Abstract
Introduction
Dental implants are a vastly used treatment option for tooth replacement. Dental implants are however susceptible to inflammatory diseases such as peri-implant mucositis and peri-implantitis, which are highly prevalent and may lead to implant loss. Unfortunately, the understanding of the pathogenesis of peri-implant mucositis and peri-implantitis is fragmented and incomplete. Therefore, the availability of a reproducible animal model to study these inflammatory diseases would facilitate the dissection of their pathogenic mechanisms. The objective of this study is to propose a murine model of experimental peri-implant mucositis and peri-implantitis.
Materials and Methods
Screw-shaped titanium implants were placed in the upper healed edentulous alveolar ridges of C57BL/6J mice eight weeks after tooth extraction. Following four weeks of osseointegration, Porphyromonas gingivalis-lipolysaccharide (LPS) injections were delivered to the peri-implant soft tissues for six weeks. No-injections and vehicle injections were utilized as controls. Peri-implant mucositis and peri-implantitis were assessed clinically, radiographically (micro-CT) and histologically following LPS-treatment.
Results
LPS-injections resulted in a significant increase in soft tissue edema around the head of the implants as compared to the control groups. Micro-CT analysis revealed significantly greater bone loss in the LPS-treated implants. Histological analysis of the specimens demonstrated that the LPS-group had increased soft tissue vascularity, which harbored a dense mixed inflammatory cell infiltrate, and the bone exhibited noticeable osteoclast activity.
Conclusion
The induction of peri-implant mucositis and peri-implantitis in mice via localized delivery of bacterial LPS has been demonstrated. We anticipate that this model will contribute to the development of more effective preventive and therapeutic approaches for these two conditions.
Keywords: Peri-implant mucositis, Peri-implantitis, Implants, Mouse, Dental Implants, Lipopolysaccharide
INTRODUCTION
Dental implants have become an increasingly popular and reliable treatment option for tooth replacement (Nickenig, Wichmann et al. 2008; Johannsen, Westergren et al. 2012). However, periodontal tissues surrounding dental implants are susceptible to inflammatory diseases such as peri-implant mucositis and peri-implantitis (Berglundh, Lindhe et al. 1992; Roos-Jansaker, Lindahl et al. 2006; Graziani, Figuero et al. 2012), which resemble gingivitis and periodontitis.
Peri-implant mucositis involves inflammation of the soft tissues surrounding a dental implant. It is present in 80% of subjects who received dental implants and in 50% of functional implants (Roos-Jansaker, Lindahl et al. 2006; Lang and Berglundh 2011; Romanos and Weitz 2012). Peri-implant mucositis is a reversible process and does not result in peri-implant bone loss. However, if left untreated, peri-implant mucositis can progress to peri-implantitis (Mombelli and Lang 1998; Lindhe and Meyle 2008; Zitzmann and Berglundh 2008; Heitz-Mayfield and Lang 2010; Sanz and Chapple 2012).
Peri-implantitis, an inflammatory condition of the soft and hard tissues around implants, includes peri-implant bone destruction and can result in implant loss (Mombelli and Lang 1998). Approximately 11%-47% of implants have some degree of peri-implantitis, and such high prevalence poses a significant clinical problem given the cumulative number of implants delivered overtime (Fransson, Lekholm et al. 2005; Roos-Jansaker, Lindahl et al. 2006). Unfortunately, there are no established effective treatment protocols for peri-implantitis, primarily because the pathogenesis of the disease is not fully understood.
Peri-implantitis shares some common features with periodontitis (Mombelli, Marxer et al. 1995). Clinically, implant fixtures affected by peri-implantitis present with increased pocket depth, inflammatory soft tissue changes including bleeding on probing, clinical attachment loss and radiographic bone loss. In addition, the biofilm in deep pockets around implants is similar to the microbiota associated with periodontitis (Leonhardt, Renvert et al. 1999). From the macroscopic and histopathological standpoints; however, periodontitis and peri-implantitis lesions are markedly different. Clinically and radiographically, peri-implantitis is often symmetric, occurring around the whole fixture perimeter, as opposed to surface-specific periodontits lesions around natural teeth. Histopathologically, inflammation in peri-implantitis penetrates deep and beyond the soft tissue around the pocket, often onto bone and its alveolar spaces (Lindhe, Berglundh et al. 1992). In contrast, inflammation in periodontitis is consistently separated from the alveolar bone from a zone of inflammation-free connective tissue that is about 1 mm wide. One of the most interesting and puzzling differences between periodontitis and peri-implantitis has been revealed in a dog study (Zitzmann, Berglundh et al. 2004). Plaque-induced lesions were initiated around natural teeth and implants via installation of ligatures, which were subsequently removed. Upon ligature removal, periodontitis and peri-implantitis lesions responded differently: periodontitis lesions tended to stabilize and present with no further progression while peri-implantitis lesions almost invariably progressed and were not self-limiting. Moreover, little biochemical and molecular information exists about the possible role of pro-inflammatory and other mediators in the pathogenesis of peri-implantitis.
Therefore, understanding the pathogenesis of peri-implant mucositis and peri-implantitis and developing a systematic approach to prevent the onset and progression of both conditions is crucial to maximize the longevity of dental implants (Graziani, Figuero et al. 2012; Romanos and Weitz 2012; Schwarz, Iglhaut et al. 2012). One of the main limiting factors in dissecting the pathogeneses of peri-implant mucositis and peri-implantitis is the absence of an easily accessible, reproducible and inexpensive animal model.
Rodent models are attractive to study the pathogenic phenomena and biological pathways associated with various diseases due to similarities that these animals share with humans. Mouse models, in particular, present with the additional advantage of the availability of genetically manipulated animals, which can serve as unique and sophisticated tools to studying disease pathogenic mechanisms. This study describes a murine model of peri-implant mucositis and periimplantitis.
MATERIALS AND METHODS
Mice
Four-week old C57BL/6J (The Jackson Laboratories, Bar Harbor, ME) male mice were utilized in this study adhering to the approved protocol and guidelines of the Chancellor’s Animal Research Committee at the University of California, Los Angeles. Mice were fed a soft diet (BioServe ®, Frenchtown, New Jersey) ad libitum for the duration of the experiments.
Tooth extraction and implant placement
Under inhalation anesthesia with 3% isoflurane, maxillary left 1st, 2nd, and 3rd molars were extracted and sockets were allowed to heal for eight weeks. Animals were given antibiotics (sulfamethoxazole and trimethoprim oral suspension, USP; 850μg/170μg/mL) orally by incorporating the medications into the drinking water for four weeks after extractions.
Custom-designed screw-shaped implants 0.5 mm in diameter by 1 mm in length were fabricated from 6AL4V titanium rods (DP Machining Inc., La Verne, CA) (Figure 1). Eight weeks after extractions and under general anesthesia, a mesial-distal incision was made with a 12D blade in the edentulous left alveolar ridge. Buccal and palatal full thickness flaps were elevated with a #5 dental explorer as to exposed the healed alveolar ridge. Implant fixture osteotomy was performed with a 0.03 mm carbide micro hand drill (BIG Kaiser Precision Tooling Inc., Hoffman Estates, Illinois) in the first/second molar area, using the teeth in the opposite side as spatial reference. One implant was placed in each mouse. Implant fixtures were inserted to the point that the implant head was leveled with the bone using a clock-wise screwing motion. Antibiotics as described above were given to the animals for four weeks after implant placement.
Figure 1.
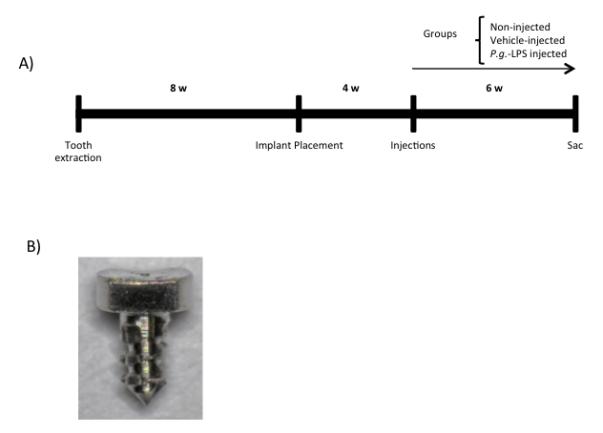
A) Schematic diagram depicting timing of the experimental design. B) Representative clinical image of the implant fixtures utilized in the development of the peri-implant mucositis and peri-implantitis murine model.
Induction of peri-implant mucositis and peri-implantitis
The induction of peri-implant mucositis and peri-implantitis was initiated four weeks after implant placement via lipopolysaccharide (LPS) injection into the peri-implant mucosa. Mice were divided into three groups: (1) no-injected control (2) vehicle-injected control (2μl injection of phosphate-buffer saline - PBS) and (3) LPS-injected, experimental group (2 μl of 10 mg/ml ultrapure LPS from Porphyromonas gingivalis [InvivoGen, San Diego, CA]), as described in a previous study (Sartori et al, 2009).
Injections were administered in the disto-palatal aspect of the peri-implant mucosa twice a week for a period of six weeks with the use of a hamilton syringe and a 33 gauge needle. At the end of the treatment period, mice were sacrificed, their maxillae harvested and fixed in 4% paraformaldehyde for 48 hours. Maxillae were then stored in 70% ethyl alcohol.
Clinical assessment of peri-implant mucositis
Macroscopic images of the specimens were obtained using a Keyence ® VHX-1000 (Osaka, Japan) digital optical microscope. Clinical images were analyzed with Aperio Image Scope software V11.1.2.752 (Vista, CA) to determine the amount of soft tissue coverage over the implant head. Soft tissue coverage was assessed by the evaluation of the exposed implant head surface area (μm2). If the head of the implant had complete soft tissue coverage the exposed surface area was considered to be 0 μm2.
Micro-CT analysis of peri-implantitis
Mouse maxillae were imaged by micro-computed tomography (micro-CT) scanning (SkyScan 1172; SkyScan, Kontich, Belgium) at 10μm resolution and x-ray energy of 55 KVp and 181 μA. Volumetric data were converted into Digital Imaging and Communications in Medicine (DICOM) format and imported into Dolphin Imaging software (Chatsworth, CA) to generate three-dimensional/multi-planar reconstructed images. Bone levels were assessed using the Dolphin software by measuring from the outermost edge of the head of the implant to the alveolar bone in the palatal aspect of the implant through the use of sagittal and coronal images.
Histology
For histological evaluation of implant osseointegration and peri-implant bone levels, undecalcified maxillae were embedded in methylmethacrylate (Pereira, Stadmeyer et al. 2007) and ground to 20 μm-thick sections in the sagittal plane, parallel to the to the long axis of the implant (EXAKT Cutting & Grinding System, Exakt Apparatebau, Norderstedt, Germany). Toluidine blue staining of the sections was performed according to standard protocols.
Additional samples were decalcified in 15% EDTA. At the end of the decalcification period, implant were removed via unscrewing motion and the specimens embedded in paraffin. Five μm-thick paraffin sections were generated. Sections were cut sagittally and stained with hematoxylin and eosin (H&E) using standard protocols.
Statistics
Data were represented as group means plus/minus the standard error of the mean (SEM). Comparisons between groups were conducted by Student’s t-tests utilizing GraphPad Instat software (La Jolla, CA).
RESULTS
Implant placement and osseointegration
Healing of the extraction sockets was uneventful in all animals (Figure 1A). To determine implant osseointegration (Figure 1B), implant mobility was evaluated four weeks after implant placement by observing a wiggling movement between two periodontal probes; 84.6% (22/26) implants were clinically osseointegrated. Additionally, the soft tissues around implant fixtures appeared to be healthy with no swelling or any other inflammatory signs (Figure 2A).
Figure 2.
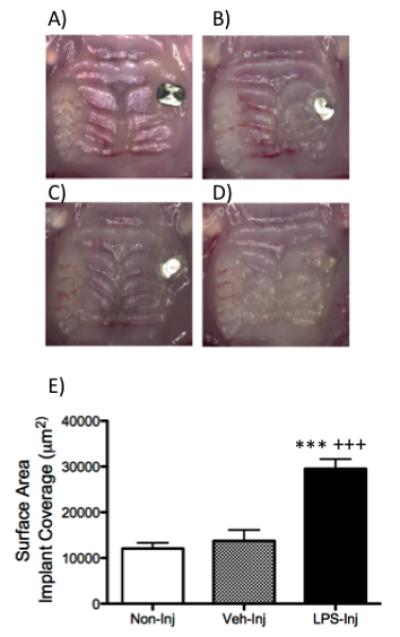
A) Representative clinical image of the mouse maxilla at four weeks after implant placement. B) Representative clinical images of a mouse maxilla in the B) non-injected, C) vehicle-injected and D) LPS-injected groups at six weeks after initiation of implant treatment. E) Graph represents surface area exposed by soft tissue of the non-injected, vehicle-injected and LPS-injected groups six weeks after injections were initiated. Data are mean ± SEM. ***p<.0001 compared to control and +++p<.0001 compared to vehicle (n≥5/group).
Induction of peri-implant mucositis and peri-implantitis
Clinically, the experimental group presented with significantly more edema around the head of the implant than the control groups (non-injected and veh-injected). The mean implant head surface was 29,518 μm2 .The mean exposed surface was 12,076 μm2 in the non-injected, 13,743 μm2 in the vehicle-injected and 3,191 μm2 in the LPS-injected group, respectively (Figure 2B-E). There was a statistically significant increase in implant coverage in the LPS group compared to the control groups (non-injected and vehicle-injected). There was no statistical difference in implant head coverage in the non-injected group compared to the vehicle-injected group.
Micro-CT measurements of the distances between the implant head and the bone (Figure 3) revealed that the measurements were significantly increased in the LPS-injected group (320 μM) as compared to the control groups, non-injected (217μM) and vehicle-injected (230 μM), suggesting increased bone loss. No significant differences were detected between the non-injected versus vehicle-injected groups.
Figure 3.
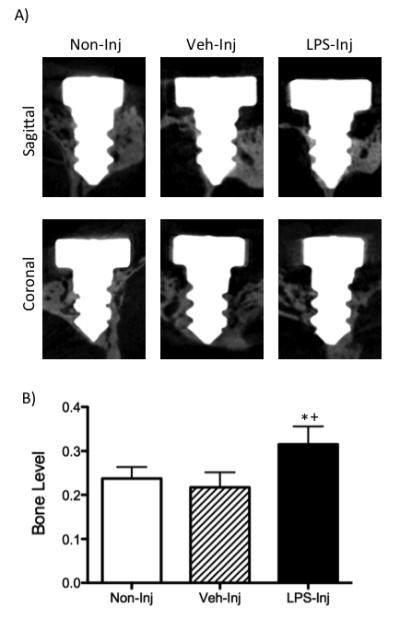
A) Representative micro-CT images - sagittal and coronal planes – six weeks after initiation of treatment (non-injected, vehicle-injected and LPS-injected groups). B) Graph represents bone levels as measured by the distance of the implant head to the alveolar bone crest on the palatal aspect of the fixtures. Data are mean ± SEM. *p<.05 compared to non-injected and +p<.05 compared to vehicle-injected (n≥7/group).
Histology
Toluidine blue staining showed that all implants in the three study groups were osseointegrated at the end of the experimental period (Figure 4). The peri-implant mucosa of the experimental group exhibited increased vascularity and harbored a dense mixed inflammatory cell infiltrate. The experimental group had vertical defects on the buccal alveolar process of the implant. The surrounding bone was porous and exhibited noticeable osteoclast activity. The porous nature of the bone was responsible for bone fragmentation after removing the implant (as shown in the H&E section)(Figure 4).
Figure 4.
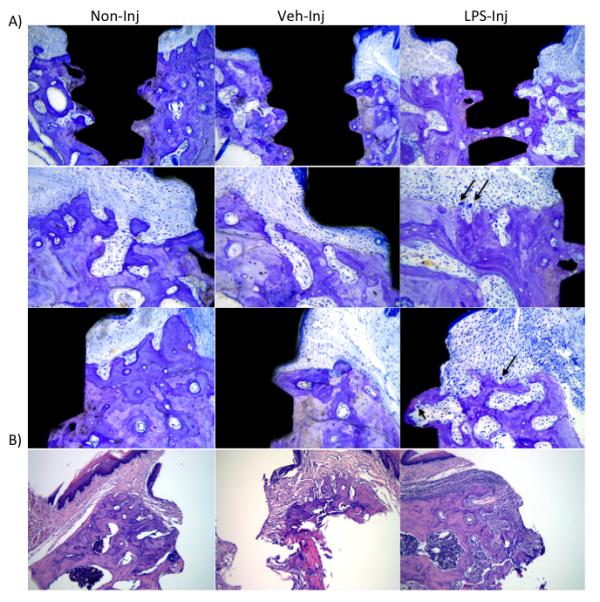
A) Representative images of toluidine blue stained sections six weeks after treatment (non-injected, vehicle-injected and LPS-injected groups); 100X magnification. Notice different bone levels between the experimental and the control/vehicle groups. Black arrows represent osteoclasts. B) Representative images of hematoxylin and eosin stained sections six weeks after treatment weeks after treatment (control, vehicle and experimental groups), 100X magnification. Notice the presence of a dense mixed inflammatory cell infiltrate in the experimental group as compared to the control/vehicle groups.
DISCUSSION
A substantial body of scientific knowledge about peri-implant mucositis and peri-implantitis has emerged through human studies. While human studies are essential in framing a problem at the clinical and epidemiological levels, they present with limitations. Human studies do not allow for the conduction of detailed specimen histological examination. Additionally, experimental protocols cannot be established with respect to disease initiation and progression. Human study protocols often cannot avoid confounding variables such as the existence of systemic conditions (i.e., diabetes), smoking habits, oral hygiene habits, small sample cohorts, and genetic heterogeneity. All together, researchers cannot solely rely on human studies to understand the pathogenesis of disease and to conduct initial treatment protocol testing for diseases. Therefore, much can be achieved buy combining data obtained from human and animal studies.
Therefore, animal models where genetic and environmental factors can be tightly controlled offer an attractive and essential complement to human studies in the process of understanding a disease or condition. As an example, mice share structural, functional and genetic traits with humans, which makes them a convenient starting point in dissecting disease pathways at the tissue and cell levels. Moreover, powerful molecular and genetic tools developed in the past two decades make mice an ideal animal model to study of diseases that may be multifactorial and complex in etiology. A major advantage of murine models is that the genetic mechanisms of disease can be dissected, facilitating the functional characterization of genes that may be important in disease initiation and development (Nguyen and Xu 2008; Flint and Eskin 2012). Hence, establishing an inflammatory-mediated peri-implant mucositis and peri-implantitis mouse model is essential for understanding disease initiation and progression.
Peri-implant mucositis and peri-implantitis are multi-factorial diseases. They are initiated by bacterial biofilm accumulation, which leads to the development of an inflammatory response. This inflammatory response activates multiple signaling pathways that lead to inflammation of the peri-implant soft tissues, which has been termed peri-implant mucositis. It is believed that some cases of chronic peri-implant mucositis do progress into peri-implantitis, which include bone destruction and clinical attachment loss (Lindhe and Meyle 2008). While some basic understanding of peri-implant mucositis and peri-implantitis is available, including the fact that they appear to be conditions initiated by bacteria, details of their pathogenesis are still elusive.
Based on the similarities that are evident between inflammatory diseases around natural teeth and dental implants, our peri-implant mucositis/peri-implantitis murine model derived from a well-characterized model of periodontal bone loss (Patil, Rossa et al. 2006; Rogers, Li et al. 2007; Sartori, Li et al. 2009; Li, Valerio et al. 2012), which involved localized P. gingivalis LPS delivery to the gingival tissues around a maxillary implant. This method was chosen because it bypasses the bacterial colonization process, allowing to focus on the inflammatory components of the disease, and avoiding variations that are inherently associated with bacterial colonization disease models (Hirschfeld, Ma et al. 2000; Bainbridge and Darveau 2001; Mahler, Janke et al. 2002; Patil, Rossa et al. 2006; Garrett, Lord et al. 2007; Rogers, Li et al. 2007; Khachatryan, Ktsoyan et al. 2008; Sartori, Li et al. 2009; Benson, Kelly et al. 2010; Vijay-Kumar, Aitken et al. 2010; von Chamier, Allam et al. 2012) .
In this study, we utilized P. gingivalis-derived LPS because P. gingivalis, a gram-negative anaerobic rod and member of the “red complex”, is widely recognized as a main contributor to periodontitis in humans and has also been implicated in human peri-implantitis (Socransky, Haffajee et al. 1998; Hajishengallis 2009) (Heitz-Mayfield and Lang 2010). Our data clearly show that the injection of P. gingivalis-derived LPS not only causes peri-implant bone loss, but also results in inflammatory changes in the peri-implant soft tissues, resembling the inflammatory sequential processes that are observed around dental implants placed in humans.
In the model presented here, threaded implants were placed in post-extraction sockets. Becker et. al also developed a murine model of periodontitis. However, Becker et al placed non-threaded implants in the palate. Although information can be gained by Baker’s model, it does not resemble clinical implant placement in humans. Nonetheless, our results corroborate those of Becker et al in that they confirm that a mouse model can be effective in the investigation of the pathogenesis of peri-implant mucositis and peri-implantitis (Becker, Foge et al. 2013). Other disease models, such as ligature-induced peri-implantitis are under investigation by our research group. Altogether, these models can be a valuable tool for the study of peri-implant mucositis an peri-implantitis.
In conclusion, a peri-implantitis model that focuses on understanding the inflammatory component of the disease may allow us to prevent and treat conditions that are highly prevalent (Fransson, Lekholm et al. 2005; Roos-Jansaker, Lindahl et al. 2006) in human kind. Despite the fact that there are treatment options for these conditions such as non-surgical and resective/reconstructive surgical therapies (Aljateeli, Fu et al. 2012), the effectiveness of these treatment methods has not been determined. We anticipate this model will contribute to the development of more effective preventive and therapeutic approaches for inflammatory conditions around dental implants.
ACKNOWLEDGEMENTS
This project was supported by the American Academy of Implant Dentistry Foundation and a seed grant from the UCLA School of Dentistry. Ms. Sarah Hiyari was supported by a training grant from NIH/NIDCR (T90 DE022734-01). The authors would like to thank Dr. Renata Pereira for her assistance with the preparation of undecalcified histological sections depicted in the manuscript and the Translational Pathology Core Laboratory at the UCLA School of Medicine for preparing the paraffin embedded sections included in the study. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
AAID RF Research grant (Camargo, PM)
REFERENCES
- Aljateeli M, Fu JH, et al. Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res. 2012;14(Suppl 1):e109–118. doi: 10.1111/j.1708-8208.2011.00387.x. [DOI] [PubMed] [Google Scholar]
- Bainbridge BW, Darveau RP. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol Scand. 2001;59(3):131–138. doi: 10.1080/000163501750266710. [DOI] [PubMed] [Google Scholar]
- Becker ST, Foge M, et al. Induction of periimplantitis in dental implants. J Craniofac Surg. 2013;24(1):e15–18. doi: 10.1097/SCS.0b013e318266fb2d. [DOI] [PubMed] [Google Scholar]
- Benson AK, Kelly SA, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107(44):18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglundh T, Lindhe J, et al. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res. 1992;3(1):1–8. doi: 10.1034/j.1600-0501.1992.030101.x. [DOI] [PubMed] [Google Scholar]
- Flint J, Eskin E. Genome-wide association studies in mice. Nat Rev Genet. 2012;13(11):807–817. doi: 10.1038/nrg3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson C, Lekholm U, et al. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005;16(4):440–446. doi: 10.1111/j.1600-0501.2005.01137.x. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131(1):33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani F, Figuero E, et al. Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri-implant diseases. J Clin Periodontol. 2012;39(Suppl 12):224–244. doi: 10.1111/j.1600-051X.2011.01832.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11(6-7):637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167–181. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Ma Y, et al. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- Johannsen A, Westergren A, et al. Dental implants from the patients perspective: transition from tooth loss, through amputation to implants - negative and positive trajectories. J Clin Periodontol. 2012;39(7):681–687. doi: 10.1111/j.1600-051X.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- Khachatryan ZA, Ktsoyan ZA, et al. Predominant role of host genetics in controlling the composition of gut microbiota. PLoS ONE. 2008;3(8):e3064. doi: 10.1371/journal.pone.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NP, Berglundh T. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(Suppl 11):178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- Leonhardt A, Renvert S, et al. Microbial findings at failing implants. Clin Oral Implants Res. 1999;10(5):339–345. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Valerio MS, et al. MAPK usage in periodontal disease progression. J Signal Transduct. 2012;2012:308943. doi: 10.1155/2012/308943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J, Berglundh T, et al. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3(1):9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- Mahler M, Janke C, et al. Differential susceptibility of inbred mouse strains to Helicobacter pylori infection. Scand J Gastroenterol. 2002;37(3):267–278. doi: 10.1080/003655202317284165. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Marxer M, et al. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22(2):124–130. doi: 10.1111/j.1600-051x.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Xu T. The expanding role of mouse genetics for understanding human biology and disease. Dis Model Mech. 2008;1(1):56–66. doi: 10.1242/dmm.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig HJ, Wichmann M, et al. Oral health-related quality of life in partially edentulous patients: assessments before and after implant therapy. J Craniomaxillofac Surg. 2008;36(8):477–480. doi: 10.1016/j.jcms.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Patil C, Rossa C, Jr., et al. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral Microbiol Immunol. 2006;21(6):392–398. doi: 10.1111/j.1399-302X.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Stadmeyer LE, et al. CCAAT/Enhancer-binding protein homologous protein (CHOP) decreases bone formation and causes osteopenia. Bone. 2007;40(3):619–626. doi: 10.1016/j.bone.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JE, Li F, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007;78(3):550–558. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos GE, Weitz D. Therapy of peri-implant diseases. Where is the evidence? J Evid Based Dent Pract. 2012;12(3 Suppl):204–208. doi: 10.1016/S1532-3382(12)70038-6. [DOI] [PubMed] [Google Scholar]
- Roos-Jansaker AM, Lindahl C, et al. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33(4):290–295. doi: 10.1111/j.1600-051X.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Sanz M, Chapple IL. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012;39(Suppl 12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- Sartori R, Li F, et al. MAP kinase phosphatase-1 protects against inflammatory bone loss. J Dent Res. 2009;88(12):1125–1130. doi: 10.1177/0022034509349306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Iglhaut G, et al. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J Clin Periodontol. 2012;39(Suppl 12):63–72. doi: 10.1111/j.1600-051X.2011.01838.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Chamier M, Allam A, et al. Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum. PLoS ONE. 2012;7(8):e44047. doi: 10.1371/journal.pone.0044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 Suppl):286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- Zitzmann NU, Berglundh T, et al. Spontaneous progression of experimentally induced periimplantitis. J Clin Periodontol. 2004;31(10):845–849. doi: 10.1111/j.1600-051X.2004.00567.x. [DOI] [PubMed] [Google Scholar]


