Abstract
We have previously demonstrated that immune responses in subjects with chronic Trypanosoma cruzi infection display features common to other persistent infections with signs of T cell exhaustion. Alterations in cytokine receptor signal transduction have emerged as one of the cell-intrinsic mechanisms of T cell exhaustion. Herein, we performed an analysis of the expression of IL-7R components (CD127 and CD132) on CD4+ and CD8+ T cells, and evaluated IL-7-dependent signaling events in patients at different clinical stages of chronic chagasic heart disease. Subjects with no signs of cardiac disease showed a decrease in CD127+CD132+ cells and a reciprocal gain of CD127-CD132+ in CD8+ and CD4+ T cells compared to either patients exhibiting heart enlargement or uninfected controls. T. cruzi infection, in vitro, was able to stimulate the downregulation of CD127 and the upregulation of CD132 on T cells. IL-7-induced phosphorylation of STAT5 as well as Bcl-2 and CD25 expression were lower in T. cruzi-infected subjects compared with uninfected controls. The serum levels of IL-7 was also increased in chronic chagasic patients. The present study highlights perturbed IL-7/IL-7R T cell signaling through STAT5 as a potential mechanism of T cell exhaustion in chronic T. cruzi infection.
Introduction
Chagas disease, caused by Trypanosoma cruzi infection, represents a major public health problem in Latin America (1). As a consequence of migration flows, the disease is also found in non-endemic countries, becoming a global health problem (2). T cells play a major role in the immune control of T. cruzi. We have shown that T. cruzi-infected subjects in the chronic phase of the infection have increased frequencies of fully differentiated and pre-apoptotic memory T cells along with decreased levels of naïve T cells in the peripheral circulation, reflecting a constant activation of the immune system in association with this decades-long infection (3-5). These immune dysfunctions of the total T cell compartment were associated with decreased frequencies of IFN-γ- and IL-2-producing T cells in response to T. cruzi antigens in patients with severe myocardiopathy (3,6,7).
Interleukin (IL)-7 plays an important role in the maintenance of naïve and memory T cell compartments, positively regulating the survival, differentiation and proliferation of T cells. The IL-7 cell-surface receptor is composed of the specific IL-7Rα (CD127) chain and the common γ-chain (CD132 or γc) which is shared with other cytokines of the γc family (8). The regulation of the two IL-7R chains is different; while CD127 is downregulated upon T-cell activation, the CD132 chain is rapidly upregulated (9). In persistent infections, reduced expression of the IL-7R and an inability to effectively respond to this cytokine has been associated with defective antigen independent homeostatic self-renewal and loss of pathogen-specific T cells (10-15).
In view of the key role of IL-7 in controlling T cell homeostasis, alterations in IL-7 signaling might also be involved in the impaired T cell responses observed in patients with chronic Chagas disease, as supported by the lower expression of CD127 on CD4+ T cells in chronically T. cruzi-infected subjects with severe cardiomyopathy (4). Herein, we performed an analysis of the expression of IL-7R components and evaluated IL-7-dependent signaling events in patients in different clinical stages of chronic Chagas heart disease. The findings reported in this work support the notion that T. cruzi infection perturbed the IL-7/IL-7R T cell signaling through STAT5 and thus represents another potential mechanism of T cell exhaustion in chronic T. cruzi infection.
Materials and methods
Selection of study population
Subjects were recruited at the Chagas disease Section, Cardiology Department, Hospital Interzonal General de Agudos “Eva Perón”, Buenos Aires, Argentina. T. cruzi infection was determined by a combination of indirect immunofluorescence assay, hemagglutination, and ELISA tests, performed at the Diagnosis Department of Instituto Nacional de Parasitología “Dr. M. Fatala Chaben” (INP). Chronic Chagas disease subjects were evaluated clinically and grouped according to a modified version of the Kuschnir grading system (16). Group 0 (G0, n=13; mean age±SD =50±6y) included seropositive individuals exhibiting a normal electrocardiogram (ECG) and normal echocardiograph; Group 1 (G1, n=12; mean age±SD =49±14y) seropositive individuals with a normal echocardiograph but abnormalities in the ECG; Group 2-3 (G2-G3, n=12; mean age±SD =56±11y) seropositive individuals with ECG abnormalities and heart enlargement (G2) and clinical or radiological evidence of heart failure (G3). All patients were untreated with respect to T. cruzi infection, the exception being one patient in the G2 patient group, who received treatment with benznidazole ten years prior to this study and who remained seropositive for T. cruzi infection. The uninfected control group (n=10; mean age±SD =47±10y) consisted of aged-matched healthy Caucasian natives from Argentina who have always resided in areas non-endemic for T. cruzi infection and who have negative serology for T. cruzi infection. T. cruzi-infected subjects and uninfected controls with hypertension, ischemic heart disease, cancer, HIV infection, syphilis, diabetes, arthritis or serious allergies were excluded from this study. This study was approved by the Institutional Review Boards of the Hospital Interzonal General de Agudos “Eva Perón”, Buenos Aires, Argentina and the University of Georgia. Signed informed consent was obtained from all individuals prior to inclusion in the study.
Collection of peripheral blood mononuclear cells (PBMCs) and sera
Approximately, 50 ml of blood were drawn by venipuncture into heparinized tubes (Vacutainer, Becton-Dickinson, San Jose, CA). PBMC were isolated by density gradient centrifugation on Ficoll-hypaque (Amershan, Sweden) and resuspended in RPMI (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated Fetal Calf Serum (FCS; Hyclone Laboratories, Logan, UT). Blood to be used for serum component analysis was allowed to coagulate at 37° C and centrifuged at 1000 g for 15 min. Non-haemolysed serum was separated, and aliquots were stored at -70° C until use.
Monoclonal Antibodies
Phycoerythrin (PE)-conjugated anti-Bcl-2 (Clone Ms IgG1), fluorescein (FITC)-conjugated anti-CD25 (Clone M-A251), PE-conjugated anti-CD132 (Clone AG184), allophycocyanin (APC; Clone RPA-T4) and Peridinin-chlorophyll proteins (PerCP; Clone RPA-T4)-conjugated anti-CD4, PerCp (Clone SK1) or FITC-conjugated anti-CD8 (Clone HIT8a), Alexa Fluor 647-conjugated (Clone HIL-7R-M21) anti-CD127 or PE (Clone HIL-7R-M21), as well as PE-conjugated anti-phosphorylated STAT5 (p-STAT5; Clone pY694) were purchased from BD Pharmingen (San Diego, CA, USA).
Expression of CD127 and CD132 in PBMC from chronically T. cruzi-infected subjects
One million PBMC were stained with anti-CD4 PerCp, anti-CD8 FITC, anti-CD127 Alexa Fluor 647 and anti-CD132 PE for 30 min on ice. Thereafter, the cells were washed and re-suspended in PBS containing 2% paraformaldehyde. Data were acquired on a FACSCalibur flow cytometer (Becton Dickinson, USA) and analyzed with Flowjo version 6.3 (Tree Star, San Carlos, CA, USA) software.
Expression of IL-7R components on the T. cruzi-specific T cells in vitro
Two ×106 PBMCs were co-cultured with VERO cell culture-derived trypomastigotes (Brazil strain) at a ratio of 1:1 cell-parasite in a 24-well plate at 37° C in 5% CO2 atmosphere during 48h. A ratio of 1:5 cell-parasites was also tested in order to set up the appropriate conditions for in vitro infection with T. cruzi. The supernatants were removed, washed with staining buffer and labeled with anti-CD4 PerCp, anti-CD8 FITC, anti-CD127 Alexa Fluor 647 and anti-CD132 PE for 30 min on ice. Cells were then washed and re-suspended in PBS containing 2% paraformaldehyde. Data were acquired on a FACSCalibur flow cytometer (Becton Dickinson, USA) and analyzed with Flowjo version 6.3 (Tree Star, San Carlos, CA, USA) software.
Intracellular p-STAT5 assay
2×106 PBMC were cultured overnight in serum-free medium (AIM-V, Invitrogen, Carlsbad, USA) followed by a 15-min incubation with 100 ng/ml recombinant human IL-7 (rhIL-7, BD Pharmigen) at 37°C, 5% CO2. Thereafter, cells were washed with staining buffer (PBS, 4% fetal bovine serum), labeled with CD4 and CD8 monoclonal antibodies for 20 min on ice and immediately fixed by adding an equal volume of pre-warmed 4% paraformaldehyde (PFA) for 10 minutes at 37°C. After centrifugation and PFA removal by aspiration, cells were permeabilized by adding 1 ml of 90% ice-cold methanol for 30 min on ice and washed twice with staining buffer. Lastly, the cells were incubated at room temperature for 30 min with anti-p-STAT5 (20μl per test according to the instructions of the manufacturer). Data were acquired on a FACScalibur flow cytometer (Becton & Dickinson) and further analyzed with Flowjo version 6.3 (Tree Star, San Carlos, CA, USA) software. IL-7-induced STAT5 phosphorylation (Δ % p-STAT5+) for CD4+ and CD8+ T cells was measured by the difference in the percentage of p-STAT5+ cells between IL-7-stimulated and unstimulated samples.
Measurement of serum IL7 cytokine
Serum levels of IL-7 (Interleukin-7) were measured, in duplicate, using an IL-7 Human SimpleStep ELISA™ Kit (Abcam) according to the instructions of the manufacturer.
Evaluation of IL-7 functional responses
2×106 PBMC were cultured in complete RPMI medium in the presence or absence of 10ng/ml rhIL-7 for 2 days. After the incubation, cells were labeled with anti-CD4 (APC), anti-CD8 (PerCp) and anti-CD25 (FITC) for 30 min on ice followed by permeabilization with Cytofix/Cytoperm solution (Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. Thereafter, cells were labeled with anti-Bcl-2 (PE) for 30 min at 4°C, washed twice with Perm/Wash solution and re-suspended in PBS containing 2% paraformaldehyde. Data were acquired on a FACScalibur flow cytometer (Becton & Dickinson) and further analyzed with Flowjo version 6.3 (Tree Star, San Carlos, CA, USA) software. Lymphocytes were gated in side scatter vs forward scatter light parameters. Induction of Bcl-2 and CD25 expression was measured by subtracting the MFI or percentages of Bcl-2 and CD25-expressing T cells in unstimulated from those in IL-7 stimulated cultures.
Statistical analysis
Differences among groups were evaluated by analysis of variance (ANOVA) followed by Bonferroni test for multiple comparisons or by a test for lineal trend. Mann-Whitney U-test was used to evaluate the differences between T. cruzi-infected and uninfected subjects. Paired t test was used to compare the expression of the IL7R components prior and after in vitro T. cruzi infection. Correlation between variables was explored with the Spearman test. Differences were considered statistically significant when p≤0.05.
Results
Expression of IL-7R components in subjects with chronic T. cruzi infections
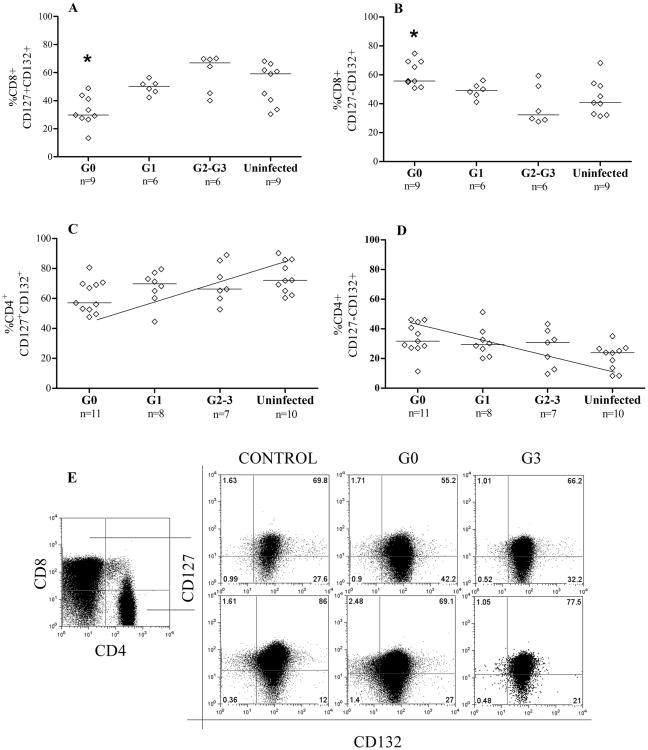
The effect of T. cruzi infection on the expression of the IL7R components, CD127 and CD132, was evaluated in CD8+ and CD4+ T cells from subjects T. cruzi infections of >20 years in length. Two main subpopulations (CD127+CD132+ and CD127-CD132+) of CD8+ and CD4+ T cells out of the 4 possible subsets based on the expression of CD127 and CD132 IL-7 receptor components were observed in chronically T. cruzi-infected subjects. In subjects in the G0 group the CD8+ T cell subset shows a decrease in CD127+CD132+, and a reciprocal gain of CD127-CD132+ cells, in comparison to patients with heart enlargement (G2 and G3 clinical groups) (Fig. 1A, 1B, 1C). Of note, no significant differences in CD8+CD127+CD132+ and CD8+CD127-CD132+ T cell populations were found between patients with severe cardiac dysfunction and uninfected controls (Fig. 1A, 1B, 1C). A similar trend was observed in the CD4+ T cell compartment, with diminished CD127+CD132+CD4+ T cells along with increased CD127-CD132+CD4+ T cells in the G0 patient group (Fig. 1C-E). One consequence of T cell activation is the downregulation of CD127. Thus, these findings suggest a differential response to T cell activation in G0 subjects relative to patients with severe disease.
Figure 1.
Cell-surface expression of IL-7 receptor components in subjects with chronic T. cruzi infection. PBMC were stained for CD8, CD4, CD127 and CD132 and analyzed by flow cytometry. Lymphocytes were gated by side scatter vs forward scatter light. Each point represents the expression of CD127 and CD132 on the total CD8+ and CD4+ T cell compartments in one patient. Median values are indicated by the horizontal lines. Statistically significantly differences (*p < 0.05) in the frequencies of CD8+CD127+CD132+ (A) and CD8+CD127-CD132+ (B) in G0 patients vs uninfected or G2-G3 clinical groups. [C-D], Positive and negative trend in the percentages of CD127+CD132+ and CD127-CD132+ CD4+ T cells, respectively, as clinical stage becomes more severe. [E] Representative dot plots of the expression of CD127 and CD132 in CD4+ and CD8+ T cells from an uninfected control, an asymptomatic patient (G0) and a severe cardiomyopathy patient (G3). The numbers in each quadrant represents the percentage of expression of each of the 4 possible subsets based on the expression of CD127 and CD132 IL-7 receptor components.
Modulation of IL-7R components in T cells after T. cruzi infection in vitro
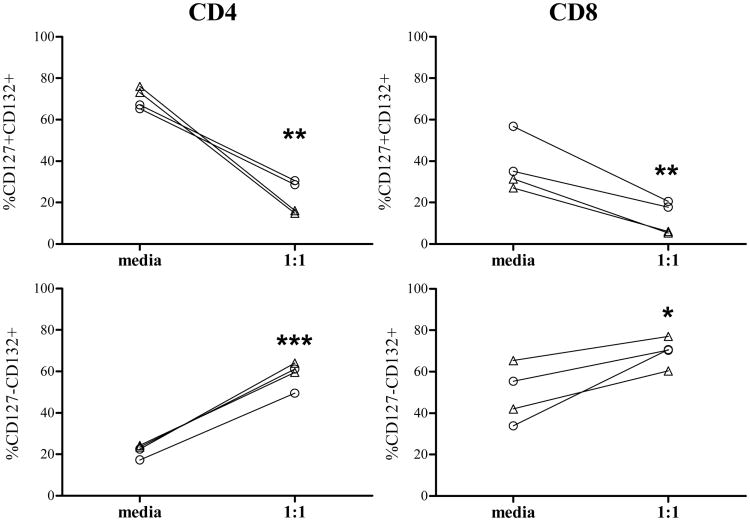
To assess whether T. cruzi infection might modulate the IL7 receptor components, the expression of CD127+ and CD132+ was measured on T cells after in vitro infection of PBMC with T. cruzi. A downregulation of CD127 and a reciprocal gain of CD132 in CD4+ and CD8+ T cells was observed in all the subjects evaluated, supporting the activation of the IL-7R (Fig. 2) after infection with T. cruzi.
Figure 2.
Modulation of the IL-7R components after in vitro infection of T cells with T. cruzi. PBMCs from two chronic Chagas disease subjects and two uninfected controls were cocultured with T. cruzi in a 1:1 ratio for 48hs, stained for CD8, CD4, CD127 and CD132 and analyzed by flow cytometry. Lymphocytes were gated by side scatter vs forward scatter light. Each point represents the expression of CD127 and CD132 on total CD8+ (right panel) and CD4+ (left panel) T cells from media and T. cruzi stimulated wells in each subject. △ Uninfected controls; ○ Chagas disease patient
Decreased IL-7-induced STAT5 phosphorylation in T cells from chronically T. cruzi-infected subjects
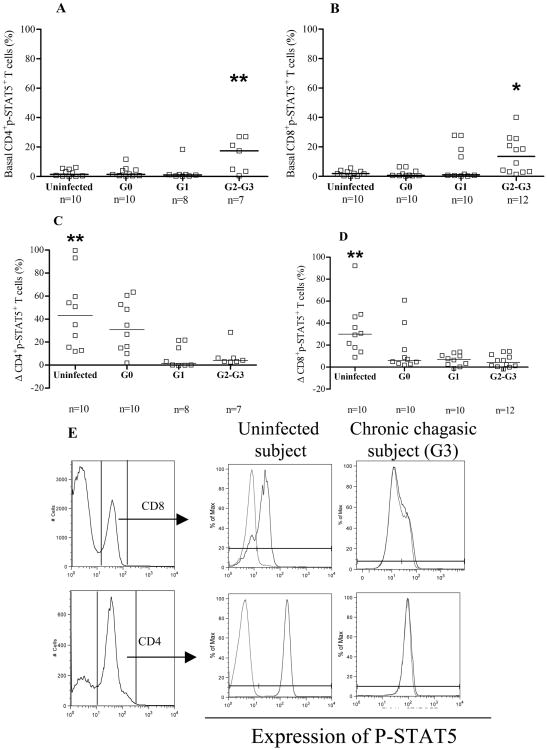
A common signaling event that occurs rapidly after ligation of γc cytokines to their receptors is the janus-kinase-dependent phosphorylation of the STAT5 (17). To evaluate potential defects in IL-7R-mediated signaling, IL-7-induced phosphorylation of STAT5 was measured in CD4+ and CD8+ T cells from T. cruzi infected subjects with different degrees of disease severity and in uninfected controls. The basal number of CD4+ and CD8+ T cells expressing p-STAT5+ in G2 and G3 patients was higher compared with G0, G1, and uninfected controls (Fig. 3A, 3B, 3E). The percentages of CD4+p-STAT5+ and CD8+p-STAT5+ T cells after IL-7 stimulation were significantly lower in all groups of T. cruzi-infected subjects compared with uninfected controls (Figure 3C-E). Of note, we observed a trend towards lower responsiveness to IL-7 in association with the increased severity of disease (CD4 p=0.0005 and CD8 p=0.0001, respectively; Fig. 3C, 3D). In contrast, the analysis of basal or IL-7-induced MFI of pSTAT5 did not show any differences between T. cruzi-infected subjects and uninfected controls (Supplemental Table I).
Figure 3.
STAT-5 activation in CD4+ and CD8+ T cells in chronic Chagas disease. PBMC were stimulated with 100 ng/ml IL-7 and evaluated for p-STAT5+ induction in CD4+ and CD8+ T cells by flow cytometry. Lymphocytes were gated in side scatter vs forward sacatter light. [A-B] Each point represents the basal levels of CD4+p-STAT5+ and CD8+p-STAT5+ T cells, respectively. Differences among groups were evaluated by ANOVA followed by Bonferroni. Median values are indicated by the horizontal lines. [C-D] Each point represents the induced expression of p-STAT5 for individual subjects, calculated as the difference in the percentage of CD4+p-STAT5+ or CD8+p-STAT5+ T cells, respectively, between IL-7-stimulated and unstimulated samples. Differences among groups were evaluated by ANOVA followed by Bonferroni. Median values are indicated by the horizontal lines. *p<0.05, **p<0.01 [E] Representative CD4+/CD8+ histogram plots of PBMC from an uninfected control and a T. cruzi-infected subject with severe cardiomyopathy. Grey lines indicate the basal expression of p-STAT5 and the black lines the expression of p-STAT5 after IL-7 stimulation.
The expression of the IL-7 receptor components (CD127 and CD132) on CD4+ or CD8+ T cells was not correlated with the levels of p-STAT5+ T cells following IL-7stimulation (data not shown). In contrast, higher basal phosphorylation levels were correlated with lower magnitudes of IL-7-induced STAT5 phosphorylation (CD4+ T cells, r = -0.6, p<0.0001 and CD8+ T cells, r = -0.75, p<0.0001; data not shown). Altogether, these findings indicate altered IL-7/IL-7R-mediated signaling in chronic T. cruzi infection, increasing as cardiac disease becomes more severe.
IL-7 serum levels in chronic chagas disease patients
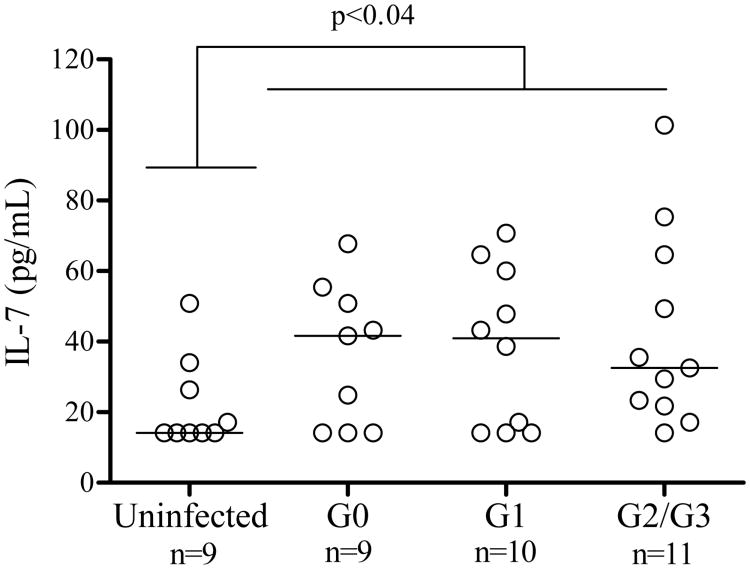
Taking into account that IL-7 is one of the STAT5 activating cytokines, the levels of circulating IL-7 were measured in 30 chronic Chagas disease patients and 9 uninfected controls. An increased level of IL7 was found in chronically T. cruzi-infected subjects compared with uninfected controls (Fig. 4). There was no correlation between the concentration of IL-7 and the basal or IL-7-induced p-STAT5 expression (data not shown).
Figure 4.
Serum IL-7 concentration in chronic Chagas disease patients. Serum levels were measured in 30 chronically T. cruzi-infected subjects with different clinical stages of the disease and 9 uninfected controls using an IL-7 a capture ELISA assay. Each point represents the IL7 serum levels in each subject. Median values are indicated by the horizontal lines.
Impairment of IL-7 functional responses downstream of STAT-5 in chronic Chagas disease patients
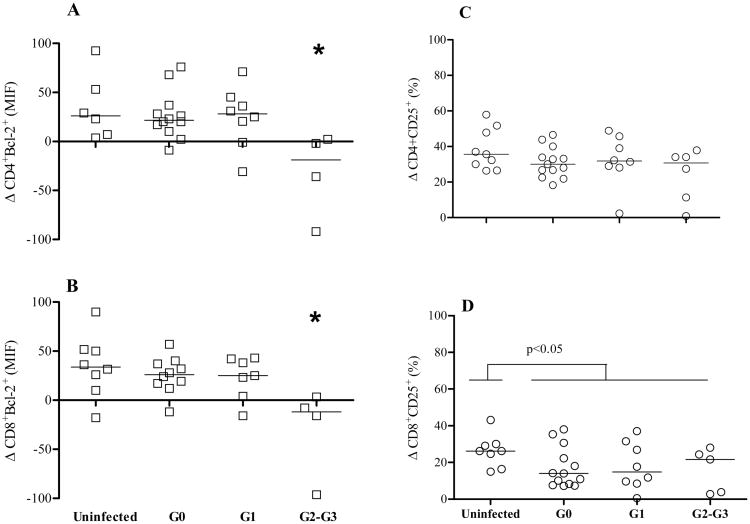
IL-7 is known to induce the expression of the survival factor Bcl-2 and the IL-2R α chain (CD25), through STAT5 (18). To evaluate the functional responses of CD4+ and CD8+ T cells to IL-7 downstream STAT5, the induced expression of Bcl-2 and CD25 was measured. As Bcl-2 is constitutively expressed, the change in MFI was used to evaluate the induction above the basal levels. The induction of Bcl-2 on CD4+ and CD8+ T cells was reduced in G2 and G3 compared with G0 and G1 patient groups and uninfected controls (Fig. 5A, 5B), whereas baseline Bcl-2 expression on T cells remained unchanged in T. cruzi-infected subjects (Supplemental Table I).
Figure 5.
Impaired IL-7 functional responses in CD4+ and CD8+ T cells from chronic Chagas disease patients. PBMC from healthy donors (n=9) and chronically T. cruzi-infected subjects (G0 n=13; G1 n=8; G2-G3 n=6) were cultured for 2 days in complete medium in the presence or absence of 10ng/ml of IL-7. Each point represents the differences in Bcl-2 MFI (Δ Blc-2 MFI) [A-B] and in the percentage of CD25+ T cells (Δ% CD25) [C-D] between IL7-stimulated and unstimulated samples in CD4+ or CD8 T cell compartments. [A-B] (*) Analysis of variance (ANOVA) followed by Bonferroni test for multiple comparisons. G2-G3 p < 0.05 compared to G0, G1 and uninfected subjects. [D] Mann-Whitney U-test between T. cruzi-infected and uninfected subjects, p < 0.05 compared to T. cruzi-infected subjects.
The percentage of CD8+ T cells expressing CD25 after the IL7-stimulation was lower in chronically T. cruzi-infected subjects, regardless of the clinical status (Fig. 5D). A trend toward higher basal percentages of CD25+CD8+ T cells (data not shown) as well as higher MFI of CD25+CD8+ T cells (Supplemental Table I) as disease becomes more severe was found. Conversely, no alterations were observed either in baseline or IL7-induced expression of CD25 on CD4+ T cells in T. cruzi-infected subjects. (Fig. 5C; Supplemental Table I). These findings support that T cell function downstream STAT5 is also perturbed in the face of the chronic infection.
Discussion
Different mechanisms of T cell exhaustion have been demonstrated during chronic infections, including overexpression of inhibitory receptors, altered chemotaxis, homing and adhesion molecules, changes in transcription factor expression, as well as metabolic deficiencies (15). We have previously demonstrated that T cells in subjects with chronic T. cruzi infection display signs of immune exhaustion, as showed by the presence of monofunctional T. cruzi-specific T cells and a high degree of differentiation o the overall T cell compartment (3,4,6,7). Alterations in signal transduction through cytokine receptors, including IL-7, IL-15 and other gamma-chain cytokine signals mediated predominantly by the kinases Jak1, Jak3, and STAT5, have emerged as one of the cell-intrinsic mechanisms of T cell exhaustion during chronic infections (15,19). The present study shows that chronic T. cruzi infection leads to a perturbation of the IL-7/IL-7R-mediated T cell signalling through the STAT5 pathway which is independent of the expression of the IL-7 receptor components.
IL-7 is a critical cytokine that regulates the transition from effector-to-memory T cells, as it is involved in survival and homeostasis of memory T cells (8, 20). Naive T cells express IL-7R, but IL-7R expression drops on activated T cells as they expand and differentiate during infection. At the peak of expansion, most effector T cells are IL-7Rlo; however, a smaller subset of IL-7Rhi cells exist that express higher levels of Bcl-2 and preferentially survive to generate long-lived memory T cells with self-renewal capacity (21-24).
T cells in patients with no signs of cardiac disease appeared to retain the capability to regulate the expression of the IL-7R components with a relative loss of CD127+CD132+ T cells along with an increase in CD127−CD132+, as expected in the context of a process of immune activation. It has been shown that CD127-CD132+ T cells are mainly comprised by short-live effector T cells (25), concurring with the high frequencies of total effector CD4 and CD8 T cells found in chronically T. cruzi-infected subjects (3,4). Despite that patients in the chronic phase of the infection, including those with more severe forms of the disease, display an activated immune status (3,4, 26-29), herein we show that the capability to modulate IL7R receptor components are impaired in more severe clinical stages. Our experiments of coculture of PBMC with T. cruzi trypomastigotes, support that T. cruzi can mediate the modulation of the IL-7R components. In our system, IL-7R might have been activated either through the interaccion of T cells with APCs displaying T. cruzi antigens on their surfaces, or through the action of cytokines released after in vitro infection.
Further evidence of the disturbances in the IL-7/IL-7R pathway is the lower levels of STAT5 phosphorylation in response to IL-7 in association with increased basal STAT5 phosphorylation in patients with cardiomyopathy. Apart from persistent antigen stimulation, upregulated expression of IL-7 and IL-15 might induce a dysregulation in the IL-7/IL-7R pathway (8,15,19). Other cytokines or growth factors, such as thymic stromal lymphopoietin, IL-3, IL-5 or IL-9 released as a result of the persistent infection, could also activate STAT5 (31) accounting for the high basal p-STAT5. This upregulation of STAT5 phosphorylation may in turn desensitize the STAT5-dependent signaling pathway resulting in the lower capacity to respond to IL-7 stimulation and to a downregulation of the IL-7R alpha chain in these patients.
Although, serum levels of IL-7 were increased in chronically T. cruzi-infected subjects, we did not observe a correlation between circulating IL-7 and basal or induced p-STAT5 expression. Fonseca et al (31) have also demonstrated increased mRNA for IL-7 and the CD132 chain of the IL-R in heart tissues from Chagas disease patients with severe cardiomyopathy, supporting a putative role for IL-7 in the alteration of IL-7R components in the chronic phase of T. cruzi infection. A role for IL-5 and IL-9 in the perturbation of the IL-7 signaling pathway cannot be ruled out since these cytokines have been also detected in sera from patients with chronic cardiomyopathy (32,33). In human HIV infection, impaired IL-7/IL-7R signaling was correlated with the levels of basal phosphorylation, viral load and activation status, which were reverted upon successful antiretroviral treatment (34,35). The reduced capacity of CD4 and CD8 T cells to upregulate Bcl-2 expression in patients with severe heart disease is in agreement with the poor STAT5 phosphorylation induced upon IL-7 stimulation found in these subjects. Although restricted to CD8+ T cells, CD25 upregulation, another STAT5 downstream function, was also impaired in subjects with chronic Chagas disease.
The alterations observed in the IL-7/IL7R system in long-term T. cruzi-infected subjects could explain the diminished frequencies of T. cruzi-specific T cells and the increased levels of T cell apoptosis, previously found in subjects in the chronic phase of the infection (3,4). Moreover, more than one mechanisms of immune exhaustion might be triggered during chronic T. cruzi infection, as indicated by our previous findings showing increased expression of CTLA-4 by IFN-γ-producing CD4+ T cells responsive to T. cruzi Ags and in tissues from patients with severe cardiomyopathy (36).
Although most of IFN-γ-responsive T cells to T. cruzi Ags express the IL-7Rα chain (D.P.-M., personal communication), it is possible that the function and expression of the IL-7R might be dampened overtime. In a mouse model of chronic T. cruzi infection, a population of CD8+CD127hiCD62L+CD122+ T cells specific for T. cruzi was maintained following transfer into naive mice (37). This T cell population was capable to produce IFN-γ upon restimulation with T. cruzi Ags and expanded in response to challenge infection, indicating these cells are functionally responsive upon antigen re-encounter (37). However, the mouse model of chronic T. cruzi infection does not lead to immune exhaustion (37,38).
In conclusion, parasite persistence in chronic Chagas disease alters the IL-7/IL-7R pathway system which might represent another potential mechanism of T cell exhaustion in chronic T. cruzi infection.
Supplementary Material
Acknowledgments
We thank the staff and patients the “Hospital Interzonal de Agudos Eva Perón who provided blood samples; the staff of Diagnosis Department of the Instituto Nacional de Parasitología Dr Mario Fatala Chaben” for serological test and Julie Nelson (Center for Tropical and Emerging Global Disease, University of Georgia, Athens, GA) for assistance with flow cytometry.
Funding: This work was supported by The National Institutes of Health (grant P01AI044979 to RLT) and National Fund for Science and Technology of Argentina (PICTO-ANLIS 2011-00116 to M.C.A.). M.C.A. and S.A.L. are members of the Scientific Career, Consejo Nacional de Investigación Científica yTécnica (CONICET), Argentina.
References
- 1.World Health Organization. Research Priorities for Chagas disease, Human African Trypanosomiasis and Leishmaniasis. Tech Rep Ser. 2012:1–100. [PubMed] [Google Scholar]
- 2.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, Tarleton RL, Postan M. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas' disease patients. Int Immunol. 2006;18:465–471. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 4.Albareda MC, Olivera GC, Laucella SA, Alvarez MG, Fernandez ER, Lococo B, Viotti R, Tarleton RL, Postan M. Chronic human infection with Trypanosoma cruzi drives CD4+ T cells to immune senescence. J Immunol. 2009;183:4103–4108. doi: 10.4049/jimmunol.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albareda MC, Olivera GC, De Rissio AM, Postan M. Short Report: Assessment of CD8 + T Cell Differentiation in Trypanosoma cruzi -Infected Children. Am J Trop Med Hyg. 2010;82:861–864. doi: 10.4269/ajtmh.2010.09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laucella SA, Postan M, Martin D, Hubby Fralish B, Albareda MC, Alvarez MG, Lococo B, Barbieri G, Viotti RJ, Tarleton RL. Frequency of interferon-g-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis. 2004;189:909–918. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez MG, Postan M, Weatherly DB, Albareda MC, Sidney J, Sette A, Olivera C, Armenti AH, Tarleton RL, Laucella SA. HLA class I-T cell epitopes from trans-sialidase proteins reveal functionally distinct subsets of CD8+ T cells in chronic Chagas disease. PLoS Negl Trop Dis. 2008;2:e288. doi: 10.1371/journal.pntd.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Bani L, Pasquier V, Kryworuchko M, Salamero J, Thèze J. Unstimulated human CD4 lymphocytes express a cytoplasmic immature form of the common cytokine receptor gamma-chain. J Immunol. 2001;167:344–349. doi: 10.4049/jimmunol.167.1.344. [DOI] [PubMed] [Google Scholar]
- 10.Golden-Mason L, Jr, Burton J, Castelblanco N, Klarquist J, Benlloch S, Wang L, Rosen HR. Loss of IL-7 receptor α-chain CD127 expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–1109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 11.Colpitts SL, Dalton NM, Scott P. IL-7 receptor expression provides the potential for long-term survival of both CD62L high central memory T cells and Th1 effector cells during Leishmania major infection. J Immunol. 2009;182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One. 2010;5:e10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Viotti R, Vigliano C, Alvarez MG, Lococo B, Petti M, Bertocchi G, Armenti A, De Rissio AM, Cooley G, Tarleton R, Laucella S. Impact of aetiological treatment on conventional and multiplex serology in chronic Chagas disease. PLoS Negl Trop Dis. 2011;5:e1314. doi: 10.1371/journal.pntd.0001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imada K, Leonard WJ. The jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 18.Leonard WJ, O'Shea JJ. Jaks and Stats: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 19.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 21.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osbornea LC, Abrahama N. Regulation of memory T cells by γc cytokines. Cytokine. 2010;50:105–113. doi: 10.1016/j.cyto.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 24.Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasson SC, Zaunders JJ, Seddiki N, Bailey M, McBride K, Koelsch KK, Merlin KM, Smith DE, Cooper DA, Kelleher AD. Progressive activation of CD127+132- recent thymic emigrants into terminally differentiated CD127-132+ T-cells in HIV-1 infection. PLoS One. 2012;7:e31148. doi: 10.1371/journal.pone.0031148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Mazliah DE, Alvarez MG, Cooley G, Lococo BE, Bertocchi G, Petti M, Albareda MC, Armenti AH, Tarleton RL, Laucella SA, Viotti R. Sequential combined treatment with allopurinol and benznidazole in the chronic phase of T. cruzi infection: a pilot study. J Antimicrob Chemother. 2013;68:424–437. doi: 10.1093/jac/dks390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutra WO, Martins-Filho OA, Cancado JR, Pinto-Dias JC, Brener Z, Freeman Júnior GL, Colley GD, Gazzinelli G, Parra JC. Activated T and B lymphocytes in peripheral blood of patients with Chagas' disease. Int Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- 28.Dutra WO, Martins-Filho OA, Cancado JR, Pinto-Dias JC, Brener Z, Gazzinelli G, Carvalho JF, Colley DG. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 29.Menezes CAS, Rocha MOC, Souza PEA, Chaves ACL, Gollob KJ, Dutra WO. Phenotypic and functional characteristics of CD28+ and CD28 cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca SG, Reis MM, Coelho V, Nogueira LG, Monteiro SM, Mairena EC, Bacal F, Bocchi E, Guilherme L, Zheng XX, Liew FY, Higuchi ML, Kalil J, Cunha-Neto E. Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic Chagas disease cardiomyopathy. Scand J Immunol. 2007;66:362–371. doi: 10.1111/j.1365-3083.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- 31.Ross JA, Nagy ZS, Cheng H, Stepkowski SM, Kirken RA. Regulation of T cell homeostasis by JAKs and STATs. Arch Immunol Ther Exp. 2007;55:231–245. doi: 10.1007/s00005-007-0030-x. [DOI] [PubMed] [Google Scholar]
- 32.Longhi SA, Atienza A, Perez Prados G, Buying A, Balouz V, Buscaglia CA, Santos R, Tasso LM, Bonato R, Chiale P, Pinilla C, Judkowski VA, Gómez KA. Cytokine production but lack of proliferation in peripheral blood mononuclear cells from chronic Chagas' disease cardiomyopathy patients in response to T. cruzi ribosomal P proteins. PLoS Negl Trop Dis. 2014;8:e2906. doi: 10.1371/journal.pntd.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poveda C, Fresno M, Gironès N, Martins-Filho OA, Ramírez JD, Santi-Rocca J, Marin-Neto JA, Morillo CA, Rosas F, Guhl F. Cytokine profiling in Chagas disease: towards understanding the association with infecting Trypanosoma cruzi discrete typing units (a BENEFIT TRIAL sub-study) PLoS One. 2014;9:e91154. doi: 10.1371/journal.pone.0091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camargo JF, Kulkarni H, Agan BK, Gaitan AA, Beachy LA, Srinivas S, He W, Anderson S, Marconi VC, Dolan MJ, Ahuja SK. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweneker M, Favre D, Martin JN, Deeks SG, McCune JM. HIV-induced changes in T cell signaling pathways. J Immunol. 2008;180:6490–6500. doi: 10.4049/jimmunol.180.10.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argüello RJ, Albareda MC, Alvarez MG, Bertocchi G, Armenti AH, Vigliano C, Meckert P, Tarleton RL, Laucella SA. Inhibitory receptors are expressed by T. cruzi-specific effector T cells and in hearts of subjects with chronic Chagas disease. PLoS One. 2012;7:e35966. doi: 10.1371/journal.pone.0035966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–2650. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin DL, Weatherly DB, Laucella SA, Cabinian MA, Crim MT, Sullivan S, Heiges M, Craven SH, Rosenberg CS, Collins MH, Sette A, Postan M, Tarleton RL. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.