Abstract
Background and aims: We aimed to investigate the effect of serum sodium level on survival in hepatocellular carcinoma (HCC) patients complicating with liver cirrhosis (LC).
Methods: A total of 1170 HCC patients with LC were analysed. We classified these patients into three groups according to serum sodium level at HCC diagnosis: group A (n=96); serum sodium ≤135 mmol/L, group B (n=520); 135 mmol/L < serum sodium ≤140 mmol/L, group C (n=554); serum sodium >140 mmol/L. We compared the baseline characteristics and overall survival (OS) among these three groups. Furthermore, we examined the factors linked to OS using univariate and multivariate analyses.
Results: In our results, decreased baseline serum sodium level was significantly associated with Child-Pugh classification and HCC stage along with several laboratory parameters in groups A, B and C. The median follow-up period was 1.1 years in group A, 2.4 years in group B and 3.3 years in group C. The 1-, 3- and 5-year cumulative OS rates in groups A, B and C were 64.8%, 46.9% and 25.7%, respectively, in group A, 85.5%, 60.5% and 41.1%, respectively, in group B and 90.7%, 66.6% and 48.2%, respectively, in group C (P<0.001). The multivariate analyses showed that Child-Pugh classification (P<0.001), HCC stage (P<0.001), serum sodium (P<0.001), aspartate aminotransferase ≥57 IU/L (P=0.002), alkaline phosphatase ≥348 IU/L (P<0.001), alpha-fetoprotein ≥29.2 ng/mL (P=0.019) and des-γ-carboxy prothrombin ≥55 mAU/mL (P<0.001) were significant independent predictors linked to OS.
Conclusion: Lower serum sodium concentration is a useful predictor in HCC patients complicating with LC.
Keywords: Hepatocellular carcinoma, Liver cirrhosis, Serum sodium, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the most common cancer worldwide. 1 In Japan, HCC ranks fifth and third in female and male as the cause of cancer related death and around 30000 patients die of this malignancy in each year. 2, 3 Treatment strategies for HCC vary depending on the hepatic functional reserve and tumor characteristics, and they include liver transplantation, surgical resection (SR), percutaneous ablative therapies (PATs) such as radiofrequency ablation or percutaneous ethanol injection, transcatheter arterial chemotherapy with or without embolization, molecular-targeted therapy (MTT) such as sorafenib and radiation therapy (RT). 4-9 HCC also often recurs even after curative therapy at the initial treatment, with any underlying liver disease and tumor status significant predictors affecting the risk of HCC recurrence. 1, 5, 6
Hyponatremia is a frequently seen complication in patients with advanced liver cirrhosis (LC) associated with arterial splanchnic vasodilatation, which involves a reduction of effective circulating blood volume. 10-14 The circulatory dysfunction leads to a non-osmotic hypersecretion of arginine vasopressin from the neurohypophysis, which causes retention of water that is disproportionate to the retention of sodium. These may cause hypo-osmolality and hypervolemic hyponatremia in patients with advanced cirrhosis. 10, 12, 13 There are few cirrhotic patients with hypovolemic hyponatremia. 10, 12, 13 In the early stage of LC, serum sodium levels are within normal range in general, however, they progressively decrease in the later stage of LC. 10, 12, 13
On the other hand, serum sodium level has been already recognized as an essential prognostic factor in patients with LC and has been shown to be associated with hepatorenal syndrome, ascites, hepatic encephalopathy, bacterial infections, and death from liver disease in many previous studies. 10, 12, 15, 16-21 Furthermore, several investigators reported that baseline serum sodium level correlates well with survival in LC patients awaiting liver transplantation, and they suggested that serum sodium concentration could be added to the calculation of model for end-stage liver disease (MELD) score for improving the accuracy of MLED scoring system in organ allocation for liver transplantation (MELD-Na). 22, 23 However, to the best of our knowledge, its prognostic role in the field of HCC with LC has been less investigated. 15 There is a paucity of available data regarding clinical outcomes of HCC patients complicating with LC focusing on serum sodium concentration. The aims of our current study were thus to examine the effect of serum sodium level on survival in HCC patients complicating with LC.
Patients and methods
Patients
A total of 1170 consecutive treatment-naïve patients diagnosed with HCC complicating with LC were admitted to the Department of Gastroenterology and Hepatology, Osaka Red Cross Hospital, Japan, between March 2004 and June 2014. LC was determined based on radiologic findings including typical computed tomography (CT) or ultrasound findings, laboratory parameters and/or histological findings obtained by surgical specimens or liver biopsy. We divided these patients into three groups according to serum sodium level at HCC diagnosis: group A (n=96); serum sodium ≤135 mmol/L, group B (n=520); 135 mmol/L < serum sodium ≤140 mmol/L, group C (n=554); serum sodium >140 mmol/L. 10, 24, 25 A recent study demonstrated that serum sodium concentration below 139 mEq/L may be predictive markers for mortality in LC patients despite being within normal ranges. 14 Thus, in this study, patients with serum sodium of >135 mmol/L were further divided into two groups using serum sodium cutoff level of 140 mmol/L. We compared the baseline characteristics and clinical outcomes among these three groups. Furthermore, we examined prognostic factors associated with overall survival (OS) using univariate and multivariate analyses.
Prior to therapy for HCC, written informed consent for HCC therapy was obtained from all subjects. The ethics committee of our department approved the protocol for this study. The present study comprised a retrospective analysis of patients' medical records in our database and all treatments were performed in an open-label manner.
Diagnosis of HCC and HCC therapy
HCC was diagnosed based on the results from abdominal ultrasound and dynamic CT scan (hyperattenuation during the arterial phase in the entire or part of the tumor, and hypoattenuation in the portal-venous phase) and/or magnetic resonance imaging mainly as recommended by the American Association for the Study of Liver Diseases. 26 Arterial and portal phase dynamic CT images were obtained approximately 30 seconds and 120 seconds after injection of contrast material. In our hospital, abdominal angiography combined with CT (angio-CT) was routinely performed before therapy for HCC after obtaining informed consent from them for performing abdominal angiography. This was performed based on the fact that this technique was useful for detecting small satellite nodules as reported by Yamasaki et al. 27 Then, we confirmed HCC using CT during hepatic arteriography (CTHA) and CT during arterial-portography (CTAP). HCC stage was determined by TNM staging system by the Liver Cancer Study Group of Japan. As for HCC therapy, the most appropriate treatment modality for each patient was selected through discussion with surgeons, hepatologists and radiologists. 8, 9 In our hospital, transcatheter arterial chemotherapies with or without embolizaiton are preferred method even for patients with portal vein thrombosis or metastatic diseases if they have preserved liver function. Sorafenib therapy is considered in HCC patients refractory to such therapies. Best supportive care was provided when treatment efficacy was considered limited or patients refused therapy for HCC. In the present analysis, there was no patient treated with liver transplantation.
Follow-up after initial therapy for HCC
Follow-up observation consisted of regularly blood tests and monitoring of tumor markers, including alpha-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), which was measured using a chemiluminescent enzyme immunoassay (Lumipulse PIVKAII Eisai, Eisai, Tokyo, Japan). Dynamic CT scan was performed every 3-4 months after initial therapy for HCC. When HCC recurrence or disease progression was detected based on radiologic findings, most appropriate therapy was performed in each patient.
Statistical analysis
The primary end point is OS. Continuous variables were compared by Mann-Whitney U test, and categorical variables were compared by Fisher's exact test. Data were analyzed using univariate and multivariate analyses. To analyze the significance of prognostic predictors, continuous variables were divided by the median values for all cases (n=1170) and treated as dichotomous covariates. The cumulative OS rate was calculated by Kaplan-Meier method and tested by log-rank test. A Cox proportional hazard model via a stepwise forward method was used for multivariate analyses of factors with P value <0.05 in univariate analyses. These statistical methods were used to estimate the interval from each date of diagnosis for HCC until the date of death or last follow up date. Data were analyzed using SPSS software (SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Data are expressed as mean ± standard deviation. A P value less than 0.05 were considered to be statistically significant.
Results
Patient demographic characteristics in groups A, B and C
Baseline demographic characteristics of patients in groups A (n=96), B (n=520) and C (n=554) are shown in Table 1.
Table 1.
Baseline characteristics among three groups of A (serum sodium ≤135 mmol/L, n=96), B (135 mmol/L < serum sodium ≤140 mmol/L, n=520) and C (serum sodium >140 mmol/L, n=554).
| Variables | A (n=96) | B (n=520) | C (n=554) |
P value A vs. B |
P value A vs. C |
P value B vs. C |
|---|---|---|---|---|---|---|
| Age (years) | 68.9 ± 9.7 | 68.6 ± 9.1 | 69.5 ± 9.7 | 0.792a | 0.580a | 0.139a |
| Gender, male / female | 70 / 26 | 351 / 169 | 303 / 251 | 0.340b | <0.001b | <0.001b |
| Causes of liver disease | ||||||
| B/C/non B non C/B and C | 9/74/13/0 | 49/355/110/6 | 62/387/92/13 | 0.213b | 0.306b | 0.111b |
| Child-Pugh, A / B / C | 41/31/24 | 342/152/26 | 421/120/13 | <0.001b | <0.001b | <0.001b |
| HCC stage, I / II / III / IV | 19/34/19/24 | 121/190/142/67 | 150/239/129/36 | 0.016b | <0.001b | 0.001b |
| Maximum tumor size (cm) | 4.1 ± 2.9 | 3.5 ± 2.8 | 2.9 ± 2.0 | 0.052a | 0.001a | 0.009a |
| Tumor number (single/multiple) | 46 / 50 | 259 / 261 | 327 / 227 | 0.740b | 0.045b | 0.003b |
| AST (IU/L) | 89.9 ± 80.0 | 71.9 ± 55.6 | 63.7 ± 40.9 | 0.040a | 0.001a | 0.015a |
| ALT (IU/L) | 61.9 ± 53.2 | 56.1 ± 44.5 | 53.3 ± 41.7 | 0.843a | 0.421a | 0.224a |
| Total bilirubin (mg/dL) | 1.75 ± 1.86 | 1.14 ± 0.93 | 1.09 ± 1.00 | 0.001a | <0.001a | 0.547a |
| Serum albumin (g/dL) | 3.23 ± 0.59 | 3.59 ± 0.54 | 3.72 ± 0.49 | <0.001a | <0.001a | <0.001a |
| ALP (IU/L) | 496.7 ± 313.5 | 422.8 ± 228.2 | 377.9 ± 218.4 | 0.047a | <0.001a | <0.001a |
| GGT (IU/L) | 152.8 ± 203.7 | 121.9 ± 150.0 | 94.9 ± 124.4 | 0.425a | 0.003a | <0.001a |
| Serum sodium (mmol/L) | 133.4 ± 2.6 | 138.8 ± 1.2 | 142.4 ± 1.5 | <0.001a | <0.001a | <0.001a |
| Diabetes mellitus, yes / no | 37 / 59 | 157 / 363 | 95 / 459 | 0.120b | <0.001b | <0.001b |
| Prothrombin time (%) | 75.6 ± 19.9 | 78.8 ± 17.1 | 80.5 ± 17.3 | 0.046a | 0.005a | 0.038a |
| Platelets (×104/mm3) | 10.8 ± 6.6 | 10.4 ± 5.1 | 9.8 ± 4.4 | 0.976a | 0.627a | 0.296a |
| AFP (ng/mL)# | 7774 ± 39861 | 6800 ± 45168 | 3453 ± 44921 | 0.715a | 0.591a | 0.091a |
| DCP (mAU/mL)## | 6807 ± 31845 | 4522 ± 21198 | 2108 ± 9370 | 0.237a | 0.002a | <0.001a |
Data are expressed as number or mean ± standard deviation. HCC; hepatocellular carcinoma, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP; alpha-fetoprotein, DCP; des-γ-carboxy prothrombin, a; Mann-Whitney U test, b; Fisher's exact test. #; missing data, n=2, ##; missing data, n=15
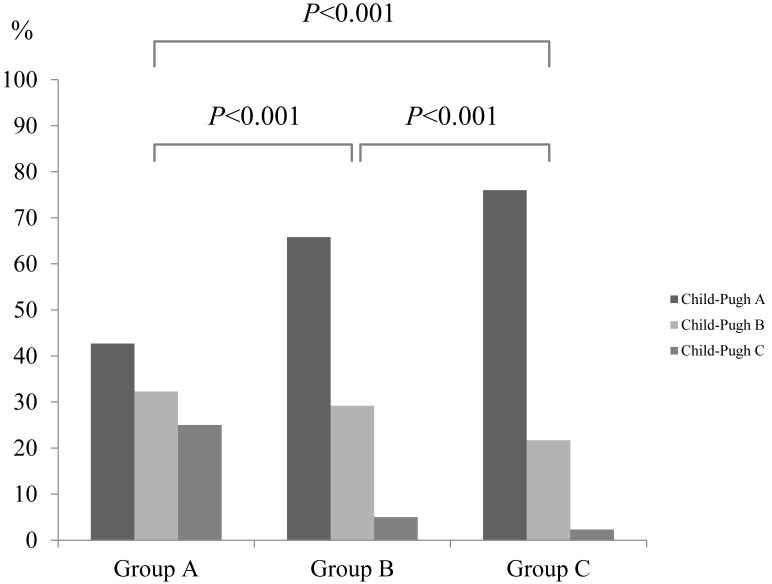
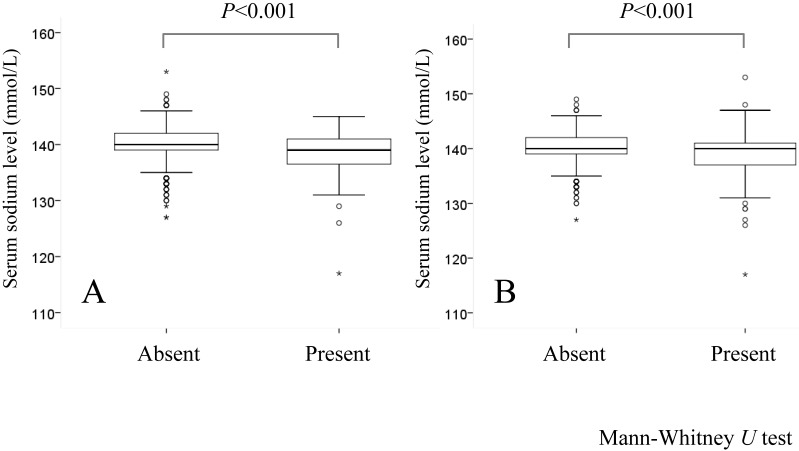
In groups A and B, decreased serum sodium level was significantly associated with Child-Pugh classification (P<0.001), HCC stage (P=0.016), aspartate aminotransferase (AST) value (P=0.040), hyperbilirubinemia (P=0.001), serum albumin (P<0.001), alkaline phosphatase (ALP) (P=0.047) and prothrombin time prolongation (P=0.046), whereas in groups A and C, decreased serum sodium level was significantly associated with gender (P<0.001), Child-Pugh classification (P<0.001), HCC stage (P<0.001), maximum tumor size (P=0.001), tumor number (P=0.045), AST value (P=0.001), hyperbilirubinemia (P<0.001), serum albumin (P<0.001), ALP (P<0.001), gamma glutamyl transpeptidase (GGT) (P=0.003), presence of diabetes mellitus (P<0.001), prothrombin time prolongation (P=0.005) and DCP (P=0.002). (Table 1 and fig. 1) Serum sodium level in patients with hepatic encephalopathy (n=79: median value; 139 mmol/L, range; 117-145 mmol/L) was significantly lower than that in patients without hepatic encephalopathy (n=1091: median value; 140 mmol/L, range; 127-153 mmol/L) (P<0.001). Similarly, serum sodium level in patients with ascites (n=252: median value; 140 mmol/L, range; 117-153 mmol/L) was significantly lower than that in patients without ascites (n=918: median value; 140 mmol/L, range; 127-149 mmol/L) (P<0.001). (Fig. 2A and 2B)
Fig 1.
The proportions of Child-Pugh A, B and C in group A (serum sodium ≤135 mmol/L, n=96), group B (135 mmol/L < serum sodium ≤140 mmol/L, n=520) and group C (serum sodium >140 mmol/L, n=554). Serum sodium concentration was significantly associated with Child-Pugh classification (P<0.001).
Fig 2.
(A) Comparison of serum sodium level in patients with (n=79) or without (n=1091) encephalopathy. (B) Comparison of serum sodium level in patients with (n=252) or without (n=918) ascites.
Initial therapy for HCC in the three groups
As an initial therapy for HCC, in group A, SR was performed in 9 patients, PATs in 37, trancatheter arterial chemotherapy with or without embolization in 37, MTT in none, RT in none and no specific therapy in 13. In group B, SR was performed in 89 patients, PATs in 270, trancatheter arterial chemotherapy with or without embolization in 140, MTT in four, RT in two and no specific therapy in 15. In group C, SR was performed in 107 patients, PATs in 325, trancatheter arterial chemotherapy with or without embolization in 104, MTT in none, RT in none and no specific therapy in 18.
Overall survival and causes of death in groups A, B and C
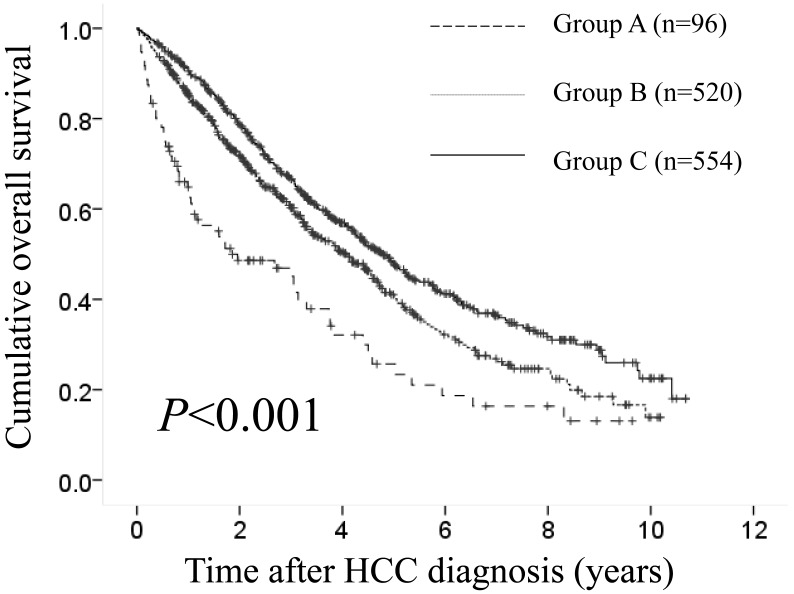
The median follow-up period was 1.1 years (range, 0.1-9.6 years) in group A, 2.4 years (range, 0.1-10.2 years) in group B and 3.3 years (range, 0.1-10.7 years) in group C. The 1-, 3- and 5-year cumulative OS rates in groups A, B and C were 64.8%, 46.9% and 25.7%, respectively, in group A, 85.5%, 60.5% and 41.1%, respectively, in group B and 90.7%, 66.6% and 48.2%, respectively, in group C (P<0.001). (Fig. 3) During follow-up period, there were 63 (65.6%), 271 (52.1%) and 291 (52.5%) deaths in groups of A, B and C, respectively. The causes of death in the three groups were HCC recurrence in 37 patients, liver failure in 20 patients and miscellaneous causes in 6 patients, respectively, in group A, HCC recurrence in 156 patients, liver failure in 89 patients and miscellaneous causes in 26 patients, respectively, in group B and HCC recurrence in 153 patients, liver failure in 95 patients and miscellaneous causes in 43 patients, respectively, in group C.
Fig 3.
Cumulative overall survival (OS) rates in group A (serum sodium ≤135 mmol/L, n=96), group B (135 mmol/L < serum sodium ≤140 mmol/L, n=520) and group C (serum sodium >140 mmol/L, n=554). The 1-, 3- and 5-year cumulative OS rates in groups A, B and C were 64.8%, 46.9% and 25.7%, respectively, in group A, 85.5%, 60.5% and 41.1%, respectively, in group B and 90.7%, 66.6% and 48.2%, respectively, in group C (P<0.001).
Univariate and multivariate analyses of factors contributing to OS
Using univariate analyses of factors contributing to OS, tumor number (P<0.001), maximum tumor size ≥2.5 cm (P<0.001), Child-Pugh classification (P<0.001), HCC stage (P<0.001), serum sodium (P<0.001), AST ≥57 IU/L (P<0.001), ALP ≥348 IU/L (P<0.001), GGT ≥64 IU/L (P<0.001), AFP ≥29.2 ng/mL (P<0.001) and DCP ≥55 mAU/mL (P<0.001) were found to be significant factors associated with OS. (Table 2) The multivariate analyses involving ten factors with P<0.05 in the univariate analysis demonstrated that Child-Pugh classification (P<0.001), HCC stage (P<0.001), serum sodium (P<0.001), AST ≥57 IU/L (P=0.002), ALP ≥348 IU/L (P<0.001), AFP ≥29.2 ng/mL (P=0.019) and DCP ≥55 mAU/mL (P<0.001) were significant independent predictors linked to OS. The hazard ratios (HRs), 95% confidence intervals (CIs) and P values for these factors are detailed in Table 2.
Table 2.
Univariate and multivariate analyses of factors contributing to overall survival (n=1170).
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Variables | n | Univariate Analysis | Hazard Ratio (95% CI) | P valuea |
| Gender, male vs. female | 724 / 446 | 0.081 | ||
| Age (years), ≥70 vs. <70 | 615 / 555 | 0.175 | ||
| Tumor number, single vs. multiple | 632 / 538 | <0.001 | ||
| Maximum tumor size (cm), ≥2.5 vs. <2.5 | 599 / 571 | <0.001 | ||
| Child-Pugh, A vs. B or C | 804 / 366 | <0.001 | 2.226 (1.877-2.640) | <0.001 |
| HCC stage, I or II vs. III or IV | 753 / 417 | <0.001 | 2.096 (1.883-2.653) | <0.001 |
| Cause of liver disease, Virus related vs. NBNC | 955 / 215 | 0.511 | ||
| Serum sodium (A / B / C) | 96 / 520 / 554 | <0.001 | ||
| Serum sodium >140 mmol/L (group C) | Reference | |||
| 135 mmol/L < serum sodium ≤140 mmol/L (group B) | 0.536 (0.403-0.714) | <0.001 | ||
| Serum sodium ≤135 mmol/L (group A) | 0.518 (0.389-0.690) | <0.001 | ||
| AST (IU/L), ≥57 vs. <57 | 593 / 577 | <0.001 | 0.767 (0.650-0.905) | 0.002 |
| ALT (IU/L), ≥44 vs. <44 | 587 / 583 | 0.132 | ||
| ALP (IU/L), ≥348 vs. <348 | 586 / 584 | <0.001 | 0.675 (0.572-0.796) | <0.001 |
| GGT (IU/L), ≥64 vs. <64 | 590 / 580 | <0.001 | ||
| Platelet count (×104 / mm3), ≥9.2 vs. <9.2 | 590 / 580 | 0.783 | ||
| Diabetes mellitus, yes vs. no | 289 / 881 | 0.478 | ||
| AFP (ng/mL), ≥29.2 vs. <29.2† | 584 / 584 | <0.001 | 0.821 (0.696-0.968) | 0.019 |
| DCP (mAU/mL), ≥55 vs. <55‡ | 580 / 575 | <0.001 | 0.504 (0.424-0.598) | <0.001 |
CI; confidence interval, HCC; hepatocellular carcinoma, NBNC; non B and non C, AST; aspartate aminotransferase, ALT; alanine aminotransferase, ALP; alkaline phosphatase, GGT; gamma glutamyl transpeptidase, AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin, a; Cox proportional hazard model, †; missing data, n=2, ‡; missing data, n=15
Subgroup analyses according to Child-Pugh classification, HCC stage and Japan Integrated Staging (JIS) score
As Child-Pugh classification and HCC stage revealed to be significant predictors linked to OS in our multivariate analysis, we conducted subgroup analyses according to Child-Pugh classification, HCC stage and Japan Integrated Staging (JIS) score. JIS score was calculated by summation of TNM stage score (stage I=0, stage II=1, stage III=2 and stage IV=3) and Child-Pugh classification (A=0, B=1 and C=2) as reported by Kudo, et al. 28
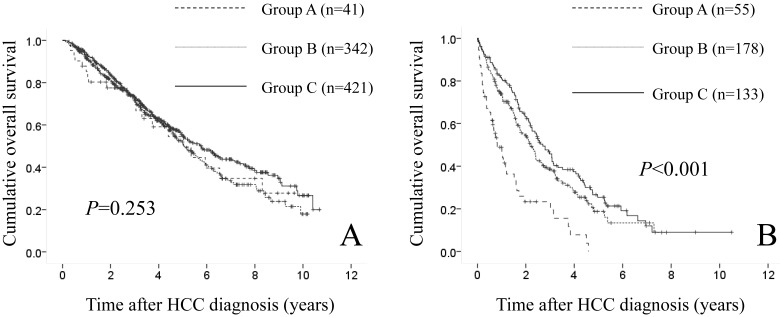
In patients with Child-Pugh A (n=804), there were 41 patients in group A, 342 in group B and 421 in group C. The difference in the three groups did not reach significance in terms of OS (P=0.253). (Fig. 4A) In patients with Child-Pugh B or C (n=366), there were 55 patients in group A, 178 in group B and 133 in group C. Significant difference was observed in the three groups in terms of OS (P<0.001). (Fig. 4B)
Fig 4.
(A) Cumulative OS rates in patients with Child-Pugh A (n=804: 41 patients in group A, 342 in group B and 421 in group C). The difference in the three groups did not reach significance in terms of OS (P=0.253). (B) Cumulative OS rates in patients with Child-Pugh B or C (n=366: 55 patients in group A, 178 in group B and 133 in group C). Significant difference was found in the three groups in terms of OS (P<0.001).
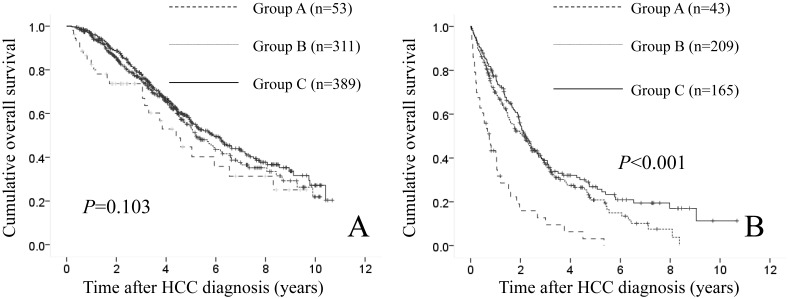
In patients with HCC stage I or II (n=753), there were 53 patients in group A, 311 in group B and 389 in group C. The difference in the three groups did not reach significance in terms of OS (P=0.103). (Fig. 5A) In patients with HCC stage III or IV (n=417), there were 43 patients in group A, 209 in group B and 165 in group C. Significant difference was observed in the three groups in terms of OS (P<0.001). (Fig. 5B)
Fig 5.
(A) Cumulative OS rates in patients with HCC stage I or II (n=753: 53 patients in group A, 311 in group B and 389 in group C). The difference in the three groups did not reach significance in terms of OS (P=0.103). (B) Cumulative OS rates in patients with HCC stage III or IV (n=417: 43 patients in group A, 209 in group B and 165 in group C). Significant difference was found in the three groups in terms of OS (P<0.001).
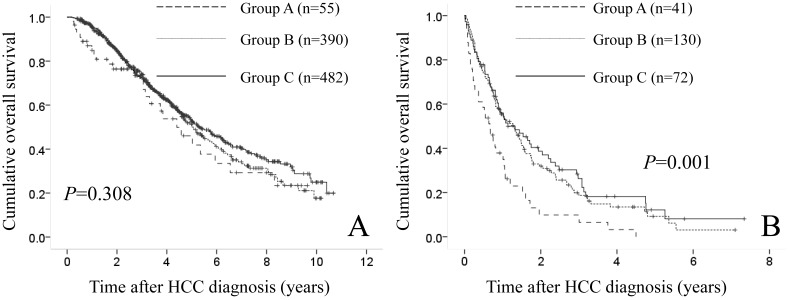
In patients with JIS score of 0, 1, or 2 (n=927), they included 55 patients in group A, 390 in group B and 482 in group C. No significant difference was found in the three groups in terms of OS (P=0.308). (Fig. 6A) In patients with JIS score of 3, 4, or 5 (n=243), they included 41 patients in group A, 130 in group B and 72 in group C. There reached significance in the three groups in terms of OS (P=0.001). (Fig. 6B)
Fig 6.
(A) Cumulative OS rates in patients with Japan Integrated Staging (JIS) score of 0, 1 or 2 (n=927: 55 patients in group A, 390 in group B and 482 in group C). The difference in the three groups did not reach significance in terms of OS (P=0.308). (B) Cumulative OS rates in patients with Japan Integrated Staging (JIS) score of 3, 4 or 5 (n=243: 41 patients in group A, 130 in group B and 72 in group C). Significant difference was found in the three groups in terms of OS (P=0.001).
Comparison of OS according to initial treatment modality
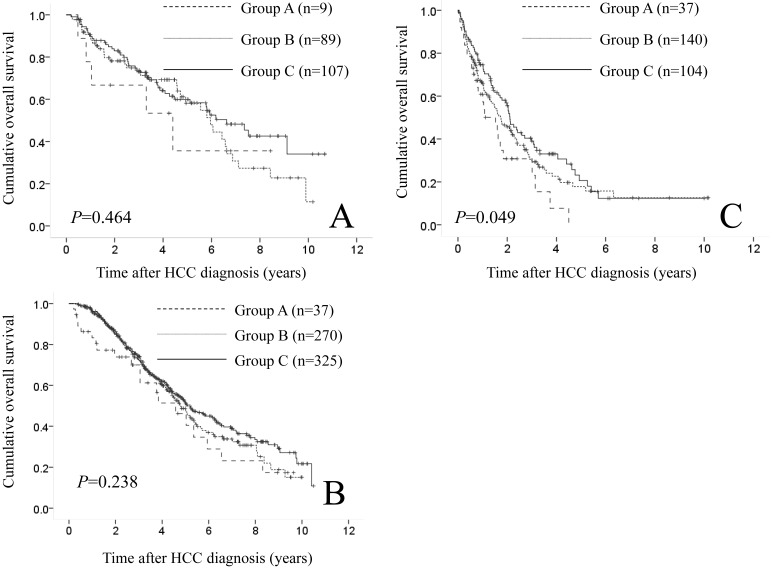
In patients treated with SR (9 patients in group A, 89 in group B and 107 in group C) and PATs (37 patients in group A, 270 in group B and 325 in group C), the difference in the three groups did not reach significance in terms of OS (P=0.464 and 0.238). (Fig. 7A and 7B) While in patients who underwent trancatheter arterial chemotherapy with or without embolization (37 patients in group A, 140 in group B and 104 in group C), significant difference was found in terms of OS (P=0.049). (Fig. 7C)
Fig 7.
Cumulative OS rates according to initial treatment modality. (A) In patients treated with surgical resection (9 patients in group A, 89 in group B and 107 in group C), the difference in the three groups did not reach significance in terms of OS (P=0.464). (B) In patients treated with percutaneous ablative therapies (37 patients in group A, 270 in group B and 325 in group C), the difference in the three groups did not reach significance in terms of OS (P=0.238). (C) In patients who underwent trancatheter arterial chemotherapy with or without embolization (37 patients in group A, 140 in group B and 104 in group C), significant difference was observed in terms of OS (P=0.049).
Discussion
To our knowledge, our study represents the largest investigation reported thus far assessing serum sodium concentration in HCC patients complicating with LC on clinical outcome and the association between serum sodium levels and baseline characteristics in our cohort. Few available data exist regarding clinical outcomes of HCC patients complicating with LC focusing on baseline serum sodium concentration. 15 There is urgent need for clarifying these issues. Thus, we conducted this observational study.
In our results, lower serum sodium level was associated with poorer survival for all cases (n=1170) and it was a significant independent predictor in the multivariate analysis (serum sodium ≤135 mmol/L: HR=0.518 and P<0.001) along with other well known predictors in the field of HCC such as Child-Pugh classification, HCC stage and tumor markers. 1-6 Our study results indicate that in not only LC patients without HCC but also LC patients with HCC, serum sodium level can be a useful predictor.
A previous study (n=997) demonstrated that if the cutoff value of hyponatremia of 135 mmol/L is used, the prevalence of hyponatremia in patients with LC is around 50%. 29 While in our data, the prevalence of patients with serum sodium level of ≤135 mmol/L was 8.2% (96/1170). HCC patients with LC and hyponatremia are poor candidates for HCC therapy in general due to the expected increased risk of treatment related complications and shorter survival. 15 Discrepancies of distribution of hyponatremia between these studies may be attributed to the fact that HCC patients with high risk of treatment related complication or severe concomitant disorders were rarely introduced to our hospital. Our hospital is a tertiary center aiming at performing HCC therapy. On the other hand, lower serum sodium level was significantly associated with Child-Pugh classification in our baseline characteristics. Our results were similar to the previous study. 29 Serum sodium levels as well as serum ammonia levels are major determinants of electroencephalographic abnormalities in patients with LC and there is evidence indicating that hyponatremia may affect brain function and predispose to hepatic encephalopathy, which are in line with our study results as shown in Fig. 2A. 13, 25, 29, 30
In our subgroup analyses, lower serum sodium was closely associated with prognosis in patients with higher Child-Pugh score, more advanced HCC stage or higher JIS score. In patients with earlier HCC stage or less advanced LC, serum sodium level may not be a useful predictor. On the other hand, in terms of comparison of OS according to treatment modality, serum sodium level was significantly associated with OS only in patients who underwent trancatheter arterial chemotherapy with or without embolization. Biolato M, et al. reported that in HCC patients treated with transcatheter arterial chemoembolization (n=270), the inclusion of baseline serum sodium level alongside the already known predictors may allow a better prognostic definition of HCC patients as candidates for transcatheter arterial chemoembolization. 15 Our results were compatible with their study results. In patients with early stage HCC who are potentially candidate for SR or PATs, curative therapy for HCC rather than hyponatremia itself may be essential for improving prognosis.
We acknowledge several limitations in our study. First, this is a retrospective observational study with heterogeneous HCC patient populations. Second, there are several missing data in the present study. However, the number of subjects with missing data was very small considering large sample of our study (n=1170), which may not effect on interpretation of our results. Third, serum sodium concentration is a labile variable and it can be influenced by concomitant treatment such as diuretics. 13, 20, 24, 25 However, our data revealed that baseline serum sodium concentration was a useful predictor in HCC patients complicating with LC.
In conclusion, there may exist prognostic significance of baseline serum sodium value in HCC patients complicating with LC. Lower serum sodium may be associated with prognosis especially in patients with higher Child-Pugh score, more advanced HCC stage or higher JIS score.
Acknowledgments
We would like to thank Haruko Takada for the data collection.
References
- 1.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2014. Jun 26. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 3.Nishikawa H, Osaki Y. Non-B, non-C hepatocellular carcinoma (Review) Int J Oncol. 2013;43(5):1333–1342. doi: 10.3892/ijo.2013.2061. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa H, Kita R, Kimura T, Osaki Y. Transcatheter Arterial Embolic Therapies for Hepatocellular Carcinoma: A Literature Review. Anticancer Res. 2014;34(12):6877–6886. Review. [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JW, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL. et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC) Liver Int. 2013;33:327–337. doi: 10.1111/liv.12083. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 10.Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterol Rep (Oxf) 2014;2(1):21–26. doi: 10.1093/gastro/got037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81–95. doi: 10.1055/s-2008-1040323. [DOI] [PubMed] [Google Scholar]
- 12.Yu C, Sharma N, Saab S. Hyponatremia: clinical associations, prognosis, and treatment in cirrhosis. Exp Clin Transplant. 2013;11(1):3–11. doi: 10.6002/ect.2012.0147. [DOI] [PubMed] [Google Scholar]
- 13.Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48(3):1002–1110. doi: 10.1002/hep.22418. [DOI] [PubMed] [Google Scholar]
- 14.Umemura T, Shibata S, Sekiguchi T, Kitabatake H, Nozawa Y, Okuhara S, Serum sodium concentration is associated with increased risk of mortality in patients with compensated liver cirrhosis. Hepatol Res. 2014. Aug 28. [Epub ahead of print] [DOI] [PubMed]
- 15.Biolato M, Miele L, Vero V, Racco S, Di Stasi C, Iezzi R. et al. Hepatocellular carcinoma treated by conventional transarterial chemoembolization in field-practice: serum sodium predicts survival. World J Gastroenterol. 2014;20(25):8158–8165. doi: 10.3748/wjg.v20.i25.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min YW, Kim J, Kim S, Sung YK, Lee JH, Gwak GY. et al. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33:197–202. doi: 10.1111/liv.12023. [DOI] [PubMed] [Google Scholar]
- 17.Jenq CC, Tsai MH, Tian YC, Chang MY, Lin CY, Lien JM. et al. Serum sodium predicts prognosis in critically ill cirrhotic patients. J Clin Gastroenterol. 2010;44:220–226. doi: 10.1097/MCG.0b013e3181aabbcd. [DOI] [PubMed] [Google Scholar]
- 18.Guevara M, Baccaro ME, Torre A, Gómez-Ansón B, Ríos J, Torres F. et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- 19.Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G. et al. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch Surg. 2009;144:57–63. doi: 10.1001/archsurg.2008.522. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT. et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeff HP, Streule GC, Drognitz O, Tittelbach-Helmrich D, Spangenberg HC, Hopt UT. et al. Early mortality and long-term survival after abdominal surgery in patients with liver cirrhosis. Surgery. 2014;155(4):623–32. doi: 10.1016/j.surg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 23.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T. et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 25.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342–1348. doi: 10.1002/1097-0142(20010401)91:7<1342::aid-cncr1137>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38(3):207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 29.Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44(6):1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi MO, Khokhar N, Saleem A, Niazi TK. Correlation of hyponatremia with hepatic encephalopathy and severity of liver disease. J Coll Physicians Surg Pak. 2014;24(2):135–137. [PubMed] [Google Scholar]