Key Points
A novel PC mutation in a healthy subject results in type II PC deficiency as diagnosed by commercial kits.
Recombinant expression and analysis reveals this is a gain-of-function mutant of PC that cannot be properly diagnosed by commercial kits.
Abstract
Protein C (PC) is a vitamin K–dependent plasma glycoprotein, which upon activation by thrombin in complex with thrombomodulin (TM), regulates the coagulation cascade through a feedback loop inhibition mechanism. PC deficiency is associated with an increased risk of venous thromboembolism (VTE). A recent cohort study aimed at establishing a normal PC range identified a healthy PC-deficient subject whose PC antigen level of 65% and activity levels of 50% (chromogenic assay) and 36% (clotting assay) were markedly low. The proband has a negative family history of VTE. Genetic analysis revealed the proband has a heterozygous missense mutation in which Thr-315 of the PC heavy chain has been substituted with Ala. We expressed this mutant in HEK-293 cells and purified it to homogeneity. A similar decrease in both anticoagulant and anti-inflammatory activities of the activated protein C mutant was observed in plasma- and cell-based assays. Interestingly, we discovered if functional assays were coupled to PC activation by the thrombin-TM complex, the variant exhibits improved activities in all assays. Sequence analysis revealed Thr-315 is a consensus N-linked glycosylation site for Asn-313 and that its elimination significantly (∼four- to fivefold) improves the maximum velocity of PC activation by the thrombin-TM complex, explaining the basis for the proband’s negative VTE pedigree.
Introduction
Protein C (PC) is a vitamin K–dependent zymogen of 62 kDa that circulates in plasma as a heterodimer consisting of a 41-kDa heavy chain and a 21-kDa light chain, held together via a single disulfide bond.1 The physiological activation of PC occurs on the surface of endothelial cells and requires 2 membrane receptors, thrombomodulin (TM) and endothelial PC receptor (EPCR), both of which function as cofactors for converting PC to activated protein C (APC) by thrombin.2,3 The binding of TM to thrombin accelerates the activation of PC by 3 orders of magnitude, and EPCR binding to the zymogen further improves the activation rate by 20-fold.2,4 APC inhibits thrombin generation (TG) by inactivating activated factor V (FVa) and activated FVIII (FVIIIa) via limited proteolysis.5-7 In addition to its anticoagulant activity, APC also exhibits potent cytoprotective and anti-inflammatory properties. The latter functions of APC are thought to be mediated through EPCR-dependent cleavage of protease-activated receptor 1 (PAR1) on endothelial cells.8,9
The importance of APC in the regulation of blood coagulation can be illustrated by the observation that heterozygous PC deficiency is associated with increased risk of venous thromboembolism (VTE). The homozygous deficiency of PC is associated with purpura fulminans, which is fatal unless treated by PC replacement therapy.10,11 A complete PC deficiency in mice results in lethal perinatal consumptive coagulopathy, as demonstrated by targeted gene disruption.12 PC deficiency exhibits an autosomal dominant pattern of inheritance and is phenotypically divided into type I deficiency, which is characterized by equally low antigen (PC:Ag) and activity (PC:A) levels, and type II deficiency, which is characterized by a lower activity level for APC.13 In clinical laboratories, PC deficiency is initially diagnosed by the PC:A level; if the activity is determined to be decreased, PC deficiency is confirmed, thus requiring an immunoassay to measure the PC:Ag level to assign the type of PC deficiency.14 Commercially available activity tests used to characterize PC deficiency include either a clotting or a chromogenic assay. In both types of assays, PC in patient’s plasma is activated with an activator derived from the venom of a specific snake (Protac) followed by evaluation of APC activity employing either a plasma-based activated partial thromboplastin time (aPTT) assay or a synthetic peptide-based chromogenic assay.14,15 In a recent study aimed at establishing the normal range of PC in the healthy population, we identified a healthy subject whose PC:Ag and PC:A levels were 65%, 50% (chromogenic assay), and 36% (clotting assay) of the normal range, respectively.15 Noting a lack of family history of VTE for the proband, we decided to determine whether a mutation in the PC gene (PROC) was responsible for this apparent type II PC deficiency. Genetic analysis revealed that the proband has a heterozygous missense mutation in PROC leading to substitution of Thr-315 of the PC heavy chain with Ala.15 In this study, we expressed this PC mutant in HEK-293 cells and purified it to homogeneity. Following activation, we characterized the anticoagulant and anti-inflammatory properties of the APC mutant in purified, plasma-based, and cell-based assay systems. Consistent with results of the proband’s plasma assays, the APC mutant exhibited significantly decreased activity in all functional assays. Interestingly, further analysis revealed that if the functional anticoagulant and anti-inflammatory assays were coupled to activation of the zymogen by the physiological activator (thrombin-TM), the PC mutant exhibits improved activities in all assays. The complementary DNA sequence analysis indicates that Thr-315 is a consensus N-linked glycosylation site for Asn-313 and that its elimination significantly improves the maximum velocity (Vmax) of PC activation by thrombin-TM. Thus, this is a novel gain-of-function mutant of PC that cannot be diagnosed by current commercial assays.
Materials and methods
Analysis of TG in plasma
TG assay was performed using previously described standard methods.16 Each assay contained 80 µL citrated test plasma incubated with 20 µL PPP reagent containing 5 pM tissue factor, 4 µM phospholipids, and 100 mM CaCl2. In some experiments, TG assay was performed in the presence of 2 to 10 nM soluble thrombomodulin (sTM). TG was determined by measuring the hydrolysis of a fluorogenic thrombin substrate as described.15,16 Three parameters—including lag time (minutes), peak height (peak, nM), and area under the curve, referred to the endogenous thrombin potential (ETP, nM⋅min)—were used to assess TG dynamics and evaluate the anticoagulant activity of PC.
Expression and characterization of APC derivatives are described in the supplemental Materials, available on the Blood Web site.
Anticoagulant assays
The anticoagulant activity of recombinant APC derivatives was evaluated in both purified and plasma-based assay systems as described.17-19 Briefly, FVa (2.5 nM) was incubated with increasing concentrations of either wild-type APC (APC-WT) or APC-T315A (0-5 nM) on 25 μM phosphatidylcholine (PC, 80%)/phosphatidylserine (PS, 20%) vesicles (PC/PS) in Tris-buffered saline (TBS)/Ca2+. Following 10 minutes incubation at room temperature, the remaining FVa activity was determined in a prothrombinase assay from the FVa-catalyzed prothrombin activation by FXa as described.17,18 The prothrombinase assay was carried out for 30 seconds using 1 μM prothrombin and 1 nM FXa. The remaining FVa activity was determined from the decrease of the FVa-dependent rate of TG as determined by an amidolytic activity assay using S2238 (100 μM). The same assay was used to monitor the inactivation of FVa by increasing concentrations of APC in the presence of protein S (100 nM) with the exception that incubation time was decreased to 1 minute. In an alternative assay, the activation of PC by thrombin-TM was coupled to the rate of FVa degradation by the generated APC. In this case, FV (2.5 nM), thrombin (1 nM), protein S (100 nM), sTM (10 nM), PC derivatives (0-100 nM), and PC/PS vesicles (25 μM) were incubated in TBS/Ca2+ for 1 minute before adding prothrombin (1 μM) and factor Xa (1 nM) and measuring TG for 1 minute. In a variation of this assay, FV (2.5 nM), protein S (100 nM), sTM (10 nM), PC derivatives (0-100 nM), prothrombin (1.4 μM), and PC/PS vesicles (25 μM) were incubated in TBS/Ca2+ and the reaction was initiated by either 0.1 nM FXa or a mixture of FX (135 nM), FVIIa (0.2 nM), and relipidated tissue factor (10 pM) and measuring TG for 1 minute.
The anticoagulant activity of wild-type APC and APC-Thr315Ala toward FVIIIa was evaluated using purified human FVIIIa as described.19 Briefly, FVIIIa (20 nM) was incubated with increasing concentrations of either wild-type or mutant APC (0-50 nM) in the absence or presence of protein S (100 nM) on PC/PS vesicles (50 µM) in TBS/Ca2+. Following 5 to 30 minutes of incubation at room temperature, the remaining FVIIIa activity was determined using an intrinsic Tenase assay from the FVIIIa-dependent factor X activation by factor IXa as described.19 Factor X activation by the intrinsic Tenase complex was carried out for 2 minutes with excess factor X (0.2 µM) and a factor IXa concentration of 1 nM on PC/PS vesicles (50 µM). The remaining activity of FVIIIa was determined from a decrease in the rate of factor Xa generation as measured by an amidolytic activity assay using Spectrozyme FXa (200 µM). In a variation of this assay, the activation of PC by the thrombin-TM complex was coupled to the rate of FVIIIa degradation by the generated APC. In this case, FVIII (5 nM), thrombin (1 nM), protein S (100 nM), sTM (10 nM), PC derivatives (0-100 nM), and PC/PS vesicles (50 μM) were incubated in TBS/Ca2+ for 8 minutes before adding FX (0.2 μM) and FIXa (2 nM) and measuring the rate of FXa generation for 10 minutes.
The anticoagulant activities of APC-WT and APC-Thr315Ala were also evaluated in plasma by an aPTT assay using a STart 4 fibrinometer (Diagnostica/Stago, Asnieres, France). Briefly, 0.05 mL TBS containing 0 to 20 nM final concentrations of APC derivatives were incubated with a mixture of 0.05 mL of normal pooled plasma plus 0.05 mL of the aPTT reagent (Alexin) for 5 minutes before the initiation of clotting by the addition of 0.05 mL of 35 mM CaCl2 at 37°C as described previously.17-19
Endothelial cell permeability assay
The intracellular signaling activity of APC derivatives (5-100 nM, 3 hours) was evaluated in an endothelial cell permeability assay in response to lipopolysaccharide (LPS) (10 ng/mL, 4 hours) using transformed human umbilical vein endothelial (EA.hy926) cells as described elsewhere.20 The endothelial cell permeability in response to LPS was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional cell monolayers using a modified 2-compartment chamber model as described elsewhere.19
A variation of the cell permeability assay was used to evaluate the intracellular signaling function of PC derivatives, activated on endothelial cells by the thrombin-W215A/E217A mutant, which is known to activate PC normally, but has no PAR1-dependent signaling activity.21 In this case, cells were initially incubated with increasing concentrations of APC or PC (0-100 nM) plus the thrombin mutant (5 nM) for 3 hours before inducing cell permeability with LPS (10 ng/mL, 4 hours).
Expression of cell surface receptors
The effect of PC (0-80 nM) + thrombin-W215A/E217A on the expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 on EA.hy926 cells in response to LPS (10 ng/mL, 4 hours) was determined by a whole-cell enzyme-linked immunosorbent assay (ELISA) as described.20
Cell adhesion assay
The interaction of THP-1 cells with LPS-stimulated endothelial cells was evaluated by fluorescent labeling of THP-1 cells as described.22 THP-1 cells were labeled with the Vybrant DiD dye and then added to LPS (10 ng/mL)-stimulated EA.hy926 cells. Cells were allowed to adhere, the nonadherent THP-1 cells were washed off, and the fluorescence of the adherent cells was measured as described.22 The effect of PC (0-80 nM) + thrombin-W215A/E217A on THP-1-endothelial cell interaction was analyzed as described previously.22
NF-κB assay
The protective effect of PC zymogens on LPS-induced activation of nuclear factor κB (NF-κB) pathway in nuclear lysates of EA.hy926 cells was evaluated in a coupled assay using an ELISA-based nonradioactive transcription factor assay kit (Abcam, Cambridge, UK) according to the manufacturer’s protocol and as described elsewhere.20,22 Briefly, confluent monolayers of endothelial cells were incubated with increasing concentrations of either PC-WT or PC-T315A (0-100 nM) plus the thrombin-W215A/E217A mutant (5 nM, 3 hours) before inducing NF-κB activation with LPS (10 ng/mL, 4 hours).
Results
Case presentation and PC assays
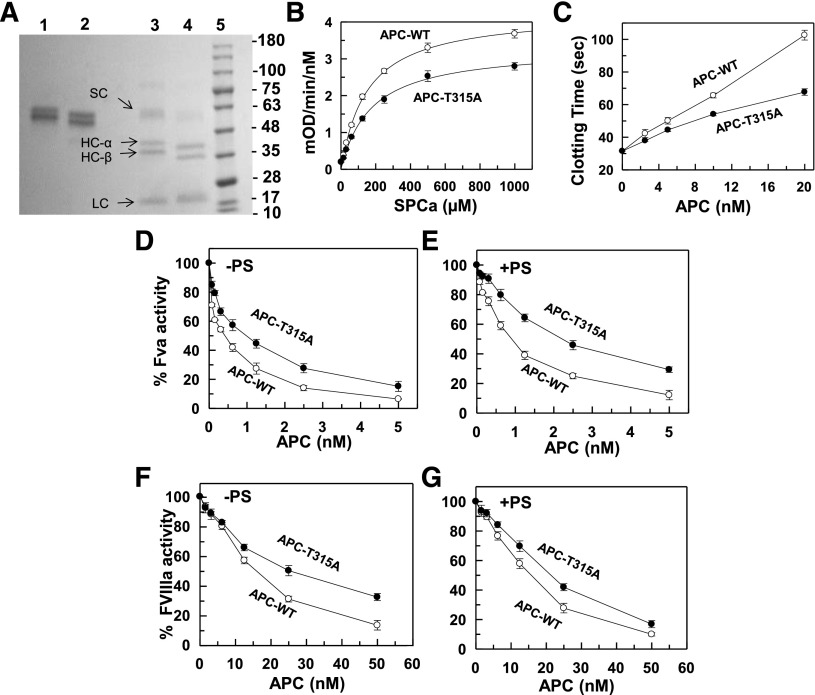
The healthy subject, a 33-year-old female with a negative personal and family history of VTE, voluntarily took part in a cohort study aimed at establishing normal ranges of anticoagulation proteins when she was diagnosed with PC deficiency. The total PC:Ag level in the proband’s plasma was 65% and the PC:A level, measured by both coagulation (aPTT) and chromogenic assays, were 36% and 50% of normal ranges, respectively. Genetic analysis identified a missense mutation in the PROC gene, resulting in substitution of Thr-315 of PC with Ala. In light of the proband’s negative VTE pedigree, we decided to express this mutant for further characterization. Thus, the PC mutant (PC-T315A) as well as its wild-type counterpart were expressed in HEK-293 cells and purified by combinations of immunoaffinity and ion exchange chromatography as described elsewhere.17 Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis under reducing and nonreducing conditions indicated that both PC derivatives have been purified to near homogeneity, with the heavy chain of the PC mutant migrating slightly faster than wild-type (Figure 1A). The heavy chain of both plasma and recombinant PC has several forms designated as α, β, and γ, which are glycosylation variants of the protein (23). The γ subform has the lowest distribution level (23). Both α and β subforms of PC-T315A migrated slightly faster than wild-type, but the light chains of both PC derivatives migrated with essentially identical apparent molecular masses (Figure 1A). The PC complementary DNA sequence analysis revealed Thr-315 constitutes the third consensus residue of an N-linked glycosylation (N-X-S/T) site located in the heavy chain of PC immediately outside the autolysis loop of the protein and that the substitution of this residue with Ala abolishes glycosylation at Asn-313, thus explaining the slightly lower molecular mass of the mutant protein on SDS-PAGE (Figure 1A). The western analysis of the proband’s plasma confirmed the ELISA data that the total PC:Ag level decreased; however, the analysis did not shed a light on the relative distribution of wild-type and T315A proteins in the heterozygous subject (data not shown).
Figure 1.
SDS-PAGE analysis of recombinant PC derivatives and their characterization in amidolytic, plasma-based clotting, and purified systems. (A) Recombinant PC-WT and PC-T315A (lanes 1 and 2, respectively) were fractionated on a 10% SDS-PAGE under nonreducing and reducing conditions (lanes 3 and 4, respectively). Lane 5 represents molecular mass standards in kDa. SC, single chain; HC-α, heavy chain α; HC-β, heavy chain β; LC, light chain. (B) Amidolytic activity of APC-WT (○) and APC-T315A (●) (5 nM each) toward the chromogenic substrate SpPCa. (C) Plasma clotting activity of APC-WT (○) and APC-T315A (●) were determined as a function of increasing concentrations of proteases (0-20 nM) at 37°C as described in “Materials and methods.” (D-E) FVa (2.5 nM) degradation by APC-WT (○) and APC-T315A (●) in the absence (D, 10 minutes) and presence (E, 1 minute) of protein S (110 nM) was analyzed by incubating increasing concentrations of each protease with the cofactor on PC/PS vesicles (25 μM) in TBS/Ca2+ in a 96-well assay plate. The remaining cofactor activity of FVa was determined by a prothrombinase (1 nM FXa and 1 μM prothrombin for 1 minute) assay as described in “Materials and methods.” (F-G) FVIIIa (20 nM) degradation by APC-WT (○) and APC-T315A (●) in the absence (F, 30 minutes) and presence (G, 5 minutes) of protein S (110 nM) was analyzed by incubating increasing concentrations of each protease with the cofactor on PC/PS vesicles (50 μM) in TBS/Ca2+ in a 96-well assay plate. The remaining cofactor activity of FVIIIa was determined by an intrinsic Tenase (1 nM FIXa and 200 nM FX for 2 minutes) assay as described in “Materials and methods.” Data in panels B-G are derived from 3 independent measurements (± standard deviation).
Wild-type and mutant PC were activated by thrombin and the resulting APC derivatives were separated from thrombin on the Mono Q column. These concentrations of APC derivatives, as determined by the active-site titration, were within 90% to 100% of those expected, based on the zymogen concentrations as determined from the absorbance at 280 nm, further confirming that the recombinant proteins have been purified to homogeneity. The amidolytic activity of the proteases toward the chromogenic substrate SpPCa was analyzed. APC-T315A exhibited ∼20% lower amidolytic activity toward SpPCa (Figure 1B). The properties of APC derivatives were further characterized in a competitive p-aminobenzamidine (PAB) binding study employing S2266 as another chromogenic substrate. In this assay, both wild-type and APC-T315A cleaved S2266 with similar kinetic parameters (Km(app) = 162-167 μM and kcat = 11-13 seconds−1), but the APC-T315A variant bound with slightly lower affinity to the S1 site-specific probe of trypsin-like serine proteases, PAB, exhibiting a Ki value of 34 μM, which is ∼20% weaker than the affinity of APC-WT for the active-site directed inhibitor (Ki = 27 μM). Analysis of the activity of the APC mutant in the plasma-based aPTT assay supported the results obtained with the proband’s plasma that the anticoagulant activity of APC-T315A is significantly impaired as evidenced by its inefficient prolongation of the clotting time (Figure 1C). Analysis of FVa (Figure 1D-E) and FVIIIa (Figure 1F-G) degradation in either the absence (Figure 1D,F) or presence (Figure 1E,G) of protein S supported results of other assays that the anticoagulant activity of APC-T315A has been negatively impacted by the mutation. The APC variant exhibited a similar half-life in plasma and an essentially identical reactivity profile toward specific plasma inhibitors (antithrombin, PC inhibitor, and α1-antithrypsin) in purified systems (data not shown).
Analysis of PC activation
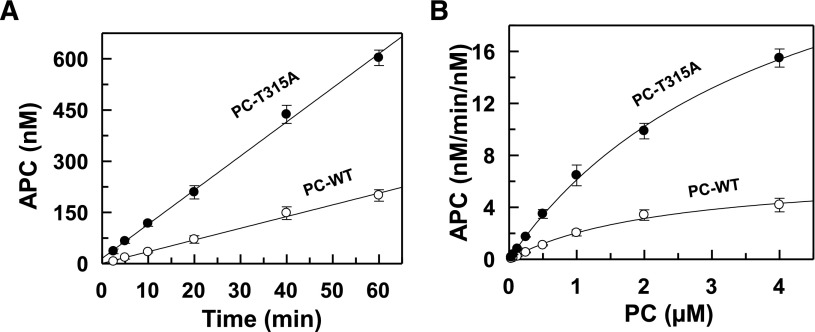
Analysis of the initial rate of PC activation indicated that the activation of the PC variant by the thrombin-TM complex has been improved four- to fivefold (Figure 2A). The PC concentration dependence of activation revealed that the improvement in the activation of the PC variant by the thrombin-TM complex is primarily due to a higher Vmax (Figure 2B). The activation of the PC variant by thrombin in the absence of TM was improved ∼60% (data not shown). These results suggest that the glycosylation of Asn-313 plays an inhibitory role in the activation of PC by the thrombin-TM complex. This is consistent with previous mutagenesis studies demonstrating that the substitution of Asn-313 of PC with a Gln (N313Q) improves the activation of the zymogen mutant by the thrombin-TM complex.23 Nevertheless, the latter variant exhibited a reduced Km in activation by the thrombin-TM complex,23 whereas the T315A mutant has a higher Vmax. As demonstrated later in the coupled functional assays, this improved Vmax for the natural T315A variant of PC activation by the thrombin-TM complex is the most likely reason for the proband’s negative VTE history.
Figure 2.
Initial rate of PC activation by thrombin. (A) The time course of PC-WT (○) and PC-T315A (●) (1 μM each) activation by thrombin (1 nM) in complex with sTM (10 nM) was monitored in TBS/Ca2+. At indicated time intervals, the activity of thrombin was inhibited by antithrombin and the rate of APC generation was determined by an amidolytic activity as described in “Materials and methods.” (B) The same as panel A except that the concentration dependence of PC activation by the thrombin-sTM complex was carried out in TBS/Ca2+ for 10 minutes and the rate of APC generation was determined as described previously. Data are derived from at 3 independent measurements (± standard deviation). The solid lines in panel A are computer fits of data to a linear equation and those in panel B are fits of data to the Michaelis-Menten equation, yielding Km and kcat values of 2.7 ± 0.2 µM and 7.8 ± 0.3 minutes−1 for APC-WT and 4.2 ± 0.4 µM and 34.5 ± 1.6 minutes−1 for APC-T315A, respectively.
Coupling PC activation to its anticoagulant function
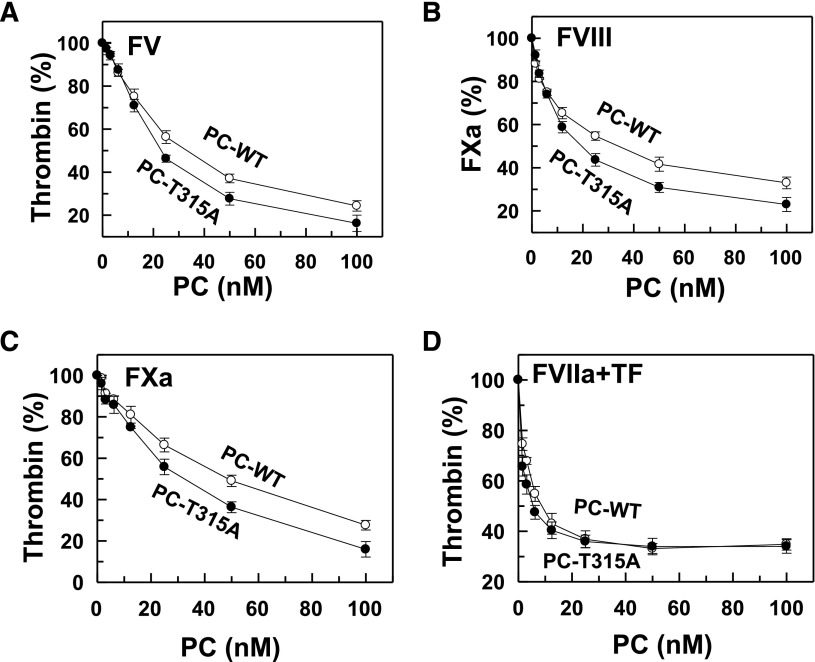
To investigate the hypothesis that the improvement in the Vmax of variant PC activation may be responsible for the proband’s negative VTE history, the activation of PC by the thrombin-TM complex was mechanistically linked to its anticoagulant function by coupling zymogen activation to protease function in both purified and plasma-based assay systems. In the purified system, FV, protein S, PC, and sTM were all incubated with 1 nM thrombin on PC/PS vesicles for 1 minute before initiating TG by the addition of FXa plus prothrombin (1.5 µM). Interestingly, it was discovered that, unlike the decreased anticoagulant effect of the APC variant, the PC variant exhibits improved anticoagulant function in this assay (Figure 3A). Thus, the APC variant inactivated FVa better than wild-type APC. The anticoagulant function of the PC variant was also evaluated in the intrinsic pathway using a similar coupled assay. Thus, FVIII, protein S, PC, and sTM were incubated with 1 nM thrombin on PC/PS vesicles for 8 minutes before initiating FXa generation by adding FIXa plus FX (Figure 3B). Similar to the inactivation of FVa, the anticoagulant function of the PC variant toward FVIIIa was higher than that of wild-type PC in this assay (Figure 3B). Similar results were obtained in variations of these assays if FV, prothrombin, protein S, PC, and sTM were all incubated on PC/PS vesicles and TG was directly monitored by addition of a low concentration of FXa (Figure 3C). This was also true if FV, prothrombin, FX, protein S, PC, and sTM were all incubated on PC/PS vesicles and TG was initiated by a low concentration of tissue factor–factor VIIa (10 pM) complex (Figure 3D). Taken together, these results suggest that the improved activation of the PC variant by the thrombin-TM complex more than compensates for its decreased catalytic function if these rather physiologically relevant coupled assay systems are employed to analyze the anticoagulant activity of the PC variant.
Figure 3.
Analysis of inhibition of thrombin generation by PC derivatives in coupled assays. (A) FV (2.5 nM), thrombin (1 nM), protein S (100 nM), sTM (10 nM), PC derivatives (0-100 nM), and PC/PS vesicles (25 μM) incubated in TBS/Ca2+ for 1 minute before adding prothrombin (1 μM) and FXa (1 nM) and measuring thrombin generation for 1 minute as described in “Materials and methods.” (B) FVIII (5 nM), thrombin (1 nM), protein S (100 nM), sTM (10 nM), PC derivatives (0-100 nM), and PC/PS vesicles (50 μM) were incubated in TBS/Ca2+ for 8 minutes before adding FX (0.2 μM) and factor IXa (2 nM) and measuring the rate of FXa generation for 10 minutes as described in “Materials and methods.” (C) The same as panel A except that thrombin generation was directly started with 0.1 nM FXa. (D) The same as panel A except that the zymogen FX (135 nM) was included in the assay and thrombin generation was initiated with the FVIIa (0.2 nM) in complex with tissue factor (10 pM) as described in “Materials and methods.”
Analysis of TG in plasma
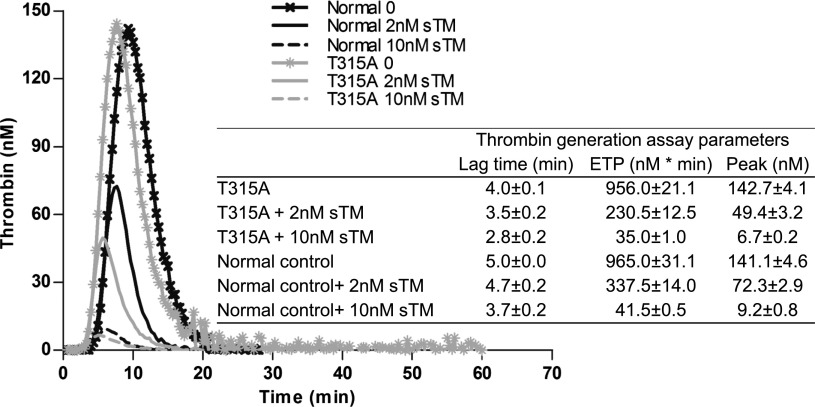
The anticoagulant activity of PC in the proband’s plasma was evaluated by TG with a commercial kit using a tissue factor concentration of 5 pM to initiate the clotting cascade. Analysis of TG in plasma was conducted in both the absence and presence of sTM (2-10 nM). The results in the presence of 10 nM sTM indicated a normal anticoagulant function for the proband’s plasma PC based on similar clotting parameters obtained in this test (Figure 4). In the absence of sTM, the values of lag time, peak, and ETP in plasma derived from the case were similar to those in normal control plasma (Figure 4). In the presence of 10 nM sTM, the proband’s plasma exhibited a similar reduction (or slightly lower) in lag time, peak, and ETP of TG. Interestingly, at a low concentration of sTM (2 nM), the T315A variant exhibited an anticoagulant advantage as evidenced by significantly lower values for these parameters (Figure 4). These results support the central hypothesis presented previously and may explain the molecular basis for the normal anticoagulant activity of the PC-Thr315Ala variant under physiological conditions.
Figure 4.
Assessment of thrombin generation in the absence and presence of sTM in normal and proband plasma. Citrated normal or test plasma (80 µL) was incubated with 20 µL PPP reagent in the absence and presence of either 2 nM or 10 nM sTM and thrombin generation was analyzed as described in “Materials and methods.”
Anti-inflammatory activity
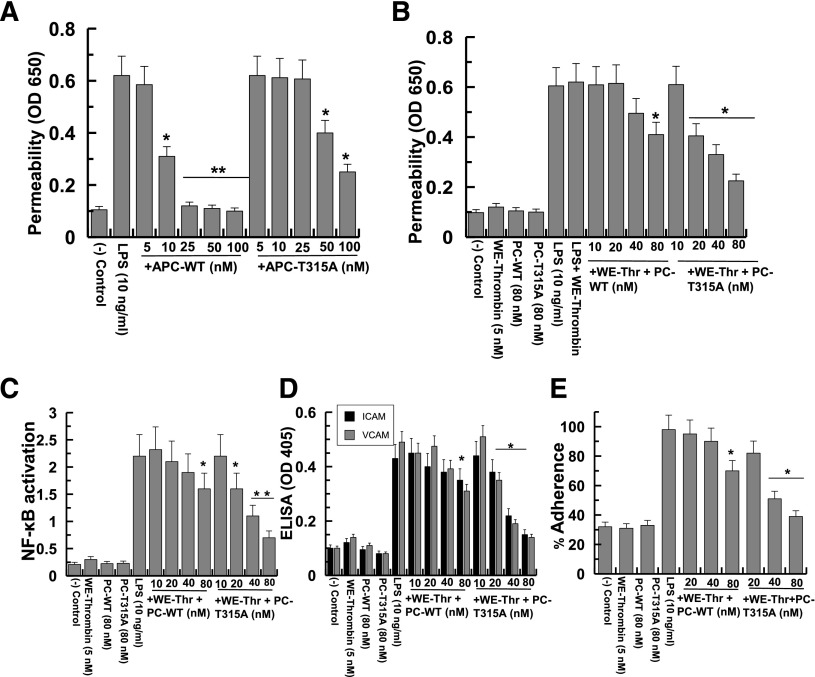
APC can elicit potent cytoprotective signaling responses in endothelial cells in response to thrombin and proinflammatory cytokines.8,9 The modulation of LPS-induced endothelial cell permeability has been frequently used to probe the cytoprotective signaling function of APC variants. Thus, we evaluated the signaling activity of APC-WT and APC-T315A in an LPS-mediated permeability assay. Analysis of concentration dependence of APC activity in this assay indicated that the barrier protective activity of the APC variant is significantly impaired (Figure 5A). Thus, in contrast to effective barrier protective activity for 10 nM APC-WT, a markedly higher concentration (50-100 nM) was required to observe a similar barrier protective function for the APC variant (Figure 5A). To determine whether, similar to anticoagulant assays, coupling of PC activation to its cytoprotective function on cell surfaces can alter the outcome, we incubated endothelial cells with different concentrations of wild-type and variant PC zymogens followed by their activation with a thrombin derivative (W215A/E217A-thrombin or WE-thrombin), which is known to activate PC normally in the presence of TM, but is incapable of activating PAR1.20 This assay facilitated specific monitoring of the PAR1-dependent protective activity of the cell surface–generated APC, without interference from thrombin. Interestingly, when the activation of the PC zymogens was coupled to their activity by this strategy, the PC variant exhibited a markedly higher barrier protective activity than did wild-type PC (Figure 5B). Thus, in contrast to wild-type PC, which only exhibited a significant barrier protective activity at 80 nM zymogen, the same extent of barrier protective activity was observed with only a 20 nM variant zymogen (Figure 5B).
Figure 5.
Cytoprotective signaling activity of APC/PC derivatives in established cellular assays. (A) The endothelial cell permeability in response to LPS (10 ng/mL for 4 hours) following their treatment with APC derivatives (5-100 nM for 3 hours) was quantitated by spectrophotometric measurement of the flux of Evans blue-bound albumin across functional cell monolayers as described in “Materials and methods.” (B) The same as panel A except that the activation of PC derivatives by the thrombin-W215A/E217A mutant (WE-Thr) was coupled to its barrier protective function. Endothelial cells were initially incubated with increasing concentrations of PC derivatives (0-100 nM) plus the thrombin mutant (5 nM) for 3 hours before inducing cell permeability with LPS (10 ng/mL for 4 hours). (C) The same as panel B except that the effect of cell surface WE-Thr–activated PC derivatives on NF-κB activation in response to LPS was measured by an ELISA. (D) The same as panel C except that the effect WE-Thr–activated PC derivatives on cell surface expression of intercellular adhesion molecule (ICAM)-1 (gray bars) and vascular cell adhesion molecule (VCAM)-1 (black bars) was monitored by a cell-based ELISA. (E) The same as panel C except that the adherence of THP-1 cells to LPS-stimulated endothelial cells was monitored. *P < .05 and **P < .01 in both panels. OD, optical density.
A similar improved cytoprotective activity for PC-T315A was observed in inhibiting NF-κB activation, cell adhesion molecule expression, and THP-1 cells binding to endothelial cells in response to LPS when the activity of the zymogen was evaluated in the same coupled assay using the thrombin-W215A/E217A mutant as the PC activator (Figure 5C-E). These results clearly suggest that the PC variant has significantly higher protective activity when its activation is mechanistically coupled to its cellular activity on cells expressing both EPCR and TM.
Discussion
In a cohort study aimed at establishing normal PC levels, we identified a healthy subject whose plasma tests of chromogenic and clotting-based assays followed by an ELISA, conducted by commercially available reagents, demonstrated that the subject may have type II PC deficiency.15 This prediction was based on results of these assays yielding significantly decreased PC activity levels of 36% (clotting) and 50% (chromogenic) and antigen level of 65% (ELISA) in the subject plasma. In light of a negative family history of VTE for the subject’s living parents and other family members, we decided to further study the underlying cause of this apparent type II PC deficiency and to understand whether it is environmentally acquired or has a genetic basis. Genetic analysis revealed a single-point mutation in the PROC gene of the subject, leading to substitution of Thr-315 of PC with an Ala. Following recombinant expression and zymogen activation, the characterization of the APC variant in various assays indicated a similar two- to threefold impairment in the catalytic activity of the variant toward the degradation of FVa and FVIIIa in the purified system, which paralleled a significant loss in the capacity of the APC variant to prolong clotting time in an aPTT assay. These results correlated with results of the assays obtained from the subject’s plasma, supporting the reliability of methods used for the characterization of recombinant proteins. The results of a cell-based assay further indicated that the anti-inflammatory signaling activity of the APC variant may also be negatively impacted because the protease mutant, unlike wild-type APC, exhibited a significant loss in its capacity to protect the barrier permeability function of APC in response to LPS in endothelial cells. Interestingly, further characterization revealed that the activation of the PC zymogen variant by the thrombin-TM complex has been markedly improved by an improvement in the Vmax of activation. This observation led us to conduct the TG tests with the subject’s plasma in the presence of soluble TM. Remarkably, despite a significantly lower PC antigen level, the subject’s plasma in the presence of TM exhibited an essentially identical TG profile and, similar to normal plasma, effectively inhibited TG with identical reduction in lag time, peak, and ETP of TG. These results suggest that the T315A is a unique gain-of-function mutant of PC that wrongly manifests itself as a type II PC deficiency when analyzed by commercially available kits. The inclusion of soluble TM in plasma samples in a TG assay can discriminate this type of variant from type II PC deficiency in clinical laboratories.
In agreement with the TG result in the presence of TM, when the activation of the PC variant by the thrombin-TM complex was coupled to its anticoagulant function in either purified or plasma-based clotting assays, the PC variant exhibited a superior anticoagulant effect, suggesting that the catalytic defect of the variant is more than compensated by its improved activation by the activator complex. Interestingly, this was also true when the anti-inflammatory effect of the PC variant was analyzed on the endothelial cell surface by employing a thrombin derivative that is known to activate PC normally in the presence of TM but is incapable of cleaving cell surface PAR1, thus allowing the assay to specifically monitor the activation of the receptor by APC generated on the cell surface. This assay has been previously used to demonstrate that the activation of PC zymogen is mechanistically linked to its anti-inflammatory function on endothelial cells.24 This is not surprising because TM together with the critical receptors EPCR and PAR1 are colocalized to lipid-raft microenvironments, which is where the signaling molecules involved in the anti-inflammatory function of APC are also localized.25 Thus, when the EPCR-bound PC is activated by the thrombin-TM complex, the resulting APC is already primed to function in the anti-inflammatory pathways.
The basis for the lower PC level in the subject’s plasma is not known. However, given the established role of posttranslationally attached N-linked glycans to the stability of protein molecules, it is possible that the lack of Asn-313 glycosylation caused by Thr-315 mutation reduces the stability of the variant PC in circulation. This modification may also be responsible for the lower catalytic function of the APC variant in the functional assays, possibly because of the carbohydrate side chain of Asn-313 playing a productive role in the interaction of the protease with its natural substrates. The observation that the variant APC exhibited a normal amidolytic activity toward 2 chromogenic substrates and also bound to the active-site probe, PAB, with a normal affinity, is consistent with the hypothesis that the defect in the catalytic activity of the APC variant is due to the loss of a secondary substrate binding site on the protease. There is a precedent for the carbohydrate side chains of APC contributing to its catalytic function; a recent study demonstrated that the mutation of another N-linked glycosylation site, Asn-329, improved the cytoprotective activity of the APC mutant.26 Thus, in addition to conferring stability, the carbohydrates of APC can modulate the catalytic function of the protease in anticoagulant and anti-inflammatory pathways. Nevertheless, the ∼twofold instability and the same extent of loss in the catalytic activity of the APC-T315A variant is compensated by ∼four- to fivefold improvement in the activation of the PC zymogen variant by the thrombin-TM complex with the net effect being the APC variant in the subject’s plasma functioning normally in both anticoagulant and anti-inflammatory pathways. These findings suggest that the TM dependence of PC activation may need to be evaluated for diagnosing all PC deficiencies.
Acknowledgments
The authors thank Dr Yongqiang Zhao from the Peking Union Medical College Hospital for providing the case sample identified by the Chinese Hemostasis Investigation on Natural Anticoagulants Study I Group, Leslie Pelc from the laboratory of Dr Enrico Di Cera for the WE-thrombin mutant, and Audrey Rezaie for proofreading the manuscript.
This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants HL 101917 and HL 68571) (A.R.R.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Q.D. designed experiments, performed research, analyzed data, and contributed to writing of the manuscript; L.Y. prepared recombinant proteins and performed research; P.D. performed the inflammatory assays; X.W. designed experiments and supervised studies conducted in Ruijin Hospital with the proband’s plasma; and A.R.R. analyzed data, wrote the article, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Department of Biochemistry and Molecular Biology, St. Louis University School of Medicine, 1100 S Grand Blvd, St. Louis, MO 63104; e-mail: rezaiear@slu.edu; and Xuefeng Wang, Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai, 200025 China; e-mail: wangxuefeng6336@hotmail.com.
References
- 1.Foster DC, Yoshitake S, Davie EW. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci USA. 1985;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 3.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269(42):26486–26491. [PubMed] [Google Scholar]
- 4.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA. 1996;93(19):10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marlar RA, Kleiss AJ, Griffin JH. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982;59(5):1067–1072. [PubMed] [Google Scholar]
- 6.Fay PJ, Smudzin TM, Walker FJ. Activated protein C-catalyzed inactivation of human factor VIII and factor VIIIa. Identification of cleavage sites and correlation of proteolysis with cofactor activity. J Biol Chem. 1991;266(30):20139–20145. [PubMed] [Google Scholar]
- 7.Dahlbäck B, Villoutreix BO. Molecular recognition in the protein C anticoagulant pathway. J Thromb Haemost. 2003;1(7):1525–1534. doi: 10.1046/j.1538-7836.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 8.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 9.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 10.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981;68(5):1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyfus M, Magny JF, Bridey F, et al. Treatment of homozygous protein C deficiency and neonatal purpura fulminans with a purified protein C concentrate. N Engl J Med. 1991;325(22):1565–1568. doi: 10.1056/NEJM199111283252207. [DOI] [PubMed] [Google Scholar]
- 12.Jalbert LR, Rosen ED, Moons L, et al. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest. 1998;102(8):1481–1488. doi: 10.1172/JCI3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitsma PH, Bernardi F, Doig RG, et al. Protein C deficiency: a database of mutations, 1995 update. On behalf of the Subcommittee on Plasma Coagulation Inhibitors of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;73(5):876–889. [PubMed] [Google Scholar]
- 14.Pabinger I, Kyrle PA, Speiser W, Stoffels U, Jung M, Lechner K. Diagnosis of protein C deficiency in patients on oral anticoagulant treatment: comparison of three different functional protein C assays. Thromb Haemost. 1990;63(3):407–412. [PubMed] [Google Scholar]
- 15.Zhu T, Ding Q, Bai X, et al. Normal ranges and genetic variants of antithrombin, protein C and protein S in the general Chinese population. Results of the Chinese Hemostasis Investigation on Natural Anticoagulants Study I Group. Haematologica. 2011;96(7):1033–1040. doi: 10.3324/haematol.2010.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Manithody C, Rezaie AR. Contribution of basic residues of the 70-80-loop to heparin binding and anticoagulant function of activated protein C. Biochemistry. 2002;41(19):6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Yang L, Hassanian SM, Rezaie AR. Expression and functional characterisation of natural R147W and K150del variants of protein C in the Chinese population. Thromb Haemost. 2013;109(4):614–624. doi: 10.1160/TH12-10-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manithody C, Fay PJ, Rezaie AR. Exosite-dependent regulation of factor VIIIa by activated protein C. Blood. 2003;101(12):4802–4807. doi: 10.1182/blood-2003-01-0126. [DOI] [PubMed] [Google Scholar]
- 20.Dinarvand P, Hassanian SM, Qureshi SH, et al. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood. 2014;123(6):935–945. doi: 10.1182/blood-2013-09-529602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pineda AO, Chen ZW, Caccia S, et al. The anticoagulant thrombin mutant W215A/E217A has a collapsed primary specificity pocket. J Biol Chem. 2004;279(38):39824–39828. doi: 10.1074/jbc.M407272200. [DOI] [PubMed] [Google Scholar]
- 22.Bae JS, Lee W, Rezaie AR. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J Thromb Haemost. 2012;10(6):1145–1151. doi: 10.1111/j.1538-7836.2012.04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinnell BW, Walls JD, Gerlitz B. Glycosylation of human protein C affects its secretion, processing, functional activities, and activation by thrombin. J Biol Chem. 1991;266(15):9778–9785. [PubMed] [Google Scholar]
- 24.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281(29):20077–20084. doi: 10.1074/jbc.M600506200. [DOI] [PubMed] [Google Scholar]
- 25.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci USA. 2007;104(8):2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ní Ainle F, O’Donnell JS, Johnson JA, et al. Activated protein C N-linked glycans modulate cytoprotective signaling function on endothelial cells. J Biol Chem. 2011;286(2):1323–1330. doi: 10.1074/jbc.M110.159475. [DOI] [PMC free article] [PubMed] [Google Scholar]