Abstract
Purpose
The aim of our study was to assess the influence of perioperative blood transfusion (PBT) on survival outcomes following radical cystectomy (RC) and pelvic lymph node dissection (PLND).
Materials and Methods
We reviewed and analyzed the clinical data of 432 patients who underwent RC for bladder cancer from 1991 to 2012. PBT was defined as the transfusion of allogeneic red blood cells during RC or postoperative hospitalization.
Results
Of all patients, 315 patients (72.9%) received PBT. On multivariate logistic regression analysis, female gender (p=0.015), a lower preoperative hemoglobin level (p=0.003), estimated blood loss>800 mL (p<0.001), and performance of neoadjuvant chemotherapy (p<0.001) were independent risk factors related to requiring perioperative transfusions. The receipt of PBT was associated with increased overall mortality (hazard ratio, 1.91; 95% confidence interval, 1.25-2.94; p=0.003) on univariate analysis, but its association was not confirmed by multivariate analysis (p=0.058). In transfused patients, a transfusion of >4 packed red blood cell units was an independent predictor of overall survival (p=0.007), but not in cancer specific survival.
Conclusions
Our study was not conclusive to detect a clear association between PBT and survival after RC. However, the efforts should be made to continue limiting the overuse of transfusion especially in patients who are expected to have a high probability of PBT, such as females and those with a low preoperative hemoglobin level and history of neoadjuvant chemotherapy.
Keywords: Blood transfusion, Cystectomy, Survival, Urinary bladder neoplasms
INTRODUCTION
Red blood cell (RBC) transfusion is a commonly performed procedure in association with the anemia level in critically ill patients with various underlying benign diseases. However, large amounts of blood transfusions may be related to unfavorable clinical outcomes, such as diminished organ function, more complications, and increased mortality risk [1,2]. Similarly, the adverse correlation of perioperative blood transfusion (PBT) with tumor recurrence or survival has been reported in a variety of malignancies, including colorectal, pancreatic, ovarian, and esophageal cancer [3,4,5,6,7,8]. Although the definite mechanism supporting this correlation is not yet fully understood, it has been considered to be attributable to the immunomodulatory effect and inflammatory response during transfusions [9,10].
Radical cystectomy (RC) with pelvic lymph node dissection (PLND), which has been recognized as the standard treatment for muscle invasive and high-risk nonmuscle invasive bladder cancer, is one of the most invasive and complicated surgeries in the urologic field. Therefore, it can be associated with significant intraoperative blood loss, which may involve a high probability of requiring PBT. In previous reports, the PBT rate in patients undergoing RC has been reported to range from 30% to 63% [11,12,13,14]. However, the association of PBT with cancer recurrence and survival outcomes after RC has shown conflicting results among previous studies [11,12,13,14].
In the current study, we sought to evaluate the clinicopathological factors associated with requiring PBT and the impact of PBT on survival outcome in patients with bladder cancer who were treated by RC and PLND.
MATERIALS AND METHODS
1. Study population
This study was approved by the Institutional Review Board of Seoul National University Hospital (approval No. H-1409-091-610) prior to initiating the study. This study was conducted according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We retrospectively reviewed the bladder cancer database from a single institution consisting of 487 patients who underwent RC with PLND from January 1991 to December 2012. Among these patients, 55 patients who underwent RC during the early period had incomplete information concerning PBT and were excluded from the study. Ultimately, 432 patients were eligible for our final analysis. A portion of the patients included in this study was also included in the study by Moon et al [15].
2. Acquisition and definition of data
RC and PLND were conducted by several surgeons during the involved period. The indications for RC included patients with muscle-invasive carcinoma and recurrent T1 disease or carcinoma in situ (CIS) that had been unresponsive to intravesical therapy. All pathological specimens were evaluated by a staff pathologist with genitourinary expertise. Assessed clinicopathological parameters included age, gender, body mass index, American Society of Anesthesiologists score, preoperative C-reactive protein (CRP) level, preoperative erythrocyte sedimentation rate (ESR) level, preoperative hemoglobin (Hb) level, estimated blood loss (EBL), receipt of PBT and number of units transfused, final tumor histology, variant histology of urothelial carcinoma, pathologic tumor (pT) stage and grade, CIS, lymphovascular invasion (LVI), perivesical margin, the extent of PLND, pathologic nodal (pN) stage, total number of removed lymph nodes, and history of neoaduvant chemotherapy (NACH) and adjuvant chemotherapy. Pathologic staging and grading were assigned according to the 2010 TNM classification of 7th American Joint Committee on Cancer and the 2004 World Health Organization system, respectively. The pT stage was categorized into organ confined disease (i.e., pT0/Ta/T1/T2/CIS) and extravesical disease (i.e., pT3/T4). Final tumor histology was divided into either urothelial carcinoma or nonurothelial carcinoma. LVI was defined as the presence of tumor cells within an endothelium-lined space without underlying muscular walls. We defined PBT as transfusion of only allogeneic RBC during RC or within the postoperative hospitalization period. Therefore, transfusions of other blood products, such as fresh frozen plasma and cryoprecipitate, were not included in this study. There were no unified institutional criteria regarding the thresholds for PBT and therefore, PBT was decided on a case-by-case basis according to the surgeons' opinions. The duration of survival was calculated from the date of surgery to the date of last follow-up or death. Patients who were alive, with or without disease, were censored from the relevant analyses. The cause of death was determined by the responsible physicians and death certificates.
3. Statistical analyses
The clinical and pathological characteristics were compared between transfused and nontransfused patients using chi-square or Fisher exact tests for categorical variables and Mann-Whitney test for continuous variables. Continuous variables were expressed as the median and interquartile range (IQR); categorical variables were expressed as absolute numbers and relative percentages. Univariate and multivariate logistic regression analysis were performed to evaluate the clinicopathological factors associated with requiring PBT. Survival outcomes were measured as overall survival (OS) and cancer specific survival (CSS), which were calculated using the Kaplan-Meier method and compared using a log-rank test among groups. To assess factors associated with survival in the entire study cohort and group of patients receiving PBT, univariate analyses using the Cox proportional hazards model were conducted and significant variables identified in the univariate analyses were finally entered into a multivariate Cox regression analysis to evaluate definitive predictors. All statistical analyses were conducted using the IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) and two-sided p-values of <0.05 were considered to be statistically significant.
RESULTS
The clinical and pathological parameters of the entire study cohort and the comparative analyses results among patients divided by the receipt of PBT are summarized in Table 1. Of all patients undergoing RC with PLND, 315 patients (72.9%) received a PBT with a median value of 4 transfused units (IQR, 2-6 units). A higher percentage of females received PBT compared to males (p<0.001). Patients who received PBT had a higher preoperative ESR level (p=0.023), lower preoperative Hb level (p<0.001), and higher frequency of advanced tumor stage (p=0.032) and NACH (p=0.001) than those who did not receive PBT. In addition, it seemed to be taken for granted that transfused patients had a higher EBL (median, 950 mL; IQR, 600-1,350 mL) in comparison with nontransfused patients (median, 500 mL; IQR, 375-700 mL; p<0.001). There were significant difference in the median in the follow-up duration between transfused and non-transfused patients (35 months vs. 44 months, p=0.001). There were no significant differences in the distribution of other parameters among these 2 groups. According to the multivariate logistic regression analysis, being female (p=0.015), having a lower preoperative Hb level (p=0.003), EBL>800 mL (p<0.001), and a history of NACH (p<0.001) were significant risk factors related to requiring PBT (Table 2).
Table 1. Clinicopathological parameters of the entire study cohort and comparative analysis results according to the presence or absence of perioperative blood transfusion (PBT).
| Parameter | Overall (n=432) | No PBT (n=117) | PBT (n=315) | p-value |
|---|---|---|---|---|
| Age (y), median (IQR) | 66 (59-71) | 65 (58.5-70) | 66 (59-73) | 0.119 |
| <60 | 119 (27.5) | 32 (27.4) | 87 (27.6) | |
| ≥60 | 313 (72.5) | 85 (72.6) | 228 (72.4) | 0.956 |
| Gender | ||||
| Male | 372 (86.1) | 113 (96.6) | 259 (82.2) | <0.001 |
| Female | 60 (13.9) | 4 (3.4) | 56 (17.8) | |
| BMI (kg/m2), median (IQR) | 23.3 (21.1-25.2) | 23.8 (21.6-25.7) | 23.2 (21.0-24.8) | 0.055 |
| <25 | 316 (73.1) | 78 (66.7) | 238 (75.6) | 0.064 |
| ≥25 | 116 (26.9) | 39 (33.3) | 77 (24.4) | |
| ASA score, median (IQR) | 2 (1-2) | 2 (1-2) | 2 (1-2) | 0.439 |
| 1 | 186 (43.1) | 52 (44.4) | 134 (42.5) | 0.863 |
| 2 | 222 (51.4) | 61 (52.1) | 161 (51.1) | |
| 3 | 21 (4.9) | 4 (3.4) | 17 (5.4) | |
| 4 | 2 (0.5) | 0 (0) | 2 (0.6) | |
| Preoperative CRP (mg/dL), median (IQR) | 0.16 (0.04-0.60) | 0.15 (0.05-0.51) | 0.16 (0.04-0.60) | 0.558 |
| Preoperative ESR (mm/hr), median (IQR) | 17 (8-32) | 14 (6-22.5) | 18 (8-34) | 0.023 |
| Preoperative Hb (g/dL), median (IQR) | 13.0 (11.5-14.0) | 13.8 (12.9-14.5) | 12.5 (11.2-13.7) | <0.001 |
| Number of transfused units, median (IQR) | 4 (2-6) | |||
| EBL(mL), median (IQR) | 800 (500-1200) | 500 (375-700) | 950 (600-1350) | <0.001 |
| Final histology | ||||
| Urothelial carcinoma (UC) | 422 (97.7) | 116 (99.1) | 306 (97.1) | 0.299 |
| Non-UC | 10 (2.3) | 1 (0.9) | 9 (2.9) | |
| Pathologic tumor stage | ||||
| Organ confined (pT0/Ta/T1/T2/CIS) | 271 (62.7) | 83 (70.9) | 188 (59.7) | 0.032 |
| Extravesical (pT3/T4) | 161 (37.3) | 34 (29.1) | 74 (40.3) | |
| Pathologic grade | ||||
| Low grade | 19 (4.4) | 3 (2.6) | 16 (5.1) | 0.554 |
| High grade | 355 (82.2) | 98 (83.8) | 257 (81.6) | |
| Not identified | 58 (13.5) | 16 (13.7) | 42 (13.3) | |
| CIS within bladder | ||||
| Absent | 302 (69.9) | 81 (69.2) | 221 (70.2) | 0.852 |
| Present | 130 (30.1) | 36 (30.8) | 94 (29.8) | |
| LVI within bladder | ||||
| Absent | 287 (66.4) | 80 (68.4) | 207 (65.7) | 0.603 |
| Present | 145 (33.6) | 37 (31.6) | 108 (34.3) | |
| Perivesical margin | ||||
| Absent | 232 (97.1) | 117 (100) | 306 (97.1) | 0.121 |
| Present | 7 (2.9) | 0 (0.6) | 9 (2.9) | |
| Variant of UC | ||||
| Absent | 381 (88.2) | 106 (90.6) | 275 (87.3) | 0.345 |
| Present | 51 (11.8) | 11 (9.4) | 40 (12.7) | |
| Extent of PLND | ||||
| Limited | 63 (14.6) | 21 (17.9) | 42 (13.3) | 0.236 |
| Standard | 278 (64.4) | 78 (66.7) | 200 (63.5) | |
| Extended | 89 (20.6) | 18 (15.4) | 71 (22.5) | |
| pN stage | ||||
| N0 | 335 (77.5) | 92 (78.6) | 243 (77.1) | 0.939 |
| N1 | 36 (8.3) | 9 (7.7) | 27 (8.6) | |
| N2/N3 | 61 (14.1) | 16 (13.7) | 45 (14.3) | |
| No. of removed lymph nodes, median (IQR) | 14 (8-20) | 15 (9.5-20.5) | 14 (8-20) | 0.286 |
| NACH | ||||
| Not done | 385 (89.1) | 114 (97.4) | 271 (86.0) | 0.001 |
| Done | 47 (10.9) | 3 (2.6) | 44 (14.0) | |
| ACH | ||||
| Not done | 323 (74.8) | 85 (72.6) | 238 (75.6) | 0.537 |
| Done | 109 (25.2) | 32 (27.4) | 77 (24.4) | |
| OS f/u duration (months), median (IQR) | 38 (21-74.5) | 44 (30-84) | 35 (17-66) | 0.001 |
| Alive | 295 (68.3) | 91 (77.8) | 204 (64.8) | 0.010 |
| Death | 137 (31.7) | 26 (22.2) | 111 (35.2) | |
| CSS f/u duration (months), median (IQR) | 38 (21-74.5) | 44 (30-84) | 35 (17-66) | 0.001 |
| Alive | 330 (76.4) | 93 (79.5) | 237 (75.2) | 0.355 |
| Death | 102 (23.6) | 24 (20.5) | 78 (24.8) |
Values are presented as number (%) unless otherwise indicated.
IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; EBL, estimated blood loss; CIS, carcinoma in situ; LVI, lymphovascular invasion; UC, urothelial carcinoma; PLND, pelvic lymph node dissection; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy; OS, overall survival; CSS, cancer specific survival; f/u, follow-up.
Table 2. Univariate and multivariate logistic regression analyses results for evaluating the risk factors associated with receiving perioperative blood transfusion.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 6.10 | 2.16-17.25 | 0.001 | 6.67 | 1.43-31.05 | 0.015 |
| BMI (continuous) | 0.93 | 0.87-0.99 | 0.032 | 0.91 | 0.81-1.03 | 0.142 |
| Preoperative ESR (continuous) | 1.02 | 1.00-1.04 | 0.010 | 1.00 | 0.98-1.02 | 0.830 |
| Preoperative Hb (continuous) | 0.61 | 0.52-0.71 | <0.001 | 0.71 | 0.57-0.89 | 0.003 |
| EBL (dichotomized) | ||||||
| ≤800 mL | Reference | Reference | ||||
| >800 mL | 8.61 | 4.79-15.46 | <0.001 | 14.07 | 5.86-33.77 | <0.001 |
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 1.64 | 1.04-2.60 | 0.032 | 1.03 | 0.48.-2.20 | 0.930 |
| No. of removed lymph nodes (continuous) | 0.97 | 0.94-0.99 | 0.022 | 0.97 | 0.93-1.00 | 0.051 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 6.17 | 1.87-20.2 | 0.003 | 5.93 | 1.26-27.93 | <0.001 |
OR, odd ratio; CI, confidence interval; BMI, body mass index; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; EBL, estimated blood loss; NACH, neoadjuvant chemotherapy.
In the Kaplan-Meier analysis with the log-rank test, transfused patients showed a significantly reduced 5-year OS rate than nontransfused patients (61% vs. 74%, respectively; p=0.002) (Fig. 1A). However, there was no significant difference in the CSS between transfused and nontransfused patients (70% vs. 75%, respectively; p=0.092) (Fig. 1B).
Fig. 1. Kaplan-Meier curves for overall survival (A) and cancer-specific survival (B) in the entire study cohort according to the administration of perioperative blood transfusion (PBT).
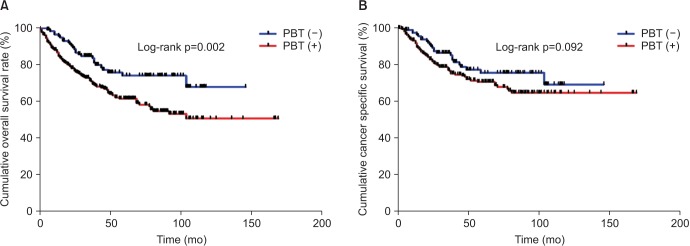
In the Cox regression analyses for the entire study cohort, PBT was significantly associated with OS in the univariate analysis (hazard ratio [HR], 1.91; 95% confidence interval [CI], 1.25-2.94; p=0.003), but not in the multivariate analysis (p=0.058) after adjusting for other clinicopathological parameters. Clinical parameters, including age (<60 years or ≥60 years), BMI (<25 kg/m2 or ≥25 kg/m2), EBL (≤800 mL or >800 mL), preoperative Hb level, and pathological parameters (i.e., pT and pN stages, LVI, and the number of lymph nodes removed) remained as independent predictors of OS in the multivariate analysis (Table 3). A significant correlation between PBT and CSS was not observed in the univariate analysis; however, similarly to the Cox regression analysis results for OS, tumor related variables (pT and pN stage, LVI, and the number of lymph nodes removed) were also independent predictors of CSS in the multivariate analysis (Supplementary Table 1).
Table 3. Univariate and multivariate Cox regression analyses results for evaluating variables associated with overall survival in the entire study cohort.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| Age (dichotomized) | ||||||
| <60 y | Reference | Reference | ||||
| ≥60 y | 1.96 | 1.27-3.02 | 0.002 | 1.98 | 1.27-3.07 | 0.002 |
| BMI (dichotomized) | ||||||
| <25 kg/m2 | Reference | Reference | ||||
| ≥25 kg/m2 | 0.57 | 0.38-0.86 | 0.008 | 0.61 | 0.40-0.93 | 0.021 |
| Preoperative Hb (continuous) | 0.84 | 0.77-0.93 | 0.001 | 0.87 | 0.79-0.97 | 0.011 |
| PBT | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.91 | 1.25-2.94 | 0.003 | 1.56 | 0.98-2.48 | 0.058 |
| EBL (dichotomized) | ||||||
| ≤800 mL | Reference | Reference | ||||
| >800 mL | 1.59 | 1.13-2.22 | 0.007 | 1.56 | 1.10-2.21 | 0.011 |
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.44 | 2.44-4.84 | <0.001 | 2.15 | 1.44-3.23 | <0.001 |
| Variant histology of UC | ||||||
| Absent | Reference | Reference | ||||
| Present | 1.67 | 1.08-2.58 | 0.020 | 1.32 | 0.82-2.15 | 0.248 |
| CIS | ||||||
| Absent | Reference | Reference | ||||
| Present | 0.64 | 0.42-0.96 | 0.033 | 0.91 | 0.59-1.41 | 0.681 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 2.77 | 1.98-3.88 | <0.001 | 1.68 | 1.13-2.50 | 0.009 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 4.13 | 1.81-9.42 | 0.001 | 1.17 | 0.48-2.83 | 0.721 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 2.58 | 1.57-4.26 | <0.001 | 2.02 | 1.20-3.40 | 0.008 |
| N2/N3 | 3.78 | 2.53-5.64 | <0.001 | 2.68 | 1.73-4.16 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.96 | 0.94-0.98 | 0.001 | 0.95 | 0.93-0.97 | <0.001 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.87 | 1.15-3.06 | 0.012 | 1.50 | 0.87-2.58 | 0.144 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.92 | 1.36-2.72 | <0.001 | 0.94 | 0.60-1.47 | 0.793 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; Hb, hemoglobin; PBT, perioperative blood transfusion; EBL, estimated blood loss; UC, urothelial carcinoma; CIS, carcinoma in situ; LVI, lymphovascular invasion; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy.
We also evaluated the variables associated with survival outcomes in patients who received PBT. Notably, a transfusion of packed RBC units>4 units (i.e., median value) was an independent predictor of OS in the multivariate Cox regression analysis controlling for the effects of other variables (HR, 1.69; 95% CI, 1.15-2.49; p=0.007) (Table 4). Furthermore, patients who had a PBT>4 units presented a lower 5 year OS rate compared to those with a PBT<4 units (49% vs. 67%, p=0.008) (Fig. 2). However, the association of the transfusion dose with CSS was not identified in the univariate Cox regression analysis (Supplementary Table 2).
Table 4. Univariate and multivariate Cox regression analyses results for evaluating variables associated with overall survival in patients receiving perioperative blood transfusions.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| Age (dichotomized) | ||||||
| <60 y | Reference | Reference | ||||
| ≥60 y | 1.90 | 1.18-3.07 | 0.008 | 2.07 | 1.27-3.38 | 0.003 |
| BMI (dichotomized) | ||||||
| <25 kg/m2 | Reference | Reference | ||||
| ≥25 kg/m2 | 0.61 | 0.38-0.98 | 0.042 | 0.61 | 0.37-0.98 | 0.041 |
| Preoperative Hb (continuous) | 0.87 | 0.78-0.96 | 0.009 | 0.95 | 0.85-1.07 | 0.452 |
| Total transfused units (dichotomized) | ||||||
| ≤4 | Reference | Reference | ||||
| >4 | 1.64 | 1.13-2.40 | 0.009 | 1.69 | 1.15-2.49 | 0.007 |
| EBL (continuous) | 1.00 | 1.00-1.001 | 0.003 | 1.00 | 1.00-1.00 | 0.883 |
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.60 | 2.44-5.29 | <0.001 | 2.40 | 1.50-3.84 | <0.001 |
| Variant histology of UC | ||||||
| Absent | Reference | Reference | ||||
| Present | 1.83 | 1.14-2.92 | 0.011 | 1.34 | 0.79-2.29 | 0.272 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 2.95 | 2.02-4.30 | <0.001 | 1.75 | 1.12-2.73 | 0.013 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 3.54 | 1.54-8.11 | 0.003 | 1.10 | 0.45-2.68 | 0.833 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 2.93 | 1.72-4.99 | <0.001 | 2.32 | 1.33-4.06 | 0.003 |
| N2/N3 | 3.53 | 2.23-5.59 | <0.001 | 2.81 | 1.70-4.65 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.95 | 0.93-0.98 | 0.001 | 0.95 | 0.92-0.97 | <0.001 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.65 | 1.11-2.44 | 0.012 | 0.75 | 0.46-1.21 | 0.241 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; Hb, hemoglobin; EBL, estimated blood loss; UC, urothelial carcinoma; LVI, lymphovascular invasion; ACH, adjuvant chemotherapy.
Fig. 2. Kaplan-Meier curves for overall survival in patients receiving perioperative blood transfusion according to transfused units of packed red blood cells. PBT, perioperative blood transfusion.

DISCUSSION
It is estimated that approximately 15 and 85 million RBC units are transfused annually into patients in the United States and worldwide, respectively [16]. Allogeneic blood transfusion (ABT) is one of the most commonly performed procedures in clinical practice for treating anemia in critically ill patients with various underlying disease [1,2,17]. Although ABT may be life-saving in many circumstances, the impact of it on the clinical outcomes of patients with a variety of diseases has been debated thus far. In general, blood transfusions may implicate significant risks, including incompatibility, transmission of infectious agents, coagulopathy, and allergic reactions [9,18]. Transfusion related immunomodulation (TRIM), which includes suppression of cytotoxic cell and monocyte activity, release of immunosuppressive prostaglandins, inhibition of interleukin-2 production, and increase in suppressor T-cell activity, has been suggested as the plausible mechanism for the association of ABT with clinical outcomes in patients with underlying malignancies [9,10]. The beneficial immunosuppressive effects of TRIM regarding ABT had been reported in kidney transplant patients and patients with Crohn disease, which include enhanced survival of renal allografts and a reduced recurrence rate of Crohn disease, respectively [19,20]. In contrast, according to a multicenter observational study, the use of ABT for treating anemia in critically ill patients was associated with diminished organ function and increased mortality [2].
In particular, the relationships between allogeneic PBT and postoperative tumor recurrence or survival outcomes have been assessed in a number of malignancies, including colorectal [3,5], ovarian [6], esophageal [7], and pancreatic [4,8]. Although these associations had conflicting results in a majority of malignancies according to previous studies, the receipt of PBT had significantly adverse effects on tumor recurrence and mortality in patients with colorectal cancer who were treated by surgery [3,5]. Recently, several studies have been published regarding the association between PBT and cancer-related outcomes in urologic malignancies, including prostate and kidney cancer [21,22,23,24]. Interestingly, it was consistently reported that PBT in patients with prostate cancer who underwent radical prostatectomy was not associated with cancer-related outcomes, including tumor progression, biochemical recurrence free survival, OS, and CSS [21,22,23]. In contrast, Linder et al. [24] demonstrated that in patients with renal cell carcinoma who were treated with partial or radical nephrectomy, both the receipt of PBT and an increased number of RBC units transfused were independent predictors of increased postoperative mortality.
In the present study, the overall PBT rate was 72.9%, which was much higher than previous reports (range, 30%-63%) [11,12,13,14]. This result may be attributable to the retrospective nature of our study and transfusion decisions, which were conducted based on the experience of each surgeon rather than using institutional standardized criteria for PBT. We identified that PBT in patients treated with RC for bladder cancer was associated with the OS in the univariate analysis, but its association was not confirmed on multivariate analysis (p=0.058). However, when analyzed for transfused patients only, increased number of allogeneic RBC units (i.e., >4 units) was a significant independent predictor of OS in the multivariate analysis (HR, 1.69; 95% CI, 1.15-2.49; p=0.007). Recently, there have been several published articles regarding similar topics as our study. Linder et al. [11] reviewed a total of 2,060 bladder cancer patients undergoing RC and reported that PBT (n=1,279, 62%) during RC was significantly associated with cancer recurrence, OS, and CSS. In addition, an increased number of transfused RBC units was also an independent predictor of decreased OS and CSS. Likewise, in a study of 350 bladder cancer patients treated with RC and PLND, Gierth et al. [13] determined that ABT (n=219, 63%) and the number of transfused packed RBC units were associated with a significant decrease in OS and progression free survival in the multivariate analysis. In another large cohort study (n=2,895) by Kluth et al. [14], it was reported that although PBT (n=1,128, 39%) was significantly related to disease recurrence, OS, and CSS in the univariate analysis, the independent association of PBT with cancer-related outcomes was not observed in the multivariate analysis.
Unlike the studies mentioned above, we did not observe any significant correlation between PBT and CSS in the univariate analysis of this study. We assumed that surgical (i.e., EBL) and tumor related factors (i.e., pT and pN stages, LVI, and number of lymph nodes removed), rather than PBT, have a critical prognostic implication with the association of CSS. Actually, these factors were significant independent predictors of CSS, as well as OS, in our study (all p<0.05), which also corresponds to findings in previous articles [11,14]. In addition, the inflammatory response is known to have an important role in cancer recurrence and progression; therefore, there have been a number of studies to evaluate the prognostic role of inflammatory makers, such as CRP and ESR, in the urologic field [25,26,27,28]. In the current study, preoperative CRP and ESR levels had no definite correlation with survival outcomes, but a lower preoperative Hb level was correlated with a lower OS and required more PBT according to the multivariate analysis. Furthermore, being female, increased EBL (i.e., >800 mL), and a history of NACH were significant factors related to requiring more PBT. Therefore, in these patients who are expected to have a higher possibility of PBT, the efforts should be continued to minimize allogeneic PBT for the improvement of postoperative OS. Alternative strategies for reducing allogeneic PBT use, which are commonly recommended in urologic surgery, include preventing severe blood loss, applying a lower Hb threshold for transfusion, preoperative autologous blood transfusions, acute normovolemic hemodilution, intraoperative blood salvage, and using iron agents and recombinant human erythropoietin [29]. However, the application of these strategies in patients who underwent RC has not yet been completely confirmed.
The current study was limited by several factors. Above all, unidentified confounding factors may have been present due to the study's retrospective nonrandomized design. Furthermore, a selection bias may have been involved because 55 patients with incomplete or unavailable clinical information had to be excluded from the study. As mentioned earlier, the decision to administer PBT was determined by the surgeon's discretion without definite criteria for PBT. Consequently, unnecessary PBT may have been conducted and adversely affected the clinical outcomes of patients enrolled in this study. Lastly, the study cohort was recruited from a single institution and included a relatively small sample size; therefore, the results derived from this study should be further validated externally using well-designed prospective and randomized clinical trials.
CONCLUSIONS
Although we couldn't observe statistically significant correlation between PBT and survival outcomes, PBT may have a negative impact on postoperative OS clinically. Given that more PBT adversely affect postoperative OS in transfused patients, it should be kept in mind that the overuse of PBT should be limited in patients who are expected to have a high probability of PBT, such as females and patients with a lower preoperative Hb level and a history of NACH, in order to improve postoperative survival. Prospective randomized controlled trial with strictly defined parameters for transfusion is needed to determine the association between transfusion at RC and survival.
ACKNOWLEDGMENTS
This study was supported by grant No. 05-2013-0010 from SNUH Research Fund.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
SUPPLEMENTARY MATERIALS
Scan this QR code to see the supplementary materials, or visit http://kjurology.org/src/sm/kju-56-295-s001.pdf.

Supplementary Table 1. Univariate and multivariate Cox regression analyses results for evaluating variables associated with cancer-specific survival in the entire study cohort.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| BMI (continuous) | 0.93 | 0.87-0.99 | 0.028 | 0.93 | 0.87-1.00 | 0.057 |
| EBL (continuous) | 1.00 | 1.000-1.0001 | <0.001 | 1.00 | 1.00-1.00 | <0.001 |
| PBT | ||||||
| Not done | ||||||
| Done | 1.47 | 0.93-2.33 | 0.095 | |||
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.66 | 2.46-5.45 | <0.001 | 1.74 | 1.09-2.78 | 0.019 |
| CIS | ||||||
| Absent | Reference | Reference | ||||
| Present | 0.57 | 0.35-0.93 | 0.025 | 0.69 | 0.41-1.17 | 0.699 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 3.64 | 2.45-5.41 | <0.001 | 2.11 | 1.32-3.37 | 0.002 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 3.74 | 1..37-10.21 | 0.010 | 0.92 | 0.31-2.67 | 0.879 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 3.30 | 1.89-5.76 | <0.001 | 2.60 | 1.45-4.66 | 0.001 |
| N2/N3 | 5.17 | 3.31-8.08 | <0.001 | 3.87 | 2.35-6.36 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.95 | 0.92-0.98 | 0.001 | 0.94 | 0.91-0.96 | <0.001 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.34 | 1.38-3.97 | 0.002 | 2.30 | 1.34-3.93 | 0.002 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.80 | 1.89-4.13 | <0.001 | 1.13 | 0.69-1.83 | 0.613 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; EBL, estimated blood loss; PBT, perioperative blood transfusion; CIS, carcinoma in situ; LVI, lymphovascular invasion; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy.
Supplementary Table 2. Univariate and multivariate Cox regression analyses results for evaluating variables associated with cancer-specific survival in patients receiving perioperative blood transfusions.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| BMI (continuous) | 0.91 | 0.85-0.98 | 0.021 | 0.89 | 0.82-0.97 | 0.014 |
| Total transfused units (dichotomized) | ||||||
| ≤4 | Reference | |||||
| >4 | 1.13 | 0.70-1.82 | 0.595 | |||
| EBL (continuous) | 1.00 | 1.000-1.0001 | 0.001 | 1.00 | 1.00-1.00 | 0.002 |
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.79 | 2.39-6.01 | <0.001 | 1.48 | 0.84-2.61 | 0.173 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 4.17 | 2.64-6.59 | <0.001 | 3.16 | 1.92-5.20 | <0.001 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 3.36 | 1.22-9.24 | 0.019 | 0.74 | 0.25-2.15 | 0.581 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 4.04 | 2.20-7.43 | <0.001 | 3.53 | 1.85-6.72 | <0.001 |
| N2/N3 | 5.33 | 3.18-8.96 | <0.001 | 4.61 | 2.56-8.32 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.95 | 0.92-0.98 | 0.002 | 0.93 | 0.90-0.96 | <0.001 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.90 | 1.07-3.36 | 0.027 | 1.93 | 1.07-3.45 | 0.027 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.58 | 1.65-4.03 | <0.001 | 1.23 | 0.73-2.09 | 0.425 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; EBL, estimated blood loss; LVI, lymphovascular invasion; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy.
References
- 1.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, et al. The CRIT Study: Anemia and blood transfusion in the critically ill: current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 3.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18:1327–1334. doi: 10.1245/s10434-010-1476-3. [DOI] [PubMed] [Google Scholar]
- 5.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and metaanalysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 6.De Oliveira GS Jr, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfus Med. 2012;22:97–103. doi: 10.1111/j.1365-3148.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu Y, Orita H, Sakurada M, Maekawa H, Hoppo T, Sato K. Intraoperative blood transfusion contributes to decreased long-term survival of patients with esophageal cancer. World J Surg. 2012;36:844–850. doi: 10.1007/s00268-012-1433-3. [DOI] [PubMed] [Google Scholar]
- 8.Sutton JM, Kooby DA, Wilson GC, Squires MH, 3rd, Hanseman DJ, Maithel SK, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18:1575–1587. doi: 10.1007/s11605-014-2567-4. [DOI] [PubMed] [Google Scholar]
- 9.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Linder BJ, Frank I, Cheville JC, Tollefson MK, Thompson RH, Tarrell RF, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013;63:839–845. doi: 10.1016/j.eururo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Morgan TM, Barocas DA, Chang SS, Phillips SE, Salem S, Clark PE, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol. 2013;31:871–877. doi: 10.1016/j.urolonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gierth M, Aziz A, Fritsche HM, Burger M, Otto W, Zeman F, et al. The effect of intra- and postoperative allogenic blood transfusion on patients' survival undergoing radical cystectomy for urothelial carcinoma of the bladder. World J Urol. 2014;32:1447–1453. doi: 10.1007/s00345-014-1257-x. [DOI] [PubMed] [Google Scholar]
- 14.Kluth LA, Xylinas E, Rieken M, El Ghouayel M, Sun M, Karakiewicz PI, et al. Impact of peri-operative blood transfusion on the outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2014;113:393–398. doi: 10.1111/bju.12439. [DOI] [PubMed] [Google Scholar]
- 15.Moon KC, Kim M, Kwak C, Kim HH, Ku JH. External validation of online predictive models for prediction of cancerspecific mortality and all-cause mortality in patients with urothelial carcinoma of the urinary bladder. Ann Surg Oncol. 2014;21:3132–3141. doi: 10.1245/s10434-014-3561-5. [DOI] [PubMed] [Google Scholar]
- 16.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 18.Rawn J. The silent risks of blood transfusion. Curr Opin Anaesthesiol. 2008;21:664–668. doi: 10.1097/ACO.0b013e32830f1fd1. [DOI] [PubMed] [Google Scholar]
- 19.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- 20.Williams JG, Hughes LE. Effect of perioperative blood transfusion on recurrence of Crohn's disease. Lancet. 1989;2:131–133. doi: 10.1016/s0140-6736(89)90185-2. [DOI] [PubMed] [Google Scholar]
- 21.Boehm K, Beyer B, Tennstedt P, Schiffmann J, Budaeus L, Haese A, et al. No impact of blood transfusion on oncological outcome after radical prostatectomy in patients with prostate cancer. World J Urol. 2014 Jul 03; doi: 10.1007/s00345-014-1351-0. [Epub]. http://dx.doi.org/10.1007/s00345-014-1351-0. [DOI] [PubMed] [Google Scholar]
- 22.Chalfin HJ, Frank SM, Feng Z, Trock BJ, Drake CG, Partin AW, et al. Allogeneic versus autologous blood transfusion and survival after radical prostatectomy. Transfusion. 2014;54:2168–2174. doi: 10.1111/trf.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeoh TY, Scavonetto F, Weingarten TN, Karnes RJ, van Buskirk CM, Hanson AC, et al. Perioperative allogeneic nonleukoreduced blood transfusion and prostate cancer outcomes after radical prostatectomy. Transfusion. 2014;54:2175–2181. doi: 10.1111/trf.12595. [DOI] [PubMed] [Google Scholar]
- 24.Linder BJ, Thompson RH, Leibovich BC, Cheville JC, Lohse CM, Gastineau DA, et al. The impact of perioperative blood transfusion on survival after nephrectomy for non-metastatic renal cell carcinoma (RCC) BJU Int. 2014;114:368–374. doi: 10.1111/bju.12535. [DOI] [PubMed] [Google Scholar]
- 25.Cross BW, Johnson TV, Derosa AB, Ogan K, Pattaras JG, Nieh PT, et al. Preoperative erythrocyte sedimentation rate independently predicts overall survival in localized renal cell carcinoma following radical nephrectomy. Int J Surg Oncol. 2012;2012:524981. doi: 10.1155/2012/524981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gakis G, Todenhofer T, Stenzl A. The prognostic value of hematological and systemic inflammatory disorders in invasive bladder cancer. Curr Opin Urol. 2011;21:428–433. doi: 10.1097/MOU.0b013e32834966fa. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, Numao N, et al. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011;78:1131–1135. doi: 10.1016/j.urology.2011.07.1416. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Kihara K. Role of C-reactive protein in urological cancers: a useful biomarker for predicting outcomes. Int J Urol. 2013;20:161–171. doi: 10.1111/j.1442-2042.2012.03121.x. [DOI] [PubMed] [Google Scholar]
- 29.Monk TG, Goodnough LT. Blood conservation strategies to minimize allogeneic blood use in urologic surgery. Am J Surg. 1995;170(6A Suppl):69S–73S. doi: 10.1016/s0002-9610(99)80063-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Univariate and multivariate Cox regression analyses results for evaluating variables associated with cancer-specific survival in the entire study cohort.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| BMI (continuous) | 0.93 | 0.87-0.99 | 0.028 | 0.93 | 0.87-1.00 | 0.057 |
| EBL (continuous) | 1.00 | 1.000-1.0001 | <0.001 | 1.00 | 1.00-1.00 | <0.001 |
| PBT | ||||||
| Not done | ||||||
| Done | 1.47 | 0.93-2.33 | 0.095 | |||
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.66 | 2.46-5.45 | <0.001 | 1.74 | 1.09-2.78 | 0.019 |
| CIS | ||||||
| Absent | Reference | Reference | ||||
| Present | 0.57 | 0.35-0.93 | 0.025 | 0.69 | 0.41-1.17 | 0.699 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 3.64 | 2.45-5.41 | <0.001 | 2.11 | 1.32-3.37 | 0.002 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 3.74 | 1..37-10.21 | 0.010 | 0.92 | 0.31-2.67 | 0.879 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 3.30 | 1.89-5.76 | <0.001 | 2.60 | 1.45-4.66 | 0.001 |
| N2/N3 | 5.17 | 3.31-8.08 | <0.001 | 3.87 | 2.35-6.36 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.95 | 0.92-0.98 | 0.001 | 0.94 | 0.91-0.96 | <0.001 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.34 | 1.38-3.97 | 0.002 | 2.30 | 1.34-3.93 | 0.002 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.80 | 1.89-4.13 | <0.001 | 1.13 | 0.69-1.83 | 0.613 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; EBL, estimated blood loss; PBT, perioperative blood transfusion; CIS, carcinoma in situ; LVI, lymphovascular invasion; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy.
Supplementary Table 2. Univariate and multivariate Cox regression analyses results for evaluating variables associated with cancer-specific survival in patients receiving perioperative blood transfusions.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| BMI (continuous) | 0.91 | 0.85-0.98 | 0.021 | 0.89 | 0.82-0.97 | 0.014 |
| Total transfused units (dichotomized) | ||||||
| ≤4 | Reference | |||||
| >4 | 1.13 | 0.70-1.82 | 0.595 | |||
| EBL (continuous) | 1.00 | 1.000-1.0001 | 0.001 | 1.00 | 1.00-1.00 | 0.002 |
| Pathologic tumor stage | ||||||
| Organ confined | Reference | Reference | ||||
| Extravesical | 3.79 | 2.39-6.01 | <0.001 | 1.48 | 0.84-2.61 | 0.173 |
| LVI | ||||||
| Absent | Reference | Reference | ||||
| Present | 4.17 | 2.64-6.59 | <0.001 | 3.16 | 1.92-5.20 | <0.001 |
| Perivesical margin | ||||||
| Negative | Reference | Reference | ||||
| Positive | 3.36 | 1.22-9.24 | 0.019 | 0.74 | 0.25-2.15 | 0.581 |
| Pathologic nodal stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 4.04 | 2.20-7.43 | <0.001 | 3.53 | 1.85-6.72 | <0.001 |
| N2/N3 | 5.33 | 3.18-8.96 | <0.001 | 4.61 | 2.56-8.32 | <0.001 |
| No. of removed lymph nodes (continuous) | 0.95 | 0.92-0.98 | 0.002 | 0.93 | 0.90-0.96 | <0.001 |
| NACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 1.90 | 1.07-3.36 | 0.027 | 1.93 | 1.07-3.45 | 0.027 |
| ACH | ||||||
| Not done | Reference | Reference | ||||
| Done | 2.58 | 1.65-4.03 | <0.001 | 1.23 | 0.73-2.09 | 0.425 |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; EBL, estimated blood loss; LVI, lymphovascular invasion; NACH, neoadjuvant chemotherapy; ACH, adjuvant chemotherapy.


