Abstract
The 10 amino acid sequence of the biologically important neutral amylo-β peptide has equally hydrophilic and hydrophobic properties, which reduces the coupling efficiency during its synthesis and reduces the final yield of the peptide, and is therefore classified as a “difficult peptide sequence.” The method presented here minimizes the synthetic problems by the introduction of improved Fmoc chemistry and effective hydroxybenzotriazole (HoBt), diisopropylcarbodiimide (DIC)-coupling and activation strategies. In addition, we developed a PS-TPGD resin as a solid support for the synthesis of specific neutral peptides, which is still a challenge to peptide chemistry. The most essential biologically active neutral amylo-β peptide (KVKRIILARS) was successfully synthesized, and some synthetic modification was performed using the Fmoc solid-phase peptide synthesis (SPPS) method for purity and yield improvement.
Graphical abstract.
ᅟ
Keywords: Polymer support, Amylo-β peptide, Fmoc SPPS, HoBt and DIC
Introduction
The general chemical requirements for the synthesis of peptide involve the blocking of carboxyl group of one amino acid and the amino group of the second amino acid [1]. The activation of the free carboxyl group resulted the formation of amide bond between the amino acids, and the selective removal of the protecting groups resulted a dipeptide [2]. The method developed by Fischer was laborious and time-consuming because the intermediate peptides have to be removed, purified, and characterized before the next coupling step [3]. The major limitation of classical solution phase synthesis of peptides is the low yield and solubility of the intermediate peptides with increase in chain length. A new approach was needed for the synthesis of larger and more complex peptides with high purity and yield [3, 4].
To overcome above limitations in peptide chemistry, R. B. Merrifield introduced solid-phase technique for peptide synthesis [2]. The invention of solid-phase peptide synthesis is the real landmark in synthetic peptide chemistry, at the time it fulfills all basic requirements to synthesize basic peptides with acceptable properties. Merrifield’s technique has undergone a series of modifications and improvements based on the nature of synthetic peptides [5]. The peptide has hydrophobic and hydrophilic nature based on the amino acid present in the target peptide; the hydrophilic nature peptide was somehow easy to synthesis by normal Fmoc chemistry methods, but the hydrophobic and neutral (i.e., 50 % hydrophobic: 50 % hydrophilic) nature peptide was difficult to synthesize due to its salvation and intermolecular aggregation property because total solid-phase peptide synthesis condition is in the homogeneous medium [6]. Even though a lot of synthetic methods are there, mainly the neutral peptide synthesis is a major goal for peptide chemist, so some specific polymer support and synthetic procedure is needed to achieve biologically active neutral peptide synthesis [7].
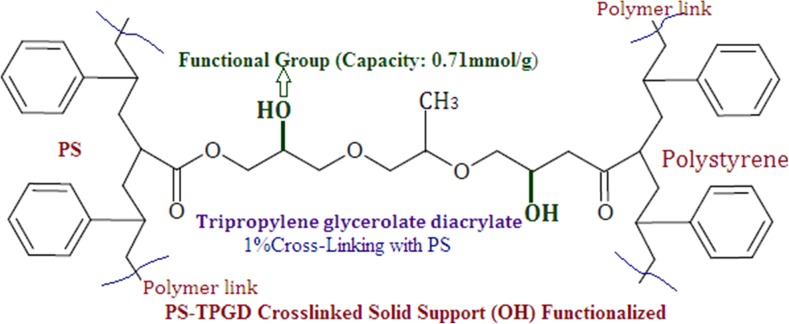
The success of solid-phase peptide synthesis (SPPS) depends on the physicochemical characteristics of the peptide bearing support. For effective swelling of the resin and salvation of the peptide, the polymer should have an optimum hydrophobic-hydrophilic balance [8]. For our condition, the developed PS-TPGD resin (Shown in Fig. 1) for neutral peptide synthesis have optimum hydrophobic-hydrophilic balance and swell like a gel in most of the organic solvents used in peptide synthesis [9]. Efficiency of these supports was demonstrated by synthesizing biologically important 50 % hydrophobic: 50 % hydrophilic peptides in high purity and yield. The new member of this series was described in this work, developed by cross-linking tripropylene glycerolate diacrylate with polystyrene [10]. This support showed very high swelling characteristics in various solvents, an effective hydrophobic-hydrophilic balance, and it was successfully used for the synthesis of neutral peptide. The optimum reactivity of these developed resin are due to the greater chain mobility of the cross-linker in solvents that enable effective interaction between the reactants and resin bound functional groups, the developed PS-TPGD polymer resin structure was shown in Fig. 1.
Fig. 1.
Structural information of PS-TPGD resin
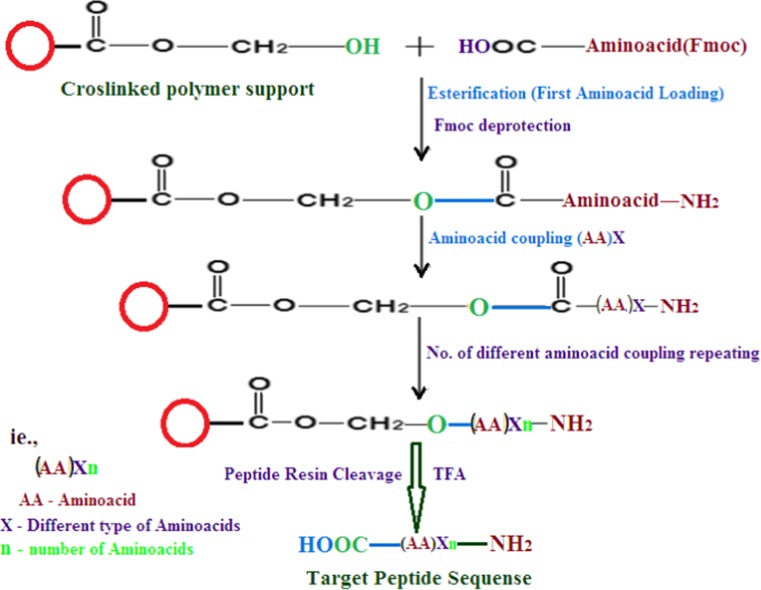
The protocol uses the 9-fluorenylmethyloxycarbonyl (Fmoc) group for Nα-amino protection. During the synthesis, Fmoc is split off by a short treatment with piperidine in dimethylformamide (DMF). For coupling reactions diisopropylcarbodiimide (DIC) can be used, and for the qualitative monitoring of the coupling reaction, Kaiser test can be used [11]. Side-chain protections that are compatible with Nα-protection are removed at the same time as the appropriate anchoring linkages typically by the use of trifluoroacetic acid (TFA) [12]. The Fmoc protocol (shown in Fig. 2) is especially recommended for the synthesis of acid sensitive peptides and derivatives. The choice of an adequate combination of protecting groups/solid support is the first step en route to achieve a successful synthesis [8]. A major, neutral peptide was purified from the venom duct of Conus spurius specimens. Amylo-β peptide H2N-Lys-Val-Lys-Arg-Ile-Ile-Leu-Ala-Arg-Ser-COOH (shown in Fig. 7) was successfully synthesized by newly developed strategy; the neutral amylo-β peptide have long held the interest of biologists, pharmacologists, and structural biologists [13]. Various solid-phase peptide synthetic methods developed till today, but it fails to give good yield and purity in final stage due to the specific nature of the peptide and its solubility in reaction condition. Here, we have chosen the specific solid support and some improvement performed in Fmoc SPPS methods especially for this type of specific peptide achievement and the improvement was presented. The improved Fmoc SPPS protocol was shown in Fig. 2.
Fig. 2.
Improved Fmoc SPPS methodology
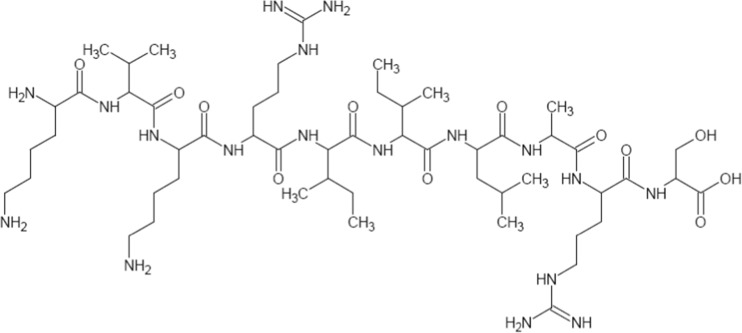
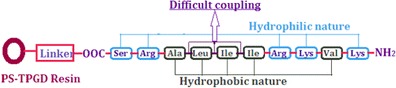
Fig. 7.
The chemical structure of H2N-Lys-Val-Lys-Arg-Ile-Ile-Leu-Ala-Arg-Ser-COOH peptide
Resin containing one –OH group on a tripropylene glycol diacrylate linker attached to 1 % of the polymer was reacted with the –COOH groups of Fmoc amino acids, resulting in strong esterification. Dimethylaminopyridine (DMAP) was used for efficient esterification, followed by reverse amidation coupling of the remaining amino acids with HOBT/DIC in DIEA, which was required for the quantitative reaction on the resin [6]. After coupling of all amino acids, the peptide-resin was treated with TFA in the presence of scavengers to cleave the peptide from the resin. The crude peptide was separated using cold diethyl ether and further purified and characterized by RP-HPLC and MALDI-TOF MS.
Experimental
Synthesis of 1 % polystyrene-tripropylene glycol glycerolate diacrylate resin
Materials
Polystyrene, divinylbenzene, tripropylene glycol glycerolate diacrylate, polyvinyl alcohol, sodium hydroxide, thionylchloride, benzoyl peroxide, toluene, benzene, chloroform, dichloromethane, dimethylformamide, and methanol are chemicals purchased from Merck Millipore (India).
Suspension polymerization
Required amount of polystyrene and divinylbenzene are destabilized by using 1 % NaOH, and the suspension polymerization reaction was conducted, 1 % PVA was prepared 0.2 g of PVA in 20 ml water (at 80 °C overall system) to that 3 ml of toluene with 0.14 ml TPGD and 0.23 g benzoyl peroxide (initiator) and 1.36 ml styrene completely dissolved and added; stirring was continued up to 8 h with 80 °C. The beaded form of polymer was obtained in the walls of the system; the system was kept such overnight and filtered washed with hot water, benzene, CHCl3, DCM, DMF, and methanol and dried. The resin was resized to 100–200 mesh and functionalized [8].
Estimation of loading (resin capacity measurement)
Above synthesized and functionalized resin capacity was measured; the measurement was done by using standard procedures. The calculated resin capacity is 0.71 mmol/g; the resin was characterized by FT-IR spectrometer and SEM analyzer; the resin size and image, functional group of the resin was analyzed [6].
Synthesis of biologically active H2N-lys-val-lys-arg-ile-ile-leu-ala-arg-ser-COOH peptide
Materials
Boc-Lys(2 cl,z)-OH, Fmoc-Val-OH, Fmoc-Lys(boc)-OH, Fmoc-Arg(pbf)-OH, Fmoc-Ile-OH, Fmoc-Leu-OH, Fmoc-Ala-OH, Fmoc-Ser(tbu)-OH amino acids were purchased from Sigma-Aldrich, Germany and Switzerland and Alfa-asear England, which the highest purity available and the purity of the amino acid was tested. Dichloromethane (DCM), dimethylformamide (DMF), methanol, acetic acid, N-methyl-2-pyrrolidone (NMP), and petroleum ether were purchased from Merck Millipore (India) and Lobachemi Mumbai.
Diisopropylethylamine (DIPEA), 4-dimethylaminopyridine (DMAP), hydroxybenzotriazole (HOBt), diisopropylcarbodiimide (DIC), trifluoroacetic acid (TFA), triisopropylsilane (TIS), pyridine, and piperidine were purchased from Sigma-Aldrich, Germany and China, Alfa Aesar England and Merck Millipore (India).
Improved Fmoc SPPS strategy
One percent polystyrene-tripropylene glycol glycerolate diacrylate resin was transferred to peptide synthesizer, to that sufficient amount of N-methyl-pyrrolidone were added and swelled for 1 h and removed the filtrate under vacuum, to that 2 eq.wt of Fmoc-Ser-(tBu)-OH, 0.1 eq.wt of DMAP with DCM, and 2 eq.wt DIC was added and shaked for 45 min and washed; the reaction was monitored by thin-layer chromatography (TLC). Fmoc amino acid dissolved in minimum quantity of NMP to that HOBt and DIC was added and shaken for 3 min, and immediately, the content was transferred in to the resin with moisture-free atmosphere and shaken well for 5 min, to that DIPEA was added and the reaction was continued for 45 min. Reaction progress was monitored by TLC. A small pinch of the resin was taken and checked for free amino group (ninhydrin test), in case positive means same amino acid coupling was repeated, in case negative means washed and deprotected. The above process was repeated until the assembly of remaining amino acids Arg, Ala, Leu, Ile, Ile, Arg, Lys, Val, and Lys.
Cleavage of peptide from resin
After the above synthetic step, the resin was washed with DCM, CHCl3, and Methanol and dried [6]. The cleavage was done with 95 %TFA: 2.5 % TIS: 2.5 % water at 2 h under nitrogen atmosphere, and resin was washed two times with TFA; the filtrate was collected and the traces of TFA was evaporated by using Rota vacuum evaporator.
The crude peptide was isolated with excess of peroxide free pure cold petroleum ether, and the peptide was washed five times with petroleum ether and centrifuged. The clear white powder form of peptide was collected in a small tube and sealed [8].
Results and discussion
The polymer support PS-TPGD (shown in Fig. 1) was designed especially for biologically active neutral peptide synthesis. The resin has excellent swelling properties and satisfied all conditions for solid-phase peptide synthesis, and the analytical report of the resin has been discussed.
SEM characteristic analysis of PS-TPGD resin
SEM characterization was done, and the analytical report clearly reveals the physical nature and size of the resin. The characteristic report was shown in Fig. 3.
Fig. 3.
The characteristic SEM image of polystyrene-tripropylene glycol glycerolate diacrylate resin
FT-IR analysis of PS-TPGD resin
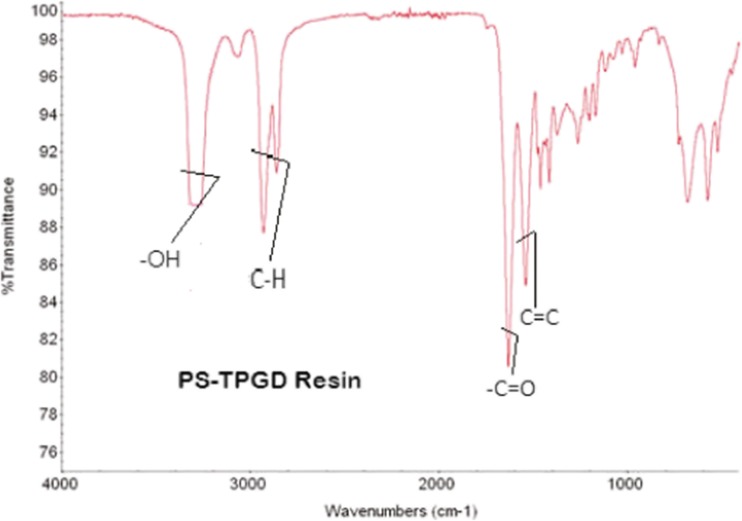
The FT-IR spectrum of the resin gave the functional group and the carbon-carbon and carbon-hydrogen environment and bonding nature information shown (Fig. 4).
Fig. 4.
Characteristic FT-IR spectrum of 1 % polystyrene-tripropylene glycol glycerolate diacrylate resin
FT-IR spectral analysis
–OH: The medium broad peak at 3428 cm−1 shows –OH group with partial hydrogen bond.
–C-H: The sp2 C-H stretch occurs at 2932 and 2993 cm−1 sharp peaks show the –C-H group in two different environment,
–C = O: The sharp peak at 1710 cm−1 as –C = O stretching lines occurs with 1686 cm−1 C = C stretch.
–C = C: The sharp peak at 1686 cm−1 clearly shows –C = C-stretching environment.
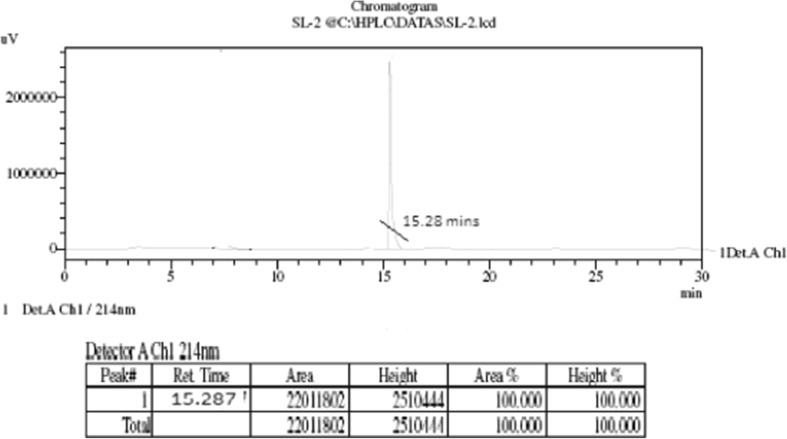
HPLC analysis of KVKRIILARS peptide
The HPLC analysis report of H2N-Lys-Val-Lys-Arg-Ile-Ile-Leu-Ala-Arg-Ser-COOH peptide was shown (Fig. 5). The sharp single peak (ret. time 15.28 min) clearly shows our target peptide with >98 % purity.
Fig. 5.
HPLC analysis report of the KVKRIILARS peptide
HPLC instrumental method
The HPLC system specially made for peptide and biological samples (make: M/s Shimadzu Corporation, Japan) RP-C18 column diameter 150 mm × 2.6 mm, 25 cm length, 5 μm particle size, run time 30 min, injection volume: 20 μL, linear gradient 5 % acetonitrile: 95 % water at 0 min, 100 % acetonitrile: 0 % water at 30 min, flow rate 1 ml per minute.
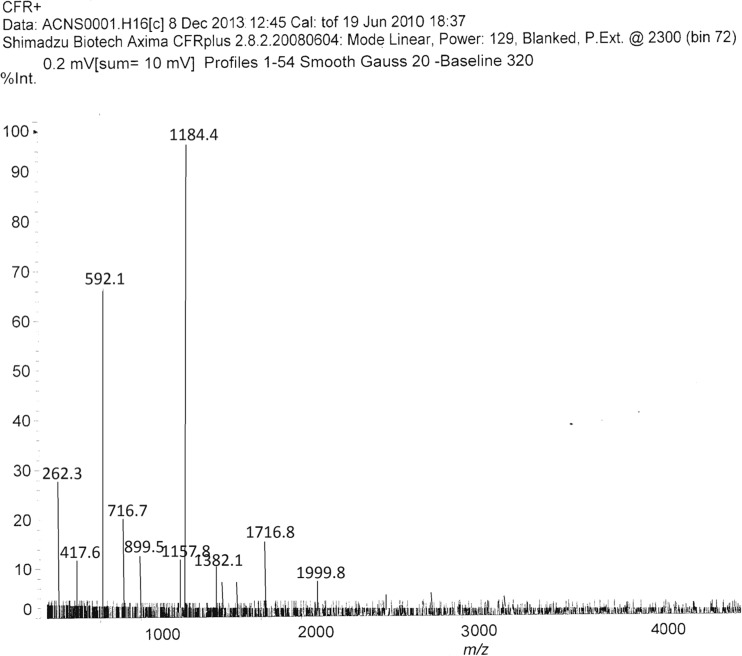
MALDI-TOF MS analysis of the neutral amylo-β peptide
The MALDI-TOF MS spectrum of the test peptide is shown in Fig. 6. Two charge states of the peptide were readily detected: m/z 1621.68 [M + H]+ and m/z 811.29 [M + 2H]2+. The obtained spectrum [M + H]+ and [M + 2H]2+ ion confirmed the expected target sequence (Fig. 7).
Fig. 6.
MALDI-TOF MS of the peptide. m/z. 1184.4 [M + H]+ and m/z. 592.1 [M + 2H]2+ verifying a molecular mass of 1183.5 Da as evidence of target peptide
Observed common properties of amylo-β peptide
Nature
Pure white color fine powder; isoelectric point pH: 12.53; net charge: +4; molecular weight: 1183.54 g/mol; hydrophobic/hydrophilic: 50/43 %; solubility: very poor water solubility and perfectly soluble in acidic solvents; purity: >99 % HPLC; yield: 89.24 % crude and 86.51 % purified.
Here, the neutral peptide was produced using an improved Fmoc SPPS strategy and suitable polymer resin was developed and analyzed. The peptide “KVKRIILARS” (Fig. 7) contains an equal number of hydrophilic and hydrophobic amino acids (5 of each). For this peptide, the developed improvement in Fmoc SPPS was highly efficient (86.5 % final yield and >99 % purity). The chemical structure of the KVKRIILARS peptide is shown in Fig. 7.
It is interesting to note that a variety of different solid-phase and solution phase peptide synthetic methodologies have been developed till today, but in the case of biologically active specific peptide, the strategies fails to give the acceptable purity and final yield. So, after discussing various parameters with peptide experts, we took this as a major research problem, and based on the natures of amino acids, the tripropylene glycol glycerolate diacrylate-cross linked polystyrene support with loading below 0.71 mmol/g was specifically developed. The polymer support shows good swelling, salvation, mechanical stability, and suitable reactivity for the application in solid-phase peptide synthesis, especially in the synthesis of biologically important peptides, which remains a challenge for peptide chemists. Some specific synthetic protocol improvements were made to the Fmoc SPPS strategy which are clearly explained and reported here along with evidence for this synthesis of biologically active amylo-β peptide (Fig. 7).
Conclusion
The evidence of this study showed that simple improvement of Fmoc solid-phase peptide synthetic strategy will eliminate the major problem in difficult peptide synthesis. Here, the successful synthesis of the amylo-β peptide, which has equal hydrophilic and hydrophobic properties, was explained using an improved Fmoc SPPS strategy that was introduced especially for this type of biologically important neutral peptide. PS-tripropylene glycol glycerolate diacrylate was also synthesized and used as a suitable polymer resin for this type of specific peptide synthesis. The biologically important amylo-β peptide was successfully synthesized by using the improved Fmoc SPPS strategy. The proposed research work encounters a difficulty in solid-phase peptide synthesis and improving both yield and purity compared to conventional methods.
Acknowledgments
We are very grateful to Dr. C. Arunan for his valuable guidance, and the authors are also thankful to University Grant Commission (UGC), New Delhi, India for providing financial support for this research work under major research scheme.
Conflicts of interest
Corresponding author R. Selvam has no conflict of interest
Co-author E. Sudha, P.R. Rajkumar and Dr. K.P. Subashchandran has no conflict of interest
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
R. Selvam, Email: selvachemist@rediffmail.com
K. P. Subashchandran, Email: kpsubhash@rediffmail.com
References
- 1.Isidro-Llobet A, Guasch-Camell J, Álvarez M (2005) F Eur J Org Chem 3031–3039. doi:10.1002/ejoc.200500167
- 2.Merrifield RB (1984) Solid phase peptide synthesis (2nd ed). Pierce Chemical Company, Rockford, pp 91–108, ISBN 0-935940-03-0
- 3.Merrifield RB. J Am Chem Soc. 1963;85(14):2149–2154. doi: 10.1021/ja00897a025. [DOI] [Google Scholar]
- 4.Hermkens PH, et al. Tetrahedron. 1997;53(16):5643–5678. doi: 10.1016/S0040-4020(97)00279-2. [DOI] [Google Scholar]
- 5.Sieber P. Tetrahedron Lett. 1987;34:1269–1270. [Google Scholar]
- 6.Selvam R, Rohini KC, Arunan C, Subashchandran KP (2013) Asian J Chem 25(15):8673–8676. doi:10.14233/ajchem.2013.14998
- 7.Atherton E, Sheppard RC. Solid phase peptide synthesis: a practical approach. Oxford: IRL Press; 1989. pp. 26–139. [Google Scholar]
- 8.Selvam R, Subashchandran KP (2014) Int J Pept Res Ther 1–7. doi:10.1007/s10989-014-9431-y
- 9.Pessi A, Mancini V, Chiappinelli L. Int J Pept Protein Res. 1992;39:58–62. doi: 10.1111/j.1399-3011.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 10.Meldal M, Bielfeldt T. Int J Pept Protein Res. 1994;43:529–536. doi: 10.1111/j.1399-3011.1994.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 11.Sieber P. Peptides. 1981;2(1):45–50. doi: 10.1016/S0196-9781(81)80010-1. [DOI] [Google Scholar]
- 12.Nilsson BL, et al. Annu Rev Biophys Biomol Struct. 2005;34:91–118. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell ARK, Kent SBH, Engelhard M, Merrifield RB. J Org Chem. 1978;43(13):2845–2852. doi: 10.1021/jo00408a022. [DOI] [Google Scholar]