Abstract
IL-35 is a newly discovered inhibitory cytokine secreted by regulatory T cells (Tregs) and may have therapeutic potential in several inflammatory disorders. Acute graft-versus-host disease (aGVHD) is a major complication of allogeneic hematopoietic stem cell transplantation and caused by donor T cells and inflammatory cytokines. The role of IL-35 in aGVHD is still unknown. Here we demonstrate that IL-35 overexpression suppresses CD4+ effector T cell activation, leading to a reduction in alloreactive T-cell responses and aGVHD severity. It also leads to the expansion of CD4+Foxp3+ Tregs in the aGVHD target organs. Furthermore, IL-35 overexpression results in a selective decrease in the frequency of Th1 cells and an increase of IL-10-producing CD4+ T cells in aGVHD target tissues. Serum levels of TNF-α, IFN-γ, IL-6, IL-22 and IL-23 decrease and IL-10 increases in response to IL-35. Most importantly, IL-35 preserves graft versus leukemia effect. Finally, aGVHD grade 2-4 patients have decreased serum IL-35 levels comparing with time-matched patients with aGVHD grade 0-1. Our findings indicate that IL-35 plays an important role in reducing aGVHD through promoting the expansion of Tregs and repressing Th1 responses, and should be investigated as the therapeutic strategy for aGVHD.
Introduction
Acute graft-versus-host disease (aGVHD) is a common and frequently lethal complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), and is induced by donor T cells and inflammatory cytokines that cause damage to host epithelial tissues, predominantly the skin, liver, and gastrointestinal tract.1-3 On the other hand, alloreactive T cells also contribute to graft-versus-leukemia (GVL) effects that may prevent disease relapse.3 The immune response is regulated by multiple subtypes of regulatory/suppressor cells.4-6 Foxp3+CD4+ regulatory T cells (Tregs) have been shown to be critically involved in tolerance induction to alloantigens.7
There are two different Treg subsets, namely natural Tregs (tTregs) and induced Tregs (pTregs).8 Several lines of evidence demonstrated that donor tTregs could reduce GVHD.7, 9-12 GVHD severity depends, in part, on the balance between the donor tTregs and the effector T cells.1, 13 Treg-based approaches to treat inflammatory conditions such as GVHD have great potential but also have limitations. The identification of a well-defined population of Tregs that can be readily generated ex vivo and are stable and potently inhibitory in vivo is a critical goal for effective cell-based immunotherapy. The effector cytokines that play important roles in Treg expansion and function could also provide novel therapeutic approaches.
Epstein-Barr virus-induced gene3 (EBI3) can heterodimerize with IL-12p35 subunit to form IL-35.14 It can be secreted by CD4+Foxp3+ Tregs or iTr35 Tregs, a regulatory T-cell population induced by IL-35.15 Treg suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner.16 Therefore, the autocrine production of IL-35 by Tregs not only induces more Tregs, but also plays an important role in the suppressive functions of Tregs. Human CD8+ Tregs can also produce IL-35.17 Shen et al recently identified IL-35-producing B cells as key players in the negative regulation of immunity.18 IL-35 has been shown to have regulatory and therapeutic potential in mouse models of several inflammatory disorders, including inflammatory bowel disease (IBD), experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA).19-21 However, whether and how IL-35 may modulate aGVHD remains unknown.
In the current study, we demonstrated that IL-35 could reduce the severity of aGVHD with retention of GVL effects after allo-HSCT. Our results also showed that IL-35 could regulate donor T cell allo-responses through expanding the Treg population and suppressing Th1 responses. Our findings indicated that exogenous IL-35 could ameliorate aGVHD and may have therapeutic potential in modulating disease progression.
Material and Method
Induction and assessment of aGVHD
Eight-to-12-wk-old female BALB/c (H-2d), male C57BL/6 (B6, H-2b) mice were purchased from SLAC Animal Laboratory (Shanghai, China). Animals were kept in specific pathogen-free conditions. All animal protocols were approved by the institutional review committee at Soochow University. BALB/c recipient mice received lethal TBI (850 cGy) from a 60Co source. Irradiation was followed by the infusion of 1×107 C57BL/6 T cell-depleted bone marrow (TCD-BM) cells and spleen cells (5×106). The severity of GVHD was assessed with a clinical GVHD scoring system as first described by Cooke et al in a blinded fashion.22
Construction of pcDNA3.1-IL-35 and hydrodynamic gene transfer
Two complementary oligonucleotides encoding for four repeats of GGGS linker were designed and used to construct single-chain IL-35 gene using overlap PCR techniques. For hydrodynamic gene transfer (HGT), the recipient mice were injected i.v. with 50μg of the recombinant plasmid in a total of 2 ml saline solution within 5s using a 23-gauge needle 24 h before transplantation.
Flow Cytometry
Lymphocytes were labeled with conjugated mAb in the presence of purified anti-CD16/32 at saturation to block unspecific staining for 30 min at 4 °C. For intracellular staining, lymphocytes were stimulated for 4 hours with phorbol-12-myristate-13-acetate (50ng/mL) and ionomycin (500ng/mL), with brefeldin A added for the final 2 hours. Tregs were detected by a mouse Treg staining kit (eBioscience, San Diego, CA). In vivo proliferation was assessed with a BD Pharmingen BrdU APC kit. Cells were analyzed on a FACS Calibur flow cytometer with CellQuest (BD Bioscience, San Diego, CA).
Serum cytokine analysis
Cytokines were quantified using cytometric bead array kit (BD Bioscience, San Diego, CA). The serum concentration of IL-35 was assessed on day 1, 3, 6, 11, 13, 16, 19, 21, 24 after allo-HSCT by ELISA (Cloud Clone, Houston, TX).
Mixed lymphocyte reaction and cytotoxicity assay
For mixed lymphocyte reaction (MLR), responders and stimulators were cultured at a final concentration of 0.5×106/ml, pulsed with tritiated thymidine (1 mCi/well) (Shanghai Institute of Physics, Chinese Academy of Sciences, Shanghai, China) 16–18 h prior to harvesting, and counted on a beta plate reader (PerkinElmer Instruments, Meriden, CT). The IL-2 levels of the supernatants from a three-day MLR were estimated by the proliferation of CTLL-2 determined by MTT assay. Cytotoxic T cell activity was determined using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Fitchburg, WI) with responding cells after a 72h MLR.
Immunohistochemistry and histopathology
The paraffin sections of liver were stained with an anti-EBI3 antibody (DNT27) (Lifespan Biosciences, Seattle, WA) and an anti-p35 antibody (Bioworld Technology, St. Louis, MN) at concentrations of 1:200 at 37°C for 30 min. The image was developed using a polymer detection system for double immunohistological staining (Zhong Shan Gold Bridge Biotechnology, Beijing, China). Representative samples of lung, liver, small intestine, and colon were obtained from transplanted recipients and fixed in 4% formalin, paraffin-embedded, sectioned, and stained with H&E. A semiquantitative scoring system was employed to account for histological changes in four organs.22-24
Bioluminescent imaging
To examine the GVL effect, 5×106 luciferase-expressing A20 lymphoma cells (A20-luc, H-2d, kindly provided by Marcel van den Brink (New York, NY)) were given along with TCD BM and splenocytes in BALB/c recipients on the day of transplantation.25 For GVL effect assessment by bioluminescence imaging (BLI), mice were given an i.p. injection of 200μg firefly luciferin and then anesthetized and imaged weekly using Xenogen, IVIS 100 Bioluminescent Imaging System (Caliper Life Sciences, Hopkinton, MA) to determine the level of tumor burden.
Patients and sample preparation
Twenty-seven patients who underwent allo-HSCT between April and December 2012 at the Center for Hematopoietic Stem Cell Transplantation at the First Affiliated Hospital of Soochow University were included in this study. The characteristics of these patients are shown in Table S1. Informed consent was obtained from all patients, with approval of the institutional review board. Peripheral blood samples were collected as soon as aGVHD was diagnosed and before therapy was begun. Levels of IL-35 in the plasma samples were determined by ELISA (Biolegend, San Diego, CA).
Results
IL-35 overexpression ameliorates aGVHD
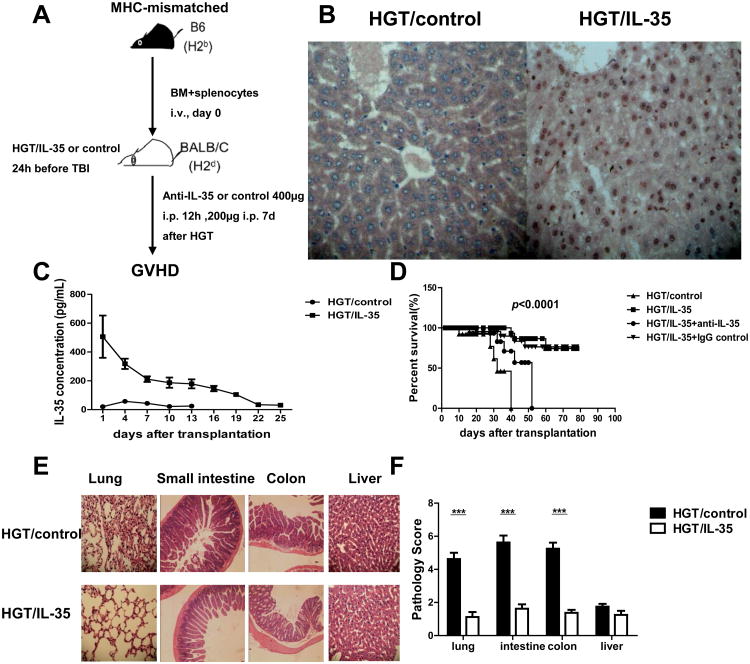
To assess the role of IL-35 in aGVHD, we used a major MHC-mismatched allogeneic BMT model (B6→BALB/c) and transplanted lethally irradiated BALB/c mice with 1×107 C57BL/6 TCD-BM cells and spleen cells (5×106). A construct encoding EBI3 and p35 linked by a glycine-serine linker was used for the expression of IL-35, and an empty vector was used as control. All recipient mice received HGT of plasmid DNA encoding IL-35 gene or control 24h before TBI (Fig. 1A). As shown in Figure 1B, liver tissues from mice that received IL-35 HGT showed a higher level of IL-35 expression compared with those of control mice. The relatively high serum levels of IL-35 were maintained for about 20 days (Fig. 1C). Therefore, the HGT could successfully mediate the expression of IL-35 in vivo. The recipients were monitored daily for clinical GVHD. We found that, 5×106 donor spleen cells induced severe aGVHD in control recipients, and all recipients died within 40 days after BMT. In contrast, the same dose of donor cells induced only moderate aGVHD in mice receiving IL-35 HGT, and 80% of them survived for more than 80 days (Fig. 1D). The protective effect of IL-35 was confirmed by the use of a unique EBI3-specific, IL-35-neutralizing antibody in vivo.19 Weights of the control mice were lower than those of IL-35 HGT group (Fig. S1A). Assessment of the clinical GVHD scores indicated markedly decreased aGVHD severity in IL-35 HGT recipients as compared to control mice (Fig. S1B).
Figure 1. IL-35 ameliorated aGVHD.
Mice received an HGT injection 24 h before TBI, lethally irradiated BALB/c recipient mice were transplanted with 1×107 B6 BM and 5×106 splenocytes. Transplanted recipients received neutralizating anti-IL-35 antibody (DV1.4C4.22) or isotype-matched control 400μg intraperitoneally on d0 and d6. Recipient mice were monitored daily. (A) The diagram illustrating the experimental design. (B) The liver sections were immunohistochemically stained with anti-EBI3 and anti-p35 antibody simultaneously. Original magnification ×400. (C) Serum IL-35 concentrations of the mice were determined by ELISA. (D) Survival data are shown. (E) A representative example of lung, small intestine, colon and liver section of BALB/c mice administrated with HGT/IL-35 or HGT/control on day 10 post allo-HSCT were collected for histological examination (× 100 magnification). (F) Histologic scores of lung, intestine, colon and liver were shown. Data are presented as the mean ± SEM. Each group consists of eight mice and the representative results were shown from at least three independent experiments. ***p<0.001.
Clinically, multiple organs could be affected by aGVHD, including liver, small intestine, colon, and lung. Ten days after transplantation, the above organs of recipient mice were harvested and histological examination showed markedly reduced inflammation in the lung, intestine and colon of IL-35 HGT recipients (Fig. 1E & F). Taken together, these results showed that exogenous IL-35 was capable of mitigating the development of aGVHD.
IL-35 overexpression leads to a reduction of CD4+ effector T cells and alloreactivity
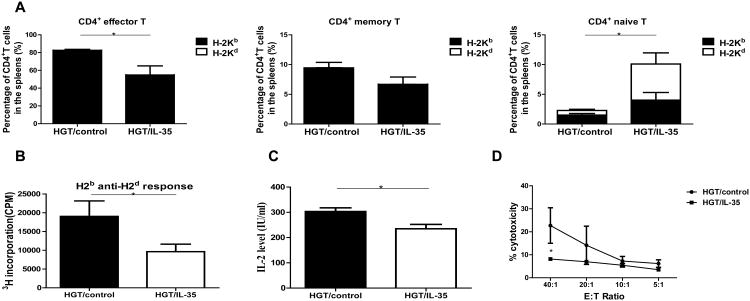
To understand the mechanisms underlying the reduction of aGVHD by overexpressing IL-35, we investigated the effect of IL-35 on T cell subsets during the development of aGVHD. The absolute numbers of CD3+, CD4+, CD8+, NKT cells were not significantly affected by IL-35 (Fig. S2A). We next investigated whether the activation status of the T cells was affected by IL-35 (Fig. 2A & Fig. S2B). The CD8+ T cells showed no significant changes in the percent of naïve, effector and memory subsets (data not shown), but the percent of naïve CD4+ T cells increased and the percent of effector CD4+ T cells decreased after IL-35 HGT compared with the control group. Almost all the effector and memory CD4+ T cells are of donor type, while about half of the naïve CD4+ T cells are from recipients in both groups of mice (Fig. 2A). These results suggest that overexpression of IL-35 impaired the activation of CD4+ T cells during GVH responses.
Figure 2. IL-35 led to a reduction of CD4+ effector T cells and reduced alloreactivity.
The experiments were performed as described in Figure 1. Lymphocytes were isolated from spleen, liver, lung, small intestine of recipient mice day 10 post allo-HCT and lymphocytes were analyzed by flow cytometry. (A) The proportions of CD4+ effector T cells, memory T cells and naive T cells were quantified. (B) MLR was performed with splenocytes from host mice as responder cells and irradiated (30 Gy) splenocytes from BALB/c mice as stimulator cells. Responders and stimulators were cultured at a final concentration of 0.5×106/ml for 3 d. [3H]thymidine added directly to the responder cells for the final 8 h of the 3-day assay (1 mCi/well), and the proliferations of the responder cells were determined. (C) Supernatants were used to measure IL-2 productions during the MLR. (D) The responder cells from the MLR were used as effector cells, and their allo-killing capacity of CT-26 (H-2d) targets was measured using CytoTox 96 nonradioactive cytotoxicity assay kit. Values were expressed as Mean ± SEM. All the experiments were performed with eight mice per group. The data shown are the representative of three experiments. *p<0.05.
Furthermore, we evaluated whether overexpression of IL-35 affected the alloreactivity of donor T cells against the host antigens by assessing MLR, IL-2 production and killing capacity against allogeneic tumor targets (Fig. 2B-D). The proliferation of the splenocytes from the recipients of IL-35 HGT was reduced to about 50% as compared to the control recipients (Fig. 2B). IL-2 concentration in the supernatant of MLR also decreased significantly (Fig. 2C). Splenocytes of the IL-35 HGT recipients showed diminished CTL activity against allogeneic tumor targets (Fig. 2D). Taken together, these data demonstrated that overexpression of IL-35 could significantly suppress allogeneic responses after allo-HSCT.
IL-35 promotes the expansion of Tregs
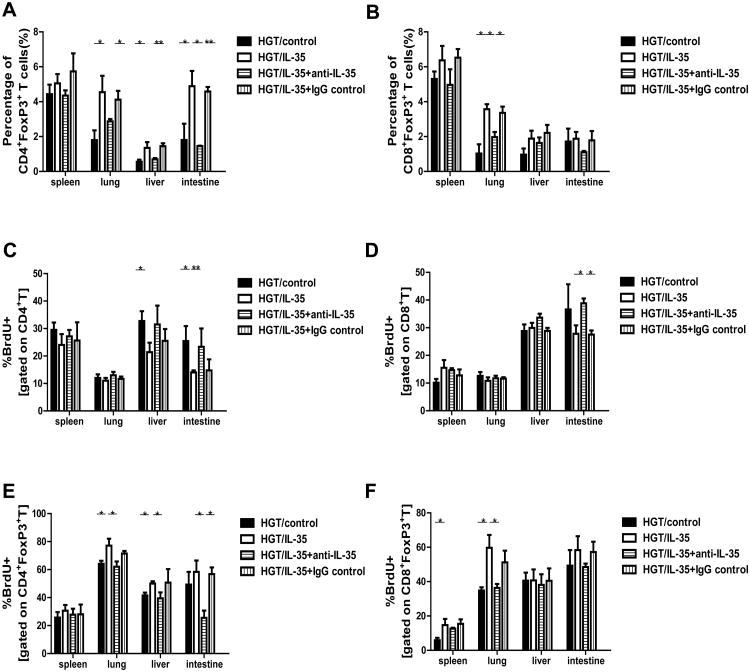
IL-35 has been shown to induce and expand the pTreg population.21 Ten days after allo-BMT, we found a significant increase in the proportion of CD4+Foxp3+ Tregs in the GVHD target organs of mice treated with IL-35 HGT compared with that of the control group (Fig. 3A and Fig. S3A). Interestingly, there was also a significant increase of CD8+Foxp3+ Tregs in the lungs of the IL-35 HGT recipients (Fig. 3B). Moreover, the percent of Tregs were reduced to similar levels as those of control groups when IL-35 was neutralized with the antibody treatment (Fig. 3A & B).
Figure 3. IL-35 promoted regulatory T cell expansion.
The percentages of (A) CD4+FoxP3+T cells and (B) CD8+FoxP3+T cells on gated CD4+ T cells in the spleen, liver, lung and small intestine of recipients administrated with HGT/IL-35, HGT/control, HGT/IL-35+anti-IL-35 and HGT/IL-35+IgG control were shown. In vivo CD4+ (C), CD8+ (D), CD4+FoxP3+ (E), and CD8+FoxP3+ (F) T-cell proliferations were assessed via BrdU incorporation after 4 h in vivo BrdU pulse in the spleen, lung, liver and small intestine of recipients. Values were expressed as Mean±SEM. All the experiments were performed with eight mice per group. The data shown are the representative of three experiments. *p<0.05, **p<0.01.
To assess the effect of IL-35 on CD4+ T cell and Treg proliferation in vivo, we performed a short 4-hour BrdU pulse and detected the BrdU incorporation. There was a substantial reduction in CD4+ T cell proliferation in the liver and small intestine of IL-35 HGT recipients and IL-35 neutralization increased CD4+ T cell proliferation to the similar levels as those of control groups (Fig. 3C and Fig. S3B). However, the CD8+ T cell proliferation was not significantly affected by IL-35 (Fig. 3D). In contrast, the frequencies of CD4+FoxP3+BrdU+ T cells in the lungs and livers were significantly elevated in IL-35 HGT recipients (Fig. 3E). This elevation was significantly reduced when IL-35 was neutralized with the antibody treatment. Moreover, there was also an increase in the frequency of CD8+FoxP3+BrdU+T cells in the spleen and lung of IL-35 HGT recipients (Fig. 3F). The proliferation of CD8+FoxP3+ cells was reduced to about similar levels as those of control groups when IL-35 was neutralized. Our results showed that IL-35 decreased the proliferation of CD4+ T cells, while increasing the proliferation of both CD4+FoxP3+ and CD8+FoxP3+ Tregs in aGVHD target organs.
IL-35 reduces the generation of Th1 cells while enhancing IL-10 production
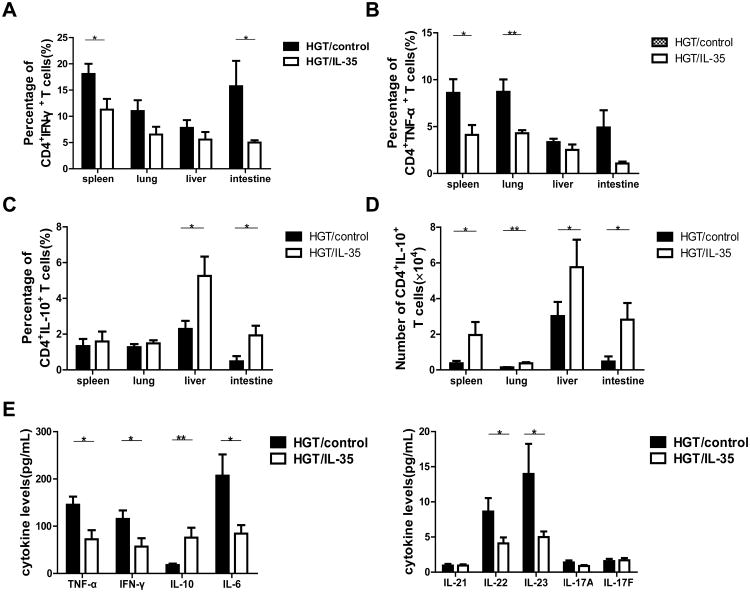
We further studied the effect of IL-35 on the cytokine productions by CD4+ T cells in different organs as well as serum cytokine levels. The result showed that IL-35 HGT resulted in a decrease in the percentage of CD4+ T cells secreting IFN-γ, with significant differences in spleen and small intestine (Fig. 4A and Fig. S4A). Furthermore, number of CD4+IFN-γ+ T cells decreased in all organs examined (Fig. S4B). Both percentage and number of CD8+IFN-γ+ T cells reduced significantly in recipient spleen, liver and small intestine with IL-35 HGT (Fig. S5A&B). Both percent and number of CD4+TNF-α+ T cells decreased with IL-35 HGT in spleen and lung (Fig. 4B and Fig. S4A & C). However, the TNF-α production by the CD8+ T cells was not affected by IL-35 (Fig. S5C&D). Interestingly, the percent of CD4+ IL-17+ T cells decreased in the liver and intestine, but increased in the lung by IL-35 HGT (Fig. S4A & D). The number of CD4+ IL-17+ T cells showed the similar results with significant decrease in the intestine and increase in the lung (Fig. S4E). The percent and number of CD8+ IL-17+ T cells exhibited similar changes (Fig. S5E&F). The percent of IL-10-producing CD4+ T cells increased in the liver and intestine, while the number of IL-10 producing CD4+ T cells was up-regulated in all the organs examined (Fig. 4C&D and Fig. S4A). The serum levels of TNF-α, IFN-γ, IL-6, IL-22 and IL-23 decreased, while IL-10 increased significantly with IL-35 HGT (Fig. 4E). These data indicated that IL-35 reduced the Th1 cytokines while increasing IL-10 production in GVHD target organs after allo-HSCT.
Figure 4. IL-35 reduced the presence of Th1 cells and enhanced IL-10 production.
Lymphocytes isolated from spleen, liver, lung, and small intestine were activated for 4h with PMA, ionomycin and Brefeldin A, and (A) the percentage of IFN-γ-secreting CD4+T cells from spleen, liver, lung, and small intestine were shown. (B) The percent of CD4+TNF-α+ T cells in the spleen, lung, liver and small intestine of recipients were shown. The percent (C) and absolute cell numbers (D) of CD4+T cells secreting IL-10 in the spleen, lung, liver and small intestine are shown. (E) Serum TNF-α, IFN-γ, IL-10, IL-6, IL-21, IL-22, IL-23, IL-17A and IL-17F levels were measured by CBA. Data are presented as the Mean± SEM. All the experiments were performed with eight mice per group. The data shown are the representative of three experiments. *p<0.05, **p<0.01.
IL-35 expression retains GVL effects
To determine whether the reduction of aGVHD lethality by IL-35 would also interfere with GVL activity, A20-luc (H-2d) lymphoma cells were injected intravenously on day 0 to generate murine GVL model. The recipients were monitored for survival and tumor growth was observed weekly using in vivo BLI. A strong signal was observed at day 21 in recipient mice receiving TCD-BM and A20 cells attesting for tumor growth (Fig. 5A). In contrast, when HGT control recipient mice received both BM and A20 cells and T cells, a low BLI signal was observed, indicating that tumor growth was controlled by allogeneic T cells. Ultimately, all recipient mice died from severe aGVHD by day 25. A strong GVL effect was mediated by expression of IL-35 as shown by the absence of BLI signal in recipient mice and was comparable to that obtained with HGT control group. Moreover, the majority of IL-35 HGT recipients survived through the 21-day observation period without or with very little BLI signal, and 33.3% (2/6) of the recipients survived for more than 35 days (p<0.0001, Fig. 5B). Altogether, these data indicated that IL-35 prevented aGVHD while retained GVL activity.
Figure 5. IL-35 expression retained GVL effects.
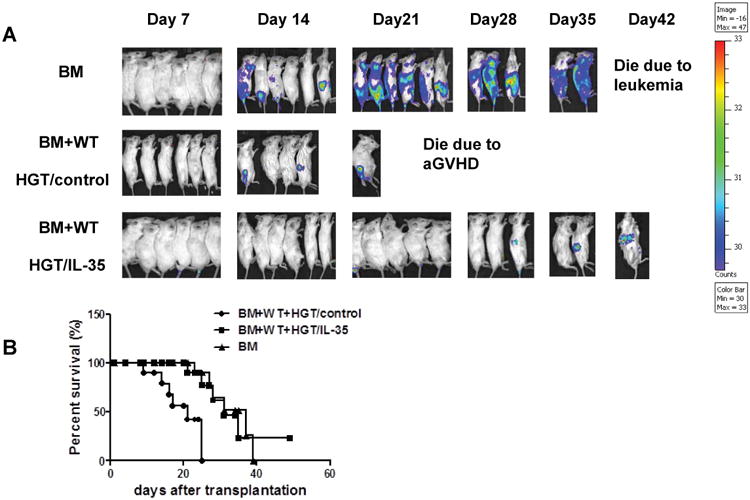
(A) Mice received an HGT injection 24 h before TBI, lethally irradiated BALB/c mice (850 cGy) were transplanted with TCD BM and 5×106 A20-luc B cell leukemia/lymphoma cells (H-2d), with or without 5×106 spleen cells from B6 mice (n=6 in each group). Tumor growth in recipients was monitored by in vivo BLI. (B) Overall survival after transplantation from all three groups was depicted. The data shown are the representative of three experiments.
aGVHD patients have reduced IL-35 serum levels
To determine the clinical relevance of IL-35 expressions in aGVHD patients, we analyzed serum IL-35 levels from 27 allo-HSCT patients (Table S1). All patients achieved a sustained and stable donor engraftment. Among the 27 allo-HSCT patients, 22 (81.5%) patients developed aGVHD. Among them, 6 (27.3%) were grade I, 6 (27.3%) were grade II, 8 (36.4%) were grade III, and 2 (9.1%) were grade IV. CD4+FoxP3+ T cells from patients with aGVHD grade 2-4 were significantly decreased compared to patients with aGVHD grade 0-1 (p<0.05) (Fig. 6A). Moreover, patients with aGVHD grade 2-4 exhibited significantly lower IL-35 levels than patients with aGVHD grade 0-1 (p<0.001) (Fig. 6B). The serum levels of IL-35 were also positively correlated with the percent of Tregs (Fig. 6C). These results demonstrated that the expression of IL-35 decreased in aGVHD patients and it was positively correlated with the percent of CD4+FoxP3+ Tregs.
Figure 6. IL-35 serum levels were reduced in aGVHD patients.
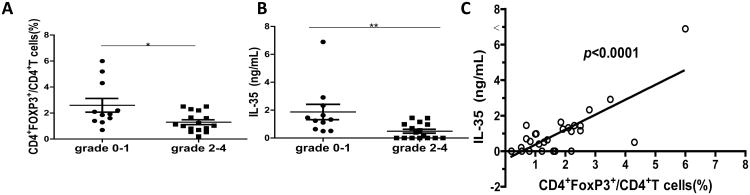
(A) The percentages of Tregs were evaluated in patients with aGVHD grade 0-1 and aGVHD grade 2-4. (B) IL-35 serum level was measured and compared between aGVHD grade 0-1 and aGVHD grade 2-4 patients. (C) Correlation between the percentage of Tregs and serum level of IL-35 was analyzed. * p<0.05, ** p<0.01.
Discussion
In this study we examined the effect of IL-35 on the development of aGVHD in a murine model. Overexpression of IL-35 prevented the development of both immunological and clinical manifestations of aGVHD. Most importantly, IL-35 preserved the GVL effect and had no impairment on the recovery of hematopoiesis and immune cells, resulting in significantly improved survival of mice after allo-HSCT and leukemia challenge. To our knowledge, this is the first report on the role of IL-35 in aGVHD after allo-HSCT.
Studies on IL-35 have been hampered by the fact that production of purified rIL-35 is challenging, because the expression level is low and the stability of rIL-35 protein is problematic. This scenario prompted us to adopt systemic plasmid DNA encoding IL-35 gene-delivery approaches to circumvent problems associated with the production of the rIL-35 protein and to provide critical proof-of-principle observations. In murine models of allo-HSCT, overexpression of IL-35 was sufficient to attenuate the development of aGVHD, suggesting that the anti-inflammatory properties of IL-35 could be a key regulatory factor in aGVHD. Although the production of rIL-35 is still challenging at the moment, with the advances in protein technology, new approaches could be developed such as generating functional mutants and stable fusion proteins to increase the production and stability of rIL-35, which would facilitate its potential clinical use.
The protective effect of Tregs on aGVHD has been already demonstrated in several models.10,12 EBI3-p35-Fc fusion protein has been shown to promote the expansion of Tregs.21 Our results demonstrated that IL-35 expression promoted the proliferation of CD4+Foxp3+ T cells in the aGVHD target organs. Interestingly, the proliferation of CD8+Foxp3+ T cells also increased in the lung. It has been shown that a population of CD8+Foxp3+ T cells that were induced early during GVHD, constituting a significant percentage of the entire Treg population.26 CD8+Foxp3+ Tregs that converts from CD8+ conventional donor T cells after allo-HSCT can protect from GVHD. 27
IL-10 is a well-known suppressive cytokine of T cell proliferation and cytokine production. Previous studies have observed an association between elevated levels of IL-10 and reduced risk of aGVHD.28 IL-35-expanded CD4+CD25+ T cell population has been shown to produce elevated levels of IL-10.21 In our study, numbers of CD4+T cells secreting IL-10 significantly increased in response to IL-35. Serum levels of IL-10 increased simultaneously, suggesting that IL-35 enhanced IL-10 production. This is consistent with the study that IL-35 protects from CIA by promotion of IL-10 production in vivo.20 Recent reports demonstrated that IL-10 provided exogenously or by CD4+Foxp3+ Tregs constrains Th17 and Th1+Th17 cell frequencies.29, 30 Collectively, our results and previous reports support a specific role for IL-10 induced by IL-35 in attenuating aGVHD in vivo. The contribution of Th17 cells in aGVHD is somewhat controversial. Studies with murine models showed conflicting results, with some data suggesting that Th17 is protective and others indicating a pathogenic role in aGVHD.31-33 In humans, there are also divergent results with retrospective reports that circulating and/or tissue localized Th17 cells can be increased or decreased in aGVHD.34-39 In our study, we observed that IL-35 reduced IL-17 production by CD4+T cells in the small intestine and liver. This is consistent with the observation by Niedbala et al that EBI3-p35-Fc fusion protein suppresses Th17 cells in vitro and in vivo.21 However, IL-17 production by CD4+T cells increased in the lung in response to IL-35. The serum level of IL-17 was not changed. Further investigations are needed to elucidate the role of IL-35 on IL-17 production and the mechanism of organ specificities.
Many studies have demonstrated that alloreactive effector T cells mediating GVHD are also important for GVL activity, although not completely overlapping.40 Therefore, inhibition of GVHD without impairing GVL effect has proven to be a difficult task. IL-35 could suppress alloreactive effector cells and alleviate aGVHD. However, this suppression still allows effective GVL effects. This could be partially mediated by donor NK cells. Another possible explanation is that the residual alloreactivity was sufficient to eliminate leukemic cells but not causing GVHD. This provides an effective therapeutic window to allow for clinical applications.
In conclusion, we present here that IL-35 alleviates aGVHD with retaining of GVL effects. IL-35 expression leads to the Treg expansion and suppression of Th1 cytokine production. Considering the challenge of generating a functional stable Treg population ex vivo for cellular therapy, IL-35 may be a promising agent to investigate and implement clinically for the prevention of aGVHD after allo-HSCT.
Supplementary Material
Acknowledgments
We thank Karen Forbes and Creg Workman for the generating and purifying the anti-Ebi3 mAb.
Financial Support: This work has been supported by the grants from National Natural Science Foundation of China (91029703, 81102271 and 81273268), the project funding from Suzhou city (SS201032, SZS201109), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Province's Key Medical Center (ZX201102), 2012 Jiangsu Provincial Special Program of Medical Science (BL2012005), National clinical key subject construction project, National Public Health Grand Research Foundation (201202017), Jiangsu Provincial Innovative Research Team, Qing Lan project of Jiangsu Province, Program for Changjiang Scholars and Innovative Research Team in University (IRT1075). D.A.A.V. was supported by the National Institutes of Health (R01 AI091977), NCI Comprehensive Cancer Center Support CORE grant (CA21765), and ALSAC.
Footnotes
Conflict of Interest: D.A.A.V. has submitted patents around IL-35 that are pending and is entitled to a share in net income generated from licensing of these patent rights for commercial development. Other authors declare no conflict of interest.
Supplementary information is available at Leukemia's website.
References
- 1.Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD) Philos Trans R Soc Lond B Biol Sci. 2005 Sep 29;360(1461):1747–1767. doi: 10.1098/rstb.2005.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006 Jan;43(1):3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007 May;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003 Mar;3(3):253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004 Nov;114(9):1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006 Aug;25(2):195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001 Jun 4;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005 Apr;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immuno- regulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002 Aug 5;196(3):401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002 Aug 5;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson BD, Konkol MC, Truitt RL. CD25+ immunoregulatory T-cells of donor origin suppress alloreactivity after BMT. Biol Blood Marrow Transplant. 2002;8(10):525–535. doi: 10.1053/bbmt.2002.v8.pm12434947. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002 May 15;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 13.Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010 Jan;45(1):1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devergne O1, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997 Oct 28;94(22):12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007 Nov 22;450(7169):566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 16.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009 May 15;182(10):6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012 Dec 15;189(12):5590–5601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014 Mar 20;507(7492):366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010 Dec;11(12):1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010 Jun 15;184(12):7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007 Nov;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 22.Cooke KR1, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996 Oct 15;88(8):3230–3239. [PubMed] [Google Scholar]
- 23.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irra-diation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997 Oct 15;90(8):3204–3213. [PubMed] [Google Scholar]
- 24.Cooke KR1, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J, Jr, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998 Nov 15;102(10):1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006 Sep;12(9):1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 26.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, Drobyski WR. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012 Jul 1;189(1):464–474. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, et al. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012 Jun 14;119(24):5898–5908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 28.Weston LE, Geczy AF, Briscoe H. Production of IL-10 by alloreactive sibling donor cells and its influence on the development of acute GVHD. Bone Marrow Transplant. 2006 Jan;37(2):207–212. doi: 10.1038/sj.bmt.1705218. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011 Apr 22;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011 Apr 22;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008 Sep 1;112(5):2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009 Jan 22;113(4):945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iclozan C, Yu Y, Liu C, Liang Y, Yi T, Anasetti C, et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Feb;16(2):170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dander E, Balduzzi A, Zappa G, Lucchini G, Perseghin P, Andre V, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009 Dec 15;88(11):1261–1272. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 35.Zhao XY, Xu LL, Lu SY, Huang XJ. IL-17-producing T cells contribute to acute graft-versus-host disease in patients undergoing unmanipulated blood and marrow transplantation. Eur J Immunol. 2011 Feb;41(2):514–526. doi: 10.1002/eji.201040793. [DOI] [PubMed] [Google Scholar]
- 36.Ratajczak P, Janin A, Peffault de Latour R, Leboeuf C, Desveaux A, Keyvanfar K, et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010 Aug 19;116(7):1165–1171. doi: 10.1182/blood-2009-12-255810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bahr F, Wehner R, Platzbecker U, Wermke M, Shayegi N, Middeke JM, et al. Reconstitution of interleukin-17-producing T helper cells after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013 Mar;19(3):357–365. doi: 10.1016/j.bbmt.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Bossard C, Malard F, Arbez J, Chevallier P, Guillaume T, Delaunay J, et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft-versus-host disease. Leukemia. 2012 Jul;26(7):1471–1474. doi: 10.1038/leu.2012.41. [DOI] [PubMed] [Google Scholar]
- 39.Malard F, Bossard C, Brissot E, Chevallier P, Guillaume T, Delaunay J, et al. Increased plasmacytoid dendritic cells and RORγt-expressing immune effectors in cutaneous acute graft-versus-host disease. J Leukoc Biol. 2013 Dec;94(6):1337–1343. doi: 10.1189/jlb.0513295. [DOI] [PubMed] [Google Scholar]
- 40.Van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002 Apr;2(4):273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.