Abstract
The St. Lawrence River (SLR) is the second largest waterway in North America. The discharge of the City of Montreal wastewater treatment plant (WWTP) represents the largest volume of treated wastewaters being released into the river. It also ranks as the largest sewage treatment plant of its kind in North America. Over the last decade, intensive multidisciplinary research has focused on assessing the impacts of Montreal wastewater effluents on the SLR. We describe the major findings of these investigations, including the determination of the fate of contaminants, bioaccumulation in fish and invertebrates, ecotoxicological measurements of aquatic animal health, evaluation of endocrine disruption, parasitism in fish, and combined effects of multiple stressors on the SLR. Impacts of the effluents from the WWTP on aquatic organisms from the SLR are both toxicological and ecological, demonstrating the need for an integrated view of the impacts of municipal effluents on aquatic ecosystems.
Keywords: Municipal effluents, Ecotoxicology, Rivers, Pollution, Contaminants, Aquatic animal health
Introduction
General characteristics of the St. Lawrence River
The St. Lawrence River–Great Lakes system (SLR–GL) is the second largest waterway in North America after the Mississippi River, spanning 3700 km from the Northeast Atlantic coast to the interior of the continent. With a watershed area of over 1 million km2 and a mean annual discharge of 12 600 m3 s−1 at Quebec City, the SLR ranks among the 20 largest rivers of the world. Approximately 40 million inhabitants within its watershed benefit from its use for drinking water, sewage disposal, hydroelectric production, commercial navigation, and countless recreational activities. Over its 550-km course between Lake Ontario and Quebec City (Fig. 1), wastewaters from approximately 5 million people living along the SLR shores are passed into the river following varying levels of treatment.
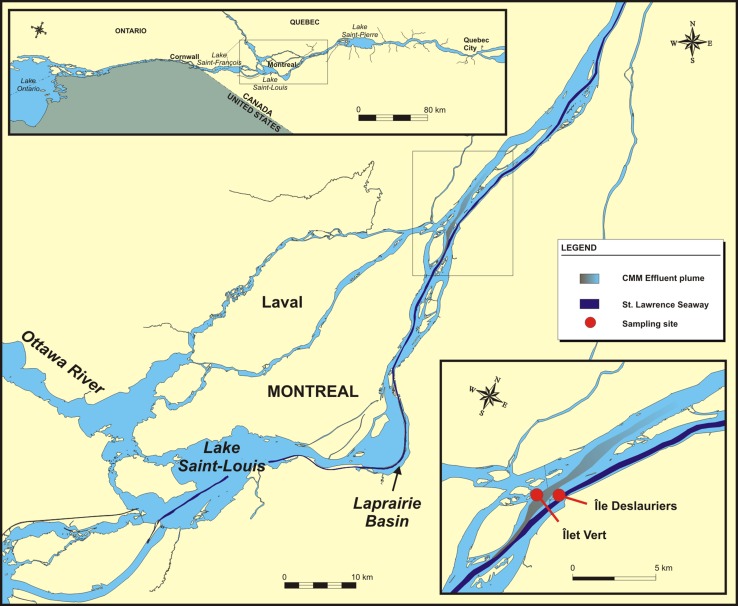
Fig. 1.
Map of the St. Lawrence River (SLR) and the effluent plume of the Montreal wastewater treatment plant. Inset at the upper left shows the fluvial lakes of the St. Lawrence River upstream and downstream of the Island of Montreal. Inset at the lower right shows sample sites and the effluent plume from the Montreal wastewater treatment plant. Direction of water flow in the SLR is from lower left to upper right
The largest urban center along the SLR is the Metropolitan Community of Montreal (CMM, http://cmm.qc.ca/), which is composed of 3.8 million inhabitants (Fig. 1). The CMM discharges about 3.5 million m3 (41 m3 s−1) of treated wastewater daily in the Montreal archipelago, representing about 66 tonnes of suspended particulate matter (SPM) and 1.5 tonnes of total phosphorus (TP). Within the CMM, the City of Montreal physical–chemical wastewater treatment plant (WWTP) alone accounts for 71 % (mean discharge 29 m3 s−1) of total CMM wastewater discharge, with 72 and 66 %, of daily SPM and TP loads, respectively (Moreira 2011), with the remainder coming from smaller municipalities.
The Montreal wastewater treatment plant (WWTP)
Initiated in the 1970s, the City of Montreal WWTP became fully operational in 1996, ensuring the complete primary treatment of all wastewaters generated on the Island of Montreal and the neighboring town of Île Bizard under dry weather conditions. The city sewer system wastewaters flow into a 15-story-deep well from which they are pumped into the WWTP (http://ville.montreal.qc.ca/portal/page?_pageid=6497,54245572&_dad=portal&_schema=PORTAL Consulted 26 January 2012). First, particles >25 mm are removed by sieving, generating annually about 750 t of residues annually which are subsequently spun-dried and buried after incineration. The plant subsequently removes about 4700 t year−1 of sand and heavy particles, concentrating and washing the waste prior to burial in landfill. Wastewaters then undergo chemical flocculation by the injection of ferrous chloride (FeCl2) combined with an anionic polymer, thus reducing the concentration of suspended solids and TP in the effluents to about 20 and 0.5 mg L−1, respectively. The effluents are then discharged to its SLR outfall near the southeastern tip of the Island of Montreal (Figs. 1, 2). This is the largest sewage treatment plant of its kind in North America with respect to the volume of treated effluent.
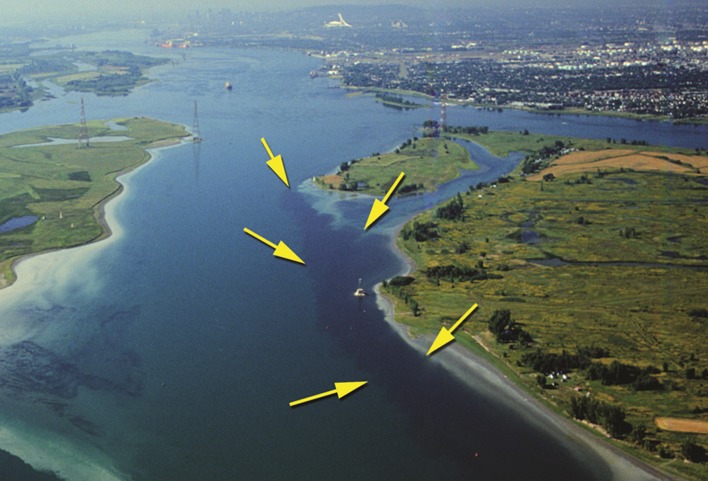
Fig. 2.
Aerial photograph of discharge of the Montreal wastewater treatment plant in the St. Lawrence River. Arrows indicate the sewage effluent, which appear as dark blue color. Photograph by Christiane Hudon
Environment Canada and academic collaborators have worked in concert to evaluate the impacts of municipal wastewater effluents on the SLR ecosystem since 1999. This involved a multidisciplinary research program combining both laboratory and in situ studies including water and sediment chemistry, bioaccumulation, and ecotoxicological analyses at different trophic levels, endocrine disruption, parasitology, and aquatic ecology. This program aimed to develop and test novel tools for assessing and improving water quality criteria for urban effluents and evaluate the impact of the WWTP directly in the SLR. The most salient results are summarized herein to help us guide future environmental research in this and other large river ecosystems.
Water chemistry in effluents
An essential step toward assessing the in situ impacts of Montreal urban effluents on the SLR involves the characterization of the effluent dispersion plume. Chemical analyses allow for the identification of contaminants, their speciation, and distribution in the SLR. Combining both domestic and industrial wastewaters, Montreal effluents contain a complex mixture of large quantities of nutrients and metals as well as several organic contaminants. Prior to 1996, neither specific studies nor monitoring programs provided adequate information on the levels of contaminants in the surface waters of the SLR in the Greater Montreal area. Legacy contaminants such as polychlorinated biphenyls (PCBs) and the combustion-derived polycyclic aromatic hydrocarbons (PAHs) were among the first contaminants targeted for analyses shortly after the WWTP was built (Pham et al. 1999). Average concentrations of summed PCBs and PAHs were 1.34 ± 0.71 and 326 ± 229 ng L−1, respectively, rendering the effluent as the principal contributor of both contaminant groups to the SLR. At the effluent discharge site, contaminant concentrations were increased by more than 170 and 300 %, respectively. Following mixing of wastewater with the surrounding river waters, congener profiles revealed that trace concentrations were gradually diluted without preferential dissipation along the effluent plume 11 km downstream (Pham et al. 1999).
Metals
Although metals constitute the most abundant contaminants measured in the effluent, the WWTP’s relative contribution to the overall metal load in the SLR is relatively small (<1 %) for most metals, most of which are naturally occurring or derived from diffuse anthropogenic sources (Gobeil et al. 2005). However, this is not the case for certain metals such as Cd, Cu, and Zn where 8–13 % of levels in the SLR originate from Montreal wastewater effluent and for Ag where as much as 25 % of the total load in the river originates from waste effluent. Although the effluent contribution to the metal contamination of the SLR appears quantitatively low, the toxicity of various forms of these metals to chronically exposed organisms remains poorly understood.
In the effluent dispersion plume, dissolved metals primarily are bound with colloidal particles, and the abundance of colloids likely modifies the bioavailability and bioaccumulation of metals. The size distributions of dissolved, colloidal, and particulate metals at different locations within the dispersion plume indicate that metal concentrations are typically high in colloidal (<0.45 µm and >10 kDa) and in the permeable (ultrafiltered <10 kDa) fractions near the effluent outfall (Gagnon and Turcotte 2007). Owing to the quantity of FeCl2 used in the wastewater treatment process, the effluent represents a major source of particulate Fe of which more than 70 % is in colloidal form. High concentrations of colloidal Fe and dissolved organic matter greatly enhance the potential of the effluent to act as a metal carrier. Colloids can influence the transport and fate of discharged metals (e.g., Al, Cd, Cu, Fe, Mn, Ag, and Zn) by changing reactivity and behavior in the effluent dispersion plume (Gagnon and Saulnier 2003). Of the metals studied, Ag followed by Cu showed the greatest association with colloids near the wastewater outfall. Given that the proportion of colloids in the plume decreases rapidly during mixing of the effluent with the receiving waters, this fraction will have limited influence on the long-range transport of metals released by the effluent (Gagnon et al. 2006).
Metal enrichment in suspended particles was also observed in the effluent dispersion plume (Gagnon and Saulnier 2003). Suspended particulate matter, sampled with sediment traps, was evaluated for solid phase speciation using selective chemical extractions (Gagnon et al. 2009). Compared to the SLR waters, the reactivities of all particulate metals from the urban effluent plume samples were much higher, with up to 65, 42, 30, 47, and 43 % for Cd, Cu, Fe, Pb, and Zn, respectively, in the two most reactive removable/mobile fractions (i.e., extractable + reducible forms). Parameters such as the organic carbon, Fe oxide, and carbonate content have different impacts on the partitioning of several metals, particularly Cd, Cu, and Zn. These results indicated that the relative distributions of metals among geochemical solid fractions changed in the effluent dispersion plume and therefore could strongly influence the metal mobility and exposure to aquatic organisms.
Emerging organic contaminants
In addition to conventional or legacy contaminants, urban effluents include a number of the so-called emerging substances impacts of which on the receiving waters remain poorly understood. Many of the these compounds, such as surfactants (0.1–144 μg L−1), flame retardants (37–1000 ng g−1), pharmaceuticals, and personal care products (PPCPs: 2–3500 ng L−1), have been measured in Montreal wastewater effluent (Sabik et al. 2004; Lajeunesse and Gagnon 2007; Lajeunesse et al. 2008; Pelletier et al. 2009).
Studies on the occurrence and fate of nonylphenols (NP) and associated ethoxylates and carboxylates (NPEOx and NPECx), which are non-ionic surfactants common in industrial usage, reveal that the Montreal WWTP effluent releases nearly 500 kg NP1-17EOs per day, mainly (75.4 %) in dissolved and particulate forms (Sabik et al. 2004). While the wastewater effluent contained essentially NP1-17EOs, the SLR surface waters downstream of the outfall contain mainly dissolved NP1-17EOs (86.7 %) and p-NPs (9.8 %), as well as a small fraction of particulate NP1-17EOs (2.1 %) and dissolved NP1-2ECs (1.3 %). This indicates that following a relatively fast-phase transfer in the receiving waters, formation of the more estrogenic p-NPs and NP1-2ECs occurred through the degradation of NP1-17EOs.
Polybrominated diphenyl ethers (PBDEs) used also as standard flame retardants are present in the SLR. Over the last decade, concentrations of the congener BDE209 have increased by five times in the SLR and doubled in sediments from the fluvial Lake Saint-Pierre (Pelletier et al. 2009). Elevated concentrations of BDE209 (up to 564 ng g−1) measured in suspended particulate matter collected within the Montreal effluent plume confirm that the effluent represents a major source of PBDE released into the SLR.
Pharmaceutical drugs such as antibiotics, anti-inflammatories, anti-depressants, anti-convulsive drugs and cytotoxics are frequently measured in urban wastewaters. Some of these drugs undergo major chemical transformation at the treatment plant and again in the receiving waters, thus affecting their bioavailability and potential toxicity. Among the first pharmaceutical classes of drugs investigated in the SLR, highly soluble acidic compounds were targeted because of the difficulty of removal in the treatment plant (typically <30 %) (Lajeunesse and Gagnon 2007; Lajeunesse et al. 2008; Gagnon and Lajeunesse 2012). Treated wastewaters contained high concentrations (>1200 ng L−1) of ibuprofen and salicylic acid along with carbazepine and triclosan which are present at non-negligible concentrations of 100 ng L−1. Pharmaceutical residues also were detected in the SLR in the effluent dispersion plume up to 8 km from the outfall at concentrations as high as 350 ng L−1 for ibuprofen and its metabolites, while others like carbamazepine and triclosan were reported at concentrations of 20 ng L−1 (Lajeunesse and Gagnon 2007). Various antibiotics, such as sulfamethoxazole, trimethoprim, ciprofloxacin and clarithromycin, were also found in wastewater samples at concentrations ranging from 39 to 276 ng L−1 (Segura et al. 2007). Large mean flows of antibiotics (e.g., clarithromycin at 830 g day−1) were estimated in the effluent discharge. Many antidepressant drugs and metabolites were present in samples collected at the Montreal WWTP at concentrations of 2–346 ng L−1 (Lajeunesse et al. 2008). The treatment of raw wastewater resulted in reductions of less than 50 % of original concentrations. Many antidepressants were thus still detected in water samples from the effluent plume in the SLR, but at lower concentrations (0.41–69 ng L−1; Lajeunesse et al. 2008).
Other therapeutic classes of pharmaceutical products detected in wastewaters included lipid regulators, chemotherapy drugs, lipase inhibitors, and hypertension drugs. Among these, the hypertension drug enalapril and the lipid regulator bezafibrate were the most abundant compounds with concentrations of 35 and 239 ng L−1 (Garcia-Ac et al. 2009a). Despite dilution of the wastewater, these compounds were still detected in surface water samples from sites in the SLR downstream of Montreal at concentrations of 8 and 63 ng L−1, respectively (Garcia-Ac et al. 2009b).
Unfortunately, there are no long-term data on water or sediment chemistry in the effluent plume. All long-term monitoring has been restricted to the fluvial lakes occurring along the river. In sediments in the downstream Lake Saint-Pierre, mean PCB concentration dropped by 94 % between 1986 and 2003 and mean Hg concentration dropped by 90 % between 1976 and 2003 (Pelletier 2008). In contrast, mean PBDE concentration in Lake Saint-Pierre sediments shows an increase since the 1990s (http://planstlaurent.qc.ca/fileadmin/site_documents/documents/SESL/PBDE_e.pdf).
Contaminants in biota
Measurement of contaminants in the various tissues of living aquatic organisms represents an indicator of significant exposure to WWTP effluent waters, either through direct contact or indirectly through the food chain. Several groups of organisms were studied for this purpose, including submerged aquatic vegetation, invertebrates, fish, humans, and birds. Each group of organism poses specific challenges to interpretation owing to their unique ecological, physiological, and behavioral characteristics.
Submerged aquatic plants
Submerged aquatic vegetation sampled downstream of the greater Montreal area in the SLR accumulates significantly higher concentrations of phosphorus and metals (Cd, Cu, Mn, Pb) than plants sampled upstream (Hudon 1998). Between 1976 and 1996, concentrations of Cd, Cr, Pb, and Zn in aquatic vegetation decreased significantly by 80–600 %, indicating the positive effects of the WWTP installation on the overall water quality of the SLR in the Montreal area. The effects of plant exposure to polluted waters is evidenced by the higher metal concentrations measured in plants exposed to deep, fast-flowing open-water in contrast with plants growing under shallow, sheltered conditions within the macrophyte bed (Hudon 1998), suggesting that exposure to contaminants may be reduced in littoral areas.
Animals
Fish
The SLR is characterized by a rich and diversified fish community with 89 freshwater fish species (Bernachez and Giroux 2012). The SLR supports both sport and commercial fishing activities, and the waters surrounding Montreal have long been recognized as a significant fishing zone. While lake sturgeon (Acipenser fulvescens) and American eel (Anguilla rostrata) were commercially exploited until recently, sport fishing now represents the major fishing activity.
A questionnaire survey conducted in 1996 reveals that half of fishermen from a local community near Montreal consumed their catch (Chan et al. 1999), attaining as much as 20 kg of SLR-caught fish per year per person (Kosatsky et al. 2000; Nadon et al. 2002). Apparently, one-third of fishermen stopped eating local fish over the previous decade, due to the perception that fish are unsafe to consume because of the presence of contaminants (Chan et al. 1999). This perception largely still holds among the general public despite the scientific evidence that both Hg and PCBs levels in fish from the Montreal area have declined by 35–50 % and by 80–99 %, respectively, between 1976 and 1997 (Laliberté 2003). Nevertheless, there exists a considerable degree of fish consumption within certain elements of the population living in proximity to the SLR (Nadon et al. 2002). Hair samples from women of childbearing age (under 45 years) who had eaten SLR fish over an 11-year period, but less than once a month, had more than fivefold higher circulating levels of blood Hg and twice the level of PCBs than non-fish eaters. It is therefore not surprising that the levels of chemical contamination in the SLR fish and the quality of fish resource always have been issues of concern for public health.
Fish bioaccumulation studies in large rivers are sometimes difficult to interpret in terms of local exposure to toxic contaminants due to fish migratory behaviour and contaminant dynamics. Within-species variability in contaminant burdens including PCBs is much higher for non-migratory species than for those which move extensively along the river (Brochu et al. 1995; Ion and Lafontaine 1998; de Lafontaine et al. 2002). Given the concentrating effects of urban effluents discharged from both Montreal and Longueuil WWTPs at two major sites at the eastern end of the Island of Montreal, it is likely that fish downstream of Montreal have higher contaminant exposure than fish located upstream. Yet, two studies conducted in 1989 comparing contaminants (Hg, trace metals, PCBs, organochlorine pesticides) in adult fish from Lake Saint-Pierre downstream of Montreal to those from fluvial lakes upstream of Montreal (Ion et al. 1997; Ion and Lafontaine 1998) did not support this hypothesis. Results suggest that contaminants from urban effluents are sufficiently diluted by the time water masses reach Lake Saint-Pierre, as resulting exposure levels are low. Laliberté (2011) noted, however, that fish caught from Lake Saint-Pierre between 2002 and 2008 had significantly higher levels of PBDE than specimens from Lake Saint-François. The author concluded that these contaminants likely originated from the discharge of Montreal urban wastewaters into the SLR.
In the absence of high-frequency and long-term monitoring data for adult fish contamination in the SLR and recognizing the limited power of spatial studies of adult fish bioaccumulation (due to fish movements within rivers) to track the potential effects of local point sources of contaminants in large river systems, the use of juvenile fish as bio-indicators of toxic substances in the SLR was assessed by comparing contaminants’ levels in young-of-the-year (YOY). Contaminants’ levels in YOY yellow perch (Perca flavescens) and spottail shiners (Notropis hudsonius) collected at two sites in proximity of the Montreal WWTP discharge were 2–5 times higher than in fish from either upstream or downstream locations in 1984 (Guay and Dandurand 1986). Urban effluents from Montreal were identified as a major source of PCBs bioaccumulated in juvenile fish at that time. More recently, a study conducted during 2009–2011 investigated the chemical contamination of muskellunge (Esox masquinongy) in relation to the Montreal WWTP discharge (Houde et al. 2014; de Lafontaine et al. personal communication). Muskellunge is a very large predatory fish which is highly territorial and exhibits little migratory behavior, permitting a better evaluation of the effect of local exposure to contaminated effluents. Comparison of contaminants’ levels in different tissues of muskellunge specimens captured in the SLR at the east end of the Island of Montreal, relative to specimens from upstream locations, reveals significantly higher concentrations of PBDEs and antidepressant pharmaceutical products in fish captured at the former site, in the vicinity of the Montreal WWTP discharge, strongly indicative of local exposure to Montreal municipal contaminants (Table 1). Significantly higher levels of iron (Fe) in liver tissues of fish east of Montreal provided further confirmation of local fish exposure to the effluent discharge, because of the addition of ferric chloride in treated waters. Similar PBDE results were found for yellow perch and northern pike (Esox lucius) (Houde et al. 2014). Higher levels of PBDEs in fish downstream of Montreal agreed with observations by Laliberté (2011), who concluded that urban effluents may represent the major source of PBDEs to the SLR. In addition, a combination of 12 perfluorinated compounds (PFCs), including the newly detected perflurooethylcyclohexane sulfonates (PFECHS), was four times higher in tissues of northern pike (Exox lucius) downstream of the effluents than they were in pike caught upstream (Houde et al. 2013).
Table 1.
Levels of different contaminants in liver tissues of muskellunge (Esox maskinongy) from three main sectors around the Island of Montreal in 2009–2010
| Contaminants | Lake Des Deux Montagnes | Lake St. Louis | St. Lawrence River east of Montreal |
|---|---|---|---|
| Hg (μg/g) | 1.92 | 3.22 | 0.62 |
| PCBs (Σ88 congeners—ng/g) | 3349.1 | 2674.5 | 6111.3 |
| Total organochlorine (Σ27 compounds—ng/g) | 386.5 | 428.2 | 550.6 |
| PBDEs (Σ15 congeners—ng/g) | 553.0 | 314.9 | 1082.2 |
| Antidepressants (Σ15 compounds—ng/g) | 14.89 | 9.24 | 19.92 |
| Fe (μg/g) | 169 | 319 | 547 |
In summary, fish contamination studies in Montreal waters remain relatively scanty despite the fact that this river sector was recognized some 40 years ago as being contaminated and despite the general public perception that fish are unsafe to consume. While contaminants residues in fish tissues indicate that SLR fish are locally exposed to the Montreal urban wastewater discharge, it remains difficult to partition the relative importance of the municipal effluents on the overall fish contaminants loadings.
Birds
Wetlands in the SLR, including the archipelago of islands downstream of Montreal through which the municipal effluents flow, harbor a high diversity of avian life and constitute an important forage area for waterfowl (DesGranges and Jobin 2003). However, there have been limited studies to date on waterfowl. A large colony of ring-billed gulls (Larus delawarensis) is found at Îles Deslauriers, located in close proximity to the municipal effluent plume (Fig. 1). In a survey of four gull species from across Canada, eggs of these birds as well as those of herring gulls have among the highest levels of perfluorinated sulfonates (PSFA) and perfluorinated carboxylates (PFCA), as well as the flame-retardant chemicals—polybrominated diphenyl ethers (PBDEs) (Gebbink et al. 2011; Chen et al. 2012). Ring-billed gulls from Îles Deslauriers possess the highest concentrations of the flame-retardant bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEHTBP; 2.16 ng g−1 wet weight) known to date among birds, and those of one PBDE (BDE-209) exceed those in apex raptors (Gentes et al. 2012). However, in that study, the source of these contaminants in birds could not be confirmed and may have involved both aquatic and terrestrial prey. In another study, PBDEs were up to ten-fold higher in great blue herons (Ardea herodeas) eggs from the Montreal area compared to areas upstream or downstream (Champoux et al. 2010), also consistent with a point source of these contaminants.
Invertebrates
Studies on bioaccumulation show that total metal concentrations in waters alone are an insufficient predictor of their bioavailability in the dispersion plumes of municipal effluents (Gagnon et al. 2006). Consequently, metal exposure and bioavailability to biota were further investigated using caged mussels exposed to the effluent dispersion plume (Gagnon et al. 2006; Fig. 3). The tissue distribution of certain metals such as Ag, Cd, and Cr in gill, with mean concentrations of 1.5, 3, and 4 mg kg−1, represented a good indicator of the metal exposure pathways (i.e., from dissolved or ingested particulate metals). These results also indicate relatively low metal bioavailability near the effluent outfall compared to reference sites and sites further downstream.
Fig. 3.
Cages used to house mussels (Elliptio complanata) for varying periods of time upstream and downstream of the Montreal wastewater treatment plant effluents in the St. Lawrence River. a Cage showing strings of mussels, b a single string of mussels. Photographs by Sylvain Trottier
Ecotoxicological effects of effluents
Laboratory bioassays
Among the different tools and approaches employed to assess the risks of effluent point source discharges, bioassays are often at the forefront of ecotoxicological investigations to determine potential (sub)lethal effects owing to their rapid and cost-effective screening applications. In this context, Table 2 highlights a variety of bioanalytic testing endeavors conducted on the Montreal WWTP effluent, with major findings described as follows.
Table 2.
Studies demonstrating ecotoxicological effects of municipal effluents from the Montreal wastewater treatment plant effluent as revealed by bioanalytic testing conducted from 1995 to 2010
| Measured endpoint(s) | Taxonomic group(s) tested | Effluent(s) investigated | Reference |
|---|---|---|---|
| Cytotoxicity (loss of cell viability); mRNA expression of metallothionein (MT) induction; cytochrome P-450 1A1 activity | Fish: rainbow trout (Oncorhynchus mykiss) primary hepatocytes | Montreal WWTP effluent | Gagné and Blaise (1995) |
| Genotoxicity (DNA damage) | Bacteria: Escherichia coli PQ 37 strain (SOS Chromotest) | Montreal WWTP effluent | White et al. (1996) |
| Acute and chronic toxicity | Algae: Selenastrum capricornutum | Montreal WWTP effluent including a series of representative municipal wastewater treatment plants (WWTPs) located in the province of Quebec | Env. Canada (1998) |
| Micro-invertebrates: Daphnia magna and Ceriodaphnia dubia | |||
| Fish: O. mykiss and Pimephales promelas | |||
| Toxic loading as determined by the PEEP effluent scalea; where several (geno)toxicity (acute, chronic) tests were conducted in the process | Bacteria: Photobacterium phosphoreum (now renamed Vibrio fisheri) | Montreal WWTP effluent | Env. Canada (1998) |
| Bacteria: E. coli PQ 37 strain (SOS Chromotest) | |||
| Algae: Selenastrum capricornutum | |||
| Micro-invertebrates: D. magna and C. dubia | |||
| Immunocompetence: assessment of phagocytosis activity of hemocytes by measuring their capacity to internalize fluorescent bacteria | Bivalves: Elliptio complanata | Montreal WWTP effluent | Blaise et al. (2002) |
| Acute and chronic toxicity assessment of pharmaceutical products | Acute/chronic toxicity testing with a suite of small-scale bioassays representing four taxonomic groups: | Montreal WWTP effluent | Blaise et al. (2006) |
| Bacteria (V. fisheri); | |||
| Algae (S. capricornutum); | |||
| Invertebrates (Hydra attenuata); | |||
| Fish (primary hepatocytes of O. mykiss) | |||
| Trophic chain transfer of carbamazepine (CBM) | Algae (primary producer): S. capricornutum; | Montreal WWTP effluent | Vernouillet et al. (2010) |
| Invertebrate (primary consumer): Thamnocephalus platyurus; | |||
| Invertebrate (secondary consumer): H. attenuata) |
aToxic loading is the product of the sum of toxic units (as determined by several bioassays representing three levels of biological organization) generated by an effluent and the effluent flow (measured in m3 h−1). The resulting PEEP index number is the log10 value of an effluent’s toxic loading (i.e., toxic loading = toxic units discharged to a receiving water system per cubic meter per hour). Refer to Costan et al. (1993) for details on the PEEP index
In the first investigation reported in 1995 on the Montreal WWTP effluent, rainbow trout (Oncorhynchus mykiss) primary hepatocytes exposed to SLR water samples collected at sites up to 8 km downstream in the effluent plume displayed a significant increase in cytotoxicity, mRNA metallothionein induction, and CYP1A1 activity in comparison with similar endpoints at the upstream site, thereby suggesting the presence of bioavailable metals and planar organic compounds (e.g., PAHs, PCBs) discharged by the WWTP effluent (Gagné and Blaise 1995). This effluent was shown to display significant genotoxic activity, as measured with the miniaturized SOS Chromotest conducted in 96-well microplates. Moreover, its genotoxic emission based on effluent flow to the SLR was estimated to be 51,297 g of Benzo [a] Pyrene equivalents day−1, a loading found to be higher than those of many industrial effluents discharging into this same aquatic ecosystem (White et al. 1996).
Acute and chronic toxicity data were later reported after conducting a suite of bioassays at different taxonomic levels on a series of representative municipal WWTPs located in the province of Québec, including the Montreal WWTP effluent. Effluents were tested for lethal toxicity on rainbow trout (Oncorhynchus mykiss) and water fleas (Daphnia magna), while effects on growth were measured in fathead minnow (Pimephales promelas), a water flea (Ceriodaphnia dubia), and an alga (Selenastrum capricornutum). In this investigation, Montreal WWTP effluent’s toxic loading to the SLR as determined by the potential ecotoxic effects probe (PEEP) index scale (see footnote, Table 2 for details) yielded a value of 5.9, indicating a relatively marked input of toxic emission to the SLR. For comparative purposes, the lowest and the highest toxic loadings among 50 major industrial effluents discharging into the SLR were 0 and 7.5, respectively (Env. Canada 1998).
Furthermore, hemocytes of bivalves exposed to the Montreal WWTP effluent for 96 h displayed a significant decrease in immunocompetence in relation to controls, suggesting the presence of immunotoxic compounds in this effluent. Hemocytes of bivalves exposed for 62 days in cages at sites located downstream of the effluent plume in the SLR displayed a significant decrease in immunocompetence in relation to the upstream site (Blaise et al. 2002), corroborating the 96-h laboratory exposure.
After demonstrating the presence in the Montreal WWTP effluents of 12 pharmaceuticals representing six classes of drugs, notably anti-inflammatory agents, lipid regulators, antibiotics, an anti-convulsant, a stimulant (caffeine), and a nicotine metabolite (cotinine), their relative toxicity levels were assessed with a suite of small-scale bioassays representing four taxonomic groups. Eleven of the 12 products examined (barring caffeine) had toxic effects ranging from “harmful” to “very toxic” based on a European toxicity scale. Their concentrations in the effluent, however, are far below those capable of eliciting acute toxicity effects to biota, although chronic effects for some could not be entirely ruled out (Blaise et al. 2006).
The bioaccumulation/biomagnification potential of the anti-convulsant carbamazepine (CBM), which is present in the Montreal WWTP effluent, was also appraised via a miniaturized experimental trophic chain developed in our laboratories. CBM, a relatively lipophilic pharmaceutical (Kow = 2.2), was shown to bio-accumulate in algae (the chlorophyte S. capricornutum) and was biomagnified to the primary consumer (the micro-crustacean Thamnocephalus platyurus) level. While CBM was then taken up by secondary consumers (the cnidarian Hydra attenuata) via dietary exposure to T. platyurus, CBM was shown to be biotransformed (degraded) by Hydra enzymatic oxidative metabolism suggesting that it would not biomagnify further (Vernouillet et al. 2010).
The Montreal WWTP effluent delivers a cocktail of chemicals (Pham et al. 1999; Sabik et al. 2004; Gobeil et al. 2005; Blaise et al. 2006). Unsurprisingly, and very likely owing to the complex chemical make-up of, and interactions within, such effluents, all bioassay results described above were in concordance in revealing their wide-ranging toxicity, evident at various taxonomic levels, and in displaying myriad acute and chronic sublethal and lethal effects (Table 2).
Field studies: Bivalves
Invertebrates comprise an important component of the aquatic fauna and are critical targets as bioindicators of pollution in aquatic ecosystems. The lifestyle of bivalves such as the unionid mussel Elliptio complanata, being a sedentary, long-lived filter feeder, makes it particularly susceptible to the effects of environmental pollutants.
This species was used extensively in cage studies in the field to evaluate the effects of the effluent on different aspects of mussel physiology and reproductive function (Figs. 3, 4a). Exposure of E. complanata to estrogenic compounds, which are present in many municipal effluents, induces the expression of vitellogenin (VTG) in both males and females. Evidence suggests that VTG expression is under the control of the estrogen receptor (estradiol-17β) and that this major egg yolk protein precursor plays a role in immunity in addition to serving as an energy reserve (Gagné et al. 2001). The long-term consequences of exposure to estrogenic compounds include intersex, feminization of males locally and general loss of male gamete quality which could indicate a risk for these and other sedentary organisms. Feral mussel samples collected at two downstream sites were 85 % female compared to 35–40 % at an upstream site adjacent to the SLR (Gagné et al. 2010). Increased VTG production was found in E. complanata of both sexes caged for one year upstream and 7 km downstream of the municipal effluent outflow, and the proportion of females increased from 41 to 67 % in mussels at the downstream exposure site compared to upstream (Blaise et al. 2003).
Fig. 4.
Schematic of multidisciplinary approaches to evaluating impacts of City of Montreal municipal effluents on aquatic fauna in the St. Lawrence River. a The unionid mussel Elliptio complanata; drawing by Aleta Karstad Schueler, © Canadian Museum of Nature. b The spottail shiner Notropis hudsonius; photograph by David J. Marcogliese
Extracts from water collected up to 5 km downstream from Montreal’s effluent outflow activated the serotonin receptor in mussels suggesting the presence of serotonin mimics in the effluents (Gagné et al. 2006). This is consistent with the presence of selective serotonin-reuptake inhibitors, which increase the effect of serotonin, in effluents (Lajeunesse et al. 2011). The estrogenic and serotonergic properties of the municipal effluents also caused important changes at the neurological level (Gagné et al. 2007, 2011a). Indeed, exposure to the treated effluent from the Montreal WWTP also led to an “excitotoxic” syndrome implicating perturbations in GABA, dopamine, and acetylcholine signaling. The enhanced turnover of dopamine also suggests disruption of opiate signaling, which may be immunosuppressive (Gagné et al. 2006, 2011a). Municipal effluents are also genotoxic to mussels and capable of blocking the production of DNA precursors, inhibiting DNA strand break repair and turnover of DNA (Gagné et al. 2011b).
Endocrine disruption in fish
In general, endocrine-disrupting chemicals (EDCs) from natural or industrial processes can be released into the aquatic ecosystem as a result of industrial (such as pulp and paper mill effluent) and municipal sewage wastewater discharge. Primary sewage wastewater effluents are known to contain high concentrations of estrogenic substances (Purdom et al. 1994; Sumpter 2005; Gregory et al. 2008), among which the most prevalent are the alkylphenols widely used as industrial surfactants and the synthetic and natural estrogens found in the urine of women (Gregory et al. 2008). Male rainbow trout (Oncorhynchus mykiss), exposed to wastewater effluent both in situ as well as under controlled laboratory conditions, show induced levels of VTG, an estrogen-regulated hepatic protein (Sumpter 2005). These estrogenic responses promote the inhibition of spermatogenesis and are suspected to be responsible for increases in hermaphroditism observed in fish living in a variety of aquatic ecosystems (Sumpter 2005; Sumpter and Johnson 2005).
In northern pike (Esox lucius) collected upstream and downstream of the Montreal effluent outfall, VTG gene expression in liver and VTG levels in plasma were correlated with PFC concentrations in plasma, suggesting their possible role as endocrine disruptors (Houde et al. 2013). In a more extensive study, spottail shiners were shown to be exposed to environmental estrogens in the SLR. This contamination begins approximately 20 km upstream from the Island of Montreal, and although it results from several inputs including the Ottawa River, sewage effluents from Montreal contribute the greatest charge in estrogenic compounds (Sabik et al. 2004; Aravindakshan et al. 2004a). Male spottail shiners captured near the WWTP discharge point exhibit elevated levels of VTG and altered reproductive functions, including delayed spermatogenesis, decreased sperm counts, altered sperm motility, and a significantly greater concentration of static sperm (Fig. 4b). Histological analyses of the testes of these fish indicate that almost 35 % of fish captured near sewage outfall exhibit intersex (Aravindakshan et al. 2004a). Spermatogenesis requires coordinated intercellular communication, as mediated by gap junctions shown to be essential for spermatogenesis in the mammalian testis. Gap junctions are composed of connexons, which are themselves formed by six transmembrane proteins termed connexins (Cxs). In order to understand the mechanism by which spermatogenesis in fish was altered by exposure to municipal wastewater effluent, rainbow trout were exposed under laboratory conditions for 4 and 12 weeks to various concentrations of municipal effluent (0, 1, 10, and 20 % v/v). Hepatic VTG mRNA levels were not different between exposure groups after 4 weeks but increased significantly after 12 weeks of exposure, suggesting that chemicals responsible for the estrogenic effects must bioaccumulate (de Montgolfier et al. 2008). After 12 weeks, spermatogenesis in the 1 % group was more advanced than in other groups, and testicular Cx43 mRNA levels were higher than controls at all doses, while Cx43.4 levels remained lower than controls. Cx31 mRNA levels were significantly lower in the 1 and 10 % groups than in the control and 20 % group. Thus, long-term exposure to environmental concentrations of wastewater effluent alters the expression of testicular Cxs and may contribute to effects observed on spermatogenesis in wild-caught spottail shiners.
Effects on fish immune function
Immune function was also altered in fish exposed naturally to Montreal municipal wastewater effluent (Ménard et al. 2010). In spottail shiners collected at upstream and downstream sites of the effluent discharge point, both resting and active phagocytic activities in leukocytes were significantly correlated with fecal coliform abundance in water (r = 0.62 and r = 0.54, respectively, at p < 0.01) suggesting that ambient bacterial concentrations stimulate phagocytic activity. A negative correlations of both resting and active phagocytosis activities against coliform levels reveal an immunosuppressive effect in shiners downstream of the city’s wastewater plume (Fig. 4b). Studies conducted under laboratory conditions in which rainbow trout were exposed to Montreal wastewater effluents confirmed the immunotoxic potential of these effluents (Salo et al. 2007; Escarné et al. 2008; Hébert et al. 2008). Tests of various fractions of effluents clearly showed that the soluble fraction from which bacteria and suspended materials were systematically removed retained immunotoxic effects, indicating that the immunotoxicity of the effluent was not due exclusively to its bacterial content (Hébert et al. 2008). Indeed, exposure to bacteria in effluents may stimulate the immune response and promote immunization against other pathogenic agents (Escarné et al. 2008; Ménard et al. 2010).
A series of experiments characterized the chronic toxicity of the effluents in juvenile female rainbow trout exposed under laboratory conditions to Montreal wastewater effluent for 1, 4, and 13 weeks to varying concentrations of sewage effluent (Salo et al. 2007; Hébert et al. 2008). Phagocytic activities of pronephric macrophages and of granulocytes were suppressed following a 1 week exposure, with the greatest suppressive effects being observed at the highest exposure concentration. Surprisingly, phagocytic activity returned to control levels or was enhanced after 4 weeks, but was reduced again at 13 weeks (Salo et al. 2007; Hébert et al. 2008). The cytotoxic activity of non-specific cytotoxic cells from the head kidney was enhanced after a 1-week exposure, especially at the lowest exposure concentration, but rebounded to control levels after 4 weeks (Salo et al. 2007). Lymphocyte proliferation in response to the mitogens lipopolysaccharide (LPS) and concanavilin A (ConA) activation was not affected following exposure to sewage effluents, but non-activated, spontaneous proliferation of lymphocytes was suppressed in a dose-dependent manner after 4 and 13 weeks of exposure (Salo et al. 2007; Hébert et al. 2008). Plasma lysozyme activity was elevated at the lowest exposure concentration after 4 weeks. The proportion of circulating granulocytes increased following exposure for 4 weeks to the low effluent concentration, but no change was observed in either the blood leukocyte/erythrocyte ratio or the proportion of lymphocytes and thrombocytes (Salo et al. 2007). Thus, various immune functions were differentially affected, depending on both the concentration and duration of exposure.
Mammalian exposure
It is known that some women of reproductive age consume considerable quantities of SLR fish (see above) and, as a result of this exposure, have elevated levels of circulating contaminants (Nadon et al. 2002). To assess the potential of EDCs to be passed up the food chain from fish to mammals, spottail shiners from a xenoestrogen-contaminated site resulting from CMM sewage effluent were fed to lactating female rats (Aravindakshan et al. 2004b). The dams were gavaged using a stomach tube three times a week with 1 % body weight of spottail shiners until weaning. When the male lactating pups reached adulthood, they displayed delayed spermatogenesis and decreased sperm counts and motility. Furthermore, there was a decrease in Cx43 in Sertoli cells suggesting decreased intercellular communication (Aravindakshan et al. 2004b). This effect on Cx43 appeared to result from exposure to alkylphenols, as opposed to an estrogenic effect of the effluent mixture (Aravindakshan and Cyr 2005). Immune function was also altered, as indicated by a significant decrease in the number of splenocytes in adult rats exposed to EDCs via maternal milk. Furthermore, phagocytic activity of the remaining splenocytes was also significantly decreased (Brousseau et al. 2007). These results suggest that EDC effects could be passed up the food chain, and therefore, represent a risk to humans and other fish-eating mammals that consume fish from xenoestrogen-contaminated aquatic ecosystems.
Multiple stressors and cumulative effects
Recent studies highlighted the potential for cumulative effects of multiple stressors in natural environments (Sih et al. 2004; Holmstrup et al. 2010). Not only are organisms in municipal effluents exposed to a smorgasbord of chemical contaminants, but they are also exposed to other natural and anthropogenic stressors in the ecosystem, including habitat modification and invasive species, among others. Parasites compose one such natural stressor, but are often ignored in studies of environmental effects. However, the synergistic effects of parasites and contaminants on hosts may be much greater than either stressor alone, with lethal or sublethal consequences (Marcogliese and Pietrock 2011).
A variety of studies in the SLR demonstrate that particular parasites have more pathological effects in polluted waters than in reference waters. In some cases, the parasites implicated did not appear to be measurably pathogenic in reference waters. Pigmented macrophages and their aggregates typically are found in greater numbers in fish tissues such as liver and spleen in polluted conditions (Agius and Roberts 2003), including spottail shiners downstream of Montreal (Thilakaratne et al. 2007). These cells and their aggregates are responsible for a variety of functions including detoxification, scavenging of materials, and responding to foreign particles or infectious agents (Agius and Roberts 2003). However, macrophage number in the spleens of spottail shiners increase with infection intensity of the gall bladder trematode Plagioporus sinitsini (Fig. 4b) in polluted waters, including those downstream of the municipal effluents, but not at reference sites (Thilakaratne et al. 2007). In that same study, fish condition also decreased with infection intensity, but again only at polluted sites.
Oxidative stress results when the accumulation of reactive oxygen species, which are toxic products induced by exposure to contaminants and inflammation, exceeds the capacity of the organism to metabolize them (Sorci and Faivre 2009). Measuring activity of different enzymes involved in oxidative stress metabolism, Marcogliese et al. (2010) found that oxidative stress increased in yellow perch infected with two different parasites, but again, only at polluted sites and not reference sites. Activity of glutathione reductase decreased in the gills at polluted sites with increasing numbers of the blackspot trematode (Apophallus brevis), but not at reference sites. Intensity of another parasite, the eyefluke Diplostomum spp., was correlated with catalase activity in the head kidney at polluted sites, including within the municipal effluents, but not at reference sites (Marcogliese et al. 2010).
Results from these studies suggest that exposure to municipal contaminants affects tolerance to parasites, but not resistance (Marcogliese et al. 2010; Marcogliese and Pietrock 2011). Essentially, resistance in a host limits the numbers of parasites, while tolerance limits the pathological damage inflicted by parasites (Råberg et al. 2009). Decreased tolerance is inferred by an increase in disease severity at a given infection level (Råberg et al. 2009). For the most part, macroparasites are not more prevalent or abundant in fishes from waters exposed to effluents in the SLR (Marcogliese et al. 2006, 2010; Krause et al. 2010). These observations suggest that resistance to parasites in fish may not be affected by exposure to municipal water pollution. However, increased pathology but not intensity of parasites implies an effect on tolerance (Råberg et al. 2009). Studies in the SLR suggest that the resistance–tolerance distinction may be a useful conceptual framework to examine immunosuppression in polluted habitats (Marcogliese et al. 2010; Marcogliese and Pietrock 2011).
Ecological effects
Most ecological studies in the SLR have concentrated on the fluvial lakes (Lake Saint-François, Lake Saint-Louis, Lake Saint-Pierre). Few studies address the fluvial corridor between the Island of Montreal and Lake Saint-Pierre, while fewer still examined organisms within the city’s effluents.
Primary producers
Free-floating (planktonic) and attached (periphytic) algae represent the first line of indicator species of nutrient enrichment originating from wastewater discharge. The plume of the WWTP effluent can be traced at least 7 km downstream, from elevated fecal coliform (23-fold increase), NH3–N (3.8-fold increase), and TP (1.4-fold increase) concentrations in comparison with waters from the navigation channel (Hudon and Sylvestre 1998; Vis et al. 1998a). In spite of exposure to the effluent, phytoplankton biomass (chlorophyll a concentration) and species composition at the same site did not differ significantly from those at other stations within the same water mass (Hudon 2000), most likely because of very short exposure time (<4 h), resulting from rapid dilution and advection of plume waters (Hudon and Sylvestre 1998).
In contrast, attached filamentous algae on navigation buoys chronically exposed to the effluent of the WWTP were dominated by the cyanobacterium Plectonema notatum, an indicator species (Vis et al. 1998a). However, the level of exposure to sewage-polluted waters was not reflected by the biomass and diversity indices of periphytic algae (Vis et al. 1998a). Yet, over the 1978–1995 period, reductions in phosphorus loadings originating from Lake Ontario resulted in a decrease in biomass of attached algae, particularly noticeable for filamentous algae (Cladophora sp.) in the greater Montreal area (Vis et al. 1998b).
Macroinvertebrates
Few studies have examined macroinvertebrate productivity or diversity within the effluent plume. Macroinvertebrate communities within the wastewater plume are characterized by the variations in the presence of certain nematodes, flatworms, poriferans, dipterans (Chironomus, Dicrotendipes, Tribelos), gastropods (Bithynia), isopods (Caecidatea, Lirceus), and tubificid oligochaetes (Hyodrilus, Quistadrilus and Limnodrilus) (Masson et al. 2010). The oligochaetes, in particular, are tolerant of organic pollution (Aston 1973). While benthic communities in the SLR are influenced by sulfur, DOC, and contaminants in sediments, as well as water mass, the communities in the effluent plume could not be distinguished from other contaminated sites located in Montreal Harbor and other areas of the SLR. Stable isotope analyses of carbon and nitrogen reveal that macroinvertebrates within the effluent plume downstream of Montreal consume sewage-derived resources, whereas those outside the plume-ingested epiphytic resources (deBruyn and Rasmussen 2002; deBruyn et al. 2003). The amphipod Gammarus fasciatus, a detritivore deposit feeder, the invasive bivalve zebra mussel (Dreissena polymorpha, a suspension feeder), and the snail Bithynia tentaculata (a detritivore/deposit feeder and facultative suspension feeder) were all found to preferentially consume sewage-derived particulate organic matter within the effluent plume (deBruyn and Rasmussen 2002). Furthermore, productivity of macroinvertebrates was 1.8–4.1 times higher within the effluents compared to nearby sites outside the effluents. Composition of the macroinvertebrate assemblage changed within the effluent, with chironomids almost doubling in importance compared to that outside the effluents (deBruyn et al. 2003).
Fish
Aside from bioaccumulation studies, the discharge of urban effluents in the Montreal area affects fish condition and fish health. The fish condition factor (K = W/L3), used as a general index of fish physiological status, indicates that fish are bigger at comparable length presumably as a result of better individual growth. Values of this index found to be significantly larger in fish exposed to Montreal urban effluents. Mikaelian et al. (2000) reported that large white sucker (Catostomus commersonii) (>350 mm; corresponding to 4 years and older) from the Montreal area had significantly higher K values (mean = 1.44) than fish collected from sites much further downstream in Lake St. Pierre (mean K = 1.27) or near Quebec City (mean K = 1.05). Similarly, Ion et al. (1997) reported that yellow perch collected at the east end of Montreal Island in 1989 had significantly higher condition factor (mean K = 1.73) than specimens collected either upstream in Lake Saint-Louis (mean K = 1.58) and the Laprairie Basin (mean K = 1.56) or downstream in Lake Saint-Pierre (mean K = 1.39). However, Ion et al. (1997) found no significant differences in both Hg and PCB levels in perch from the various sectors and could not relate the variation in K values to contaminant levels. In contrast, analyses of six fish species collected in 1989 revealed no significant differences in K-values among fluvial lakes in any of the species (Ion and Lafontaine 1998).
Results from these three studies suggest that higher fish condition factor appears to be spatially confined to fish captured in the vicinity of the urban effluents discharged at the eastern end of Montreal, and does not extend far downstream. Evidence of higher K-values indicates that fish are heavier at a similar size, which suggests that the presence of urban effluents enhance fish growth rate and productivity relative to other sectors. Such an increase in fish condition therefore would be explained by enhanced productivity in urban effluents waters rather than by toxic effects, although enhanced growth could increase exposure to contaminants. However, a link between greater condition factor (and thus, growth) and higher contaminant concentration is not obvious, because contaminant levels in fish are routinely standardized to eliminate size and weight effects for comparative purposes.
Sewage wastewater effluent appears to affect the size distribution of fishes within the SLR, with greater productivity and larger individuals predominating at sewage-impacted sites compared to other sites downstream of Montreal (deBruyn et al. 2002). However, this effect appeared to be limited to larger size classes of fish. Only the largest size classes of fish have higher densities in sewage-enriched waters. Daily fish production increases in the effluent 1.3–4.4 times for algivores and detritivores, 1.7–10 times for invertivores, and 11–73 times for piscivores (deBruyn et al. 2003). Secondary productivity increases overall fivefold within the effluent plume versus outside the plume.
Fish parasites
Numerous studies over the last two decades suggest that parasites may be useful indicators of environmental stress, food web structure, and biodiversity (Marcogliese 2004, 2005; Lafferty 2008). Parasite communities of different fishes in the SLR show a common pattern with respect to the municipal effluents. Species richness in spottail shiners and mean number of parasite species per fish tend to be low at Îlet Vert immediately downstream of the effluent outflow compared to other localities in the river (Marcogliese et al. 2006; Fig. 4b). The pattern is even more pronounced in johnny darters (Etheostoma nigrum), where the mean species richness and number of parasites per fish are significantly lower than those at other sites (Krause et al. 2010). Species richness in yellow perch at Îlet Vert is markedly lower than that at other localities (Marcogliese et al. 2010). A number of factors may be at play here. Wetland area is limited in the channel downstream of Montreal, and diversity of both parasites (Marcogliese et al. 2006) and their fish hosts (La Violette 2004; Environment Canada, Biodiversity Portrait of the St. Lawrence http://www.qc.ec.gc.ca/faune/biodiv/en/fish/fw_local_richness.html) tends to be higher in Lake Saint-Louis, upstream of Montreal, which includes extensive wetlands (Marcogliese et al. 2006, 2010; Krause et al. 2010). However, food webs are trophically compressed within the municipal effluents, with detritivory being a common mode of feeding among invertebrates and fish (deBruyn et al. 2003). Such a food web structure also may contribute to limiting parasite diversity by restricting opportunities for transmission.
In contrast to the macroparasites (helminths and arthropods), the myxozoans show a very different pattern. Prevalence of infection and mean species richness per host in spottail shiners increase in waters exposed to municipal effluents downstream of Montreal compared to those in fish collected upstream (Marcogliese et al. 2009). Myxozoans typically have complex life cycles that include both oligochaetes and fish. Oligochaete density increases at sites exposed to municipal effluents downstream of Montreal compared to those upstream (Marcogliese et al. 2009; Masson et al. 2010). Thus, myxozoans are good indicators of organic pollution and eutrophication. However, the situation is further complicated by river hydrology. When pooling across sites on an annual basis, the mean number of parasite species per fish is inversely proportional to the mean water flow 1 month prior to sampling. Low water levels, and thus low flow rates, promote transmission of myxozoans (Marcogliese et al. 2009). Thus, hydrological conditions in the SLR, which are dictated by climate, superimpose their effects regionally over pollution-induced effects that manifest on a local scale.
Conclusions, recommendations, and future perspectives
New information details acquired over the last decade suggest that exposure to the Montreal municipal effluents induces a variety of long-term or chronic effects, likely inter-related, across multiple physiological systems in both vertebrates and invertebrates. However, information gaps still remain concerning fish contamination and fish ecotoxicology in the SLR and Montreal area. While the levels of some persistent contaminants (i.e., Hg, PCBs) have significantly declined over the last 40 years, many other classes of toxic contaminants are still poorly studied or even unquantified (e.g., new pharmaceuticals, perfluorinated compounds, and nanotechnology products). As a result, actual bioaccumulation data do not provide strong evidence to support the hypothesis of severe and detrimental effects of contaminant loadings from the discharge of urban wastewaters on SLR fish. For example, while endocrine disruption can be considered high in fish downstream of the Montreal area, the evidence for significant effects at the population level is lacking, due in part to the absence of adequate population-monitoring studies. Further research is required to confirm mechanisms of toxicity and bioaccumulation of pharmaceuticals and other emerging contaminants in aquatic organisms.
An important avenue of research, highlighted by some of the results described herein, manifests itself in a need to develop a better understanding of the distinction between ecological and ecotoxicological impacts of the municipal effluents on the SLR ecosystem and the inter-relationship between the two. Without this knowledge, it is possible to misinterpret the cause of particular effects and ignore their potential interactions. For example, the increased prevalence and species richness of myxozoan parasites downstream could mistakenly be attributed to a compromised immune response in the fish, and not organic enrichment. Such misunderstandings may impede the decision-making process for better management of the SLR ecosystem. Indeed, higher level effects observed on populations and communities of organisms exposed to WWTP effluents in the SLR may actually be ecological, rather than toxicological, or a combination of the two.
Research on the cumulative effects of urban pollution in the context of climate change surely represents an area of relevant interest. We need to develop a better understanding of the cumulative effects of environmental stressors on aquatic life at scales ranging from the cell to the ecosystem to better protect aquatic organisms, populations, and communities. Indeed, organisms have to cope with many anthropogenic stressors such as contaminants, eutrophication, habitat loss, and invasive species, in addition to normal natural stressors such as predation, competition, parasitism, and disease. Climate change will alter SLR hydrology, with cascading impacts on water levels and flow rates, water chemistry, toxicity, habitat structure, and biotic interactions.
In coming years, the City of Montreal will be upgrading its sewage treatment plant to include disinfection and decontamination, at least to some extent, of effluents using ozonation processes. While ozonation will modify the effluent, at this time, it proves difficult to predict how the effluent will be modified with respect to its overall toxicity and concomitant impact on the SLR. Regardless, these changes will certainly have a powerful ecological impact on the SLR downstream of Montreal. The series of studies described herein provide important groundwork for before-and-after studies on the effects of the improved effluent treatment on the SLR ecosystem. Such studies are imperative in order to protect and conserve this majestic river ecosystem and its natural resources for future generations.
Acknowledgments
Funding for this research was provided by Environment Canada’s St. Lawrence Action Plan and Chemical Management Plan, the Canadian Network of Toxicology Centers, and the Canada Research Chair in Immunotoxicology. Numerous technical staff, undergraduate students, and graduate students worked on the various studies. In particular, the authors wish to thank Michel Arseneau, Germain Brault, Jean-Pierre Cloutier, Simon-Pierre Despatie, Andrée Gendron, Andre Lajeunesse, Claude Lessard, Sophie Trépanier, and Patrice Turcotte for their technical and professional assistance in the field and in the laboratory. The comments of two anonymous reviewers are greatly appreciated.
Biographies
David Marcogliese
is a research scientist at the St. Lawrence Centre, Environment Canada, in Montreal, Canada. His research interests include the effects of pollution and anthropogenic activity on host–parasite systems.
Christan Blaise
is a research scientist (emeritas) at the St. Lawrence Centre, Environment Canada, in Montreal, Canada. His research interests include the use of bioassays to evaluate effects of pollution.
Daniel Cry
is a professor at INRS-Institut Armand Frappier in Laval, Canada. His research interests include the effects of pollution on endocrine function in fish and mammals.
Yves de Lafontaine
is Sciences Director, Quebec region, Department of Fisheries and Oceans Canada, in Mont-Joli, Canada. His research interests include the ecology of large river ecosystems.
Michel Fournier
is a professor at INRS-Institut Armand Frappier in Laval, Canada. His research interests include the effects of pollution on immune function in aquatic animals.
François Gagné
is a research scientist at the St. Lawrence Centre, Environment Canada, in Montreal, Canada. His research interests include the biochemical ecotoxicology of pollution in aquatic ecosystems.
Christian Gagnon
is a research scientist at the St. Lawrence Centre, Environment Canada, in Montreal, Canada. His research interests include the study of the fate and transformation of metals and emerging substances in aquatic ecosystems.
Christiane Hudon
is a research scientist at the St. Lawrence Centre, Environment Canada, in Montreal, Canada. Her research interests include the study of primary production and nutrient flow in large river ecosystems.
References
- Agius C, Roberts RJ. Melano-macrophage centres and their role in fish pathology. Journal of Fish Diseases. 2003;26:499–509. doi: 10.1046/j.1365-2761.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- Aravindakshan J, Cyr DG. Nonylphenol alters connexin 43 levels and connexin 43 phosphorylation via an inhibition of the p38-MAP kinase pathway. Biology of Reproduction. 2005;72:1232–1240. doi: 10.1095/biolreprod.104.038596. [DOI] [PubMed] [Google Scholar]
- Aravindakshan J, Gregory M, Marcogliese DJ, Fournier M, Cyr DG. Consumption of xenoestrogen-contaminated fish during lactation alters adult male reproductive function. Toxicological Sciences. 2004;81:179–189. doi: 10.1093/toxsci/kfh174. [DOI] [PubMed] [Google Scholar]
- Aravindakshan J, Paquet V, Gregory M, Dufresne J, Fournier M, Marcogliese DJ, Cyr DG. Consequences of xenoestrogen exposure on male reproductive function in spottail shiners (Notropis hudsonius) Toxicological Sciences. 2004;78:156–165. doi: 10.1093/toxsci/kfh042. [DOI] [PubMed] [Google Scholar]
- Aston RJ. Tubificids and water quality: a review. Environmental Pollution. 1973;5:1–10. doi: 10.1016/0013-9327(73)90050-5. [DOI] [Google Scholar]
- Bernachez, L. and M. Giroux. 2012. Les poissons d’eau douce du Québec et leur répartition dans l’est du Canada. Editions Broquet Inc., Saint-Constant, QC, 348 pp.
- Blaise C, Trottier S, Gagné F, Lallement C, Hansen PD. Immunocompetence of bivalve hemocytes by a miniaturized phagocytosis assay. Environmental Toxicology. 2002;17:160–169. doi: 10.1002/tox.10047. [DOI] [PubMed] [Google Scholar]
- Blaise C, Gagné F, Salazar M, Salazar S, Trottier S, Hansen P-D. Experimentally-induced feminisation of freshwater mussels after long-term exposure to a municipal effluent. Fresenius Environmental Bulletin. 2003;12:865–870. [Google Scholar]
- Blaise C, Gagné F, Eullaffroy P, Férard JF. Ecotoxicity of selected pharmaceuticals of urban origin discharged to the Saint-Lawrence River (Québec, Canada): A review. Brazilian Journal of Aquatic Sciences and Technology. 2006;10:29–51. doi: 10.14210/bjast.v10n2.p29-51. [DOI] [Google Scholar]
- Brochu C, Hamelin G, Moore S, Laliberté D, de Lafontaine Y. Contribution to PCDD/PCDFs and ortho-substituted PCB congeners to the total TCDD-equivalent concentration in fish fillet from the St. Lawrence River. Organohalogen Compounds. 1995;26:293–298. [Google Scholar]
- Brousseau P, Ménard L, Aravindakshan JP, Gregory M, Marcogliese D, Cyr DG, Fournier M. Effets d’une diète de poissons provenant d’un milieu contaminé par des xénoestrogènes sur le développement post-natal du système immunitaire de rats mâles. Bulletin de la Société zoologique de France. 2007;132:147–158. [Google Scholar]
- Champoux L, Moisey J, Muir DCG. Polybrominated diphenyl ethers, toxaphenes, and other halogenated organic pollutants in great blue heron eggs. Environmental Toxicology and Chemistry. 2010;29:243–249. doi: 10.1002/etc.37. [DOI] [PubMed] [Google Scholar]
- Chan HM, Trifonopoulos M, Ing A, Receveur O, Johnson E. Consumption of freshwater fish in Khanawake: Risks and benefits. Environmental Research Section A. 1999;80:S213–S222. doi: 10.1006/enrs.1998.3930. [DOI] [PubMed] [Google Scholar]
- Chen DRJ, Letcher NM, Burgess L, Champoux JE, Elliott CE, Hebert P, Martin M, Wayland M, Weseloh DVC, Wilson L. Flame retardants in eggs of four gull species (Laridae) from breeding sites spanning Atlantic to Pacific Canada. Environmental Pollution. 2012;168:1–9. doi: 10.1016/j.envpol.2012.03.040. [DOI] [PubMed] [Google Scholar]
- Costan G, Bermingham N, Blaise C, Férard JF. Potential ecotoxic effects probe (PEEP): A novel index to assess and compare the toxic potential of industrial effluents. Environmental Toxicology and Water Quality. 1993;8:115–140. doi: 10.1002/tox.2530080202. [DOI] [Google Scholar]
- de Lafontaine Y, Gilbert NL, Dumouchel F, Brochu C, Moore S, Pelletier E, Dumont P, Branchaud A. Is chemical contamination responsible for the decline of the copper redhorse (Moxostoma hubbsi), an endangered fish species in Canada? Science of the Total Environment. 2002;298:25–44. doi: 10.1016/S0048-9697(02)00166-3. [DOI] [PubMed] [Google Scholar]
- de Montgolfier B, Fournier M, Audet C, Marcogliese D, Cyr DG. Influence of municipal effluents on connexin expression in the brook trout (Salvelinus fontinalis) testis. Aquatic Toxicology. 2008;86:38–48. doi: 10.1016/j.aquatox.2007.09.013. [DOI] [PubMed] [Google Scholar]
- deBruyn AMH, Rasmussen JB. Quantifying assimilation of sewage-derived organic matter by riverine benthos. Ecological Applications. 2002;12:511–520. doi: 10.1890/1051-0761(2002)012[0511:QAOSDO]2.0.CO;2. [DOI] [Google Scholar]
- deBruyn AMH, Marcogliese DJ, Rasmussen JB. Altered body size distributions in a large river fish community enriched by sewage. Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:819–828. doi: 10.1139/f02-056. [DOI] [Google Scholar]
- deBruyn AMH, Marcogliese DJ, Rasmussen JB. The role of sewage in a large river foodweb. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1332–1344. doi: 10.1139/f03-114. [DOI] [Google Scholar]
- DesGranges J-L, Jobin B. Knowing, mapping and understanding St. Lawrence biodiversity, with special emphasis on bird assemblages. Environmental Monitoring and Assessment. 2003;88:177–192. doi: 10.1023/A:1025564922626. [DOI] [PubMed] [Google Scholar]
- Environnement Canada et Ministère de l’Environnement et de la Faune du Québec. 1998. Evaluation de la toxicité des effluents des stations d’épuration municipales du Québec. Saint-Laurent Vision 2000, Montreal, Canada, 88 pp + annexes.
- Escarné R, Gagné F, Dautremepuits C, Cyr DG, Finnson K, Marcogliese DJ, Bernier J, Cejka P, Fournier M. Effects of municipal sewage effluent on non-specific immune and thyroid functions of rainbow trout (Oncorhynchus mykiss) Trends in Comparative Biochemistry & Physiology. 2008;13:27–39. [Google Scholar]
- Gagné F, Blaise C. Fluorescent in situ hybridization En Suspension (FISHES) procedure using biotin-labelled DNA probes for measuring genetic expression of metallothionein and cytochrome P-450 IA1 in rainbow trout hepatocytes exposed to wastewaters. In: Richardson M, editor. Environmental toxicology assessment. London: Taylor & Francis; 1995. pp. 41–54. [Google Scholar]
- Gagné F, Blaise C, Salazar M, Hansen P. Evaluation of estrogenic effects of municipal effluents to the freshwater mussel Elliptio complanata. Comparative Biochemistry and Physiology, Part C. 2001;128:213–225. doi: 10.1016/S1096-4959(00)00312-2. [DOI] [PubMed] [Google Scholar]
- Gagné F, Blaise C, André C, Salazar M. Effects of municipal effluents and pharmaceutical products on temperature-dependent mitochondrial electron transport activity in Elliptio complanata mussels. Comparative Biochemistry and Physiology, Part C. 2006;143:388–393. doi: 10.1016/j.cbpc.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gagné F, Cejka P, André C, Hausler R, Blaise C. Neurotoxicological effects of a primary and ozonated treated wastewater on freshwater mussels exposed to an experimental flow-through system. Comparative Biochemistry and Physiology, Part C. 2007;146:460–470. doi: 10.1016/j.cbpc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Gagné F, Bouchard B, André C, Farcy E, Fournier M. Evidence of feminization in wild Elliptio complanata mussels in the receiving waters downstream of a municipal effluent outfall. Comparative Biochemistry and Physiology, Part C. 2010;153:99–106. doi: 10.1016/j.cbpc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Gagné F, André C, Cejka P, Hausler R, Fournier M. Evidence of neuroendocrine disruption in freshwater mussels exposed to municipal wastewaters. Science of the Total Environment. 2011;409:3711–3718. doi: 10.1016/j.scitotenv.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Gagné F, André C, Cejka P, Hausler R, Fournier M. Alterations in DNA metabolism in Elliptio complanata mussels after exposure to municipal effluents. Comparative Biochemistry and Physiology, Part C. 2011;154:100–107. doi: 10.1016/j.cbpc.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Lajeunesse A. Low removal of acidic and hydrophilic pharmaceutical products by various types of municipal wastewater treatment plants. Xenobiotics Journal. 2012;2:e3. doi: 10.4081/xeno.2012.e3. [DOI] [Google Scholar]
- Gagnon C, Saulnier I. Distribution and fate of metals in the dispersion plume of a major municipal effluent. Environmental Pollution. 2003;124:47–55. doi: 10.1016/S0269-7491(02)00433-5. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Turcotte P. Role of colloids in the physical speciation of metals in the dispersion plume of a major municipal effluent. Journal of Water Science. 2007;20:275–285. [Google Scholar]
- Gagnon C, Gagné F, Turcotte P, Saulnier I, Blaise C, Salazar M, Salazar S. Metal exposure to caged mussels in a primary-treated municipal wastewater plume. Chemosphere. 2006;62:998–1010. doi: 10.1016/j.chemosphere.2005.06.055. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Turcotte P, Vigneault B. Comparative study of the fate and mobility of metals discharged in mining and urban effluents using sequential extractions on suspended solids. Environmental Geochemical and Health. 2009;31:657–671. doi: 10.1007/s10653-008-9223-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Ac A, Segura PA, Gagnon C, Sauvé S. Determination of bezafibrate, methotrexate, cyclophosphamide, orlistat and enalapril in waste and surface waters using on-line solid solid-phase extraction liquid chromatography coupled to polarity-switching electrospray tandem mass spectrometry. Journal of Environmental Monitoring. 2009;11:830–838. doi: 10.1039/b817570e. [DOI] [PubMed] [Google Scholar]
- Garcia-Ac A, Segura PA, Viglino L, Fürtos A, Gagnon C, Prévost M, Sauvé S. On-line solid-phase extraction of large-volume injections coupled to liquid chromatography-tandem mass spectrometry for the quantification and confirmation of 14 selected trace organic contaminants in drinking and surface water. Journal of Chromatography A. 2009;1216:8518–8527. doi: 10.1016/j.chroma.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Letcher RJ, Burgess NM, Champoux L, Elliot JE, Hebert CE, Martin P, Wayland M, Weseloh DVC, Wilson L. Perfluoralkyl carboxylates and sulfonates and precursors in relation to dietary source tracers in the eggs of four species of gulls (Larids) from breeding sites spanning Atlantic to Pacific Canada. Environment International. 2011;37:1175–1182. doi: 10.1016/j.envint.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Gentes ML, Letcher RJ, Caron-Beaudoin É, Verreault J. Novel flame retardants in urban-feeding Ring-billed gulls from the St. Lawrence River, Canada. Environmental Science & Technology. 2012;46:9735–9744. doi: 10.1021/es302099f. [DOI] [PubMed] [Google Scholar]
- Gobeil C, Rondeau B, Beaudin L. Contribution of municipal effluents to metal fluxes in the St. Lawrence River. Environmental Science and Technolology. 2005;39:456–464. doi: 10.1021/es049335x. [DOI] [PubMed] [Google Scholar]
- Gregory M, Aravindakshan J, Nadzialeck S, Cyr DG. Effects of endocrine disrupting chemicals on male reproduction. In: Alavi H, Raffiee R, Cosson J, Coward K, editors. Fish spermatology. New Delhi: Narosa Publishing House; 2008. pp. 161–214. [Google Scholar]
- Guay, G., and J. Dandurand. 1986. Utilisation des jeunes poissons de l’année comme bioindicateurs des substances toxiques dans le fleuve Saint-Laurent (Tronçon fluvial: Cornwall/Portneuf). Environnement Canada, Direction générale des Eaux Intérierues, Région du Québec. 133 pp.
- Hébert N, Gagné F, Cejka P, Cyr D, Marcogliese DJ, Blaise C, Pellerin J, Fournier M. The effects of a primary-treated municipal effluent on the immune system of rainbow trout (Oncorhynchus mykiss): Exposure duration and contribution of suspended particles. Comparative Biochemistry and Physiology, Part C. 2008;148:258–264. doi: 10.1016/j.cbpc.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Holmstrup M, Bindesbøl A-M, Oostingh GJ, Duschl A, Scheil V, Köhler H-R, Loureio S, Soares AMVM, et al. Interactions between effects of environmental chemicals and natural stressors: A review. Science of the Total Environment. 2010;408:3746–3762. doi: 10.1016/j.scitotenv.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Houde M, Douville M, Despatie S, De Silva AO, Spencer C. Induction of gene responses in St. Lawrence River northern pike (Esox lucius) environmentally exposed to perfluorinated compounds. Chemosphere. 2013;92:1195–1200. doi: 10.1016/j.chemosphere.2013.01.099. [DOI] [PubMed] [Google Scholar]
- Houde M, Berryman D, de Lafontaine Y, Verreault J. Novel brominated flame retardants and dechloranes in three fish species from the St. Lawrence River, Canada. Science of the Total Environment. 2014;479–480:48–56. doi: 10.1016/j.scitotenv.2014.01.105. [DOI] [PubMed] [Google Scholar]
- Hudon C. Metal accumulation in American wild celery (Vallisneria americana Michx.) in the St. Lawrence River: Effects of water depth and exposure to current. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:2317–2328. doi: 10.1139/f98-114. [DOI] [Google Scholar]
- Hudon C. Phytoplankton assemblages in the St. Lawrence River, downstream of its confluence with the Ottawa River, Quebec, Canada. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:16–30. doi: 10.1139/f99-228. [DOI] [Google Scholar]
- Hudon, C., and A. Sylvestre. 1998. Qualité de l’eau en aval de l’île de Montréal, 1994–1996. Environment Canada, St. Lawrence Centre, Scientific and Technical Report ST-170, Montreal, 338 pp + app.
- Ion, J., and Y. de Lafontaine. 1998. Spatial variation of contaminant levels in six species of fish from the St. Lawrence River. Environment Canada—Quebec Region, Environmental Conservation, St. Lawrence Centre, Scientific and Technical Report ST-165E, Montreal, 74 pp + app.
- Ion J, de Lafontaine Y, Dumont P, Lapierre L. Contaminant levels in the St. Lawrence River yellow perch (Perca flavescens): spatial variation with implications for monitoring. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:2930–2946. doi: 10.1139/f97-198. [DOI] [Google Scholar]
- Kosatsky T, Przybysz R, Armstrong B. Mercury exposure in Montrealers who eat St. Lawrence River sportfish. Environmental Research. 2000;84:36–43. doi: 10.1006/enrs.2000.4073. [DOI] [PubMed] [Google Scholar]
- Krause RJ, McLaughlin JD, Marcogliese DJ. Parasite fauna of Etheostoma nigrum (Percidae: Etheostomatinae) in localities of varying pollution stress in the St. Lawrence River, Quebec, Canada. Parasitology Research. 2010;107:285–294. doi: 10.1007/s00436-010-1862-6. [DOI] [PubMed] [Google Scholar]
- La Violette N. Les communautés de poissons d’eau douce: un indicateur de l’état du fleuve Saint-laurent. Vecteur Environnement. 2004;37:28–33. [Google Scholar]
- Lafferty KD. Ecosystem consequences of fish parasites. Journal of Fish Biology. 2008;73:2083–2093. doi: 10.1111/j.1095-8649.2008.02059.x. [DOI] [Google Scholar]
- Lajeunesse A, Gagnon C. Determination of acidic pharmaceuticals and carbamazepine in roughly treated sewage by solid phase extraction and gas chromatography–tandem mass spectrometry. International Journal of Environmental Analytical Chemistry. 2007;87:565–578. doi: 10.1080/03067310701189083. [DOI] [Google Scholar]
- Lajeunesse A, Gagnon C, Sauvé S. Determination of antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography–tandem mass spectrometry. Analytical Chemistry. 2008;80:5325–5333. doi: 10.1021/ac800162q. [DOI] [PubMed] [Google Scholar]
- Lajeunesse A, Gagnon C, Gagné F, Louis S, Cejka P, Sauvé S. Distribution of antidepressants and their metabolites in brook trout exposed to municipal wastewaters before and after ozone treatment. Evidence of biological effects. Chemosphere. 2011;83:564–571. doi: 10.1016/j.chemosphere.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Laliberté, D. 2003. Évolution des teneurs en mercure et en BPC de quatre espèces de poissons du Saint-Laurent, 1976–1997. Québec, ministère de l’Environnement, Direction du suivi de l’état de l’environnement, envirodoq no EN/2003/0287, Quebec City, 85 pp. + 6 annexes.
- Laliberté, D. 2011. Teneurs en polybromodiphényléthers (PBDE) dans les poissons du fleuve Saint-Laurent et des lacs et rivières du Québec (2002–2008). Québec, ministère du Développement durable, de l’Environnement et des Parcs, Direction du suivi de l’état de l’environnement, Quebec City, 48 pp. ISBN 978-2-550-60987-2.
- Marcogliese DJ. Parasites: Small players with crucial roles in the ecological theatre. EcoHealth. 2004;1:151–164. doi: 10.1007/s10393-004-0028-3. [DOI] [Google Scholar]
- Marcogliese DJ. Parasites of the superorganism: Are they indicators of ecosystem health? International Journal of Parasitology. 2005;35:705–716. doi: 10.1016/j.ijpara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ, Pietrock M. Combined effects of parasites and contaminants on animal health: Parasites do matter. Trends in Parasitology. 2011;27:123–130. doi: 10.1016/j.pt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ, Gendron AD, Plante C, Fournier M, Cyr D. Parasites of spottail shiners (Notropis hudsonius) in the St. Lawrence River: Effects of municipal effluents and habitat. Canadian Journal of Zoology. 2006;84:1461–1481. doi: 10.1139/z06-088. [DOI] [Google Scholar]
- Marcogliese DJ, Gendron AD, Cone DK. Impact of municipal effluents and hydrological regime on myxozoan parasite communities of fish. International Journal of Parasitology. 2009;39:1345–1351. doi: 10.1016/j.ijpara.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ, Dautremepuits C, Gendron AD, Fournier M. Interactions between parasites and pollutants in yellow perch (Perca flavescens) in the St. Lawrence River, Canada: Implications for resistance and tolerance to parasites. Canadian Journal of Zoology. 2010;88:247–258. doi: 10.1139/Z09-140. [DOI] [Google Scholar]
- Masson S, Desrosiers M, Pinel-Alloul B, Martel L. Relating macroinvertebrate community structure to environmental characteristics and sediment contamination at the scale of the St. Lawrence River. Hydrobiologia. 2010;647:35–50. doi: 10.1007/s10750-009-9915-5. [DOI] [Google Scholar]
- Ménard L, Escarné R, Marcogliese DJ, Cyr D, Fournier M, Gagné F. The impacts of urban pollution on the immune system of spottail shiners (Notropis hudsonius) in the St. Lawrence River. Fresenius Environmental Bulletin. 2010;19:1369–1374. [Google Scholar]
- Mikaelian, I., Y. de Lafontaine, P. Gagnon, C. Ménard, Y. Richard, L. Pelletier, Y. Mailhot, P. Dumont, and D. Martineau. 2000. Prevalence of naturally occurring lip neoplasms of white suckers (Catostomus commersoni) in the St. Lawrence River basin. Canadian Journal of Fisheries and Aquatic Sciences 57: 174–181.
- Moreira, J. 2011. Évaluation de la performance des ouvrages municipaux d’assainissement des eaux pour l’année 2010. Ouvrages de surverse et stations d’épuration. Rapport du Ministère des Affaires Municipales, Régions et occupation du territoire (MAMROT), Québec, 194 pp.
- Nadon S, Kosatsky T, Przybysz R. Contaminant exposure among women of childbearing age who eat St. Lawrence River sport fish. Archives of Environmental Health. 2002;57:473–481. doi: 10.1080/00039890209601440. [DOI] [PubMed] [Google Scholar]
- Pelletier, M. 2008. Évolution spatiale et temporelle de la dynamique et de la géochimie des sédiments du lac Saint-Pierre. Environnement Canada—Sciences et technologie de l’eau, Monitoring et surveillance de la qualité de l’eau au Québec. Rapport scientifique et technique ST-240, Montreal, Canada, 82 pp.
- Pelletier, M., B. Rondeau, C. Gagnon, and F. Messier. 2009. Polybrominated diphenyl ethers (PBDEs) in the St. Lawrence River/Les polybromodiphényléthers (PBDE) dans le fleuve Saint-Laurent. In Proceedings of the 36th aquatic toxicity workshop, LaMalbaie, Canada.
- Pham T, Proulx S, Brochu C, Moore S. Composition of PCBs and PAHs in the Montreal urban community wastewater and in the surface water of the St. Lawrence River (Canada) Water, Air, and Soil Pollution. 1999;111:251–270. doi: 10.1023/A:1005090309906. [DOI] [Google Scholar]
- Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chemical Ecology. 1994;8:275–285. doi: 10.1080/02757549408038554. [DOI] [Google Scholar]
- Råberg L, Graham AL, Read AF. Decomposing health: Tolerance and resistance to parasites in animals. Philosophical Transactions of the Royal Society, Series B. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabik H, Gagnon C, Houde F, Deblois C. Distribution, fate and behaviour of nonylphenol ethoxylates and degradation products in the dispersion plume of a major municipal wastewater effluent. Environmental Forensics. 2004;5:1–10. doi: 10.1080/15275920490454120. [DOI] [Google Scholar]
- Salo H, Hébert N, Dautremepuits C, Fortier M, Cejka P, Cyr D, Fournier M. Treated Montreal municipal effluents affects immune responses in rainbow trout (Oncorhynchus mykiss) Aquatic Toxicology. 2007;84:406–414. doi: 10.1016/j.aquatox.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Segura PA, Garcia-Ac A, Lajeunesse A, Ghosh D, Gagnon C, Sauvé S. Determination of six anti-infectives in wastewater using tandem solid phase extraction and liquid chromatography–tandem mass spectroscopy. Journal of Environmental Monitoring. 2007;9:307–313. doi: 10.1039/b618801j. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Kerby JL. Two stressors are far deadlier than one. Trends in Ecology & Evolution. 2004;19:274–276. doi: 10.1016/j.tree.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host-parasite systems. Philosophical Transactions of the Royal Society Series B. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter JP. Endocrine disrupters in the aquatic environment: An overview. Acta Hydrochimie Hydrobiologie. 2005;33:9–16. doi: 10.1002/aheh.200400555. [DOI] [Google Scholar]
- Sumpter JP, Johnson AC. Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environmental Science and Technology. 2005;39:4321–4332. doi: 10.1021/es048504a. [DOI] [PubMed] [Google Scholar]
- Thilakaratne IDSIP, McLaughlin JD, Marcogliese DJ. The effects of pollution and parasites on biomarkers of fish health in spottail shiners Notropis hudsonius (Clinton) Journal of Fish Biology. 2007;71:519–538. doi: 10.1111/j.1095-8649.2007.01511.x. [DOI] [Google Scholar]
- Vernouillet G, Eullaffroy P, Lajeunesse A, Blaise C, Gagné F, Juneau P. Toxic effects and bioaccumulation of carbamazepine evaluated by biomarkers measured in organisms of different trophic levels. Chemosphere. 2010;80:1062–1068. doi: 10.1016/j.chemosphere.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Vis C, Hudon C, Cattaneo A, Pinel-Alloul B. Periphyton as an indicator of water quality in the St. Lawrence River (Quebec, Canada) Environmental Pollution. 1998;101:13–24. doi: 10.1016/S0269-7491(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Vis C, Cattaneo A, Hudon C. Periphyton in the clear and coloured water masses of the St. Lawrence River (Quebec, Canada): A 20-year overview. Journal of the Great Lakes Research. 1998;24:105–117. doi: 10.1016/S0380-1330(98)70803-2. [DOI] [Google Scholar]
- White P, Rasmussen J, Blaise C. Comparing the presence, potency and potential hazard of organic compounds extracted from a broad range of industrial effluents. Environmental Molecular Mutagenesis. 1996;27:116–139. doi: 10.1002/(SICI)1098-2280(1996)27:2<116::AID-EM7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]