Abstract
Purpose
To investigate the potential benefits of testosterone administration to elderly men (>65 years) with late-onset hypogonadism (LOH) in comparison with younger men and to assess the safety of testosterone administration to elderly men.
Materials and Methods
A total of 561 hypogonadal men from two registry studies were divided into age groups of ≤65 years (group Y, n=450; range, 32-65 years) and >65 years (group O, n=111; range, 66-84 years). Following an initial 6-week interval, all men were treated with 3-month injections of parenteral testosterone undecanoate for up to 6 years.
Results
Over the 6 years, there was a progressive decrease of body weight and waist circumference. Beneficial effects on lipids and other metabolic factors and on psychological and sexual functioning progressed over the first 24 to 42 months and were sustained. Rather than a deterioration, there was an improvement of urinary parameters. Prostate volume and prostate-specific antigen increased moderately. Hematocrit levels increased but remained within safe margins.
Conclusions
The benefits of restoring serum testosterone in men with LOH were not significantly different between men older than 65 years of age and younger men. There were no indications that side effects were more severe in elderly men. The effects on prostate and urinary function and hematocrit were within safe margins. Age itself need not be a contraindication to testosterone treatment of elderly men with LOH.
Keywords: Aging, Body weight, Erectile dysfunction, Hypogonadism, Testosterone
INTRODUCTION
Over the last decades numerous publications have highlighted the decline of serum testosterone levels in aging men [1]. This decline has been termed late-onset hypogonadism (LOH). Rather than chronological age per se, obesity, but also impaired general health, are the more common causes of low testosterone in aging men [1]. It has become abundantly clear that the decline of serum testosterone with aging is not an academic question but has important implications for elderly men. Severe LOH is associated with substantially higher risks of all-cause and cardiovascular mortality, to which both the level of testosterone and the presence of sexual symptoms contribute independently [2]. A recent literature review on the risk factors, comorbidities, and consequences of LOH demonstrated that advanced age, obesity, a diagnosis of metabolic syndrome, and poor general health status were predictors of LOH. Diabetes mellitus was correlated with hypogonadism in most studies but was not established as a risk factor. Although diseases such as coronary heart disease, hypertension, stroke, and peripheral arterial disease did not predict hypogonadism, they did correlate with the incidence of low testosterone. Low levels of testosterone may have important long-term negative health consequences [3]. In the European Male Ageing Study, more than 50% of subjects with LOH reported the presence of one or more common morbidities. Overall, hypertension (29%), obesity (24%), and heart diseases (16%) were the most prevalent conditions [4]. Particularly, sexual symptoms might indicate LOH. In one study, an inverse relationship between an increasing number of sexual symptoms and a decreasing testosterone level was observed. The authors concluded that LOH can be defined by the presence of at least three sexual symptoms associated with a total testosterone level of less than 11 nmol/L (3.2 ng/mL) and a free testosterone level of less than 220 pmol/L (64 pg/mL) [5].
Testosterone treatment has a number of beneficial effects, particularly on weight, fat distribution, and metabolic factors [6,7]. Testosterone might be a powerful tool in weight reduction and its associated benefits [8,9,10].
In the present study, we analyzed whether the age of subjects receiving testosterone for the treatment of LOH is a determinant of the beneficial effects of testosterone administration. There is as yet almost no information on this question.
MATERIALS AND METHODS
In this cumulative registry study, 561 hypogonadal mainly elderly men from two urology offices were followed through observational registry studies. A total of 450 men were ≤65 years of age (mean±standard deviation [SD], 56.10±6.29 years) and 111 men were >65 years (mean±SD, 68.45±2.91 years). All subjects had sought urological consultation for various urological conditions. A number of subjects, for instance, men with osteoporosis, had been referred by other specialists who suspected the men might have testosterone deficiency. We performed a pooled analysis of men ≤65 years of age (group Y) and men >65 years (group O) of age from both registries. A threshold for testosterone deficiency of 12.1 nmol/L was used, as confirmed by an international group of authors [11]. All men also had clinical symptoms of hypogonadism as assessed with the Aging Males' Symptoms (AMS) scale [12]. All men received treatment with parenteral testosterone undecanoate 1,000 mg (Nebido, Bayer Pharma, Berlin, Germany), administered at baseline and 6 weeks and every 12 weeks thereafter for at least 72 months.
Exclusion criteria for testosterone administration were a previous diagnosis of primary or secondary hypogonadism; previous treatment with androgens; prostate cancer or any suspicion thereof, such as prostate-specific antigen (PSA) levels>4 ng/mL; International Prostate Symptom Score (IPSS)>19 points; breast cancer; or severe untreated sleep apnea.
Measurements of anthropometric parameters were performed at baseline (height, weight, waist circumference) and at each visit, and blood samples were drawn at each or every other visit before the next injection of testosterone. Therefore, testosterone levels were trough levels at the end of an injection interval. Waist circumference provides a unique indicator of body fat distribution, which can identify patients who are at increased risk of obesity-related cardiometabolic disease, above and beyond the measurement of body mass index (BMI). The significance of waist circumference to assess the accumulation of abdominal fat has been well documented [13]. The AMS scale [12] and the International Index of Erectile Function (IIEF) were used as self-administered quality of life and erectile function instruments [14]. The AMS questionnaire is a 17-item scale assessing health-related quality of life and symptoms in aging men (e.g., somato-vegetative, psychological, and sexual symptoms) on a 5-point Likert scale (1=not applicable to 5=very severe). Higher total scores represent having more symptoms in aging men [15]. The IIEF erectile function domain (IIEF-EF; maximum score, 30; according to Cappelleri et al. [14]) was assessed at baseline and every 3 months. Liver enzymes were measured because reductions in liver enzymes may suggest reductions in liver fat content, as observed by Hoyos et al. [16]. Reductions in C-reactive protein indicate the anti-inflammatory properties of testosterone [17].
Postvoid residual bladder volume and prostate volume were measured by using a Sonoace SA 8000 SE with 3 ultrasound probes. For abdominal measurement of residual bladder urine volume, a probe with 3-7 MHz was used, and for prostate volume, a transrectal probe of 5-12 MHz was used. The IPSS was assessed.
The methodology of statistical analysis has been described elsewhere [8].
Ethical guidelines as formulated by the German "Ärzte kammer" (the German Medical Association) for observational studies in patients receiving standard treatment were followed. All subjects consented to be included in the research of their treatment protocol, which was in accordance with the Declaration of Helsinki (http://www.wma.net). All procedures were carried out with the adequate understanding and written consent of the subjects.
RESULTS
The group ≤65 years of age was labeled as group Y (young: n=450: mean age, 56.10±6.29 years; range, 32-65 years), and the group >65 years of age as group O (old: n=111; mean age, 68.45±2.91 years; range, 66-84 years). All together, we are reporting 3,320 patient-years: 2,587 for group Y and 733 for group O.
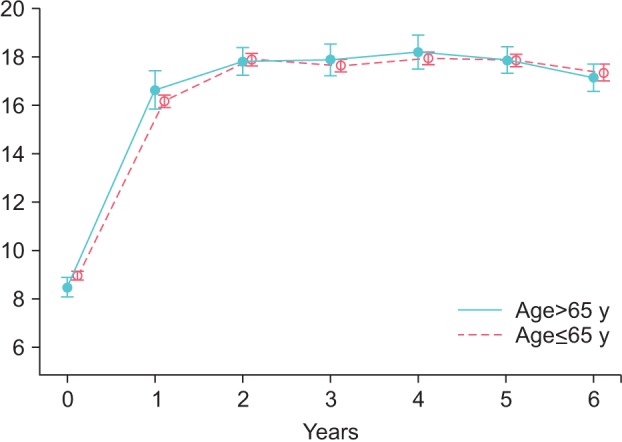
The results of 6 years of testosterone administration are presented in Table 1. Trough levels of total testosterone (nmol/L) rose from 8.96±1.95 to 16.18±2.73 nmol/L after 1 year and stabilized at 17-18 nmol/L in group Y. In group O, total testosterone rose from 8.46±2.26 to 16.64±4.23 nmol/L after 1 year and remained stable at 17-18 nmol/L thereafter (Fig. 1).
Table 1. Results of 6 years testosterone administration to men with late-onset hypogonadism, ≤65 years of age (young) and >65 years (old).
| Variable | Young (n=450) | Old (n=111) | ||||
|---|---|---|---|---|---|---|
| Baseline, mean±SD | 6 Years, mean±SD | Change, mean±SE | Baseline, mean±SD | 6 Years, mean±SD | Change, mean±SE | |
| Age (y), mean±SD (range) | 56.10±6.29 (32-65) | 68.45±2.91 (66-84) | ||||
| Anthropometry | ||||||
| Weight (kg) | 102.52±15.56 | 90.15±9.69 | -14.78±0.35a | 102.83±15.64 | 95.35±9.03 | -15.14±0.71a |
| Body mass index (kg/m2) | 32.58±5.08 | 29.02±3.02 | -4.72±0.11a | 32.84±4.86 | 30.35±2.61 | -4.81±0.22a |
| Weight loss (%) | - | 13.56±7.56a | - | - | 13.28±7.14a | - |
| Waist circumference (cm) | 106.54±9.03 | 98.26±7.1 | -9.34±0.2a | 108.95±10.75 | 100.72±9.45 | -10.45±0.47a |
| Testosterone (nmol/L) | 8.96±1.95 | 17.33±2.39 | 8.22±0.19a | 8.48±2.26 | 17.12±1.78 | 8.98±0.47a |
| Prostate parameters | ||||||
| Prostate volume (mL) | 26.77±9.5 | 31.58±10.9 | 2.77±0.16a | 33.85±8.67 | 39.95±7.6 | 3.37±0.36a |
| PSA (ng/mL) | 1.33±0.91 | 1.65±0.83 | 0.36±0.02a | 1.44±0.98 | 1.88±0.84 | 0.38±0.05a |
| Urinary function | ||||||
| Residual voiding volume (mL) | 34.87±23.16 | 16.72±6.74 | -22.17±0.73a | 41.46±23.48 | 27.25±1.58 | -18.84±5.76a |
| IPSS | 7.74±5.00 | 3.89±3.12 | -4.4±0.15a | 10.7±4.07 | 4.63±2.63 | -5.89±0.30a |
| IIEF-EF | 14.94±7.34 | 24.21±4.39 | 8.53±0.26a | 11.87±7.58 | 22.12±6.41 | 8.12±0.59a |
| Quality of life, AMS | 53.53±9.47 | 21.68±6.88 | -32.01±0.49a | 54.99±9.02 | 23.45±7.91 | -31.57±0.98a |
| Glycemic control | ||||||
| Fasting glucose (mg/dL) | 104.18±22.17 | 96.44±8.44 | -7.57±1.01a | 119.07±40.01 | 101.74±19.37 | -19.21±3.64a |
| HbA1c (%) | 6.64±1.32 | 5.66±0.65 | -1.15±0.04a | 7.00±1.38 | 5.94±0.82 | -1.37±0.09a |
| Lipids | ||||||
| Total cholesterol (mg/dL) | 268.92±45.95 | 193.56±16.58 | -76.89±2.3a | 268.44±52.69 | 191.69±21.8 | -82.55±5.66a |
| HDL (mg/dL) | 48.91±17.33 | 59.55±17.66 | 11.99±0.66a | 51.64±16.56 | 61.99±16.87 | 9.15±1.75a |
| LDL (mg/dL) | 159.87±36.70 | 119.81±34.87 | -34.63±1.62a | 162.48±31.63 | 120.86±33.56 | -40.25±3.58a |
| Triglycerides (mg/dL) | 262.35±73.16 | 192.10±34.40 | -71.04±3.64a | 266.9±84.37 | 192.27±32.16 | -98.3±8.12a |
| Ratio total cholesterol: HDL | 6.15±2.42 | 3.54±1.04 | -2.78±0.10a | 5.67±2.09 | 3.32±0.91 | -2.4±0.2a |
| Erythropoiesis/blood count | ||||||
| Hemoglobin (g/dL) | 14.39±0.89 | 15.13±0.52 | 0.77±0.05a | 14.09±1.16 | 15.12±0.81 | 1.06±0.14a |
| Hematocrit (%) | 42.98±3.27 | 48.42±2.25 | 5.56±0.21a | 42.71±3.67 | 48.58±2.28 | 5.76±0.50a |
| Leukocytes (109/L) | 7.38±2.48 | 6.29±0.83 | -0.45±0.14a | 7.08±2.02 | 6.3±0.87 | -0.8±0.26b |
| Liver transaminases | ||||||
| AST (U/L) | 35.80±16.04 | 22.83±6.43 | -12.41±0.84a | 31.33±14.71 | 22.39±6.47 | -8.8±2.03a |
| ALT (U/L) | 38.17±18.92 | 24.64±10.66 | -11.91±1.05a | 37.5±22.77 | 24.73±9.52 | -11.71±2.63a |
| Inflammation, CRP (mg/dL) | 4.04±6.34 | 0.75±1.06 | -3.49±0.22a | 2.65±3.75 | 0.71±0.51 | -2.5±0.29a |
| Blood pressure | ||||||
| Systolic (mmHg) | 145.51±17.73 | 132.39±10.41 | -17.21±0.497a | 147.01±15.78 | 132.13±10.53 | -19.57±1.15a |
| Diastolic (mmHg) | 87.33±11.69 | 78.47±5.36 | -9.88±0.61a | 89.36±10.54 | 78.70±6.22 | -13.02±0.95a |
SD, stdndard deviation; SE, standard error; PSA, prostate-specific antigen; IPSS, International Prostate Symptom Score; IIEF-EF, International Index of Erectile Function, erectile function domain; AMS, Aging Males' Symptoms score; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein.
a:p<0.0001 vs. baseline. b:p=0.0021 vs. baseline.
Fig. 1. Serum total testosterone levels in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
Mean weight decreased from 102.52±15.56 to 90.15±9.69 kg in group Y and from 102.83±15.64 to 95.35±9.03 kg in group O. Model-adjusted mean change from baseline was -14.78±0.35 and -15.14±0.71 kg, respectively. Percentage change from baseline was -13.56%±7.56% in group Y and -13.28%±7.14% in group O. Waist circumference decreased from 106.54±9.03 to 98.26±7.1 cm in group Y and from 108.95±10.75 to 100.72±9.45 cm in group O. The mean change from baseline was 9.34±0.2 cm in group Y and 10.45±0.47 cm in group O. BMI (kg/m2) decreased from 32.58±5.08 to 29.02±3.01 in group Y and from 32.84±4.86 to 30.35±2.61 in group O. The mean change from baseline was -4.72±0.11 and -4.81±0.22, respectively (p<0.0001 for all).
Total cholesterol decreased from 268.92±45.95 to 193.56±16.58 mg/dL in group Y and from 268.44±52.69 to 191.69±21.8 mg/dL in group O. Low-density lipoprotein decreased from 159.87±36.7 to 119.81± 34.87 mg/dL in group Y and from 162.48±31.63 to 120.86±33.56 mg/dL in group O. Triglycerides decreased from 262.35±73.16 to 192.1±34.4 mg/dL in group Y and from 266.9±84.37 to 192.27 ±32.16 mg/dL in group O. High-density lipoprotein (HDL) increased from 48.91±17.33 to 59.55±17.66 mg/dL in group Y and from 51.64± 16.56 to 61.99±16.87 mg/dL in group O. The ratio of total cholesterol to HDL improved from 6.15±2.42 to 3.54±1.04 in group Y and from 5.67 ±2.09 to 3.32±0.91 in group O (p<0.0001 for all).
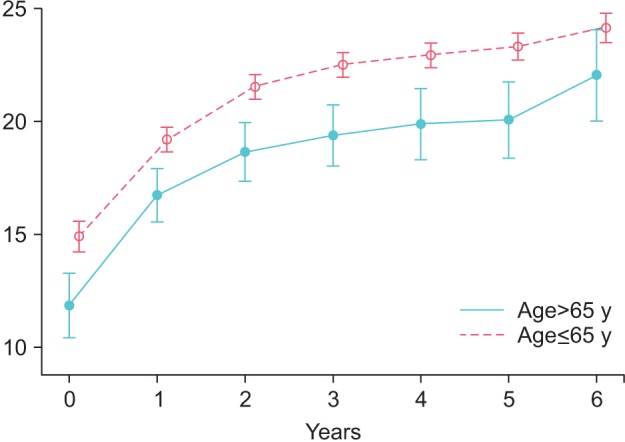
In group Y, IIEF-EF improved from 14.94±7.34 at baseline to 19.23±5.94 after 1 year, 21.58±5.72 at 2 years, 22.57±5.53 at 3 years, 22.98±5 at 4 years, 23.39±4.96 at 5 years, and 24.21±4.39 at 6 years. Changes were statistically significant versus baseline at each year (p<0.0001) and at 2 years vs. 1 year (p<0.0001), 3 years vs. 2 years (p<0.0001), and 4 years vs. 3 years (p=0.0071) and were sustained thereafter. The model-adjusted mean change from baseline was 8.53±0.26.
In group O, IIEF-EF improved from 11.87±7.58 at baseline to 16.79±6.35 after 1 year, 18.71±6.75 at 2 years, 19.46±6.62 at 3 years, 19.95±7.19 at 4 years, 20.16±7.11 at 5 years, and 22.12±6.41 at 6 years. Changes were statistically significant versus baseline at each year (p<0.0001) and at 2 years vs. 1 year (p<0.0001) and were sustained thereafter. The model-adjusted mean change from baseline was 8.12±0.59 (Fig. 2).
Fig. 2. International Index of Erectile Function in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
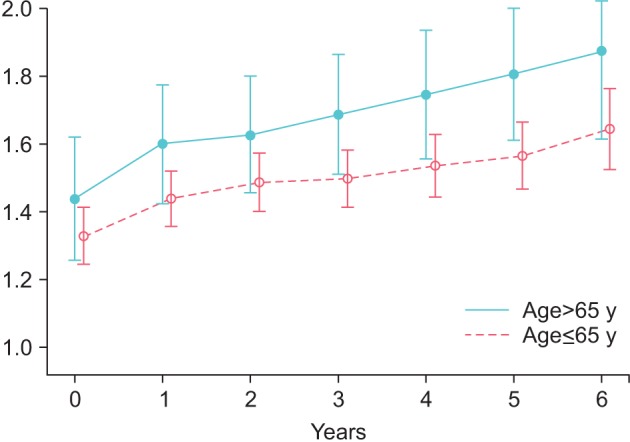
Prostate volume increased in group Y from 26.77±9.4 to 31.58±10.9 mL (p<0.0001) and in group O from 33.85±8.66 to 39.95±7.6 mL (p<0.0001) (Fig. 3). PSA increased by 0.36±0.02 ng/mL in group Y, from 1.33±0.91 to 1.65±0.83 ng/mL (p<0.0001), and by 0.38±0.05 ng/mL in group O, from 1.44±0.98 to 1.88±0.84 ng/mL (p<0.0001) (Fig. 4).
Fig. 3. Prostate volume (mL) in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
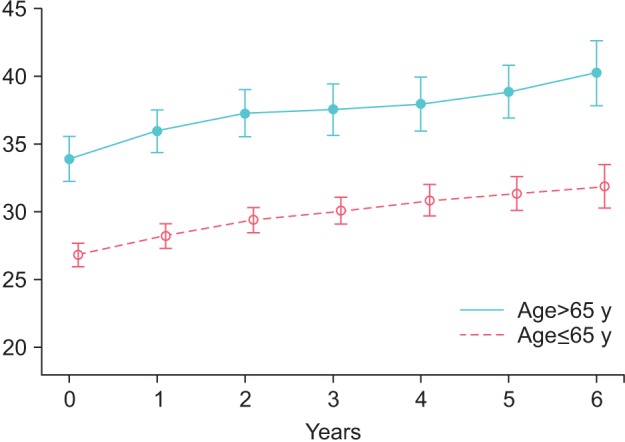
Fig. 4. Serum levels of prostate specific antigen in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
IPSS improved in group Y from 7.74±5 to 3.89±3.12 (p<0.0001) and in group O from 10.7±4.07 to 4.63±2.63 (p<0.0001). These changes were statistically significant versus the previous year for all 6 years in group Y and the first 5 years in group O and were then maintained throughout the observation period (Fig. 5).
Fig. 5. International Prostate Symptom Score in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
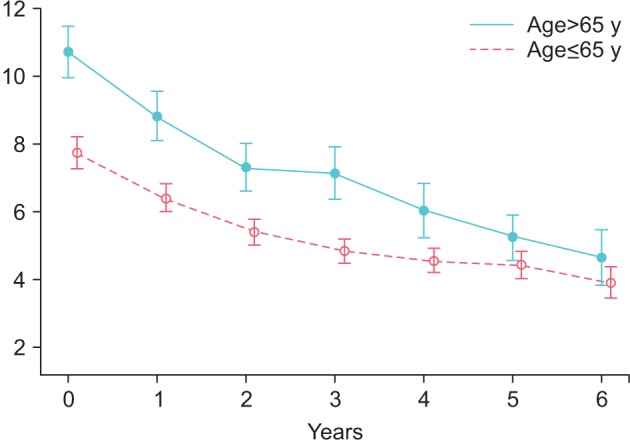
Postvoiding residue declined in group Y from 34.87±23.12 to 16.72±6.74 mL (p<0.0001) and in group O from 41.46±23.48 to 18.84±5.75 mL (p<0.0001) (Fig. 6).
Fig. 6. Postvoiding residual volume in young (≤65 y) and old men (>65 y) receiving treatment with testosterone undecanoate over six years.
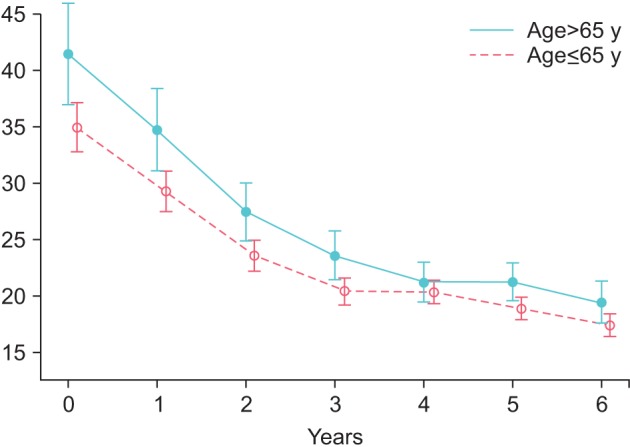
AMS improved in group Y from 53.53±9.47 to 21.68±6.88 (p<0.0001) and in group O from 54.99±9.02 to 23.45±7.91. These changes were statistically significant versus the previous year for the first 3 years in both groups and were then maintained throughout the observation period. C-reactive protein as a marker of inflammation dropped in group Y from 4.04±6.34 to 0.75±1.06 mg/dL (p<0.0001) and in group O from 2.65±3.75 to 0.71±0.51 mg/dL.
The changes in both groups over a period of 6 years are summarized in Table 1. There were no major adverse cardiovascular events during the observation time.
Prostate cancer occurred in 11 men: 7 men in group Y (1.6%) and 4 men in group O (3.6%). The incidence per 10,000 patient years was 27.1 in the younger and 54.6 in the older group.
DISCUSSION
LOH is increasingly recognized as a clinical entity. Age need not be a contraindication against treatment with testosterone. As the guidelines of the International Society for the Study of the Aging Male specify, age is not a contraindication to initiate testosterone treatment. Individual assessment of comorbidities (as possible causes of symptoms) and potential risks versus benefits of testosterone treatment are particularly important in elderly men [18].
There is a paucity of information on the benefits of testosterone treatment for elderly men with proven LOH. In this report we provide data on a large cohort of men receiving testosterone for up to 6 years. The benefits for men older than 65 years of age were compared with those of younger men, and the improvements in body weight, metabolic factors, psychological functioning, and sexual functioning were of the same magnitude in both age groups. Although weight loss was progressive over the 6-year period, effects of testosterone on lipids and on psychological and sexual functioning reached a plateau after approximately 3 years and these effects were sustained. Effects of testosterone on hematopoiesis, on the prostate, and on bladder function were not more severe in older men than in younger men. We did observe a mild increase in prostate volume and serum PSA over time, which is a normal finding in aging men. Maybe somewhat surprising, postvoiding residue and the IPSS did not deteriorate with aging but showed a degree of improvement. Some patients received treatment with α-blockers (tamsulosin) or 5α-reductase inhibitors, but these drugs were already being taken before testosterone administration started. There are now studies showing that testosterone treatment of elderly hypogonadal men improves these parameters [19,20]. This may be a direct effect on tissues or may be an indirect effect: the severity of the metabolic syndrome is associated with the severity of lower urinary tract symptoms [21]. The symptoms of the metabolic syndrome improve upon testosterone treatment and testosterone may thus have a favorable effect on lower urinary tract symptoms [22]. Therefore, it seems reasonable to conclude that the risks of testosterone administration to elderly men are not disproportionately higher in elderly men than in younger men.
Our data collection was not large enough to draw solid conclusions as to the risks of prostate cancer in an elderly population of men receiving testosterone treatment, which is, however, an important issue. Despite evidence to the contrary, physicians still harbor a wrongful association between testosterone and the development of prostate pathology (prostate cancer and benign prostate hyperplasia). In the combined group of younger and older patients, 11 cases of prostate cancer occurred. Not surprisingly, the incidence of prostate cancer was higher in older men; however, it was lower than expected in both groups. All cases were low-grade prostate cancers. These cases have been reported in a recent paper [23]. These observations suggest that the incidence of prostate cancer in patients receiving testosterone therapy, both in the younger and in the older group, was not greater than in the general population not receiving testosterone treatment [24]. Of the 11 cases of prostate cancer, 7 were ≤65 years of age when testosterone therapy was initiated, and 4 were older than 65 (66, 67, 67, and 73 years of age). Meanwhile, all 11 patients have resumed testosterone treatment. In so far as these data permit conclusions, it was not the oldest men who developed prostate cancer.
The historical fear that raising testosterone levels will result in more prostate cancer has been dispelled, particularly by the work of Abraham Morgentaler. Higher serum testosterone levels fail to show an increased risk of prostate cancer, and supraphysiological testosterone does not increase prostate volume or PSA in healthy men. This apparent paradox is explained by the "saturation model," which posits a finite capacity of androgen to stimulate the growth of prostate cancer. Recent studies indicate no increased risk of prostate cancer among men with serum testosterone in the therapeutic range [25]. Because age is a strong prognosticator of prostate malignancy, follow-up of elderly men receiving testosterone remains mandatory.
Another concern about the safety of testosterone administration regards cardiovascular disease. Two studies showing a relationship between testosterone administration and cardiovascular disease in elderly men have stirred up concerns. However, these studies have been heavily criticized for methodological flaws and have invited a much needed critical analysis of pro and con arguments [26]. The relationship between testosterone and cardiovascular disease has recently been extensively reviewed [27]. In the present observational study, no cases of major adverse cardiovascular events occurred.
A very important aspect is that the benefits of testosterone therapy are fully achieved only by long-term treatment [8,9,10,28]. To achieve maximal benefits, good patient adherence is a prerequisite. Some recent reports have raised questions about patient compliance to testosterone therapy [29,30]. In our registry study of 561 patients, however, there were only 4 dropouts (0.7%): 2 patients moved to different locations and 2 patients were lost to follow-up for unknown reasons.
Our study provides important research findings. Observations were carried out over 6 years in a large population. However, there are some limitations. The study was not blinded and was not designed to monitor specific benefits and side effects of testosterone administration to elderly men (>65 years). A limitation of our study was that we did not record any changes in use of concomitant medications, for instance, phosphodiesterase type 5 (PDE5) inhibitors. At baseline, 55 patients (10%) were taking a PDE5 inhibitor. Nevertheless, some conclusions are warranted: we found that restoring testosterone levels to normal had similar beneficial effects in men older than 65 years of age as in younger men. In addition, the risks of administration of testosterone to this age group were not higher than in younger men.
CONCLUSIONS
In medicine, the attention paid to LOH is increasing. It is now recognized as a form of hypogonadism that responds well to testosterone treatment. There is, however, a certain hesitation on the part of physicians to prescribe testosterone to men of advanced age. Are the benefits as great as in younger hypogonadal men? Are the risks disproportionately higher? The results of the present study show that benefits of testosterone treatment are equal in men older than 65 years and in men younger than 65 years. The men studied were too few in number to make a reliable assessment of the risks in elderly men, but there are now several publications indicating that the risks are acceptable [23].
ACKNOWLEDGMENTS
Financial support: Bayer Pharma AG provided partial funding for data entry and statistical analyses.
Footnotes
CONFLICTS OF INTEREST: Saad F is a full-time employee of Bayer Pharma, manufacturer of testosterone products; Yassin A and Haider A have received partial compensation for data entry from Bayer Pharma; Doros G has received payment for performing statistical analyses from Bayer Pharma; Gooren L has nothing to disclose.
References
- 1.Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, Tajar A, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99:1357–1366. doi: 10.1210/jc.2013-2052. [DOI] [PubMed] [Google Scholar]
- 3.Zarotsky V, Huang MY, Carman W, Morgentaler A, Singhal PK, Coffin D, et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2:819–834. doi: 10.1111/andr.274. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Lee DM, Forti G, O'Connor DB, Maggi M, O'Neill TW, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS) J Sex Med. 2010;7:1362–1380. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27:557–579. doi: 10.1016/j.beem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16:146–152. doi: 10.4103/1008-682X.122346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014;8:e339–e349. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013;21:1975–1981. doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 10.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329. doi: 10.1111/ijcp.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemann LA. Aging Males' Symptoms scale: a standardized instrument for the practice. J Endocrinol Invest. 2005;28(11 Suppl to no. 11):34–38. [PubMed] [Google Scholar]
- 13.Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in korean adults. Endocrinol Metab. 2014;29:418–426. doi: 10.3803/EnM.2014.29.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–351. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann LA, Saad F, Zimmermann T, Novak A, Myon E, Badia X, et al. The Aging Males' Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15. doi: 10.1186/1477-7525-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–541. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 17.Maggio M, Snyder PJ, De Vita F, Ceda GP, Milaneschi Y, Lauretani F, et al. Effects of transdermal testosterone treatment on inflammatory markers in elderly males. Endocr Pract. 2014;20:1170–1177. doi: 10.4158/EP13357.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12:5–12. doi: 10.1080/13685530802389628. [DOI] [PubMed] [Google Scholar]
- 19.Yassin DJ, El Douaihy Y, Yassin AA, Kashanian J, Shabsigh R, Hammerer PG. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014;32:1049–1054. doi: 10.1007/s00345-013-1187-z. [DOI] [PubMed] [Google Scholar]
- 20.Ko YH, Moon du G, Moon KH. Testosterone replacement alone for testosterone deficiency syndrome improves moderate lower urinary tract symptoms: one year follow-up. World J Mens Health. 2013;31:47–52. doi: 10.5534/wjmh.2013.31.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YW, Kim SB, Kwon H, Kang HC, Cho K, Lee KI, et al. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology. 2013;82:674–679. doi: 10.1016/j.urology.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 22.Kwon H, Kang HC, Lee JH. Relationship between predictors of the risk of clinical progression of benign prostatic hyperplasia and metabolic syndrome in men with moderate to severe lower urinary tract symptoms. Urology. 2013;81:1325–1329. doi: 10.1016/j.urology.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193:80–86. doi: 10.1016/j.juro.2014.06.071. [DOI] [PubMed] [Google Scholar]
- 24.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65:115–123. doi: 10.1016/j.eururo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Morgentaler A. Testosterone deficiency and cardiovascular mortality. Asian J Androl. 2015;17:26–31. doi: 10.4103/1008-682X.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1–20. doi: 10.1159/000360553. [DOI] [PubMed] [Google Scholar]
- 28.Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165:675–685. doi: 10.1530/EJE-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. J Sex Med. 2013;10:1401–1409. doi: 10.1111/jsm.12114. [DOI] [PubMed] [Google Scholar]
- 30.Donatucci C, Cui Z, Fang Y, Muram D. Long-term treatment patterns of testosterone replacement medications. J Sex Med. 2014;11:2092–2099. doi: 10.1111/jsm.12608. [DOI] [PubMed] [Google Scholar]


