Abstract
Tocotrienols, members of the vitamin E family, are natural compounds found in a number of vegetable oils, wheat germ, barley and certain types of nuts and grains. Vegetable oils provide the best sources of these vitamin E forms, particularly palm oil and rice bran oil contain higher amounts of tocotrienols. Other sources of tocotrienols include grape fruit seed oil, oats, hazelnuts, maize, olive oil, buckthorn berry, rye, flax seed oil, poppy seed oil and sunflower oil. Tocotrienols are of four types, viz. alpha (α), beta (β), gamma (γ) and delta (δ). Unlike tocopherols, tocotrienols are unsaturated and possess an isoprenoid side chain. A number of researchers have developed methods for the extraction, analysis, identification and quantification of different types of vitamin E compounds. This article constitutes an in-depth review of the chemistry and extraction of the unsaturated vitamin E derivatives, tocotrienols, from various sources using different methods. This review article lists the different techniques that are used in the characterization and purification of tocotrienols such as soxhlet and solid–liquid extractions, saponification method, chromatography (thin layer, column chromatography, gas chromatography, supercritical fluid, high performance), capillary electrochromatography and mass spectrometry. Some of the methods described were able to identify one form or type while others could analyse all the analogues of tocotrienol molecules. Hence, this article will be helpful in understanding the various methods used in the characterization of this lesser known vitamin E variant.
Keywords: Tocotrienols, Extraction, Saponification, Chromatography, Electrochromatography, Mass spectrometry
Introduction
In the beginning of twentieth century, it was clearly understood that the diets containing purified carbohydrate, protein, fat and minerals were not adequate to maintain the growth and health of experimental rats, which the natural foods such as milk could do. Hopkins [1] coined the term accessory food factors to the unknown and essential nutrients present in the natural foods. Funk [2, 3] isolated an active principle (an amine) from rice polishing and, later in yeast, which could cure beri-beri in pigeons. He coined the term “vitamine” (Greek, vita-life) to the accessory factors with a belief that all of them were amines. The term, vitamin, is however continued without the suffix final letter “e”. Vitamins are classified by their biological and chemical activity, not their structure. Since, the early 1920s, it was found that rats fed on cow’s milk could not produce offspring. The active principle from wheat germ that could rectify this deficiency in both male and female rats was named as vitamin E.
Evans and associates [4] isolated the compounds of vitamin E family and named them tocopherols (Greek: Tocos—child birth; pheros—to bear; ol—alcohol). While alpha-tocopherol was the first vitamin E analogue to be recognized, eight chemically distinct analogues are now known, consisting of alpha (α), beta (β), gamma (γ) and delta (δ)-tocopherols (T) and α, β, γ and δ-tocotrienols (T3), all of them are referred to as vitamin E. The tocopherols are saturated forms of vitamin E, whereas the tocotrienols are unsaturated and possess an isoprenoid side chain.
In 1943, Joffe and Harris demonstrated varying potencies of the eight forms of vitamins [5]. Each form of the vitamin has slightly different biological activity. In general, the L-isomers of tocotrienols lack almost all vitamin activity and the naturally occurring forms are the D-forms. The name “tocotrienol”, to denote a tocopherol with a true isoprenoid side chain, was first suggested by Bunyan et al. [6] and tocotrienols were described in nature when isolated from the latex of the rubber plant, Hevea brasiliensis [7]. Tocotrienols attracted no real attention until the 1980s and 1990s when their cholesterol-lowering potential [8] and anticancer effects were described [9, 10].
Tocochromanols: tocopherols and tocotrienols
Vitamin E is not a single compound but is at least eight “vitamers”, named tocochromanols and can be either “tocopherols” or “tocotrienols”. Vitamin E is exclusively synthesized by photosynthetic eukaryotes and other photosynthetic organisms such as cyanobacteria. In order to prevent lipid oxidation, the plants mainly accumulate tocochromanols in oily seeds and fruits or in young tissues undergoing active cell divisions [11]. Vitamin E is an interesting group of compounds, able to exert many and different biological activities in plant, animal and human cells, but the physiological and/or pharmacological role in the cell is not fully described. Vitamin E deficiency is rare in humans, although it may develop in premature infants and in persons with a chronic malabsorption of fats, as well as mild anemia, ataxia and pigmentary changes in the retina. Hence, the vitamin E compounds have to be better evaluated and characterized for understanding their properties [11]. There is a great interest in the natural forms of tocochromanols because they are considered promising compounds able to maintain a healthy cardiovascular system and blood cholesterol levels. Some evidence suggests that the potency of the antioxidant effects may differ between natural or synthetic source of tocochromanols [11].
Vitamin E is a collective term for fat soluble 6-hydroxychroman compounds that have biological activity. Tocol (2-methyl-2-(4,8,12-trimethyltridecyle)-chroman-6-ol) is generally referred to both the parent compounds of tocopherols and tocotrienols. The tocopherols are characterized by the 6-chromanol ring structure methylated at varying degrees at the 5, 7 and 8 positions. At position 2, there is a C16 saturated side chain. The tocotrienols are unsaturated at the 3, 7 and 11 positions of side chain. The specific tocotrienols differ by number and positions of methyl groups on 6-chromanol rings: α-tocotrienol is 5,7,8-trimethyl; β-tocotrienol is 5,8-dimethyl; γ-tocotrienol is 7,8-dimethyl and δ-tocotrienol is 8-monomethyl (Fig. 1). The tocotrienols have the same methyl structure at the ring and methyl notation, but differ from the analogous tocopherols by the presence of three double bonds in the hydrophobic side chain (Table 1). The unsaturation of the tails gives tocotrienols only a single stereoisomeric carbon (and thus two possible isomers per structural formula, one of which occurs naturally). Of the stereoisomers which retain activity, increasing methylation particularly full methylation to the α-form increases vitamin activity. Tocotrienols arising from 2-methyl-2-(4,8,12-trimethyltrideca-3,7,11-trienyl) chroman-6-ol (non-methylated ring structure) have only one chiral centre at position 2. Consequently, only 2R and 2S stereoisomers are possible (3, 7 of phytyl side chains permit four cis/trans geometric isomers). But only 2R, 3-trans, 7-trans isomer exists in nature.
Fig. 1.
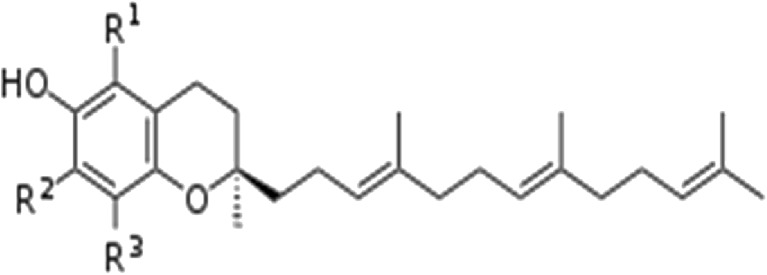
Chemical structure of tocotrienols
Table 1.
Structures of various homologs of tocotrienols

Hence, the four tocopherols (α-T, β-T, δ-T and γ-T) and four tocotrienols (α-T3, β-T3, δ-T3 and γ-T3) are collectively called vitamin E. All this eight forms contain a chromanol ring and a hydrophobic side chain, a phytyl in the case of tocopherols and an isoprenyl with three double bonds in tocotrienols. Chemically, T3 and T are closely related: both contain a polar chromanol ring linked to an isoprenoid-derived 16-carbon hydrocarbon chain. The number and position of methyl substituents in the chromanol ring give rise to α-, β-, γ- and δ-isomers. The only difference in the structures of T3 and T is in their isoprenoid chain: while T has a saturated isoprenoid chain, that of T3 contains three isolated double bonds [12]. Therefore, the term vitamin E is commonly used for α-tocopherol (d-α-tocopherol, RRR-α-tocopherol) since it has the highest bioavailability [13]. It is the said to be the gold standard against which all the other similar tocochromanols must be compared. However, it is only one out of eight natural forms of vitamin E. Tocotrienols are similar to tocopherols except that they have an isoprenoid tail with three unsaturations instead of a saturated phytyl tail (Fig. 1).
Tocopherols are the major vitamin E components present in most vegetable oils, while tocotrienols are present especially in palm oil [14]. As the essential vitamin, vitamin E cannot be produced by the human body and needs to be obtained from food. Palm fruit is one of the best sources for tocopherols and tocotrienols besides rice grain and annatto seed. Palm fruit is the richest source of tocotrienols among all vegetable oils. Tocotrienols make up almost 70 % of vitamin E in palm oil, with the remaining 30 % being tocopherols [15]. The most important T3 isomer involved in degradation in T3-rich foods is α-T3 despite the fact that it has the highest absolute antioxidant activity by virtue of its hydrogen-donating power toward free radicals. However, in fats and oils, α-T3 shows the fastest degradation and the lowest relative antioxidant activity to protect against lipid peroxidation in relation to the other T3 isomers. This phenomenon may be due to the fact that at high temperature, high concentration and in the presence of oxygen, α-tocochromanols can easily act as a prooxidant by undergoing side reactions, leading to the loss of antioxidant efficiency [16].
All tocochromanols are potent antioxidants with lipoperoxyl radical-scavenging activities. Most of the research on vitamin E has primarily focused on αT [17] because αT is the predominant form of vitamin E in tissues, and low intake of this form results in vitamin E deficiency-associated ataxia [18]. However, many human and animal studies on αT supplementation have yielded disappointing results regarding its protective role in prevention or treatment of chronic diseases including cardiovascular diseases and cancer [19, 20]. On the other hand, recent mechanistic studies combined with preclinical animal models have indicated that other forms of vitamin E appear to have different and superior biological properties that may be useful for prevention and therapy against chronic diseases. Furthermore, emerging evidence suggests that some long-chain vitamin E metabolites have even stronger anti-inflammatory effects than their vitamin precursors. These metabolites may be novel anti-inflammatory agents and may contribute to beneficial effects of vitamin E forms in vivo.
Tocotrienols
The tocotrienols (T3) are members of the vitamin E family, have excellent antioxidant properties and are able to prevent the autocatalytic lipid peroxidation process [21]. While T have been intensively investigated, in recent years, interest in T3 has increased due to their special health benefits. Unlike T, T3 are reported to inhibit cholesterol biosynthesis and to have neuroprotective properties [22, 23]. Moreover, α-T3 has outstanding antioxidant activity in liver microsomes, 40–60 times greater than that of α-tocopherol (α-T) [24], and shows special anti-carcinogenic properties [16].
Tocotrienols possess neuroprotective, antioxidant, anticancer and cholesterol-lowering properties that often differ from the properties of tocopherols [25]. Micromolar amounts of tocotrienol suppress the activity of HMG-CoA reductase, the hepatic enzyme responsible for the synthesis of cholesterol [26, 27]. Tocotrienols are thought to have more potent antioxidant properties than α-tocopherol [28, 29]. The unsaturated side chain of tocotrienol allows for more efficient penetration into tissues that have saturated fatty layers such as the brain and liver [30]. Experimental research examining the antioxidant, free radical scavenging, effects of tocopherol and tocotrienols have found that tocotrienols appear superior due to their better distribution in the lipid layers of the cell membrane [30]. One major conclusion often used to undermine tocotrienol research is the relative inferiority of the bioavailability of orally taken tocotrienols as compared to that of α-tocopherol. The hepatic α-tocopherol transfer protein (α-TTP), together with the tocopherol-associated proteins (TAP), is responsible for the endogenous accumulation of natural α-tocopherol. Although these systems have a much lower affinity to transport tocotrienols, it has been evident that orally supplemented tocotrienol results in plasma tocotrienol concentration in the range of 1 μM [31]. Despite such promising potential of tocotrienol, the experimental analysis accounts for about 1 % of all vitamin E research. The unique vitamin action of α-tocopherol, combined with its prevalence in the human body and the similar efficiency of tocopherols as chain-breaking antioxidants, led biologists to almost completely discount the “minor” vitamin E molecules as topics for basic and clinical research. However, recent findings have enforced a serious reconsideration of this traditional perception [32, 33].
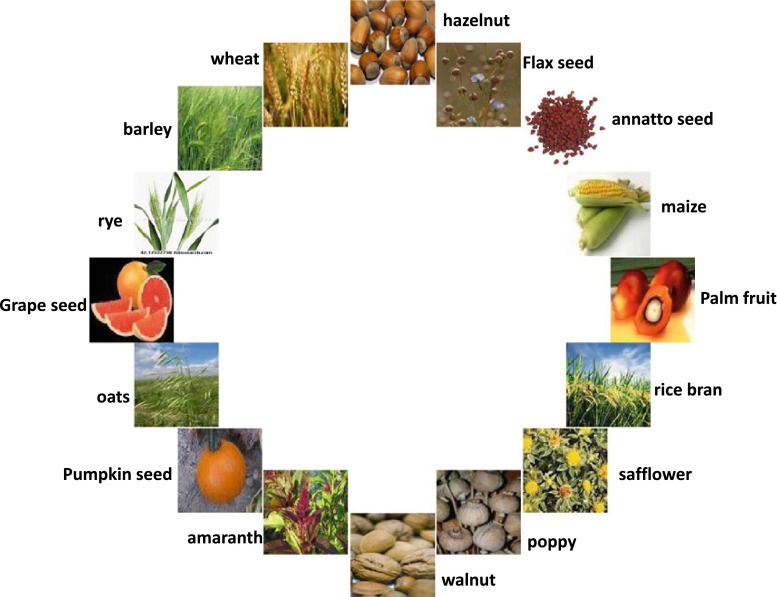
This review article takes a closer look at the methods for the purification and characterization of the lesser known forms of vitamin E, i.e. tocotrienols from different plant sources (Tables 2 and 3). Tocotrienols are found in certain cereals and vegetables such as palm oil, rice bran oil, coconut oil, barley germ, wheat germ and annatto [34, 35]. Palm oil and rice bran oil contain particularly higher amounts of tocotrienols (940 and 465 mg/kg, respectively) [36]. Other sources of tocotrienols include grape fruit seed oil, oats, hazelnuts, maize, olive oil, Buckthorn berry, rye, flax seed oil, poppy seed oil and sunflower oil (Fig. 2) [37].
Table 2.
Presence of different tocotrienols in various vegetable oils (adapted from Liu et al. 2008) [38].
| α-Tocotrienol (mg/L) | γ-Tocotrienol (mg/L) | δ-Tocotrienol (mg/L) | Total tocotrienols (mg/L) | |
|---|---|---|---|---|
| Palm | 205 | 439 | 94 | 738 |
| Rice bran | 236 | 349 | – | 585 |
| Wheat germ | 26 | – | – | 26 |
| Coconut | 5 | 1 | 19 | 25 |
| Cocoa | 2 | 0 | 0 | 2 |
Table 3.
Tocotrienol content of various palm-derived oil materials (adapted from Maarasyid et al. [15])
| Raw material | α-Tocotrienol (%) | γ-Tocotrienol (%) | δ-Tocotrienol (%) |
|---|---|---|---|
| Crude palm oil | 20–25 | 36–45 | 7–10 |
| Palm pressed fiber oil | 13–20 | 18–23 | 8–10 |
| Refined bleaching deodorized palm oil | 23–29 | 36–50 | 6–10 |
| Palm fatty acid distillate | 23–24 | 36–38 | 13–15 |
| Palm olein | 26–28 | 25–40 | 6–10 |
| Palm phytonutrient concentrate | 17–21 | 55–60 | 6–7 |
The remaining percentage is tocopherols
Fig. 2.
Natural sources of tocotrienols (reprinted from [37], with kind permission from Springer Science and Business Media)
Tocotrienols: methods of extraction, identification and characterization
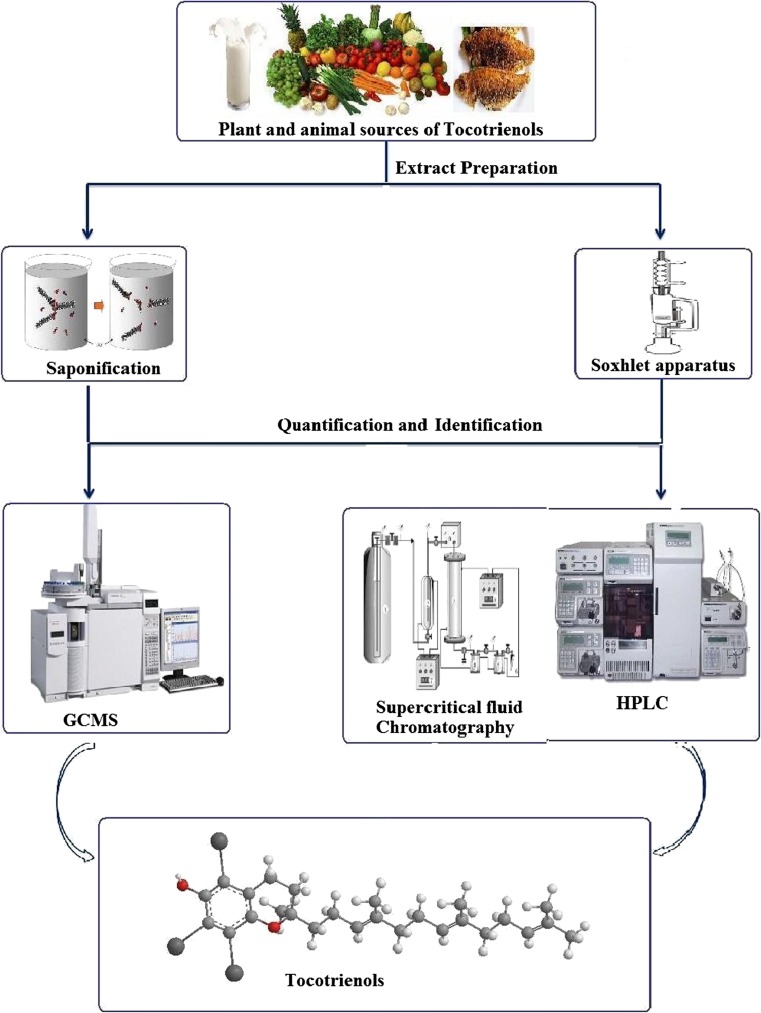
Tocotrienols occur in photosynthetic plants in varying amounts, and the vegetable oils such as sunflower, corn, safflower and cottonseed provide a useful source for these vitamin E forms. A number of researchers have developed methods and techniques for extraction, analysis, identification and quantification of tocotrienols from various sources such as solvent extraction, supercritical fluid extraction (SFE), column chromatography, thin layer chromatography, normal and reversed high-performance liquid chromatography (HPLC), etc. The different methods that have been employed in the purification and characterization of tocotrienols have been summarized in the following in Fig. 3. Several different methods have been used for the extraction of vitamin E such as solvent extraction (direct, soxhlet, pressure-liquid and supercritical-fluid), enzymatic method, chemical method (saponification, esterification), adsorption, molecular distillation, microwave-assisted extraction and membrane technology [15].
Fig. 3.
Different methods for the extraction of tocotrienols
The efficiency, economy and sufficient degree of purity and quantity are the common primary objectives of any purification procedure. It applies equally well to preparation of sample for characterization and to large-scale production. It is, therefore, necessary to set objectives for purity, amount, retention of biological activity and economy for any process. Although HPLC methods have been proven to be suitable for the separation, determination and isolation of αT oxidation products, only a few methods allow the simultaneous separation of polar and nonpolar oxidation products, although both the normal phase and the reversed phase are used for separation as the stationary phase. The detection of αT oxidation products is generally achieved by diode array detection (DAD) and mass spectroscopy (MS) because of the additional spectroscopic information provided by these methods, and by fluorescence (F) and electrochemical detection. However, no method has yet been developed for the simultaneous determination of αT3 oxidation products with other tocochromanol isomers [16]. Some of the most common methods mentioned have been discussed in detail in this review article.
The determination of tocochromanols in vegetable oils includes the classical physico-chemical techniques to the chromatographic-, spectroscopic- and molecular-based methodologies [14], among others such as liquid-liquid extraction without saponification or solvent extraction after saponification before a chromatographic analysis by HPLC or gas chromatography (GC) [39]. HPLC using both normal (NP) and reversed phases (RP) is the most common methodology used for the analysis of tocochromanols. When comparing RP to NP columns for separation, the latter show the main advantage over the former by completely separating all isomers [39, 40]. HPLC used in the analysis of these compounds includes UV, fluorescence, ELSD, electrochemical and amperometric detection. Fluorescence detection is described as more sensitive and selective than UV. ELSD has been successfully applied in the analysis of different compounds and, consequently, has been increasingly used in many analytical laboratories [14, 40].
Soxhlet extraction
Soxhlet extraction is a process in which the ground plant parts are packed in a Soxhlet apparatus and then extracted with the solvent of choice. In an experiment, tocopherols and tocotrienols were simultaneously determined from hazelnuts. The chopped hazelnuts were extracted on a Soxhlet apparatus (Buchi, Switzerland) with light petroleum ether (b.p. 40–60 °C) during 1.5 h, and the solvent that remained was removed under a stream of nitrogen. An accurately weighted sample of the obtained oil was diluted in hexane, filtered through a 0.22-μm disposable LC filter disc and then directly injected in the HPLC system [41]. In another method, a sample of chopped hazelnuts (∼300 mg) was accurately weighed in glass screw cap tubes (Supelco, Bellefonte, PA, USA) and homogenized with 2 mL of ethanol by mixing. Subsequently, 4 mL of hexane was added and again vortex mixed for 1 min. After that, 2 mL of saturated NaCl aqueous solution was added and the mixture was homogenized (1 min), centrifuged (2 min, 5,000×g), and the clear upper layer was carefully transferred to another glass screw cap tube. The sample was re-extracted twice with hexane. The combined extracts were taken to dryness under a nitrogen stream, at room temperature, on a Reacti-Therm module (Pierce, Rockford, IL, USA), transferred to microcentrifuge tubes with 1.5 mL of hexane and, finally, dehydrated with anhydrous sodium sulfate. The extract was centrifuged (10,000×g, 20 s), transferred into a dark injection vial and analysed by HPLC [41]. Hence, the above methods can be used for the characterization of tocotrienols from hazelnuts normal phase liquid extraction methods.
Saponification
Saponification has been regarded as one of the best method for the isolation of tocotrienols from plant as well as animal sources [42]. In one experiment, the method involved an alkaline digestion prior to extraction of the unsaponifiable compounds with hexane. Ethanol (2.5 mL), water (2.5 mL) and 10 M NaOH (0.5 mL) were added to each sample of chopped hazelnuts accurately weighted in a glass screw cap tube and homogenized for 1 min, by vortex mixing. After that, the tubes were flushed with nitrogen and closed. Saponification was performed at 60 °C during 20 min on a Reacti-Therm module. After the addition of water (2.5 mL) and hexane (5.0 mL), it was vortex mixed for 1 min. The tubes were then centrifuged (5 min, 5,000×g) and the clear upper layer was carefully transferred to another glass screw cap tube. The sample was re-extracted twice with hexane and processed as described in method II [41].
Chromatography
Chromatography is a separation process based on distribution between two phases, a solid or liquid stationary phase and a liquid or gas mobile phase. The sample is propelled by fluid mobile phase which percolates the stationary phase. Different chromatographic processes based on characteristic principles may be used for the separation of a wide variety of substances. A given chromatographic process may be run both in low and high pressure systems, although the basic principle remains same.
Thin layer chromatography (TLC) is one of the oldest and widely used chromatographic techniques for the identification, isolation, purification and quantification of assay samples. Tocotrienols from plant origin have been separated efficiently by TLC. Several researchers have developed TLC methods for the isolation, purification and quantification of tocotrienols [42, 43]. In one method, a plant extract was spotted on a silica gel and developed either with hexane-ethylacetate (92.5:7.5) or with chloroform in one dimension and hexane-isopropyl ether (80:20) in another dimension leading to the separation of six compounds; α, β, δ-tocopherols and α, γ, δ-tocotrienols. Visualization of samples is usually carried out under UV light [43]. Liquid–solid column chromatography (CC) has been used by various researchers for the identification and isolation of tocotrienols as it separates the undesirable impurities with high precision as compared to TLC method [44, 45]. Silica gel, Keiselgel and hydrated florisil have been widely used as stationary matrix so as to afford quantitative recovery of tocols.
A number of scientists have developed GC techniques for the accurate measurement of tocols in various oils [46–50]. GC instrument consists of a packed capillary column to afford a high degree of detection, sensitivity and component resolution. A GC column is also attached to a flame ionization detection system (FID) to monitor common effluents or to a mass spectrometer for structural identification and quantification. Some of the reported methods for the analysis of tocopherols and tocotrienols in vegetable oils and related samples are summarized in Table 4. Capillary column possesses some advantages over packed column, as it allows the detection of tocols in nanogram quantities with higher precision, resolution and sensitivity. It also possesses thermal stability [46]. To volatilize the hydroxyl-containing GC detectants, these compounds are more frequently analysed as their ester [47, 48] or trimethylsilane [49, 50] derivatives.
Table 4.
Gas chromatographic (GC) analysis of tocotrienols
| Sample no. | Source | Column | Detector | Eluted form | Reference |
|---|---|---|---|---|---|
| 1 | Vegetable oils | Packed, 8 ft × 4 mm, 6 % SE-52 on chromosorb W AW-DMCS | Flame ionization detector | Tocotrienol propionates | [47] |
| 2 | Vegetable oils | Packed, 6 ft × 5 mm, 3 % SE-30 on Gaschrom Q | Flame ionization detector | Tocotrienol butyrates | [48] |
| 3 | Palm oil | Packed, 3 ft × 4 mm, 2 % silicon oil on Chromosorb W AW- DMCS | Flame ionization detector | Native tocotrienols | [51] |
| 4 | Fats and oils | Capillary, 150 ft × 0.25 mm, Dexsil 400 | Flame ionization detector | Tocotrienol (TMS ether) | [50] |
| 5 | Oil distillate | Capillary, 90 ft × 0.25 mm, 0.25 μm DB-5 | Flame ionization detector | Tocol (TMS ether) | [49] |
| 6 | Edible oils | Capillary, 30 ft × 0.53 mm, HP-1 | Flame ionization detector | Tocol acetate | [46] |
Supercritical fluid chromatography (SFC) employs the use of an inert, low temperature supercritical carbon dioxide as mobile phase eluent which allows it to be advantageous over gas chromatography and HPLC in food industry. SFC allows sample extraction, pre-concentration, chromatographic quantification and preparative fractionation in a single operation. Various tocopherols and tocotrienols have been isolated using SFC [52–57]. Capillary SFC methods for the analysis of tocopherols and other compounds in edible oils and fish oils have been reported [58]. In one method, a successful isolation of tocotrienols from palm oil using SFC was performed [58]. The experiment was performed on JASCO Model SUPER-200 SFC system with a UV–Vis detector equipped with high-pressure flow cells. Columns used consisted of Lichrosorb silica 4.6 × 250 mm and Macherey-Nagel EC 250/4.6 Nucleosil 100-50 H diol column. SFC conditions included a temperature of 60 °C, pressure of 180 kg/cm2 and a flow rate at 3.0 mL/min for CO2 and 0.12 mL/min for ethanol. The supercritical fluid in the study was supercritical CO2. The column yielded a complete separation of tocol isomers following the sequence: αT, αT3, βT, βT3, γT, γT3 and δT3.
HPLC (sometimes also referred to as high-pressure liquid chromatography) is a chromatographic technique used to separate a mixture of compounds for various analytical and preparative purposes. HPLC finds enormous number of uses including those in medicine, research and industry. HPLC is distinguished from traditional (“low pressure”) liquid chromatography as it operates at significantly higher pressures (50–350 bar), while ordinary liquid chromatography typically relies on the force of gravity and operates at normal atmospheric pressure. Also HPLC columns are made with very fine sorbent particles (2–5 μm in average particle size) that can withstand high pressure generated during the separation. The schematic of an HPLC instrument typically includes a sampler, pumps and a detector. The sampler brings the sample mixture into the mobile phase stream which carries it into the column. The pumps deliver the desired flow and composition of the mobile phase through the column. The detector generates a signal proportional to the amount of sample component emerging from the column, hence allowing for quantitative analysis of the sample components. Some models of mechanical pumps in a HPLC instrument can mix multiple solvents together in ratios changing in time, generating a composition gradient in the mobile phase. Though the principle of any chromatographic procedure remains same whether in normal or in high pressure mode, HPLC offers several distinct advantages over the normal pressure chromatography such as high separation capacity, enabling the batch analysis of multiple components, superior quantitative capability and reproducibility, moderate analytical conditions, generally high sensitivity, low sample consumption easy preparative separation and purification of samples. With the advent of HPLC technology, a majority of researchers have shifted their attention towards the use of HPLC methods for the analysis of tocols. It is a very simple technique and can be performed in both normal phase and reverse phase. Both normal and reverse phase HPLC techniques have been extensively used for the analysis of tocols in various sample matrices. Normal phase HPLC possesses some shortcomings such as long equilibration times and employment of hazardous volatile organic solvents. Different silica-based stationary phases have been employed by different researchers for the analysis of tocopherols and tocotrienols such as Lichrosorb Si 60 [59–61], Zorbax Si [10, 35], Ultrasphere Si [62], etc. Table 5 summarizes the various normal phase and reverse phase HPLC methods used by various researchers to separate the tocols from different origins.
Table 5.
High-performance liquid chromatographic (HPLC) analysis of tocotrienols
| Sample no. | Source | Stationary/mobile phase | Detector | Elution order | Reference |
|---|---|---|---|---|---|
| Foods, tissues | LiChrosorb Si 60, 5 μm 250 × 3.2 mm, hexane/isopropanol (99.8:0.2) | Florescence, 290Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT | [61] | |
| Palm oils | ZorbaxSil, 5 μm 250 × 4.6 mm, hexane/tetrahydrofuran/methanol (97.25:2.5:0.25) | Florescence, 298Excitation
325Emission |
αT → αT3γT3 → δT3 | [10] | |
| Corn grain | Ultrasphere Si, 5 μm 250 × 4.6 mm, hexane/isopropanol (98.8:1.2) | Florescence, 205Excitation
330Emission |
αT → αT3 → γT → γT3 → δT | [62] | |
| Seed oils | Partisil PAC, 5 μm 250 × 4.6 mm, hexane/tetrahydrofuran (94:6) | Florescence, 210Excitation
325Emission |
αT → αT1 → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [63] | |
| Seed oils | Naphthylethylsilica, 5 μm, 750 × 0.53 mm, hexane/hexafluoroisopropanol (99.9:0.1) | UV 295 nm | αT → γT → T → δT | [64] | |
| Palm oils | ZorbaxSil, 5 μm 250 × 4.6 mm, hexane/isopropanol (99:1) | UV 295 nm | αT → αT3 → βT → γT → γT3 → δT → δT3 | [35] | |
| Seed oils, Cereals | LiChrospher 100 Diol, 5 μm 250 × 4.0 mm, hexane/t-butylmethyl ether (96:4) | Florescence, 295Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γ3 → δT → δT3 | [65] | |
| Stillingia oil | Nucleosil 50 Si, 5 μm 250 × 4.0 mm, hexane/dioxane (95:5) | Florescence, 295Excitation
330Emission |
αT3 → βT → γT3 → δT3 | [66] | |
| Foods | LiChrospher 100 Diol, 5 μm 250 × 4.0 mm, hexane → hexane/t-butylmethyl ether (97:3) → hexane/t-butylmethyl ether (95:5) (gradient elution) | Florescence, 295Excitation
330Emission |
αT → AC -αT → αT3 → βT → βT3 → γT3 → δT → δT3 | [65] | |
| Rice bran | Supelcosil LC-Si, 5 μm 250 × 4.6 mm: (i) isooctane–ethyl acetate (97.5:2.5), (ii) isooctane/ethyl acetate/acetic acid/2,2-dimethoxypropane (98.15:0.9:0.85:0.1) | Florescence, 290Excitation
330Emission |
αT → αT3 → βT → γT-βT3 → γT3 → δT → δT3 | [60] | |
| Margarine | Hypersil Si, 5 μm 100 × 2.1 mm hexane/isopropanol (99.8:0.2) | Florescence, 290Excitation
330Emission |
αT → βT → γT → δT | [67] | |
| Vegetable oils | Cyclobond I, 5 μm 250 × 4.6 mm, cyclohexane/diisopropyl ether (95:5) | Florescence, 298Excitation
345Emission |
αT → ζT → βT → γT → δT | [44] | |
| Tissues | LiChrosorb Si 60, 5 μm 125 × 4.6 mm, hexane/dioxane (97:3) | Florescence, 295Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [59] | |
| Soybean oil, wheat bran | Econosil Si, 10 μm 250 × 10 mm, hexane/tetrahydrofuran (gradient 0 → 15 % tetrahydrofuran) | Florescence, 290Excitation
330Emission |
(i) αT → βT → γT → δT ii) αT3 → βT3 → γT3 → δT3 |
[60] | |
| Vegetable oils | LiChrosorb Si 60, 5 μm 250 × 4.0 mm, hexane/isopropanol (99.7:0.3) | Florescence, 290Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [68] | |
| Foods | Nucleosil 100-5 NO2, 5 μm 250 × 4.0 mm, hexane → hexane/t-butylmethyl ether (98:2) → hexane/t-butylmethyl ether (98:2) → hexane/t-butylmethyl ether (85:15) (gradient elution) | Florescence, 295Excitation
330Emission |
αT → AC → αT → αT3 → βT → βT3 → γT3 → δT → δT3 | [69] | |
| Foods | Chromega Diol, 5 μm 250 × 4.6 mm, hexane/diisopropyl ether (95:5) | FL, 298Excitation
345Emission |
αT → ζT → βT → γT → δT | [44] | |
| Tissues, diet | Supelcosil LC-Diol, 5 μm 250 × 4.6 mm, hexane/isopropanol (99:1) | Florescence, 290Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [70] | |
| Foods | Vydac C8, 10 μm 250 × 3.2 mm, methanol/water (95:5) + acetic acid | Florescence, 295Excitation
330Emission |
δT → (β + γ)T → αT | [71] | |
| Feeds | Yanapack ODS-T C18, 5 μm 250 × 4.0 mm, methanol + 50 mM NaClO4 | ED, +0.8 V | δT → (β + γ)T → αT | [72] | |
| Vegetable oils | Spheri-5 RP-18, 5 μm 100 × 2.1 mm, methanol/water (95:5) | UV, 300 nm | δT → (β + γ)T → αT | [73] | |
| Tissues | Ultrasphere ODS, 5 μm 250 × 4.6 mm, methanol/ethanol (1:9) + 20 mM LiClO4 | ED, +0.8 V | δT → γT → αT | [74] | |
| Tissues | Spherisorb ODS II, 3 μm 150 × 4.6 mm, methanol/water (96:4) + NaClO4 | ED, +0.6 V | δT → γT → αT | [75] | |
| Tissues | Ultrasphere ODS, 5 μm 150 × 4.6 mm, isopropanol/acetonitrile/water/tetraethyl ammonium hydroxide/acetic acid (60:20:19.4:0.5:0.1) | ED, +0.3 V | δT → γT → αT | [76] | |
| tissues | Polymeric C18, 5 μm 750 × 0.53 mm, acetonitrile/hexane (91.5:8.5) | UV, 295 nm | δT → βT → γT → αT | [64] | |
| Palm oil | Zorbax ODS, 5 μm 250 × 4.6 mm, acetonitrile/methanol/CH2Cl2 (60:35:5) | UV, 295 nm | δT3 → γT3 → αT3 → δT → (β + γ)T → αT | [35] | |
| Tissues | YMCPack-A-ODS, 5 μm 150 × 4.6 mm, isopropanol/water (65:35) | Florescence, 298Excitation
325Emission |
δT → γT → βT → αT | [77] | |
| Tissues | Resolve C18, 5 μm 300 × 3.9 mm, acetonitrile/CH2Cl2/methanol/octanol (90:15:10:0.1) | UV, 300 nm | γT → αT | [78] | |
| Foods | Bakerbond C18, 5 μm 250 × 4.6 mm, acetonitrile/methanol + ammonium acetate/ethyl acetate (gradient elution) | Florescence, 295Excitation
335Emission |
ϵT → δT → γT → αT | [79] | |
| Tissues | Superspher 100RP-18, 4 μm 250 × 4 mm, methanol/ethanol (1:9) + 2.5 mM HClO4 + 7.5 mM NaClO4 | ED, 0.35 V | δT → (β + γ)T → αT | [80] | |
| Tissues | Ultrasphere ODS, 5 μm 250 × 4.6 mm, acetonitrile/tetrahydrofuran/methanol/1 % ammonium acetate (684:220:68:28) | Florescence, 298Excitation
328Emission |
δT3 → (β + γ)T3 → αT3 → δT → (β + γ)T → αT | [59] | |
| Vegetable oils | Taxsil PFP, 5 μm, methanol/water (92:8) | UV, 290 nm | δT → βT → γT → αT | [81] | |
| Tissues | C18 Vydac201TP54, 5 μm 250 × 4.6 mm, methanol/acetonitrile (9:1) | PDA, 200 nm → 800 nm | δT → αT | [82] | |
| Vegetable oils | Spherisorb ODS, 5 μm 250 × 4.5 mm, methanol/water (90:10) + NaClO4 | ED, +0.6 V | (β + γ)T3 → (β + γ)T → αT | [68] | |
| Vegetable oils | Asahipak ODP, 5 μm 250 × 4.6 mm, acetonitrile/water (85:15) | Florescence, 298Excitation
345Emission |
δT → ζT → βT → γT → αT | [44] | |
| Vegetable oils | YMCPack-ODS-A, 5 μm 250 × 4.6 mm, methanol/water (95:5) | Florescence, 298Excitation
345Emission |
δT-AC → ζT-AC → βT-AC → γT-AC → αT-AC | [44] | |
| Tissues | Suplex pKb-100, 5 μm 250 × 4.6 mm, methanol/t-butylmethyl ether/water (80:20:5) | PDA, 200 nm → 800 nm | αT-AC → γT → αT | [83] | |
| Foods | YMCPack-C30, 3 μm 250 × 4.6 mm, acetone/water + AgClO4 (90:10) → (100:0) (gradient elution) | UV, 295 nm MS | δT → γT → βT → αT → αT-AC | [84] | |
| Tissues | Microsorb MV-C18, 3 μm 100 × 4.6 mm A: methanol/water (3:1) + ammonium acetate B: methanol/CH2Cl2 (4:1) A → B → B (gradient elution) |
PDA, 200 nm → 800 nm | γT → αT | [85] | |
| Tissues | Asahipak ODP, 5 μm 250 × 4.6 mm, acetonitrile–water (70:30) | Florescence, 290Excitation
330Emission |
δ1T3 → δ2T3 → δ3T3 → δ4T3 → β1T3 → γ1T3 → β2T3 → γ2T3 → β3T3 → γ3T3 → β4T3 → γ4T3 → α1T3 → α2T3 → α3T3 → α4T3 | [86] | |
| Palm oil | YMCPack-C30, 3 μm 250 × 4.6 mm, methanol | UV, 295 NMR. MS | δT3 → γT3 → βT3 → αT3 → αT1 → αT | [87] | |
| Tissues | Ultrasphere ODS, 5 μm 250 × 4.6 mm, methanol/water/ethanol + 0.2 % LiClO4 (gradient elution) | ED, 0.5 V | γT3 → αT3 → γT → αT | [88] | |
| Tissues | Hypersil ODS, 5 μm 150 × 4.6 mm, methanol/water (96:4) | Florescence, 296Excitation
340Emission |
ϵT → δT → γT → αT | [89] | |
| Tissues | SuperPacPeP-S RPC2/C18, 5 μm 250 × 4.6 mm, methanol/ethanol/isopropanol (88:24:10) + 13 mM LiClO4 | ED, +0.6 V | γT3 → γT → αT | [90] | |
| Vegetable oils | Silica Sep-pak, mu-Bondapak RPC2/C18 column 5 μm 250 × 4.6 mm, methanol/water (95:5) | Florescence, 296Excitation
340Emission |
δT3 → γT3 → βT3 → αT3 → αT1 → αT | [91] | |
| Synthetic α-tocotrienol | Chiralcel OD-H column (250 × 4.6 mm, 5 mm particle size, adsorbent cellulose derivated with 3,5-dimethyl phenyl carbamate), 0.05 % 2-propanol in isohexane | Fluorescence, 295Excitation
330Emission |
RS, Z-Z-αT3 → E/Z-αT3 → E/Z-αT3 → E-E-αT3 → E-E-αT3 → E/Z-αT3 → E/Z-αT3 → Z-Z-αT3 → f E/Z-αT3 → S, E-E-αT3 → R, E-E-αT3 | [92] | |
| Vegetable oils | (i) polymethacrylic adsorbent I.D. column 150 × 4.6 mm, 10 μm (ii) silica-based I.D. column 250 × 4.6 mm, 5 μm: (i) hexane–EtOH (98:2), (ii) hexane–EtOH (99:1) |
UV, 254 nm | αT → αT3 → βT → βT3γT → γT3 → δT → δT3 | [93] | |
| Tissues | MicrosorbuMV C18, 12 cm, 3 μm, 100 A column, methanol/ethanol (1:3) containing 20 mM lithium perchlorate | LC-4B amperometric detector, 500 mV | αT3 → γT3 → αT → γT | [94] | |
| Chicken meat | Lichrosphere Si 100 silica column (5 μm, 250 mm × 4.6 mm) | Fluorescence, 295Excitation 330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [95] | |
| Hazelnuts | Inertsil 5 SI column (250 × 3 mm), hexane/1,4-dioxane (95.5:4.5) | Diode array detector (DAD) connected in series with an FP-920 fluorescence detector, 290Excitation 330Emission |
αT → αT3 → βT → βT3 → γT → γT3 → δT → δT3 | [41] | |
| Olive oils | Inertsil 5 SI normal-phase column (250 mm × 3 mm I.D.), 1,4-dioxane/n-hexane (3.5:96.5, v/v) | (i) Diode array detector (DAD) connected in series with an FP-920 fluorescence detector, 290Excitation
330Emission, (ii) UV, 295 nm; (iii) ELSD (evaporator temperature 40 °C; air pressure 3 bar; and photomultiplier sensitivity 4) |
αT → βT → γT → δT → αT3 | [96] | |
| Sea Buckthorn | Phenomenex Luna 5 μm silica (2) 100A, 250 × 4.60 mm column with a Security Guard silica 4 mm L × 3.0 mm precolumn, n-hexane/ethyl acetate/acetic acid (97.3:1.8:0.9) |
Fluorescence detector, 290Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [97] | |
| Simvastatin-tocotrienolrich fraction nanoparticle | RP-C18 column (4.6 mm × 100 mm), water/methanol (15:85, gradient elution) | UV, 238 nm, 295 nm | δT3 → γT3 → αT3 → αT | [98] | |
| Vegetable oils | Nano-C18 silica monolithic column (150 mm × 0.1 mm), acetonitrile/methanol/water (acidified with 0.2 % acetic acid) | UV, 295 nm | αT → γT → δT | [99] | |
| Rice bran | Inertsil CN-3, SIL-100A 5 μM (4.6 mm × 250 mm) column hexane/isopropanol/ethylacetate/acetic acid (97.6:0.8:0.8:0.8) | UV, 295 nm | αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [100] | |
| Palm oils | Silica column, 4.6 mm I.D. × 250 mm: (i) hexane/THF/IPA (95:4:1), (ii) heptane/ethyl acetate (95:5) | Photodiode array detector, 280 nm | αT, αT3, γT, and γT3, and δT3 | [52] | |
| Rose hips | Phenomenex Luna 5 μm silica (2) 100A, 250 × 4.60 mm column with a Security Guard silica 4 mm L × 3.0 mm precolumn, n-hexane/ethyl acetate/acetic acid (97.3:1.8:0.9) |
Fluorescence detector, 290Excitation
330Emission |
αT → αT3 → βT → γT → βT3 → γT3 → δT → δT3 | [101] |
Capillary electrochromatography (CEC) is a hybrid technique combining aspects of both HPLC and capillary electrophoresis (CE). As in the case of CE, the separation is performed in a capillary column with electro-osmotic flow as the driving force for the mobile phase and solutes transported along the column containing the stationary phase either packed or bound to the capillary wall. The advantages of CEC include the ability to analyse both neutral and changed solutes, low sample requirements and reduced organic solvent consumption. Moreover, it has been reported that CEC provides higher efficiencies compared to HPLC, better resolution, higher peak capacity and shorter analysis time. CEC has proven to be a successful technique in the separation of neutral compounds, pharmaceuticals, food/biological samples and environmental chemicals. It has also been successfully used by various researchers to separate the tocotrienols from their different sources. The technique allows high speed microanalysis with minimal solvent consumption. The instrument includes a CEC apparatus equipped with a diode array detector (290 nm) and a HP Chem Station software for system control. Three commercial CEC capillary columns (25 cm × 100 μm I.D.) packed with 3 μm alkyl or aryl bonded silica and one 3 μm silica column obtained from a different source is widely used. These columns include CEC Hyposil C8, CEC-Hypersil C18, CEC-Hypersil phenyl and Unimicre CEC silica, 25 cm × 75 μm I.D. The samples are run electrokinetically onto the column at 10 kV for 10 s. Column temperature and separation voltage were maintained at 25 kV and 30 °C, respectively. CEC coupled with calibration data enables speedy sample analysis with adequate component resolution and enhanced detection sensitivity. CEC method can serve as a viable alternative to existing RP-HPLC method and can be used in the routine analysis of tocopherols and tocotrienols in oil samples [42, 102–104].
Mass spectrometry
Spectroscopic methods represent one of the main tools of modern chemistry for the determination of the authenticity of edible oils. Most spectroscopic methods for the detection are based on NMR spectroscopy of 1H and 13C, infrared spectroscopy, Raman spectroscopy, fluorescence spectroscopy and mass spectrometry. The primary advantage of these methods is related to the nondestructive character, simplicity (relative easy sample preparation and adaptation for the use by untrained personnel), rapidness and moderate cost instrumentation [14].
Mass spectrometry has not been widely used for the analysis of vitamin E owing to the difficulty involved in the ionization of non-polar molecules. Atmospheric pressure chemical ionization (APCI) has been used as an ionization technique for LC–MS analysis of tocopherols in biological samples. Unlabeled α-T and deuterated tocopherols have been quantified in human plasma [105], and retinol, α-T and β-carotene in human serum by normal phase liquid chromatography–atmospheric pressure chemical ionization-tandem mass spectrometry (LC–APCI-MS–MS) [106]. LC–APCI-MS–MS has also been proposed to determine α-tocopherol and carotenoids [107] in plant materials. It was reported by Bustamante-Rangel et al. [108] that detection using APCI mode is 100 times more sensitive than that with an electrospray (ESI) mode. ESI is the most frequently used ionization technique for LC–MS. However, the lack of a site for protonation or deprotonation on non-polar substances such as fat-soluble vitamins hinders their ionization. The addition of a metal salt to themobile phase enhances the ionization of tocopherols; this ionization technique is called coordinated ion spray (CIS). Silver is the metal usually added, forming Ag+-tocopherol adducts that facilitate ionization. This approach has been used for the detection and identification of tocopherols and carotenoids in several food products [84], and tocotrienol isomers, α-tocoenol and α-T in a crude palm oil extract rich in vitamin E homologues [87]. Using the same method, α-T, α-tocopheryl succinate and α-tocopheryloxyacetic acid in mouse serum were analysed [109]. Some authors have enhanced the ESI, in positive ion mode, of tocopherols by the addition of an acid to the mobile phase to produce, [M + H]+ species. Thus, α-T and α-tocopherol quinone have been quantified in human plasma by the addition of formic acid to the mobile phase without problems due to the large amounts of this compound in human plasma [110]. Negative ion mode electrospray ionization was used for the determination of unlabelled and deuterium-labelled α-T in blood components [111]. ESI is not usually used in food samples because food matrices are generally more complex than clinical samples for the quantification of tocopherols [108].
Bustamante-Rangel et al. [108] have developed a rapid and simple method for the determination of tocopherols and tocotrienols in cereals. The analytes were extracted from cereals using pressurized liquid extraction (PLE) and separation was carried out by liquid chromatography, and detection was performed with electrospray ionization mass spectrometry (LC–ESI-MS). MS was chosen as the detection technique because it can provide additional information about analytes and hence allows confirmation of the presence of tocotrienols in the samples analysed. Extraction of analytes from the sample using PLE allowed their direct injection into the chromatographic system, which afforded simplicity, speed and the possibility of automation of the methodology. PLE provides some advantages over other extraction techniques, such as low solvent consumption, the possibility of automation and speed. Moreover, the reduction in sample manipulation leads to a decrease in error propagation. Negative ion mode electrospray ionization was used for the ionization of tocopherols and tocotrienols. The difficulty involved in the ionization of these molecules was avoided by adding a base, specifically the presence of ammonia in the mobile phase was essential to enhancing their ionization. The sensitivity of this LC–ESI-MS method is higher than other LC–APCI-MS. The proposed method was found to be reliable for the determination of tocopherols and tocotrienols in cereals. The repeatability and accuracy of the proposed procedure was verified by analysing the vitamin E content of wheat and rye samples [108].
Therefore, PLE-LC-ESI MS is a technique that uses solvents at high pressure and temperature above their boiling point to extract substances from solid matrix. PLE offers several advantages over other extraction techniques, such as possibility of automation, low solvent volume and reduced extraction times. PLE has been used for the extraction of vitamin E prior to chromatographic determination. Thus, Bustamante-Rangel et al. [108] developed a rapid and simple method including PLE followed by LC-ESI-MS for the determination of tocopherols and tocotrienols in cereals. Extraction of analytes from the sample using PLE allowed their direct injection into the chromatographic system, which afforded simplicity, speed and possibility of automation of the methodology. Separation was carried out by LC and detection was performed with ESI mass spectrometry. Negative ion mode electrospray ionization was used for the ionization of tocopherols and tocotrienols. The difficulty in the ionization of these molecules was avoided by adding a base. Almost all of the tocopherols and tocotrienols were analysed successfully by this method.
Conclusion
Tocotrienols, members of the vitamin E family, are natural compounds found in a number of vegetable oils, wheat germ, barley and certain types of nuts and grains. The unsaturated chain of tocotrienol allows an efficient penetration into tissues that have saturated fatty layers such as the brain and liver. Recent mechanistic studies indicate that other forms of vitamin E, such as γ-T, δ-T and γ-T3, have unique antioxidant and anti-inflammatory properties that are superior to those of α-tocopherol against chronic diseases. Tocotrienols are detectable at appreciable levels in the plasma after supplementations. However, there is inadequate data on the plasma concentrations of tocotrienols that are sufficient to demonstrate significant physiological effect and biodistribution studies show their accumulation in vital organs of the body. Considering the wide range of benefits that tocotrienols possesses against some common human ailments and having a promising potential, the current state of knowledge deserves further investigation into this lesser known forms of vitamin E.
In recent years, the basic research on vitamin E has expanded from primarily focusing on αT and its antioxidant effects to investigation of different tocopherols and tocotrienols, their metabolism and their non-antioxidant activities including anti-inflammatory property [8, 9]. In contrast to αT, accumulating evidence suggests that γT, δT and tocotrienols seem to have unique properties that are superior to αT and relevant to prevention and therapy against chronic diseases even under conditions with adequate αT status [112]. In spite of the promising potential, the experimental analysis of tocotrienols accounts for only a small portion of total vitamin E research.
Thus, the literature shows a very broad spectrum of medicinal properties of these vitamin E variants. α-T3, γ-T and δ-T3 have emerged as vitamin E molecules with functions in health and disease that are clearly distinct from that of α-T. An expanding body of evidence support that members of the vitamin E family are functionally unique. The current state of knowledge deserves further investigation on tocotrienols. Therefore, it becomes imperative that an assessment in order to regulate the quality, authenticity and consistency of delivery of the compound product to the pharmaceutical industry should be carried out. Hence, this review article containing the methods for analysis and characterization of tocotrienols will be helpful in fulfilling the experimental and methodological information on this vitamin E variant.
Acknowledgments
The authors would like to acknowledge and thank the reviewers for their critical reading, comments and suggestions for the improvement of manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Hopkins FG. Feeding experiments illustrating the importance of accessory factors in normal dietaries. J Physiol. 1912;44(5–6):425–460. doi: 10.1113/jphysiol.1912.sp001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funk C. The etiology of the deficiency diseases. Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra. J State Med. 1912;20:341–368. [Google Scholar]
- 3.Funk C. The Journal of State Medicine. Volume XX: 341-368, 1912. The etiology of the deficiency diseases, beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra. Nutr Rev. 1975;33(6):176–177. doi: 10.1111/j.1753-4887.1975.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 4.Evans HM, Emerson OH, Emerson GA. The isolation from wheat germ oil of an alcohol, a-tocopherol, having the properties of vitamin E. J Biol Chem. 1936;113(1):319–332. [Google Scholar]
- 5.Joffe M, Harris P. The biological potency of the natural tocopherols and certain derivatives. J Am Chem Soc. 1943;65:925–927. [Google Scholar]
- 6.Bunyan J, Mc HD, Green J, Marcinkiewicz S. Biological potencies of epsilon and zeta-1-tocopherol and 5-methyltocol. Brit J Nutr. 1961;5:253–257. doi: 10.1079/bjn19610030. [DOI] [PubMed] [Google Scholar]
- 7.Whittle KJ, Dunphy PJ, Pennock JF. The isolation and properties of delta-tocotrienol from Hevea latex. Biochem J. 1966;100:138–145. doi: 10.1042/bj1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi AA, Burger WC, Peterson DM, Elson CE. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem. 1986;261(23):10544–10550. [PubMed] [Google Scholar]
- 9.Guthrie N, Gapor A, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr. 1997;127(3):544S–548S. doi: 10.1093/jn/127.3.544S. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Gapor A, Tanabe K, Yamaoka M, Mamuro H. Esterified & alpha-tocopherol and tocotrienols in palm oils. J Jpn Oil Chem Soc. 1981;30:590–591. [Google Scholar]
- 11.Colombo ML. An update on vitamin E, tocopherol and tocotrienol—perspectives. Molecules. 2010;15:2103–2113. doi: 10.3390/molecules15042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamal Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 13.Vagni S, Saccone F, Pinotti L, Baldi A. Vitamin E bioavailability: past and present insights. Food Nutr Sci. 2011;2:1088–1096. [Google Scholar]
- 14.Cunha SC, Amaral JS, Oliveira MB (2011) Authentication of vegetable oils. Current topics on food authentication: ISBN: 978-81-7895-510-0.ed: M. Beatriz PP Oliveira, Isabel Mafra, JS Amaral
- 15.Maarasyid C, Muhamad II, Supriyantob E. Potential source and extraction of vitamin E from palm-based oils: a review. J Teknologi. 2014;69(4):43–50. [Google Scholar]
- 16.Büsing A, Ternes W. Separation of α-tocotrienol oxidation products and eight tocochromanols by HPLC with DAD and fluorescence detection and identification of unknown peaks by DAD, PBI-EIMS, FTIR, and NMR. Anal Bioanal Chem. 2011;401(9):2843–2854. doi: 10.1007/s00216-011-5352-1. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74(6):714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 18.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13(10):1145–1155. [PubMed] [Google Scholar]
- 19.Moya-Camarena SY, Jiang Q. The role of vitamin E forms in cancer prevention and therapy—studies in human intervention trials and animal models. In: Sarkar FH, editor. Nutraceuticals and cancer. New York: Springer; 2012. pp. 323–354. [Google Scholar]
- 20.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ, Korean Meta-Analysis Study Group Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seppanen CM, Song QH, Csallany AS. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J Am Oil Chem Soc. 2010;87(5):469–481. [Google Scholar]
- 22.Khanna S, Parinandi NL, Kotha SR, Roy S, Rink C, Bibus D, Sen CK. Nanomolar vitamin E α-tocotrienol inhibits glutamate induced activation of phospholipase A2 and causes neuroprotection. J Neurochem. 2010;112(5):1249–1260. doi: 10.1111/j.1471-4159.2009.06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Asp Med. 2007;28(5–6):692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10(5):263–275. doi: 10.1016/0891-5849(91)90033-y. [DOI] [PubMed] [Google Scholar]
- 25.Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce BC, Parker RA, Deason ME, Dischino DD, Gillespie E, Qureshi AA, Volk K, Wright JJ. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. J Med Chem. 1994;37(4):526–541. doi: 10.1021/jm00030a012. [DOI] [PubMed] [Google Scholar]
- 27.Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1992;35(20):3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- 28.Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Rad Biol Med. 1991;10(5):263–275. doi: 10.1016/0891-5849(91)90033-y. [DOI] [PubMed] [Google Scholar]
- 29.Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Meth Enzymol. 1994;234:354–366. doi: 10.1016/0076-6879(94)34105-2. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, Packer L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32(40):10692–10699. doi: 10.1021/bi00091a020. [DOI] [PubMed] [Google Scholar]
- 31.O’Byrne D, Grundy S, Packer L, Devaraj S, Baldenius K, Hoppe PP, Kraemer K, Jialal I, Traber MG. Studies of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate supplementation of hypercholesterolemic humans. Free Rad Biol Med. 2000;29(9):834–845. doi: 10.1016/s0891-5849(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 32.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Rad Biol Med. 2004;36(1):1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Das S, Lekli I, Das M, Szabo G, Varadi J, Juhasz B, Bak I, Nesaretam K, Tosaki A, Powell SR, Das DK. Cardioprotection with palm oil tocotrienols: comparison of different isomers. Am J Physiol Heart Circ Physiol. 2008;294:H970–H978. doi: 10.1152/ajpheart.01200.2007. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi AA, Mo H, Packer L, Peterson DM. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000;48:3130–3140. doi: 10.1021/jf000099t. [DOI] [PubMed] [Google Scholar]
- 35.Tan B, Brzuskiewicz L. Separation of tocopherol and tocotrienol isomers using normal- and reverse-phase liquid chromatography. Anal Biochem. 1989;180:368–373. doi: 10.1016/0003-2697(89)90447-8. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannappan R, Gupta SC, Kim JH, Aggarwal BB. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012;7(1):43–52. doi: 10.1007/s12263-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu DH, Shi J, Rodríguez Posada L, Kakuda Y, Xue SJ (2008) Separating Tocotrienols from Palm Oil by Molecular Distillation. Food Reviews International, 24(4):376–391
- 39.Cert A, Moreda W, Pérez-Camino MC. Chromatographic analysis of minor constituents in vegetable oils. J Chromatogr A. 2000;881(1–2):131–148. doi: 10.1016/s0021-9673(00)00389-7. [DOI] [PubMed] [Google Scholar]
- 40.Cunha SC, Amaral JS, Fernandes JO, Oliveira MB. Quantification of tocopherols and tocotrienols in Portuguese olive oils using HPLC with three different detection systems. J Agric Food Chem. 2006;54(9):3351–3356. doi: 10.1021/jf053102n. [DOI] [PubMed] [Google Scholar]
- 41.Amaral JS, Casal S, Torres D, Seabra RM, Oliveira BP. Simultaneous determination of tocopherols and tocotrienols in hazelnuts by a normal phase liquid chromatographic method. Anal Sci. 2005;21:1545–1548. doi: 10.2116/analsci.21.1545. [DOI] [PubMed] [Google Scholar]
- 42.Abidi SL. Chromatographic analysis of tocol-derived lipid antioxidants. J Chromatogr A. 2000;881:197–216. doi: 10.1016/s0021-9673(00)00131-x. [DOI] [PubMed] [Google Scholar]
- 43.Chow CK, Draper HH, Saari Csallany A. Method for the assay of free and esterified tocopherols. Anal Biochem. 1969;32:81–90. doi: 10.1016/0003-2697(69)90106-7. [DOI] [PubMed] [Google Scholar]
- 44.Abidi SL, Mounts TL. Separations of tocopherols and methylated tocols on cyclodextrin-bonded silica. J Chromatogr A. 1994;670:67–75. [Google Scholar]
- 45.Kormann AW. Routine assay for determination of alpha-tocopherol in liver. J Lipid Res. 1980;21:780–783. [PubMed] [Google Scholar]
- 46.Ballesteros E, Gallego M, Valcarcel M. Gas chromatographic determination of cholesterol and tocopherols in edible oils and fats with automatic removal of interfering triglycerides. J Chromatogr A. 1996;719:221–227. doi: 10.1016/0021-9673(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 47.Feeter DK. Determination of tocopherols, sterols, and steryl esters in vegetable oil distillates and residues. J Am Oil Chem Soc. 1974;51:184–187. doi: 10.1007/BF02639736. [DOI] [PubMed] [Google Scholar]
- 48.Hartman K. A simplified gas liquid chromatographic determination for vitamin E in vegetable oils. J Am Oil Chem Soc. 1977;54:421–423. doi: 10.1007/BF02671024. [DOI] [PubMed] [Google Scholar]
- 49.Marks C. Determination of free tocopherols in deodorizer distillate by capillary gas chromatography. J Am Oil Chem Soc. 1988;65:1936–1939. [Google Scholar]
- 50.Slover HT, Thompson RH, Merola GV. Determination of tocopherols and sterols by capillary gas chromatography. J Am Oil Chem Soc. 1983;60:1524–1528. [Google Scholar]
- 51.Meijboom PW, Jongenotter GA. A quantitative determination of tocotrienols and tocopherols in palm oil by TLC-GLC. J Am Oil Chem Soc. 1979;56:33–35. doi: 10.1007/BF02671757. [DOI] [PubMed] [Google Scholar]
- 52.Han NM, May CY. Chromatographic analyses of tocopherols and tocotrienols in palm oil. J Chromatogr Sci. 2012;50:283–286. doi: 10.1093/chromsci/bms002. [DOI] [PubMed] [Google Scholar]
- 53.Choo YM, Ng MH, Ma AN, Chuah CH, Hashim MA. Application of supercritical fluid chromatography in the quantitative analysis of minor components (carotenes, vitamin E, sterols, and squalene) from palm oil. Lipids. 2005;40:429–432. doi: 10.1007/s11745-006-1400-6. [DOI] [PubMed] [Google Scholar]
- 54.King JW, Favati F, Taylor SL. Production of tocopherol concentrates by supercritical fluid extraction and chromatography. Sep Sci Technol. 1996;31:1843–1857. [Google Scholar]
- 55.Manninen P, Laakso P, Kallio H. Method for characterization of triacylglycerols and fat-soluble vitamins in edible oils and fats by supercritical fluid chromatography. J Am Oil Chem Soc. 1995;72:1001–1008. [Google Scholar]
- 56.Saito M, Yamauchi Y. Isolation of tocopherols from wheat germ oil by recycle semi-preparative supercritical fluid chromatography. J Chromatogr A. 1990;505:257–271. [Google Scholar]
- 57.Yarita T, Nomura A, Abe K, Takeshita Y. Supercritical fluid chromatographic determination of tocopherols on an ODS-silica gel column. J Chromatogr A. 1994;679:329–334. [Google Scholar]
- 58.Han NM, May CY, Ngan MA, Hock CC, Ali Hashim M. Isolation of palm tocols using supercritical fluid chromatography. J Chromatogr Sci. 2004;42:536–539. doi: 10.1093/chromsci/42.10.536. [DOI] [PubMed] [Google Scholar]
- 59.Schüep W, Rettenmaier R (1994) Analysis of vitamin E homologs in plasma and tissue: high-performance liquid chromatography. In: Lester, P. (Ed.), Methods Enzymol. Academic Press, pp. 294-302 [DOI] [PubMed]
- 60.Shin TS, Godber JS. Improved high-performance liquid chromatography of vitamin E vitamers on normal-phase columns. J Am Oil Chem Soc. 1993;70:1289–1291. [Google Scholar]
- 61.Thompson JN, Hatina G. Determination of tocopherols and tocotrienols in foods and tissues by high performance liquid chromatography. J Liq Chromatogr Relat Technol. 1979;2:327–344. [Google Scholar]
- 62.Weber E. High performance liquid chromatography of the tocols in corn grain. J Am Oil Chem Soc. 1984;61:1231–1234. [Google Scholar]
- 63.Rammell CG, Hoogenboom JJL. Separation of tocols by HPLC on an amino-cyano polar phase column. J Liq Chromatogr. 1985;8:707–717. [Google Scholar]
- 64.Wahyuni WT, Jinno K. Separation of tocopherols on various chemically bonded phases in microcolumn liquid chromatography. J Chromatogr A. 1988;448:398–403. [Google Scholar]
- 65.Balz M, Schulte E, Thier HP. Trennung von Tocopherole n und Tocotrienolen durch HPLC. Lipid/Fett. 1992;94:209–213. [Google Scholar]
- 66.Aitzetmüller K, Xin Y, Werner G, Grönheim M. High-performance liquid chromatographic investigations of stillingia oil. J Chromatogr A. 1992;603:165–173. [Google Scholar]
- 67.Micali G, Lanuzza F, Currò P. Analysis of tocopherols in margarine by on-line HPLC–HRGC coupling. J High Res Chromatogr. 1993;16:536–538. [Google Scholar]
- 68.Dionisi F, Prodolliet J, Tagliaferri E. Assessment of olive oil adulteration by reversed-phase high-performance liquid chromatography/amperometric detection of tocopherols and tocotrienols. J Am Oil Chem Soc. 1995;72:1505–1511. [Google Scholar]
- 69.Balz M, Schulte E, Thier HP. Simultaneous determination of retinol esters and tocochromanols in foods using nitro-column HPLC. Lipid/Fett. 1995;97:445–448. [Google Scholar]
- 70.Kramer JG, Blais L, Fouchard R, Melnyk R, Kallury KR. A rapid method for the determination of vitamin E forms in tissues and diet by high-performance liquid chromatography using a normal-phase diol column. Lipids. 1997;32:323–330. doi: 10.1007/s11745-997-0040-1. [DOI] [PubMed] [Google Scholar]
- 71.DeVries JW, Egberg DC, Heroff JC (1979) Concurrent analysis of vitamin A and vitamin E by reversed phase high performance liquid chromatography. In: George, C. (Ed.) Liquid chromatographic analysis of food and beverages. Academic Press, pp. 477-497
- 72.Ueda T, Ichikawa H, Igarashi O. Determination of α-tocopherol stereoisomers in biological specimens using chiral phase high-performance liquid chromatography. J Nutr Sci Vitaminol. 1993;39:207–219. doi: 10.3177/jnsv.39.207. [DOI] [PubMed] [Google Scholar]
- 73.Mulholland M. Linking low dispersion liquid chromatography with diode-array detection for the sensitive and selective determination of vitamins A, D and E. Analyst. 1986;111:601–604. doi: 10.1039/an9861100601. [DOI] [PubMed] [Google Scholar]
- 74.Lang JK, Gohil K, Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 75.Pascoe GA, Duda CT, Reed DJ. Determination of α-tocopherol and α-tocopherylquinone in small biological samples by high-performance liquid chromatography with electrochemical detection. J Chromatogr B: Biomed Sci Appl. 1987;414:440–448. doi: 10.1016/0378-4347(87)80071-3. [DOI] [PubMed] [Google Scholar]
- 76.Murphy ME, Kehrer JP. Simultaneous measurement of tocopherols and tocopheryl quinones in tissue fractions using high-performance liquid chromatography with redox-cycling electrochemical detection. J Chromatogr B: Biomed Sci Appl. 1987;421:71–82. doi: 10.1016/0378-4347(87)80380-8. [DOI] [PubMed] [Google Scholar]
- 77.Satomura Y, Kimura M, Itokawa Y. Simultaneous determination of retinol and tocopherols by high-performance liquid chromatography. J Chromatogr A. 1992;625:372–376. doi: 10.1016/0021-9673(92)85224-h. [DOI] [PubMed] [Google Scholar]
- 78.Barua AB, Furr HC, Janick-Buckner D, Olson JA. Simultaneous analysis of individual carotenoids, retinol, retinyl esters, and tocopherols in serum by isocratic non-aqueous reversed-phase HPLC. Food Chem. 1993;46:419–424. [Google Scholar]
- 79.Epler KS, Ziegler RG, Craft NE. Liquid chromatographic method for the determination of carotenoids, retinoids and tocopherols in human serum and in food. J Chromatogr B: Biomed Sci Appl. 1993;619:37–48. doi: 10.1016/0378-4347(93)80444-9. [DOI] [PubMed] [Google Scholar]
- 80.Sarzanini C, Mentasti E, Vincenti M, Nerva M, Gaido F. Determination of plasma tocopherols by high-performance liquid chromatography with coulometric detection. J Chromatogr B: Biomed Sci Appl. 1993;620:268–272. doi: 10.1016/0378-4347(93)80015-v. [DOI] [PubMed] [Google Scholar]
- 81.Richheimer SL, Kent MC, Bernart MW. Reversed-phase high-performance liquid chromatographic method using a pentafluorophenyl bonded phase for analysis of tocopherols. J Chromatogr A. 1994;677:75–80. [Google Scholar]
- 82.Ben-Amotz A. Simultaneous profiling and identification of carotenoids, retinols, and tocopherols by high performance liquid chromatography equipped with three-dimensional photodiode array detection. J Liq Chromatogr. 1995;18:2813–2825. [Google Scholar]
- 83.Lane JR, Webb LW, Acuff RV. Concurrent liquid chromatographic separation and photodiode array detection of retinol, tocopherols, all-trans-α-carotene, all-trans-β-carotene and the mono-cis isomers of β-carotene in extracts of human plasma. J Chromatogr A. 1997;787:111–118. doi: 10.1016/s0021-9673(97)00655-9. [DOI] [PubMed] [Google Scholar]
- 84.Rentel C, Strohschein S, Albert K, Bayer E. Silver-plated vitamins: a method of detecting tocopherols and carotenoids in LC/ESI-MS coupling. Anal Chem. 1998;70(20):4394–4400. doi: 10.1021/ac971329e. [DOI] [PubMed] [Google Scholar]
- 85.Barua AB, Olson JA. Reversed-phase gradient high-performance liquid chromatographic procedure for simultaneous analysis of very polar to nonpolar retinoids, carotenoids and tocopherols in animal and plant samples. J Chromatogr B: Biomed Sci Appl. 1998;707:69–79. doi: 10.1016/s0378-4347(97)00614-2. [DOI] [PubMed] [Google Scholar]
- 86.Abidi SL. Reversed-phase retention characteristics of tocotrienol antioxidants. J Chromatogr A. 1999;844:67–75. [Google Scholar]
- 87.Strohschein S, Rentel C, Lacker T, Bayer E, Albert K. Separation and identification of tocotrienol isomers by HPLC-MS and HPLC-NMR coupling. Anal Chem. 1999;71(9):1780–1785. doi: 10.1021/ac981089i. [DOI] [PubMed] [Google Scholar]
- 88.Podda M, Weber C, TraberMG, Milbradt R, Packer L (1999) Sensitive high-performance liquid chromatography techniques for simultaneous determination of tocopherols, tocotrienols, ubiquinols, and ubiquinones in biological samples. In: Lester, P. (Ed.), Method Enzymol. Academic Press, pp. 330-341 [DOI] [PubMed]
- 89.Huo JZ, Nelis HJ, Lavens P, Sorgeloos P, De Leenheer A. Determination of E vitamers in microalgae using high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1997;782:63–68. [Google Scholar]
- 90.Finckh B, Kontush A, Commentz J, Hübner C, Burdelski M, Kohlschütter A (1999) High-performance liquid chromatography-coulometric electrochemical detection of ubiquinol 10, ubiquinone 10, carotenoids, and tocopherols in neonatal plasma. In: Lester, P. (Ed.) Method Enzymol. Academic Press, pp. 341-348 [DOI] [PubMed]
- 91.Bonvehi JS, Coll FV, Rius IA. Liquid chromatographic determination of tocopherols and tocotrienols in vegetable oils, formulated preparations, and biscuits. J AOAC Int. 2000;83:627–634. [PubMed] [Google Scholar]
- 92.Drotleff AM, Ternes W. Determination of RS, E/Z-tocotrienols by HPLC. J Chromatogr A. 2001;909:215–223. doi: 10.1016/s0021-9673(00)01110-9. [DOI] [PubMed] [Google Scholar]
- 93.Adachi T, Isobe E. Use of synthetic adsorbents in preparative normal-phase liquid chromatography. J Chromatogr A. 2003;989:19–29. doi: 10.1016/s0021-9673(02)01657-6. [DOI] [PubMed] [Google Scholar]
- 94.Fuchs J, Weber S, Podda M, Groth N, Herrling T, Packer L, Kaufmann R. HPLC analysis of vitamin E isoforms in human epidermis: correlation with minimal erythema dose and free radical scavenging activity. Free Rad Biol Med. 2003;34:330–336. doi: 10.1016/s0891-5849(02)01293-5. [DOI] [PubMed] [Google Scholar]
- 95.Hewavitharana AK, Lanari MC, Becu C. Simultaneous determination of vitamin E homologs in chicken meat by liquid chromatography with fluorescence detection. J Chromatogr A. 2004;1025:313–317. doi: 10.1016/j.chroma.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 96.Cunha SC, Amaral JS, Fernandes JO, Oliveira MB. Quantification of tocopherols and tocotrienols in Portuguese olive oils using HPLC with three different detection systems. J Agric Food Chem. 2006;54:3351–3356. doi: 10.1021/jf053102n. [DOI] [PubMed] [Google Scholar]
- 97.Andersson SC, Rumpunen K, Johansson E, Olsson ME. Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J Agric Food Chem. 2008;56:6701–6706. doi: 10.1021/jf800734v. [DOI] [PubMed] [Google Scholar]
- 98.Ali H, Nazzal S. Development and validation of a reversed-phase HPLC method for the simultaneous analysis of simvastatin and tocotrienols in combined dosage forms. J Pharm Biomed Anal. 2009;49:950–956. doi: 10.1016/j.jpba.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Cerretani L, Lerma-Garcia MJ, Herrero-Martinez JM, Gallina-Toschi T, Simo-Alfonso EF. Determination of tocopherols and tocotrienols in vegetable oils by nanoliquid chromatography with ultraviolet-visible detection using a silica monolithic column. J Agric Food Chem. 2010;58:757–761. doi: 10.1021/jf9031537. [DOI] [PubMed] [Google Scholar]
- 100.Huang SH, Ng LT. An improved high-performance liquid chromatographic method for simultaneous determination of tocopherols, tocotrienols and gamma-oryzanol in rice. J Chromatogr A. 2011;1218:4709–4713. doi: 10.1016/j.chroma.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 101.Andersson SC, Olsson ME, Gustavsson KE, Johansson E, Rumpunen K. Tocopherols in rose hips (Rosa spp.) during ripening. J Sci Food Agric. 2012;92:2116–2121. doi: 10.1002/jsfa.5594. [DOI] [PubMed] [Google Scholar]
- 102.Abidi SL, Rennick KA. Capillary electrochromatographic evaluation of vitamin E-active oil constituents: tocopherols and tocotrienols. J Chromatogr A. 2001;913:379–386. doi: 10.1016/s0021-9673(00)01068-2. [DOI] [PubMed] [Google Scholar]
- 103.Abidi SL, Thiam S, Warner IM. Elution behavior of unsaponifiable lipids with various capillary electrochromatographic stationary phases. J Chromatogr A. 2002;949:195–207. doi: 10.1016/s0021-9673(01)01272-9. [DOI] [PubMed] [Google Scholar]
- 104.Aturki Z, D’Orazio G, Fanali S. Rapid assay of vitamin E in vegetable oils by reversed-phase capillary electrochromatography. Electrophoresis. 2005;26:798–803. doi: 10.1002/elps.200410181. [DOI] [PubMed] [Google Scholar]
- 105.Lauridsen C, Leonard SW, Griffin DA, Liebler DC, McClure TD, Traber MG. Quantitative analysis by liquid chromatography-tandem mass spectrometry of deuterium-labeled and unlabeled vitamin E in biological samples. Anal Biochem. 2001;289(1):89–95. doi: 10.1006/abio.2000.4913. [DOI] [PubMed] [Google Scholar]
- 106.Andreoli R, Manini P, Poli D, Bergamaschi E, Mutti A, Niessen WM. Development of a simplified method for the simultaneous determination of retinol, alpha-tocopherol, and beta-carotene in serum by liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization. Anal Bioanal Chem. 2004;378(4):987–994. doi: 10.1007/s00216-003-2288-0. [DOI] [PubMed] [Google Scholar]
- 107.Kalman A, Mujahid C, Mottier P, Heudi O. Determination of alpha-tocopherol in infant foods by liquid chromatography combined with atmospheric pressure chemical ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2003;17(7):723–727. doi: 10.1002/rcm.970. [DOI] [PubMed] [Google Scholar]
- 108.Bustamante-Rangel M, Delgado-Zamarreno MM, Sanchez-Perez A, Carabias-Martinez R. Determination of tocopherols and tocotrienols in cereals by pressurized liquid extraction-liquid chromatography-mass spectrometry. Anal Chim Acta. 2007;587:216–221. doi: 10.1016/j.aca.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 109.Al-Talla ZA, Tolley LT. Analysis of vitamin E derivatives in serum using coordinated ion spray mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(16):2337–2342. doi: 10.1002/rcm.2057. [DOI] [PubMed] [Google Scholar]
- 110.Mottier P, Gremaud E, Guy PA, Turesky RJ. Comparison of gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry methods to quantify alpha-tocopherol and alpha-tocopherolquinone levels in human plasma. Anal Biochem. 2002;301(1):128–135. doi: 10.1006/abio.2001.5486. [DOI] [PubMed] [Google Scholar]
- 111.Hall WL, Jeanes YM, Pugh J, Lodge JK. Development of a liquid chromatographic time-of-flight mass spectrometric method for the determination of unlabelled and deuterium-labelled alpha-tocopherol in blood components. Rapid Commun Mass Spectrom. 2003;17(24):2797–2803. doi: 10.1002/rcm.1263. [DOI] [PubMed] [Google Scholar]
- 112.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72C:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]