Abstract
Purpose
To describe the clinical characteristics of celiac disease (CD) among Saudi children and to determine the adherence rate to gluten free diet (GFD) and its determinant factors among them.
Methods
A cross-sectional study was conducted, in which all the families registered in the Saudi Celiac Patients Support Group were sent an online survey. Only families with children 18 years of age and younger with biopsy-confirmed CD were included.
Results
The median age of the 113 included children was 9.9 years, the median age at symptom onset was 5.5 years and the median age at diagnosis was 7 years, the median time between the presentation and the final diagnosis was 1 year. Sixty two of the involved children were females. Ninety two percent of the patients were symptomatic at the diagnosis while eight percent were asymptomatic. The commonest presenting symptoms included: chronic abdominal pain (59.3%), poor weight gain (54%), abdominal distention, gases, bloating (46.1%) and chronic diarrhea (41.6%). Sixty percent of the involved children were reported to be strictly adherent to GFD. Younger age at diagnosis and shorter duration since the diagnosis were associated with a better adherence rate.
Conclusion
CD has similar clinical presentations among Saudi children compared to other parts of the ward; however, the adherence to GFD is relatively poor. Younger age at diagnosis and shorter duration since the diagnosis were associated with a better adherence rate.
Keywords: Celiac disease, Gluten-free diet, Child, Saudi Arabia
INTRODUCTION
Celiac disease (CD) is a multi-systemic autoimmune disorder that leads to enteropathy. It is caused by immunological reaction to gluten, the major protein component of wheat.
The prevalence of CD in the western countries ranges between 1-2% [1]. There is no national data about its prevalence in Saudi Arabia; however, scattered small studies from different regions of the country have estimated the sero-prevalence of CD to be between 1-4% [2,3,4]. The largest screening study was done by Aljebreen et al. [5] that included 1,167 healthy adolescents from three different regions in the country and found a sero-prevalence rate of 2.2%.
CD is managed successfully with a strict lifelong gluten free diet (GFD) which is essential to cure the symptoms and to prevent the long-term complications such as failure to thrive, osteoporosis [6] and gastrointestinal tract malignancies [7].
Though it is very effective, GFD is significantly restrictive, it is not easily available, more expensive compared to the regular gluten containing diet, and is not as palatable compared to the regular gluten containing diet. For all of these reasons, there is a significant negative impact on the adherence rate to strict GFD. Furthermore, GFD may impose several psycho-social and financial stresses on the patients and their families which consequently might reduce their adherence to GFD even more [8,9,10,11,12,13].
We conducted this study to describe the clinical characteristics of CD among Saudi children and to determine the adherence rate to GFD and its determinant factors among them.
MATERIALS AND METHODS
A cross-sectional study was conducted, in which all families registered in the Saudi Celiac Patients Support Group (SCPSG), which is the largest support group under the umbrella of the Saudi Gastroenterology Association, were invited to participate in this survey. We sent an online link of the questionnaire to the entire email list of the SCPSG on November 2013 and recruitment continued till January 2014. Only families with children 18 years of age and younger with biopsy-confirmed CD were eligible.
Instrument design and testing
We designed a questionnaire with four sections; the first section contained questions about the demographic data of the child and the family, the second section covered the details of the diagnosis, while the third section examined the family knowledge about GFD, its effect on the child's symptoms and the compliance of the child. Finally, the forth section focused on the socio-economic impacts of GFD on these children and their families (interaction with other children, family social activities, travel, and dining out).
The contents of the survey were reviewed by two local expert pediatric gastroenterologists. Pretesting of the questionnaire was done on ver 2.0 patients to check its readability and comprehensiveness.
Statistical analysis
Mean and standard deviation were calculated for continuous variables and proportions for categorical variables. One wazy ANOVA and post hoc Tukey test was conducted for continuous variables and chi squared test (or its alternative Fisher Exact test if any cell count less than 5) was conducted for categorical variables to measure the difference between children of age group 1-6.9 years, 7-12.9 years and 13-18 years. Logistic regression modeling was done to study the factors associated with the adherence to GFD. Adjusted odds ratios (aOR) and its corresponding 95% confidence intervals (CI) were calculated for the model. A p-value of <0.05 was considered as significant. IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis of the data.
Ethical considerations
The study protocol was approved by the institutional research board at King Khalid University Hospital, King Saud University (KSU) (IRB No. 13/3903). The completion and return of the questionnaire was regarded as consent. All responses were anonymous.
RESULTS
Participants' characteristics
One hundred and twenty families completed the questionnaires; 113 (94%) children had a biopsy-confirmed diagnosis of CD and 7 (5.8%) reported the diagnosis based on serology testing only. The latter group was excluded from the analysis.
Participants were divided into three groups to examine the difference in the compliance rate among them: group 1 (preschool children); 1-6 years, n=21 (18.6%), group 2 (elementary school children); 7-12 years, n=67 (59.3%) and group 3 (high school children/adolescents); 13-18 years, n=25 (22.1%). We believe that each group of these groups has specific factors affecting their compliance, for instance preschoolers stayed usually at home with their mother compared to the elementary school group who attend school unsupervised by their families and the third group is the adolescents that typically are unique in their compliance in general.
The median age of the involved children was 9.9 years (mean 10±3.7 years, range 1.2-18 years), the median age at symptom onset was 5.5 years (mean 5.8±3.7 years, range 0.6-17 years) and the median age at diagnosis was 7 years (mean 7±3.8 years, range 1-18 years). The median time between the presentation and the final diagnosis was 1 year (mean 1.2±1.9 years, range 0-12 years). The study sample was predominately females (62.8%).
Thirty three (29.2%) of the children had a family history of CD in the 1st and/or 2nd degree relatives. Insulin dependent diabetes mellitus (IDDM) was found in 30 of them (26.5%). The baseline characteristics of the involved children were presented in Table 1.
Table 1. Baseline Characteristics of the Involved Children.
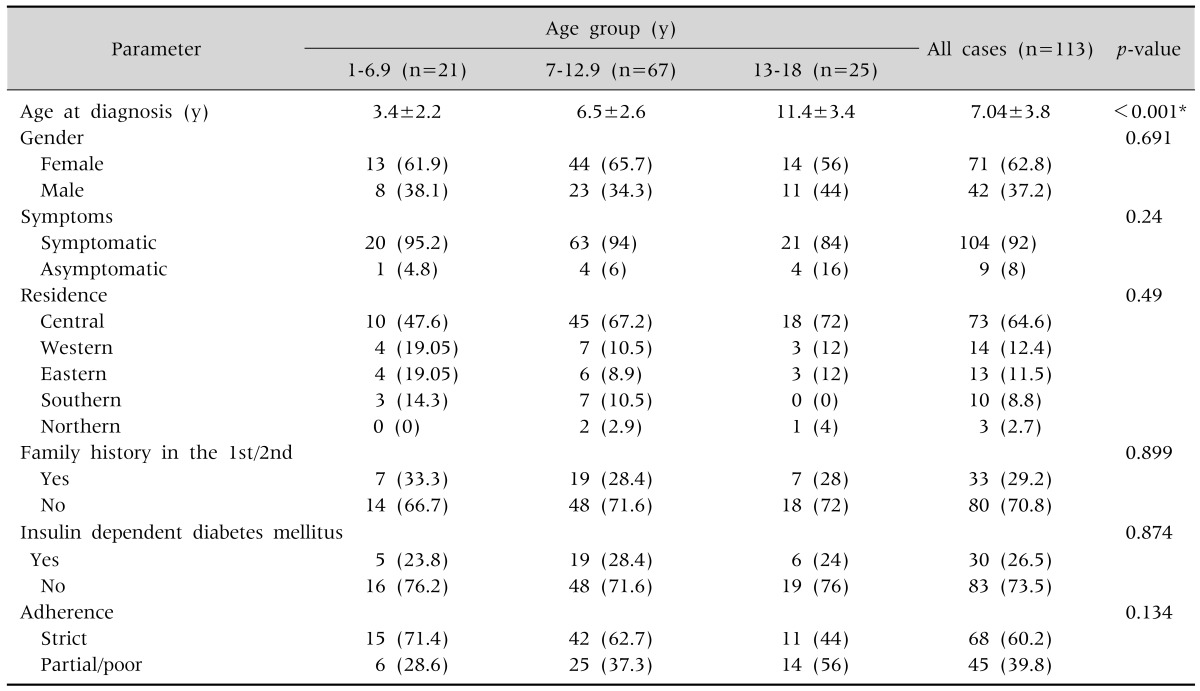
Values are presented as mean±standard deviation or number (%).
*Statistically significant, post-hoc Tukey: age at diagnosis statistically higher for children in the age group of 13-18 years followed by that in the age group 7-12.9 years and 1-6.9 years.
Clinical presentations
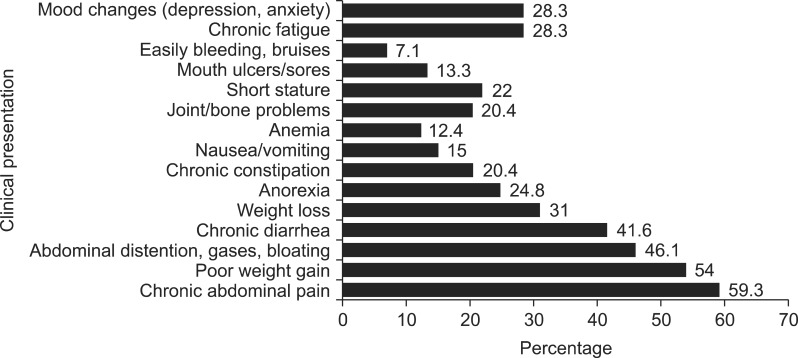
Most of the participants in our sample had symptoms at the time of diagnosis (104=92%), while 9 (8%) were asymptomatic and discovered through screening tests. The most common presentation was chronic abdominal pain (59.3%), followed by poor weight gain (54%), and a wide variety of other manifestations were also reported (Fig. 1). Among the extra-intestinal manifestations, short stature and joint/bone problems were the commonest presentations (22% and 20.4% respectively). Around one third of the children had mood changes (anxiety/depression) and chronic fatigue at the time of presentation.
Fig. 1. Clinical presentation prior to diagnosis.
Education about GFD
Ninety five (84%) of the participated families reported that they got some dietary information about GFD through their gastroenterologist and/or their dieticians. Eighty four (88.4%) of them felt that the information were enough, while 11 (11.6 %) reported that the information were not enough.
Eighty three (73.5%) of the families reported that they have been seen by a dietician at some point during their follow up, whereas 30 (26.5%) reported that they have never been seen by a dietician prior to the survey. Among those seen by dieticians; 90.4% said that the follow up with dietician was useful.
Availability of GFD
One hundred (88.5%) of the participated families reported that GFD is either difficult to find (81=72%) or it is not available at all (19=16.8%) in their local area. Only 24 (21%) of the participated families get free GFD products on prescriptions which provide the families with the basic ingredients for making gluten free meals at home.
The primary source of getting GFD as reported by the participated families included large supermarkets (39%), specialized diet centers (39%), purchasing from outside the city (16%), homemade (8%), and via the internet (3.5%).
Adherence to GFD
Ninety nine percent of the participated families were advised to follow a lifelong GFD by their pediatric gastroenterologists. Strict adherence to GFD was reported in only 68 (60.2%) as perceived by their families, while 45 (39.8%) were non-adherent (either totally or partially). Seventy percent of the children with strict adherence (48/68) showed significant improvement of their symptoms after starting GFD and 29.4% (20/68) showed partial improvement.
We found no statistically significant difference in the adherence rate between the three groups (Table 1).
Multivariable regression showed that if the age at diagnosis increases by one year, the adherence to GFD will decrease by 15% (aOR, 0.85; 95% CI aOR, 0.74-0.98) and if the time since diagnosis increases by one year, the adherence to GFD decrease by 29% (aOR, 0.71; 95% CI aOR, 0.57-0.90), adjusting for gender, mother's education, family history, residence, IDDM, follow up with dietician, availability of GFD, hospital supply of GF ingredients, pricing of GFD and monthly budget spending on GFD (Table 2). Though it was barely statistically significant, enough follow up with dietician was associated with a 3-folds increase in the adherence among our patients (Table 2).
Table 2. Factors Associated with Adherence to Gluten Free Diet (GFD).
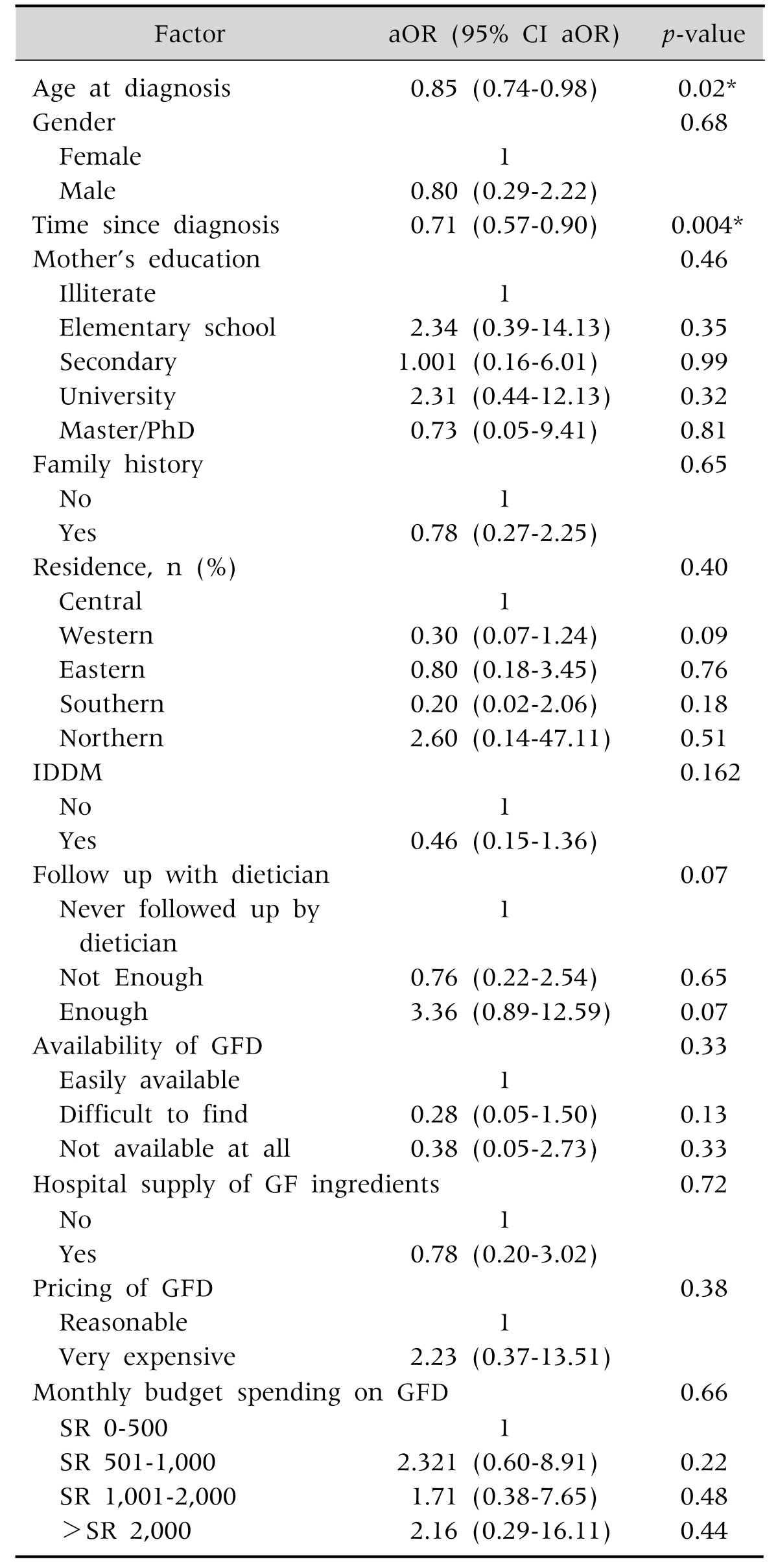
aOR: adjusted odds ratio, CI: confidence interval, IDDM: insulin dependent diabetes mellitus, SR: Saudi Riyal.
*Statistically significant association.
DISCUSSION
We examined in the present study the clinical characteristics of CD among Saudi children and the adherence rate to GFD and its determinant factors among them. In the present study, the mean age of children at diagnosis was 7 years, which is comparable to the published report from Italy (7.7 years) [13], but higher than that reported from Canada (4.8 years) [11]. The mean time between initial presentation and diagnosis in the present study was 1 year which is consistent with the reports from the Canadian study (12 months) and the Italian study (14 months) [11,13].
The commonest clinical presentation in our study was chronic abdominal pain (59%) followed by poor weight gain (54%). Constipation reported in 20% of our study sample, previous studies reported constipation between 25-30% [9,10,11]; this highlights the importance of keeping CD in the differential diagnosis of constipation, particularly in cases resistant to the standard therapy. Extra-intestinal manifestations were seen in a considerable number of our patients, these included mouth ulcers, joints/bone complaints, chronic fatigue and mood changes, the percentage of these manifestations were comparable to that reported elsewhere [11,12,13].
Previous reports estimated that only 1 : 3 to 1 : 7 of the CD patients are symptomatic at the diagnosis [14] while the rest are typically detected during screening tests for the high risk population. In our study, most of the participated patients were symptomatic (92%) and only 8% were detected from the screening tests, this could be related to the fact that the symptomatic patients were more interested to participate in the survey compared to the asymptomatic patients.
The prevalence of CD ranges between 2-4% among the 1st and the 2nd degree relatives [15], one third of our study sample has a positive family history of CD in the 1st and/or 2nd degree family members, similar high percentage was reported previously from the western region of the country by Saadah [3]. This high percentage from our study and Saadah's study are far higher than that reported from Canada (8%) [11], and from Iran (18%) [16], this might be related to the high prevalence of consanguinity marriage observed in our community (up to 60%) [17].
Strict adherence to GFD is the primary and the most effective therapeutic intervention we have so far for CD management. Strict adherence to GFD was reported by 60% of our sample, which is close to that report by Saadah [3], 56% adherence rate, in his study from the western region of the country; these percentages of adherence are lower than that reported from India (75%), Italy (87%) and Canada (95%) [11,13,18] but higher than the reported from Greek (44%) [19].
When we looked to the effect of different factors on the adherence to GFD, we found that if the age at diagnosis increases by one year, the adherence to GFD decrease by 15%, this result is similar to that reported by Charalampopoulos et al. [19], this indicate the importance of early diagnosis and early introduction of GFD as children tend to used to the diet if started early. In addition, we found that if the time since diagnosis increases by one year, the adherence to GFD decrease by 29%, this could be explained by different reasons such as increased outdoor activities as they grow up with less family supervision, peers pressure. In addition, conflict with parents and increased risk taking behaviour in the teenagers [18].
The observed relatively low compliance rate in the present study is likely due to the combination of multiple factors, these include the unavailability of commercially trusted GFD products, as indicated by the majority (88.5%) of the participated families. The issue becomes even worse in the areas away from the major cities where GFD is totally unavailable as reported by 17% of the participated families. In fact, some families forced to travel outside their cities to purchase GFD. Even if GFD is available, its price is expensive as reported by 94% of the participated families. The issue of the availability and the high cost of GFD is a worldwide problem and it has been shown, from previous studies, that it contributes to the poor compliance among CD patients [8,20].
Dietary education is the major determinant factor in improving the patient's understanding and adherence to GFD [19]; only two third of the participated families have been seen by a dietician at one point during their follow, this may contribute, to some extent, to the observed low adherence rate to GFD in the present study. A recent Canadian survey reported that families with CD patients found the dieticians, in addition to the internet, to be the preferred education resources for them [21], therefore, CD patients should have an access to an experienced dietician in CD management initially after the diagnosis, then at least annually as recommended by the British Society of Gastroenterology [22].
Limitations
Our study is not without limitations; Sixty five percent of our sample is coming from the central region of the country; this may reflect that most of the members registered in SCPSG website were from the central region and this might limit the generalizability of our results for the whole country. As our participants are coming from families involved in the support group, this may impose some population bias as it may attract the higher educated families or the families with more access to the social networks, fortunately, the participated families in the present study came from different socio-economic and educational backgrounds, and this might minimize the effect of this bias.
Our study showed that CD has similar clinical presentations among Saudi children compared to the other parts of the world; however, the adherence to GFD is relatively poor among them. Younger age at diagnosis and shorter duration since the diagnosis were associated with a better adherence rate. This reflects the importance of early diagnosis and the early introduction of GFD on improving the adherence among these patients.
ACKNOWLEDGEMENTS
We thank the families that participated in answering the survey and helped us in conducting this study. We also thank Ms. Norah Al-Otaiby from SCPSG for her assistance in collecting the data. This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
References
- 1.Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–595. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 2.Al Attas RA. How common is celiac disease in Eastern Saudi Arabia? Ann Saudi Med. 2002;22:315–319. doi: 10.5144/0256-4947.2002.315. [DOI] [PubMed] [Google Scholar]
- 3.Saadah OI. Celiac disease in children and adolescents at a singe center in Saudi Arabia. Ann Saudi Med. 2011;31:51–57. doi: 10.4103/0256-4947.75779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khayyat YM. Serologic markers of gluten sensitivity in a healthy population from the western region of Saudi Arabia. Saudi J Gastroenterol. 2012;18:23–25. doi: 10.4103/1319-3767.91733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aljebreen AM, Almadi MA, Alhammad A, Al Faleh FZ. Seroprevalence of celiac disease among healthy adolescents in Saudi Arabia. World J Gastroenterol. 2013;19:2374–2378. doi: 10.3748/wjg.v19.i15.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantaleoni S, Luchino M, Adriani A, Pellicano R, Stradella D, Ribaldone DG, et al. Bone mineral density at diagnosis of celiac disease and after 1 year of gluten-free diet. ScientificWorldJournal. 2014;2014:173082. doi: 10.1155/2014/173082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostami Nejad M, Aldulaimi D, Ishaq S, Ehsani-Ardakani MJ, Zali MR, Malekzadeh R, et al. Geographic trends and risk of gastrointestinal cancer among patients with celiac disease in Europe and Asian-Pacific region. Gastroenterol Hepatol Bed Bench. 2013;6:170–177. [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens L, Rashid M. Gluten-free and regular foods: a cost comparison. Can J Diet Pract Res. 2008;69:147–150. doi: 10.3148/69.3.2008.147. [DOI] [PubMed] [Google Scholar]
- 9.Wagner G, Berger G, Sinnreich U, Grylli V, Schober E, Huber WD, et al. Quality of life in adolescents with treated coeliac disease: influence of compliance and age at diagnosis. J Pediatr Gastroenterol Nutr. 2008;47:555–561. doi: 10.1097/MPG.0b013e31817fcb56. [DOI] [PubMed] [Google Scholar]
- 10.Kokkonen J, Viitanen A, Similä S. Coping with a coeliac diet after adolescence. Helv Paediatr Acta. 1989;43:261–265. [PubMed] [Google Scholar]
- 11.Rashid M, Cranney A, Zarkadas M, Graham ID, Switzer C, Case S, et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics. 2005;116:e754–e759. doi: 10.1542/peds.2005-0904. [DOI] [PubMed] [Google Scholar]
- 12.Kurppa K, Collin P, Mäki M, Kaukinen K. Celiac disease and health-related quality of life. Expert Rev Gastroenterol Hepatol. 2011;5:83–90. doi: 10.1586/egh.10.81. [DOI] [PubMed] [Google Scholar]
- 13.Altobelli E, Paduano R, Gentile T, Caloisi C, Marziliano C, Necozione S, et al. Health-related quality of life in children and adolescents with celiac disease: survey of a population from central Italy. Health Qual Life Outcomes. 2013;11:204. doi: 10.1186/1477-7525-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustalahti K, Sulkanen S, Holopainen P, Laurila K, Collin P, Partanen J, et al. Coeliac disease among healthy members of multiple case coeliac disease families. Scand J Gastroenterol. 2002;37:161–165. doi: 10.1080/003655202753416812. [DOI] [PubMed] [Google Scholar]
- 15.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 16.Ganji A, Esmaielzadeh A, Aafzal Aghayee M, Goshayeshi L, Ghaffarzadegan K. The clinical presentation of celiac disease: experiences from northeastern iran. Middle East J Dig Dis. 2014;6:93–97. [PMC free article] [PubMed] [Google Scholar]
- 17.El-Mouzan MI, Al-Salloum AA, Al-Herbish AS, Qurachi MM, Al-Omar AA. Regional variations in the prevalence of consanguinity in Saudi Arabia. Saudi Med J. 2007;28:1881–1884. [PubMed] [Google Scholar]
- 18.Chauhan JC, Kumar P, Dutta AK, Basu S, Kumar A. Assessment of dietary compliance to gluten free diet and psychosocial problems in Indian children with celiac disease. Indian J Pediatr. 2010;77:649–654. doi: 10.1007/s12098-010-0092-3. [DOI] [PubMed] [Google Scholar]
- 19.Charalampopoulos D, Panayiotou J, Chouliaras G, Zellos A, Kyritsi E, Roma E. Determinants of adherence to gluten-free diet in Greek children with coeliac disease: a cross-sectional study. Eur J Clin Nutr. 2013;67:615–619. doi: 10.1038/ejcn.2013.54. [DOI] [PubMed] [Google Scholar]
- 20.Singh J, Whelan K. Limited availability and higher cost of gluten-free foods. J Hum Nutr Diet. 2011;24:479–486. doi: 10.1111/j.1365-277X.2011.01160.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajani S, Sawyer-Bennett J, Shirton L, DeHaan G, Kluthe C, Persad R, et al. Patient and parent satisfaction with a dietitian- and nurse- led celiac disease clinic for children at the Stollery Children's Hospital, Edmonton, Alberta. Can J Gastroenterol. 2013;27:463–466. doi: 10.1155/2013/537160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson M, Mendoza N, McGough N. A survey of provision of dietetic services for coeliac disease in the UK. J Hum Nutr Diet. 2007;20:403–411. doi: 10.1111/j.1365-277X.2007.00813.x. [DOI] [PubMed] [Google Scholar]