Systemic lupus erythematosus (SLE), like rheumatoid arthritis (RA) and other connective tissue diseases (CTDs), does not develop on the day it is diagnosed. Rather, as rheumatologists know, the onset can be days to years before SLE is clinically diagnosed. The recognition of SLE, a prototypic autoimmune disease with a striking heterogeneity and range of manifestations, can be a challenge1, 2. In some individuals, full-blown disease, with a “full house” of autoantibodies and aggressive, life-threatening disease, such as lupus nephritis or vasculitis, seems to develop overnight. In others, however, the onset is insidious and difficult to recognize and to distinguish from other conditions. The diagnosis of SLE among patients seen in consultation, most often for a positive antinuclear antibody (ANA) and one or two other non-specific symptoms, such as arthralgias, myalgias, fatigue or rashes, is one of the common clinical challenges faced by rheumatologists. The challenge is compounded by the fact that SLE is likely a spectrum of related diseases that share immunologic and clinical features.
Despite advances in our understanding of the pathogenesis of SLE, distinguishing what is SLE, what is not SLE, and what could become SLE, continues to be an important clinical problem for rheumatologists. The American College of Rheumatology (ACR), and the more recent Systemic Lupus International Collaborating Clinics (SLICC) group criteria for the classification of SLE, were developed for classifying patients as having SLE for the purpose of clinical trials and studies3–5. While they were not intended to be used for diagnosis, from medical school on clinicians know these criteria, and often rely upon them to aid in diagnosis. However, they do not classify most patients with early or potential SLE. Therefore, a variety of descriptive terminology has been developed over the past 30 years to refer to patients thought likely to be predisposed to the development of SLE. The terms “latent lupus”, “incomplete lupus erythematosus”, and other terms have been used to refer to individuals with some SLE signs and symptoms, but not meeting ACR classification criteria or diagnosable SLE. (Table 1)
Table 1.
Terms used to Describe Early and Non-Classical Systemic Lupus Erythematosus
| Lupus-like syndrome (“overlap”, subset and/or variant syndrome) |
| Undifferentiated Connective Tissue Disease |
| Antiphospholipid antibody syndrome with SLE features |
| Mixed Connective Tissue Disease with SLE features |
| Occult lupus |
| Pseudo-lupus |
| Borderline lupus or suspected lupus |
| Latent lupus |
| Incipient lupus |
| Incomplete lupus |
| Possible lupus |
| Probable lupus |
| Potential lupus |
| Adapted from Panush RS et al. What is Lupus? What is not Lupus? Controversies in Clinical Rheumatology, 1993; 19(1), 223–234. |
In addition to the clinical challenge these patients present and our desire to be able to give them more accurate prognoses and more appropriate treatment, there is growing research interest in identifying and targeting the earliest phases of autoimmune disease development, with the hope that treatment of very early disease could head off complications and lead to less irreversible damage. We know that in SLE, as in RA, autoantibodies may be present in the blood years prior to diagnosis. Testing of banked serial serum samples from 130 military recruits who subsequently developed SLE revealed that 88% had one or more SLE-associated autoantibody present prior to diagnosis, and auto-antibodies were detectable up to 9.4 years (mean 3.3 years) before diagnosis. ANAs, anti-phospholipid, anti-Ro, and anti-La antibodies were elevated in the earliest samples (mean of 3.2 years pre-diagnosis), while anti-dsDNA, anti-Sm and anti-RNP antibodies were elevated in banked serum samples closer to the time of diagnosis6, 7. The idea was posited that “benign autoimmunity” may occur during an earlier phase of autoantibody presence during the preclinical period, whereas more dangerous, specific pathogenic autoantibodies may appear in the time interval closer to the onset of clinical symptoms. A recent study utilizing samples from a Swedish blood bank has found that concentrations of interferon-gamma inducible protein-10 (correlated with the cytokine interferon-alpha) are also elevated several years prior to SLE diagnosis8.
Cohorts of varying size of “incomplete lupus” patients (thought to be at elevated risk for developing SLE, but not clearly yet diagnosable or < 4 ACR criteria), have been studied over periods of time ranging from a few months to 7–8 years. In most of these studies, only a small proportion of patients (approximately 20%) evolve to either SLE classification or SLE diagnosis9,10 The term “undifferentiated connective tissue disease” (UCTD) has been used to refer to individuals presenting with clinical symptoms suggestive of some autoimmune connective tissue disease (SLE, Sjögren’s syndrome, scleroderma, mixed connective tissue disease or RA), but not clearly meeting ACR classification criteria for any of these diseases, a common clinical scenario. Follow-up studies of UCTD patients have revealed the rates of progressing to SLE are very similar to those in incomplete lupus cohorts, approximately 20%9–19. Additionally, those patients who have a prolonged clinical onset of their SLE tend to have a mild disease course, without severe organ involvement10.
Most of these descriptive terms, including latent lupus, incomplete lupus, probable lupus, possible lupus, and UCTD, are not mutually exclusive and likely contain a mix of patients in different phases of disease development. A patient with a +ANA, +anti-Ro, Raynaud’s and arthritis, for example, could be said to have latent lupus, incomplete lupus, or UCTD by different clinicians, and none of these terms would be wrong. We recently published a review of the medical records of 264 patients seen in consultation at our Lupus Center for possible SLE12. We referred to them as “potential SLE” patients; some could have been classified as UCTD and most as incomplete lupus at the time of their first evaluation12. Over a mean of 6.3 (SD 4.3) years of follow-up, 21% progressed to be diagnosed with SLE, while 18% were thought not to have SLE and 61% were still considered to have potential SLE at the most recent follow-up. Several past studies, including ours, have tried to identify clinical predictors for the development of SLE among these potential patients. No clear “high risk for SLE” predictors have emerged, although the presence of photosensitivity, malar rash, oral ulcerations, anti-dsDNA antibodies, and low levels of C3, have been identified in a few different studies.11, 12 Reasons for the lack of clear predictors of SLE risk likely include the heterogeneity of our terms to used describe this patient population with early potential symptoms of SLE or other CTD, as well as the heterogeneity of SLE itself, the small size of studies, their retrospective and heterogeneous study designs, and the lack of very specific biomarkers for disease pathogenesis at a molecular level.
Most of these past studies of patients at risk for the development of SLE have investigated routinely collected clinical factors, mainly the ACR criteria, in relation to the risk of transitioning to diagnosed or classified SLE. One small prospective study from the Dallas Regional Autoimmune Disease Registry investigated more novel biomarkers among 22 incomplete lupus patients (<4 ACR criteria) followed for an average of 2.4 years. Only three patients (14%) transitioned to fulfill ACR classification criteria and these three were more likely to have overall IgG autoreactivity on an autoantigen array, autoantibodies against proliferating cell nuclear antigen, beta 2 microglobulin, C1q, and hemocyanin at baseline compared to those who remained incomplete lupus20. In a separate study in the Dallas Regional Autoimmune Disease Registry, elevated levels of one or more IgG autoantibodies were detected in 19% of incomplete lupus patients compared to 26% of SLE. Additionally, the IgG to IgM autoantibody ratios steadily increased from healthy controls, compared to incomplete lupus and then SLE21,22. A set of interferon genes was upregulated in 83% of SLE patients and 50% of incomplete lupus patients, while among controls no interferon upregulation was detected23.
An essential point about this group of past studies is that none of the subjects (except for family members in the Dallas Registry) had “pre-clinical” disease. All had at least some clinical features of SLE. Studies in which subjects who are at high risk by virtue of their family history of SLE or other autoimmune disease, genetic or environmental risk factors, or are asymptomatic with autoantibodies detected, are followed are truly preclinical studies. However, we know that very few of these patients will develop SLE and that their likelihood of SLE depends on how long they are followed.
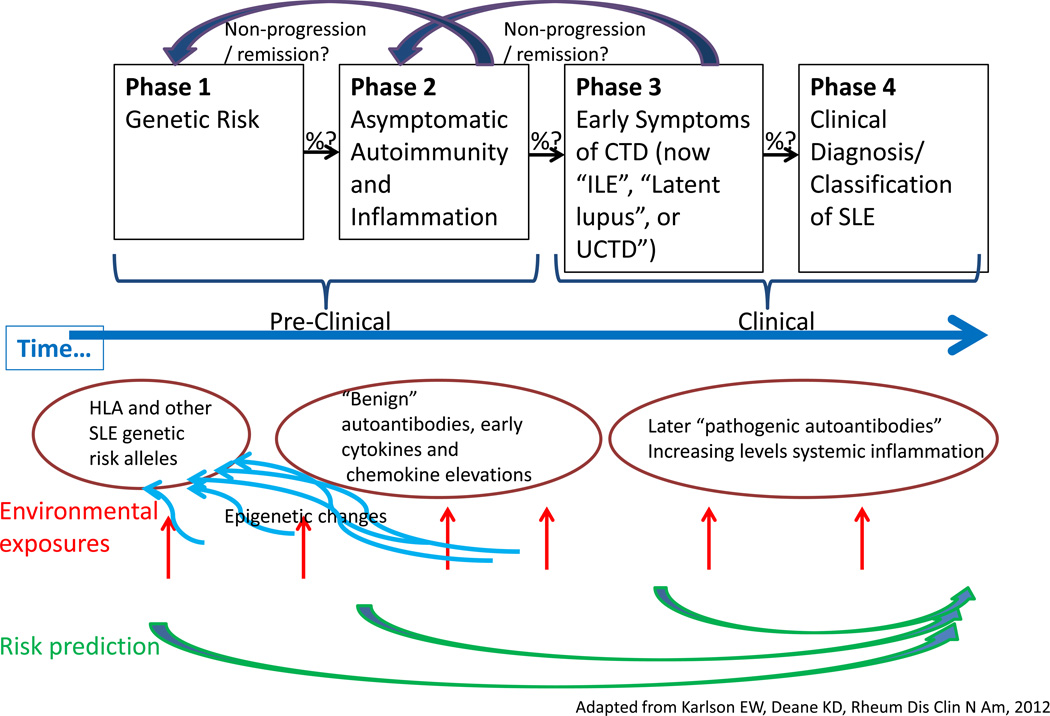
The study of the natural history and pathogenesis of RA has had similar terminology challenges over the years, with a variety of clinical descriptive entities, including “early and very early arthritis”, “undifferentiated arthritis”, and “early inflammatory polyarthritis”24. A natural history of RA timeline that has been proposed could be adapted to fit the evolution of SLE as well25. (Figure 1) Scientists studying RA have proposed standardized terminology to refer to the phase prior to the diagnosis/classification of RA24. A group from European League Against Rheumatism (EULAR) has recommended that individuals in prospective studies of RA natural history and pathogenesis be described as individuals with:
genetic risk factors for RA
environmental risk factors for RA
systemic autoimmunity associated with RA
symptoms without clinical arthritis or
unclassified arthritis
and that terms a–e may be used in combination24. The terms a–e above would also seem to fit individuals in studies of SLE very well, with e) changed to “unclassified CTD”. Moreover, they have made the important, yet subtle point that the term “pre-RA” can be employed with any combination of a–e, but only retrospectively to describe a phase that an individual had progressed through once it was known that they have developed RA. Thus, studies prospectively following individuals at risk for developing SLE, without eventual knowledge of their development of SLE, should not refer to these patients as “pre-SLE”.
Figure 1.
Phases Preceding SLE Diagnosis
As physicians and caregivers, it is essential that we can communicate and know which types of patients we are discussing. Moreover, for research purposes, we need to get our classification and terminology for potential and pre-SLE subjects straight in order to ask and possibly answer some very interesting questions, including but not limited to:
When does SLE start?
How does it start? Does it start in the same or different ways in different patients—and is that important?
How can we identify the earliest immune changes of SLE?
What determines the rapidity of onset and severity of disease at onset? (Do younger patients, male patients and non-Caucasian patients have a more explosive disease onset from the first detectable immune abnormalities to diagnosable SLE?)
Is the rapidity of onset different for different manifestations and subsets of SLE?
Why are some patients non-progressors? What can we learn from these individuals?
Is it possible to move “backwards” through the phases, as well as forwards?
Among those who have a positive ANA and one or two other non-specific symptoms (e.g. arthralgias and Raynaud’s), how can we predict who will develop SLE vs. MCTD vs. RA vs. scleroderma vs. Sjögren’s syndrome vs. no CTD?
If we could identify the earliest changes of SLE, could we “turn it off”?
In the study of the natural history and pathogenesis of SLE, individuals at increased SLE risk include those who are truly “pre-clinical”, such as those with family history of SLE and related autoimmune diseases, those who have had environmental exposures, and those with asymptomatic but elevated levels of autoantibodies. In the current confusing terminology, individuals at increased SLE risk also include those with early clinical symptoms, such as those with UCTD and the overlapping group of incomplete or latent lupus patients. The current ACR and SLICC classification criteria do not apply to any of these people who may, or may not, develop SLE in the future. Perhaps we should adopt SLE terminology similar to the proposed EULAR RA (a–e) terminology above, or perhaps the SLE research world should develop and test their own terminology for classifying patients at risk for developing SLE. With the growing interest in studying the time window prior to SLE diagnosis for clues to the etiology, pathogenesis and natural history of disease, and an eye to identifying targets for early intervention to abrogate disease, it is ever more imperative that we get our terminology for this research straight. In our opinion, the development and validation of better standardized terminology for, “people at risk of SLE and those in the process of developing a disease along the SLE spectrum”, should be placed high on the SLE research agenda.
References
- 1.Panush RS, Schur PH. It is lupus? Bull Rheum Dis. 1997;46(6):3–8. [PubMed] [Google Scholar]
- 2.Panush RS, Greer JM, Morshedian KK. What is lupus? What is not lupus? Rheum Dis Clin North Am. 1993;19(1):223–234. [PubMed] [Google Scholar]
- 3.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 4.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sanchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Jr, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinlen LD, Ritterhouse LL, McClain MT, Keith MP, Neas BR, Harley JB, James JA. Ribosomal P autoantibodies are present before SLE onset and are directed against non-C-terminal peptides. J Mol Med (Berl) 2010;88(7):719–727. doi: 10.1007/s00109-010-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, James JA. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56(7):2344–2351. doi: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson C, Rantapaa-Dahlqvist S. Cytokines in relation to autoantibodies before onset of symptoms for systemic lupus erythematosus. Lupus. 2014 doi: 10.1177/0961203314523869. [DOI] [PubMed] [Google Scholar]
- 9.Greer JM, Panush RS. Incomplete lupus erythematosus. Arch Intern Med. 1989;149(11):2473–2476. [PubMed] [Google Scholar]
- 10.Olsen NJ, Yousif M, Mutwally A, Cory M, Elmagboul N, Karp DR. Organ damage in high-risk patients with systemic and incomplete lupus syndromes. Rheumatol Int. 2013;33(10):2585–2590. doi: 10.1007/s00296-013-2783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vila LM, Mayor AM, Valentin AH, Garcia-Soberal M, Vila S. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus. 2000;9(2):110–115. doi: 10.1191/096120300678828073. [DOI] [PubMed] [Google Scholar]
- 12.Al Daabil M, Massarotti EM, Fine A, Tsao H, Ho P, Schur PH, Bermas BL, Costenbader KH. Development of SLE among "potential SLE" patients seen in consultation: long-term follow-up. Int J Clin Pract. 2014 doi: 10.1111/ijcp.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osnes LT, Nakken B, Bodolay E, Szodoray P. Assessment of intracellular cytokines and regulatory cells in patients with autoimmune diseases and primary immunodeficiencies - novel tool for diagnostics and patient follow-up. Autoimmun Rev. 2013;12(10):967–971. doi: 10.1016/j.autrev.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Mosca M, Tani C, Carli L, Bombardieri S. Undifferentiated CTD: a wide spectrum of autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26(1):73–77. doi: 10.1016/j.berh.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Calvo-Alen J, Alarcon GS, Burgard SL, Burst N, Bartolucci AA, Williams HJ. Systemic lupus erythematosus: predictors of its occurrence among a cohort of patients with early undifferentiated connective tissue disease: multivariate analyses and identification of risk factors. J Rheumatol. 1996;23(3):469–475. [PubMed] [Google Scholar]
- 16.Danieli MG, Fraticelli P, Salvi A, Gabrielli A, Danieli G. Undifferentiated connective tissue disease: natural history and evolution into definite CTD assessed in 84 patients initially diagnosed as early UCTD. Clin Rheumatol. 1998;17(3):195–201. doi: 10.1007/BF01451046. [DOI] [PubMed] [Google Scholar]
- 17.Bodolay E, Csiki Z, Szekanecz Z, Ben T, Kiss E, Zeher M, Szucs G, Danko K, Szegedi G. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD) Clin Exp Rheumatol. 2003;21(3):313–320. [PubMed] [Google Scholar]
- 18.Vaz CC, Couto M, Medeiros D, Miranda L, Costa J, Nero P, Barros R, Santos MJ, Sousa E, Barcelos A, Ines L. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clin Rheumatol. 2009;28(8):915–921. doi: 10.1007/s10067-009-1175-2. [DOI] [PubMed] [Google Scholar]
- 19.Dijkstra S, Nieuwenhuys EJ, Swaak AJ. The prognosis and outcome of patients referred to an outpatient clinic for rheumatic diseases characterized by the presence of antinuclear antibodies (ANA) Scand J Rheumatol. 1999;28(1):33–37. doi: 10.1080/03009749950155751. [DOI] [PubMed] [Google Scholar]
- 20.Olsen NJ, Li QZ, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther. 2012;14(4):R174. doi: 10.1186/ar3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, Karp D, Wakeland EK, Olsen NJ. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27(3):153–160. doi: 10.1016/j.jaut.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clinical & Experimental Immunology. 2007;147(1):60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159(3):281–291. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlag DM, Raza K, van Baarsen LG, Brouwer E, Buckley CD, Burmester GR, Gabay C, Catrina AI, Cope AP, Cornelis F, Dahlqvist SR, Emery P, Eyre S, Finckh A, Gay S, Hazes JM, van der Helm-van Mil A, Huizinga TW, Klareskog L, Kvien TK, Lewis C, Machold KP, Ronnelid J, van Schaardenburg D, Schett G, Smolen JS, Thomas S, Worthington J, Tak PP. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71(5):638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlson EW, Deane K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(2):405–426. doi: 10.1016/j.rdc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]