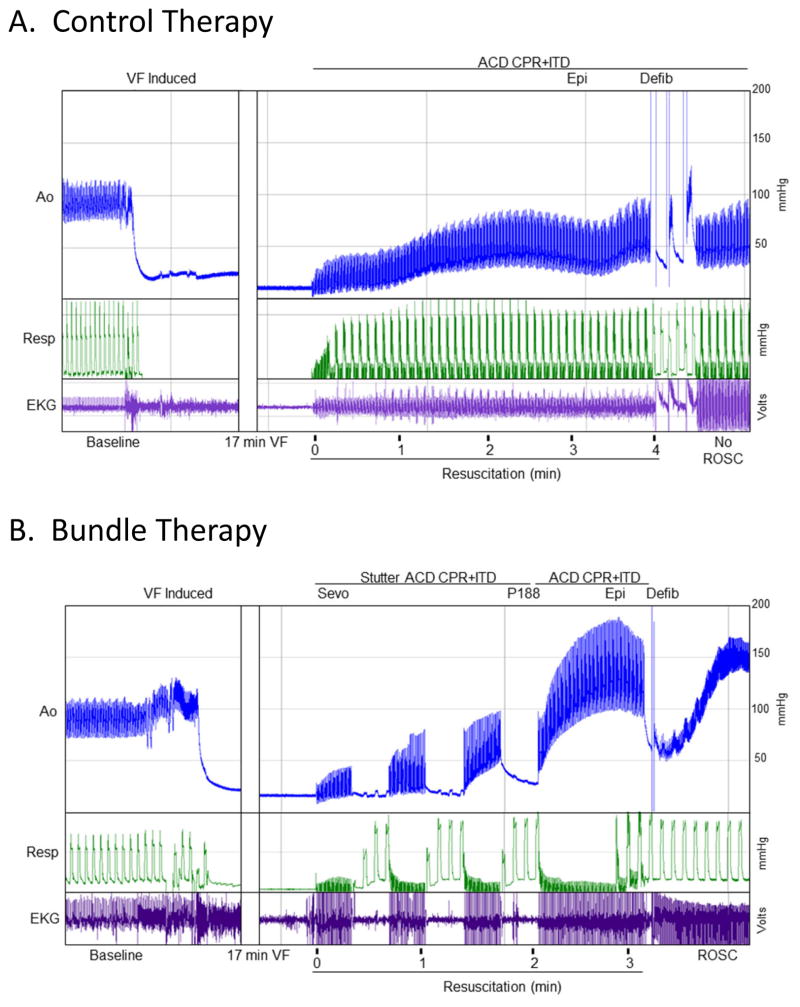
Experimental Groups (Fig. 1)
Fig. 1. Bundle therapy and Control protocols with associated representative hemodynamic tracings.
Therapy was initiated after 17 minutes of untreated ventricular fibrillation (VF). (A) Control therapy included active compression decompression CPR, application of an impedance threshold device (ACD CPR+ITD), epinephrine (Epi), and defibrillation (Defib). Return of spontaneous circulation (ROSC) was not achieved after the initial defibrillation in this representative study leading to continuation of the resuscitation. (B) The Bundle therapy protocol included sevoflurane (Sevo), poloxamer 188 (P188), epinephrine (Epi), stutter ACD CPR+ITD, and defibrillation (Defib). ROSC is achieved after a single defibrillation in this representative study.
Control Therapy
Active compression/decompression (ACD) CPR was delivered using a pneumatically driven automated piston (Pneumatic Compression Controller, Ambu International) with chest compressions occurring at a rate of 100 per minute. Compression depth was maintained at 25% of the anteroposterior chest diameter. Compression force, rate, and depth were continuously recorded and controlled. An impedance threshold device (ITD; ResQPOD, Advanced Circulatory Systems Inc.) was used in addition to ACD CPR. Asynchronous positive-pressure ventilation was delivered during CPR with room air. A tidal volume of 10 mL/kg was delivered at 10 breaths/min. Isoflurane was not restarted until return of spontaneous circulation (ROSC) was achieved. At minute 3 of CPR all animals received 0.015 mg/kg of epinephrine. Up to three 275 J biphasic shocks were delivered at minute 4. If ROSC was not achieved, CPR continued with epinephrine administered every 3 minutes. Defibrillation was attempted every 2 minutes until ROSC was achieved or a total of 15 minutes of CPR was complete. If ventricular arrhythmias developed after ROSC was achieved, defibrillation was attempted and amiodarone 40 mg IV was given. Sodium bicarbonate (50 meq) was given to all animals at 5 minutes of resuscitation.
Bundle Therapy
After 17 minutes of untreated VF, resuscitation began with ischemic postconditioning including stutter ACD CPR + ITD composed of 20 seconds of compressions followed by a 20 second pause. Sevoflurane was delivered during each pause at an end-tidal concentration of 2.0 Vol% with 3 positive pressure ventilations at a rate of 10 breaths/min. No ventilations were delivered while compressions were performed. Once three cycles of stutter ACD CPR + ITD were complete, continuous chest compressions were performed similar to the Control group. Continuous ventilation with 10 breaths/min was initiated 20 seconds later. P188 (250 mg/kg) was delivered at minute 2 of resuscitation. Epinephrine (0.015 mg/kg) was given at minute 3 of the resuscitation. Defibrillation was attempted at minute 4 as discussed above. If ROSC was achieved, P188 (460 mg/kg) was infused over 4 hours.
2.4 Post-ROSC Care
The details of post-ROSC care have been described in detail elsewhere [12]. Both groups received post-resuscitation therapeutic hypothermia to simulate best practice and optimize neurologic recovery. All animals received 1L chilled saline (8–10°C) immediately post-ROSC followed by surface cooling with ethanol soaked towels. Target temperature (34°C) was maintained for 4 hours with the use of external cooling (Arctic sun, Medivance Inc.). Animals were rewarmed at 0.5°C/h to 36°C.
In the event of an adverse event meeting predetermined criteria including status epilepticus, severe cardiopulmonary distress with evidence of agonal breathing, cyanosis, pulmonary edema, or deep coma at 24 hours with the inability to respond to painful stimuli based on the judgment of the veterinarian blinded to the intervention, animals were euthanized.
2.5 Echocardiographic Evaluation
A transthoracic echocardiogram was obtained at baseline and again at 15 minutes, 1, 4, 24, and 48 hours post-ROSC. Parasternal long and short axis views were obtained and analyzed by clinical echocardiographers blinded to the intervention as described previously [12].
2.6 Cardiac Biomarkers, Liver Function Tests, and Renal Function Assessment
Arterial blood was collected at baseline and from all survivors 4 hours post-ROSC. Cardiac specific troponin I (cTnI) and creatinine phosphokinase MB (CK-MB) were quantified via a two-site sandwich assay (Stratus CS Acute Care, Siemens). Aspartate aminotransferase, alanine aminotransferase, total bilirubin, and creatinine were measured with standard human assays. Personnel performing the analyses were blinded to the treatment.
2.7 Neurological Assessment
Neurologic function was assessed using a swine-specific cerebral performance category (CPC) at 24 and 48 hours post-ROSC by a certified veterinarian blinded to the treatment. Clinical assessment of behaviors, reflexes, and coordination are assessed as previously described [12] using the following scoring system: 1 = normal, 2 = slightly disabled, 3 = moderately disabled but not comatose, 4 = coma, 5 = dead.
2.8 Statistical Analysis
Values are expressed as means ± standard error of the mean. The primary end points were the incidence of major adverse outcomes at 48 hours and the CPC at 24 and 48 hours. Secondary endpoints included hemodynamics, need for epinephrine and defibrillations, LVEF post-ROSC, and biomarkers. Baseline data, hemodynamic parameters, epinephrine and defibrillation requirements, LVEF, biomarkers, and mean CPC scores were compared using 2-tailed unpaired t-tests. Kaplan-Meier survival curves were analyzed via Mantel-Cox test. A p-value of <0.05 was considered statistically significant.
3. RESULTS
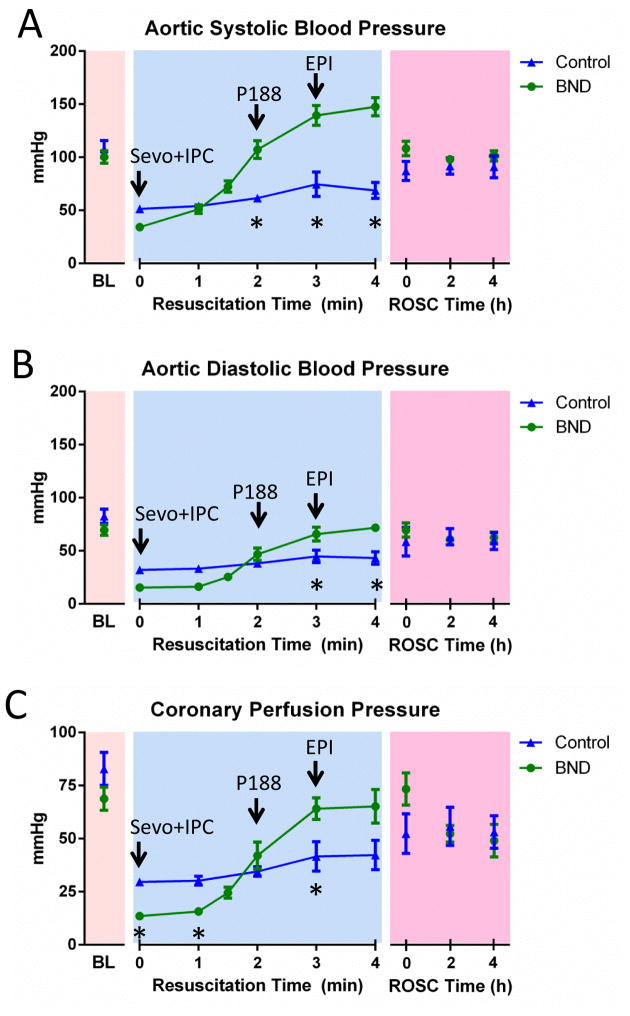
All results were obtained after 17 minutes of untreated VF. There were no significant differences in baseline hemodynamic parameters between treatment groups (Fig 2).
Fig. 2. Hemodynamic monitoring during resuscitation.
Bundle (BND; n=12) and Control (n=8) therapy are compared. (A) Aortic systolic blood pressure measured at baseline (BL), throughout the resuscitation, and after return of spontaneous circulation (ROSC) was achieved. (B) Aortic diastolic blood pressure measured during all phases of resuscitation. (C) Coronary perfusion pressure is shown as measured during all phases of resuscitation. Error bars represent SEM. Asterisks (*) indicate statistical significance (p<0.05).
3.1 Resuscitation Hemodynamics
Systolic and diastolic blood pressures were greater with Bundle therapy vs. Controls at min 2–4 of CPR (Fig 2A). This difference did not persist once ROSC was achieved. CPP was significantly lower with Bundle therapy compared to Control therapy early in the resuscitation while the increasing aortic pressures observed with Bundle therapy translated into higher CPP at minute 3 of the resuscitation (Fig 2B). The duration of CPR was significantly reduced in the Bundle therapy group (5.0 ± 0.04 min vs. 11.3 ± 1.5 min, p=0.0001).
3.2 Hemodynamic and Rhythm Support
The Bundle therapy group required less epinephrine (0.57 ± 0.08 mg vs. 1.34 ± 0.23 mg, p=0.002; Fig 3A) and fewer shocks to achieve and maintain sinus rhythm compared to the Control group (4.75 ± 0.6 vs. 7.5 ± 1.0, p=0.019; Fig 3B). The use of amiodarone was not significantly different between the groups (13.33 ± 5.7 mg vs. 30 ± 6.6 mg, p=0.07).
Fig. 3. Epinephrine and defibrillation requirements during resuscitation.
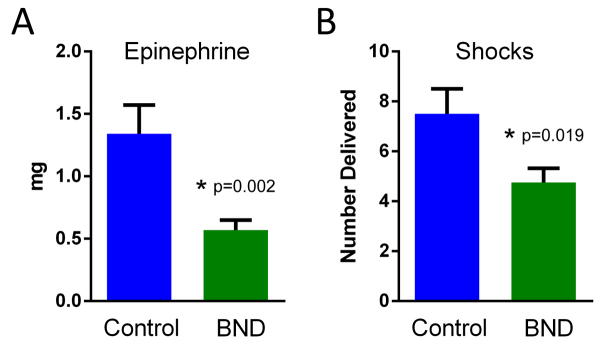
Animals receiving Bundle (BND; n=12) and Control (n=8) therapy are shown. (A) The mean of the total epinephrine (mg) required during resuscitation. (B) The mean number of shocks delivered during resuscitation. Error bars indicate SEM. P values are as indicated.
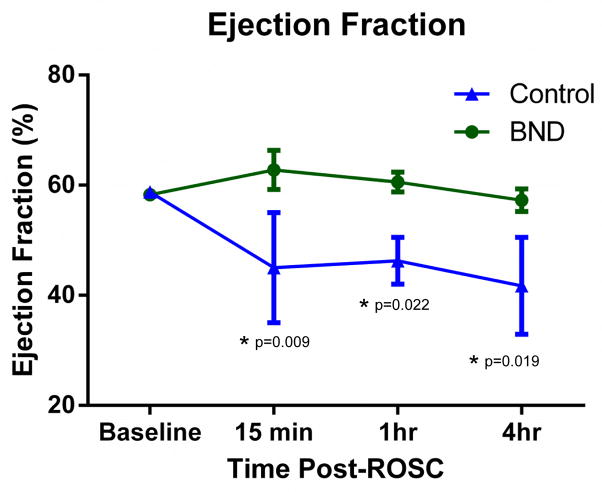
3.3 Left Ventricular Ejection Fraction
LVEF was higher with Bundle therapy compared to Control therapy at 15 minutes post-ROSC (63 ± 3.6% vs. 45 ± 10%, p=0.009; Fig 4). Bundle animals maintained a significantly higher LVEF at 4-hours post-ROSC compared to Control animals (57.3 ± 2.1% vs. 42 ± 8.8%). LVEF remained normal in Bundle therapy animals that survived 48 hours. No Control animals survived to 48-hours for comparison. Baseline LVEF was similar between the groups (58.3 ± 0.7% vs. 58.8 ± 0.8%, p=0.89).
Fig. 4. Serial measurement of the left ventricular ejection fraction (LVEF) for animals receiving Bundle versus Control therapy as determined by echocardiography.
All animals that remained free of major adverse events were assessed with echocardiography at the time points noted (BND n=12 and Control n=4). Mean LVEF was compared at each time point with asterisks noting statistically significant differences with p-values as indicated.
3.4 Biomarkers
CK-MB, cTnI, creatinine, and the transaminases were significantly reduced with Bundle therapy compared to Controls at 4 hours post-ROSC (Table 1). All baseline values were similar between groups and within normal limits.
Table 1.
Serum biomarkers assessed 4 hours post-ROSC.
| Biomarkers 4h Post-ROSC
| ||
|---|---|---|
| Control | Bundle | |
| CK-MB | 59.2 ± 13.6 | 28.2 ± 4.2* |
| TpnI | 27.7 ± 7.7 | 12.0 ± 2.7* |
| Tbili | 0.1 ± 0.0 | 0.3 ± 0.1 |
| ALT | 61.8 ± 2.0 | 40.6 ± 4.9* |
| AST | 258.3 ± 67.3 | 124.6 ± 24.0* |
| Creatinine | 1.9 ± 0.1 | 1.4 ± 0.1* |
p < 0.05
Values are shown as mean ± SEM. Asterisks indicate statistical significance (p<0.05). CK-MB = creatine kinase MB; TpnI = troponin I; Tbili = total bilirubin; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
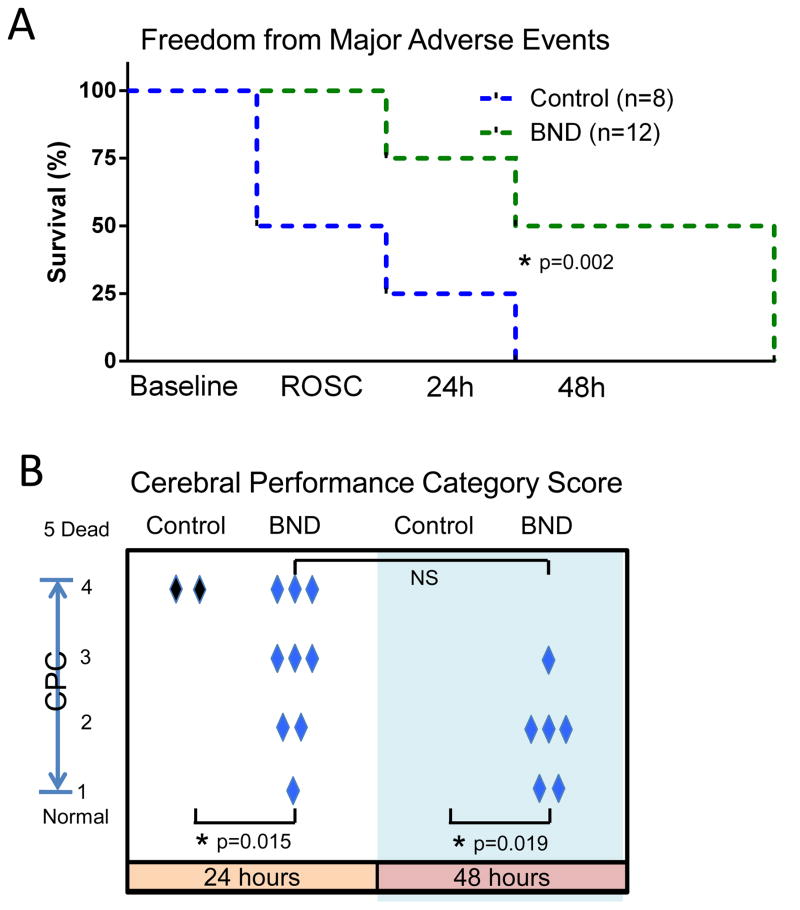
3.5 ROSC and Freedom from Major Adverse Events
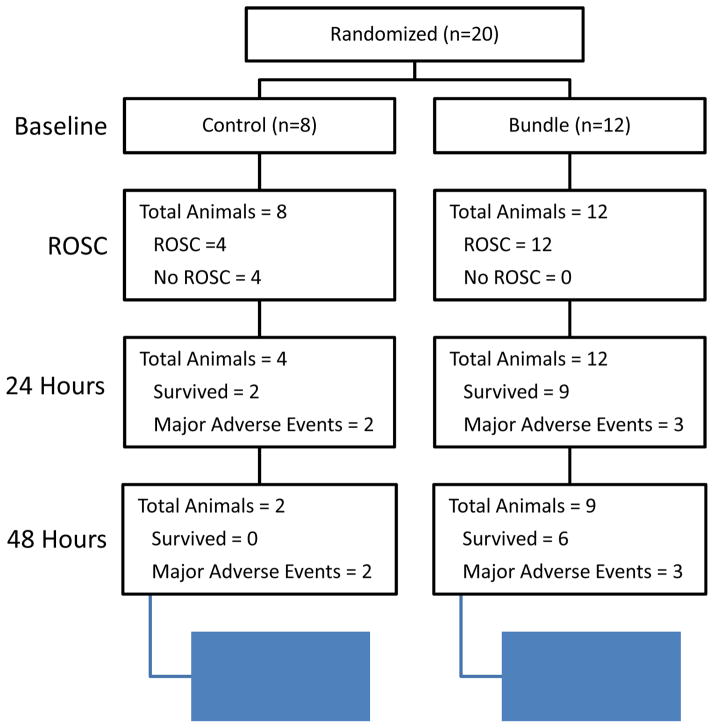
ROSC was achieved in 100% of Bundle animals and 50% of Controls (Fig 5A and Fig 6). 75% of Bundle animals remained free of major adverse events at 24 hours compared to 25% of Controls. Overall, 50% of all Bundle therapy animals remained free of major adverse events at 48 hours, whereas no Control animals remained free of adverse events at 48 hours (p=0.005).
Fig. 5. Clinical outcomes.
(A) Kaplan-Meier curve demonstrating freedom from major adverse events during the survival study including baseline, ROSC, and 24 and 48 hours. Animals receiving Bundle (BND; n=12) and Control (n=8) therapy are compared. (B) Cerebral performance category score for each animal that remained free from major adverse events at 24 or 48 hours as indicated (1=normal, 2=mild deficit, 3=moderate deficit but conscious, 4=coma). Mean CPC scores are compared with p-values as indicated. NS indicates that statistical significance was not reached.
Fig. 6. Survival at all stages of the study.
Flow chart describing the survival of animals at the time of randomization, ROSC, 24 and 48 hours.
3.6 Neurologic function at 24 and 48 hours
Bundle therapy animals demonstrated significantly better neurologic function at 24 hours (Fig 5B; mean CPC score: 3.4 ± 0.4 vs. 4.8 ± 0.2, p=0.015) and 48 hours (mean CPC score: 3.4 ± 0.5 vs. 5.0 ± 0.0, p=0.019). Both Control animals had severe disability (CPC score of 4) at 24 hours while neither of them remained free of major adverse events at 48 hours. 33% of Bundle animals that remained free of major adverse events at 24 hours had good neurologic function (CPC 1–2) while 33% had moderate disability. 67% of Bundle animals free of major adverse events at 24 hours remained free from major adverse events at 48 hours with 50% of those demonstrating improved neurologic function between 24 and 48 hours. Of all Bundle animals, 42% had good neurologic function (CPC 1–2) at 48 hours post-ROSC.
4. DISCUSSION
This study shows, for the first time, successful resuscitation resulting in good cardiac and neurologic function after 17 minutes of untreated VF cardiac arrest using a combination of CPR and medications that could be incorporated into basic and advanced life support protocols. Bundle therapy improved hemodynamics during resuscitation, reduced the need for epinephrine and defibrillation, preserved cardiac ejection fraction, reduced biomarkers of cardiac ischemia, improved freedom from major adverse events, and improved the cerebral performance post-resuscitation. Some animals achieved complete recovery to normal neurologic function.
The most important findings of this study are the large improvement in freedom from major adverse events and the extent of neurologic recovery observed despite 17 minutes of untreated VF. ROSC rates were significantly lower in Control animals despite 15 minutes of high quality CPR and continued defibrillation attempts. Of the 12 Bundle animals, 42% had good neurologic function with only slight disability while 17% recovered to have normal neurologic function within 48 hours of resuscitation. Thus, 17 minutes of ischemia was not sufficient to preclude recovery to normal neurologic function. Instead, it was the reperfusion process that determined the potential for neurologic recovery. This implicates reperfusion injury as an important factor in damage from prolonged cardiac arrest.
We have previously shown normal cardiac and neurologic function after 15 minutes of untreated VF arrest using stutter CPR [12]. Allen et al. [16, 17] also showed almost complete neurologic recovery after 30 minutes of untreated isolated cerebral ischemia using a pig model with controlled reperfusion using high pressure and a reperfusate that was hyperosmolar, alkalotic, depleted of calcium, and enriched with magnesium [18]. However, systemic ischemia may significantly alter the pathology.
Normalization of neurologic function with Bundle therapy may be related to hemodynamic improvements. Intracranial pressure was not measured in this study; however, prior studies using this model observed intracranial pressures between 20 and 30 mmHg [19]. Given the elevated aortic pressures achieved in the animals receiving Bundle therapy, cerebral perfusion pressures in excess of 70 mmHg may have been achieved which may contribute to the improved neurologic recovery observed.
Stutter CPR has previously been shown to improve hemodynamics after 15 minutes of VF arrest [12]. However, the mean systolic blood pressure of 150 mmHg in the current study exceeds that previously seen suggesting synergism with sevoflurane and P188. Bundle therapy resulted in intra-resuscitation systolic pressures that exceeded baseline and post-ROSC pressures. This has not been observed with other resuscitation protocols. The etiology is unknown. The improvement in hemodynamics reduced the need for epinephrine. Epinephrine can reduce microcirculatory blood flow including cerebral perfusion [20] while also increasing myocardial oxygen demand [21] and ventricular ectopy [22]. Therefore, reducing epinephrine use may provide additional benefits to post-resuscitation cardiac and neurologic function.
Diastolic blood pressure also increased to baseline levels with Bundle therapy which likely improved cardiac function by increasing CPP. This may contribute to the normal post-ROSC LVEF and lower biomarkers of cardiac ischemia observed in Bundle animals.
Improved hemodynamics are unlikely to account for all benefits observed with Bundle therapy, as ischemic postconditioning, sevoflurane, and P188 are known to have effects on cellular processes including membrane stability and mitochondrial function. Ischemic postconditioning is believed to protect mitochondria from the effects of prolonged ischemia by inhibiting the opening of the mitochondrial permeability transition pore which is known to trigger apoptosis in the setting of ischemia and reperfusion [23–26]. Improved myocardial function has been observed when ischemic postconditioning was used in the setting of myocardial infarction and cardiac arrest [12, 27, 28]. Improved neurologic function has also been observed with use of ischemic postconditioning in the setting of ischemic stroke [29] and more recently in global ischemia such as that experienced during cardiac arrest [12, 27, 30].
Sevoflurane has been shown to confer endothelial protection by reducing leukocyte activation while also affecting vascular tone [31]. Though the specific mechanisms remain unclear, sevoflurane has previously been shown to improve myocardial function, prevent apoptosis, and reduce cytokine production post-cardiac arrest [14].
The mechanism of P188 in cardiac arrest is entirely untested. P188 is hypothesized to fill ischemia-induced pores in the plasma membrane [15]. This may prevent unregulated exchange of ions between the extracellular and intracellular compartments thereby preventing cellular injury and apoptosis [32, 33]. P188 has been shown to protect neurons [34] and skeletal muscle [35, 36] against ischemia/reperfusion injury. It also preserves blood-brain barrier integrity during traumatic brain injury thereby reducing brain edema and neuronal apoptosis [37]. P188 has shown mixed results during myocardial infarction in humans. Schaer et al. [38] showed reduced infarct size, increased LV function, and reduced in-hospital re-infarction rates when P188 was infused immediately after thrombolytic therapy. However, a large follow-up study showed no benefit of P188 following acute myocardial infarction [39]. This discrepancy is not fully understood but it may relate to proximity of the infusion to reperfusion.
Studies of acute myocardial infarction noted that P188 worsened renal function acutely in up to 4% of patients [39]. It has been hypothesized that creatinine, a marker of glomerular filtration rate, is artificially increased by P188 as they compete for excretion in the kidneys. However, renal function in this study, indicated by creatinine levels, was significantly improved in the pigs receiving Bundle therapy including P188. This improvement may be due to improved hemodynamics leading to improved renal perfusion.
This study has important limitations. The role of individual components of Bundle therapy cannot be determined. The Bundle strategy was chosen based on the observation that treatment of complex pathology (e.g. heart failure, acute myocardial infarction, and stroke) often requires multiple agents. Agents with known or probable benefits were chosen for inclusion in Bundle therapy with a preference for those that may be integrated into standard advanced life support. However, further study will now be necessary to more fully understand the contribution and mechanism of each individual constituent. In addition, the dose-response relationships are unknown for the individual components. The specific regimen of stutter CPR was chosen based on prior published experience [12]. The doses of sevoflurane and P188 were extrapolated from animal studies and human trials. Further dose-response trials will be necessary to understand the pharmacokinetics and maximal effect achievable in the setting of cardiac arrest. Similarly, further study is necessary to understand the role of these agents in cardiac arrests of very long or short duration, as the benefit of one or more of these therapies may depend on the duration of ischemia. Lastly, these studies are inherently limited by use of a porcine model. The benefits for humans cannot be certain until human trials are conducted.
5. CONCLUSION
Bundle therapy, combining stutter ACD CPR, an ITD, sevoflurane, and P188, significantly improved neurologic and cardiac function in a porcine model including 17 minutes of untreated ventricular fibrillation cardiac arrest. Compared to Control animals, animals receiving Bundle therapy demonstrated significant improvement in hemodynamics during CPR, reduction in biomarkers of cardiac ischemia, normalization of ejection fraction, and improved 48 hour survival with favorable neurologic function.
Supplementary Material
Acknowledgments
7. FUNDING SOURCES
Dr. Yannopoulos is funded by an Institutional, Division of Cardiology grant at the University of Minnesota and the following NIH grants for CPR and resuscitation research (R01HL108926-01and R01HL123227). Dr. Riess received research funding from institutional funds, Department of Veterans Affairs (CARA-026-10F to MLR) and the National Institutes of Health.
Footnotes
6. CONFLICT OF INTEREST
None of the authors have any financial or personal relationships with people or organizations that could have inappropriately influenced this work.
8. ETHICAL APPROVAL
The study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center (protocol #12–11), and all animals received treatment in compliance with the National Research Council’s 1996 Guide for the Care and Use of Laboratory Animals.
All studies were performed with approval from the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation (protocol #12–11).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shultz JJ, Mianulli MJ, Gisch TM, Coffeen PR, Haidet GC, Lurie KG. Comparison of exertion required to perform standard and active compression-decompression cardiopulmonary resuscitation. Resuscitation. 1995;29:23–31. doi: 10.1016/0300-9572(94)00812-t. [DOI] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circulation Cardiovascular quality and outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 3.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA: the journal of the American Medical Association. 2012;307:1161–8. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 4.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–11. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummins RO. From concept to standard-of-care? Review of the clinical experience with automated external defibrillators. Annals of emergency medicine. 1989;18:1269–75. doi: 10.1016/s0196-0644(89)80257-4. [DOI] [PubMed] [Google Scholar]
- 6.Hogler S, Sterz F, Sipos W, Schratter A, Weihs W, Holzer M, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81:1577–83. doi: 10.1016/j.resuscitation.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Janata A, Bayegan K, Sterz F, Weihs W, Holzer M, Sipos W, et al. Limits of conventional therapies after prolonged normovolemic cardiac arrest in swine. Resuscitation. 2008;79:133–8. doi: 10.1016/j.resuscitation.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Janata A, Weihs W, Schratter A, Bayegan K, Holzer M, Frossard M, et al. Cold aortic flush and chest compressions enable good neurologic outcome after 15 mins of ventricular fibrillation in cardiac arrest in pigs. Critical care medicine. 2010;38:1637–43. doi: 10.1097/CCM.0b013e3181e78b9a. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nature reviews Cardiology. 2011;8:619–29. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- 10.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. The New England journal of medicine. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? The Journal of clinical investigation. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal N, Matsuura T, Caldwell E, Sarraf M, McKnite S, Zviman M, et al. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp J, Bergmann G, Bruckner T, Russ N, Bottiger BW, Popp E. Pre- and postconditioning effect of Sevoflurane on myocardial dysfunction after cardiopulmonary resuscitation in rats. Resuscitation. 2013 doi: 10.1016/j.resuscitation.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Meybohm P, Gruenewald M, Albrecht M, Muller C, Zitta K, Foesel N, et al. Pharmacological postconditioning with sevoflurane after cardiopulmonary resuscitation reduces myocardial dysfunction. Critical care. 2011;15:R241. doi: 10.1186/cc10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maskarinec SA, Hannig J, Lee RC, Lee KY. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophys J. 2002;82:1453–9. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen BS, Ko Y, Buckberg GD, Tan Z. Studies of isolated global brain ischaemia: III. Influence of pulsatile flow during cerebral perfusion and its link to consistent full neurological recovery with controlled reperfusion following 30 min of global brain ischaemia. Eur J Cardiothorac Surg. 2012;41:1155–63. doi: 10.1093/ejcts/ezr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen BS, Ko Y, Buckberg GD, Tan Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia - the importance of perfusion pressure. Eur J Cardiothorac Surg. 2012;41:1147–54. doi: 10.1093/ejcts/ezr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen BS, Ko Y, Buckberg GD, Sakhai S, Tan Z. Studies of isolated global brain ischaemia: I. A new large animal model of global brain ischaemia and its baseline perfusion studies. Eur J Cardiothorac Surg. 2012;41:1138–46. doi: 10.1093/ejcts/ezr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yannopoulos D, McKnite S, Aufderheide TP, Sigurdsson G, Pirrallo RG, Benditt D, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64:363–72. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ristagno G, Tang W, Huang L, Fymat A, Chang YT, Sun S, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Critical care medicine. 2009;37:1408–15. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 21.Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78:382–9. doi: 10.1161/01.cir.78.2.382. [DOI] [PubMed] [Google Scholar]
- 22.Tovar OH, Jones JL. Epinephrine facilitates cardiac fibrillation by shortening action potential refractoriness. J Mol Cell Cardiol. 1997;29:1447–55. doi: 10.1006/jmcc.1997.0387. [DOI] [PubMed] [Google Scholar]
- 23.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–7. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 24.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 26.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–74. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Yannopoulos D, Segal N, Matsuura T, Sarraf M, Thorsgard M, Caldwell E, et al. Ischemic postconditioning and vasodilator therapy during standard cardiopulmonary resuscitation to reduce cardiac and brain injury after prolonged untreated ventricular fibrillation. Resuscitation. 2013 doi: 10.1016/j.resuscitation.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cour M, Gomez L, Mewton N, Ovize M, Argaud L. Postconditioning: from the bench to bedside. Journal of cardiovascular pharmacology and therapeutics. 2011;16:117–30. doi: 10.1177/1074248410383174. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–85. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–90. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- 31.Lucchinetti E, Ambrosio S, Aguirre J, Herrmann P, Harter L, Keel M, et al. Sevoflurane inhalation at sedative concentrations provides endothelial protection against ischemia-reperfusion injury in humans. Anesthesiology. 2007;106:262–8. doi: 10.1097/00000542-200702000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–9. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 33.Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, et al. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. The Journal of clinical investigation. 2010;120:1140–50. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu JH, Ge JB, Li M, Xu HD, Wu F, Qin ZH. Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PloS one. 2013;8:e61641. doi: 10.1371/journal.pone.0061641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AD, McCormack MC, Bichara DA, Nguyen JT, Randolph MA, Watkins MT, et al. Poloxamer 188 protects against ischemia-reperfusion injury in a murine hind-limb model. Plastic and reconstructive surgery. 2010;125:1651–60. doi: 10.1097/PRS.0b013e3181ccdbef. [DOI] [PubMed] [Google Scholar]
- 36.Walters TJ, Mase VJ, Jr, Roe JL, Dubick MA, Christy RJ. Poloxamer-188 reduces muscular edema after tourniquet-induced ischemia-reperfusion injury in rats. The Journal of trauma. 2011;70:1192–7. doi: 10.1097/TA.0b013e318217879a. [DOI] [PubMed] [Google Scholar]
- 37.Bao HJ, Wang T, Zhang MY, Liu R, Dai DK, Wang YQ, et al. Poloxamer-188 attenuates TBI-induced blood-brain barrier damage leading to decreased brain edema and reduced cellular death. Neurochemical research. 2012;37:2856–67. doi: 10.1007/s11064-012-0880-4. [DOI] [PubMed] [Google Scholar]
- 38.Schaer GL, Spaccavento LJ, Browne KF, Krueger KA, Krichbaum D, Phelan JM, et al. Beneficial effects of RheothRx injection in patients receiving thrombolytic therapy for acute myocardial infarction. Results of a randomized, double-blind, placebo-controlled trial. Circulation. 1996;94:298–307. doi: 10.1161/01.cir.94.3.298. [DOI] [PubMed] [Google Scholar]
- 39.Effects of RheothRx on mortality, morbidity left ventricular function, and infarct size in patients with acute myocardial infarction. Collaborative Organization for RheothRx Evaluation (CORE) Circulation. 1997;96:192–201. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.