Abstract
Most of the SOD mimics thus far developed belong to the classes of Mn-(MnPs) and Fe porphyrins(FePs), Mn(III) salens, Mn(II) cyclic polyamines and metal salts. Due to their remarkable stability we have predominantly explored Mn porphyrins, aiming initially at mimicking kinetics and thermodynamics of the catalysis of O2•− dismutation by SOD enzymes. Several MnPs are of potency similar to SOD enzymes. The in vivo bioavailability and toxicity of MnPs have been addressed also. Numerous in vitro and in vivo studies indicate their impressive therapeutic efficacy. Increasing insight into complex cellular redox biology has been accompanied by increasing awareness of complex redox chemistry of MnPs. During O2•− dismutation process, the most powerful Mn porphyrin-based SOD mimics reduce and oxidize O2•− with close to identical rate constants. MnPs reduce and oxidize other reactive species also (none of them specific to MnPs), acting as reductants (antioxidant) and pro-oxidants. Distinction must be made between the type of reactions of MnPs and the favorable therapeutic effects we observe; the latter may be of either anti- or pro-oxidative nature. H2O2/MnP mediated oxidation of protein thiols and its impact on cellular transcription seems to dominate redox biology of MnPs. It has been thus far demonstrated that the ability of MnPs to catalyze O2•− dismutation parallels all other reactivities (such as ONOO− reduction) and in turn their therapeutic efficacies. Assuming that all diseases have in common the perturbation of cellular redox environment, developing SOD mimics still seems to be the appropriate strategy for the design of potent redox-active therapeutics.
Keywords: Mn-porphyrin-based SOD mimics; Fe porphyrin-based SOD mimics; Mn-porphyrin-based non-SOD mimics; Reactivities of Mn porphyrins in aqueous solutions, cells and animals; Mechanism(s) of action(s) of Mn porphyrins; Therapeutic effects
Graphical abstract
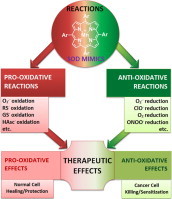
Mn porphyrins, initially developed as SOD mimics, undergo in vivo diverse pro- and antioxidative reactions which may be demonstrated as either anti- (mostly in normal cell) or pro-oxidative (mostly in cancer cell) therapeutic effects.
Highlights
-
•
Potent Mn porphyrin (MnP)-based SOD mimics oxidize and reduce O2•− during dismutation process.
-
•
During dismutation process, MnP oxidizes and reduces O2•−.
-
•
SOD-like activity clearly demonstrates that MnPs can in vivo act as oxidant and reductant.
-
•
Redox-properties that make them potent SOD mimics allow MnPs to undergo diverse, therapeutically relevant, reactions in vivo.
-
•
Anti- and pro-oxidative reactions of MnPs may result in either antioxidative or pro-oxidative therapeutic effects.
-
•
H2O2 and cellular reductants play major roles in the actions and in turn therapeutic effects of MnPs.
Introduction
The necessity of SOD enzymes for all aerobic life [1,2] led to the development of SOD mimics as therapeutics [3]. The macrocyclic structure of a porphyrin ring, by the analogy to the structure of the critical porphyrin-containing proteins, such as hemoglobin, myoglobin, nitric oxide synthases and cyt P450 family of enzymes, provides limitless stability to a metal complex and preserves integrity of the metal site where all actions occur. Consequently, porphyrin appears to be the most appropriate ligand for an SOD mimic. Further, the porphyrin structure allows for limitless modifications whereby the redox property, bioavailability and toxicity could be optimized. Over years in search for the best therapeutics, metalloporphyrins with high kcat(O2•−) reaching that of SOD enzyme and with high stability towards the loss of Mn, have been synthesized and characterized. Such properties give them the advantage over other classes of SOD mimics. Most recent research indicate that SOD-like activity parallels ability of MnPs to undergo other reactions (such as ONOO− reduction) and their therapeutic efficacy. None of the reactions appear specific to MnPs. For additional insight see also other articles in Antioxidant & Redox Signaling 2014 Forum Issues on “Superoxide dismutases” (vol. 20/10) and “SOD therapeutics” (vol.20/15).
O2•− dismutation process
The dismutation process consists of two steps indicated below for Mn porphyrin-based SOD mimics. It is critical to note that: (i) SOD enzyme or MnP-based mimic acts as a (pro)oxidant in a 1st step, and as an anti-oxidant in a 2nd step, whereby closing the catalytic cycle, and (ii) an oxidant, H2O2, is produced in a 2nd step. Under physiological conditions it is taken care of. Thus, H2O2 is either dismuted to O2 and H2O2, or H2O2 is reduced to H2O, with enzymatic systems such as catalases, glutathione peroxidases (GPx), peroxiredoxins, etc. Therefore both SOD and its mimic could be considered an antioxidative defense only when coupled to H2O2 removing systems. Growing data indicate that in cancer such systems fail. Reports indicate downregulation or inactivation of enzymes such as GPx and peroxiredoxin, while up-regulation of SOD during cancer progression [4–6]. In turn, H2O2 gets accumulated and used by cancer cell for its proliferation; under such conditions both SOD and its mimic will increase oxidative stress and cannot be anymore considered an antioxidative defense [3,5,6].
Design of SOD mimics
MnP-based SOD mimics have been developed with the goal to mimic the kinetics and thermodynamics of the catalysis of O2•− dismutation by SOD enzymes (Scheme II and III). MnPs, which kcat(O2•−) is close to that of enzyme, were synthesized: Br8MnTM-3-PyP4+(log kcat(O2•−) ≥8.85), Br8MnTM-4-PyP4+(log kcat(O2•−) ≥8.67) and Cl5MnTE-2-PyP4+(log kcat(O2•−)=8.41) (Scheme VI). Such results teach us that even with low-molecular weight compounds the properties of an enzyme could be mimicked. The log kcat(O2•−) of SOD enzymes in the range 8.84–9.30 were reported [1,9]. Yet the protein structure assures the specificity of SOD enzymes to O2•−. Despite favorable thermodynamics, the SOD enzymes react with other species, such as ONOO−, orders of magnitude slower than cationic MnTE-2-PyP5+.
Scheme I.
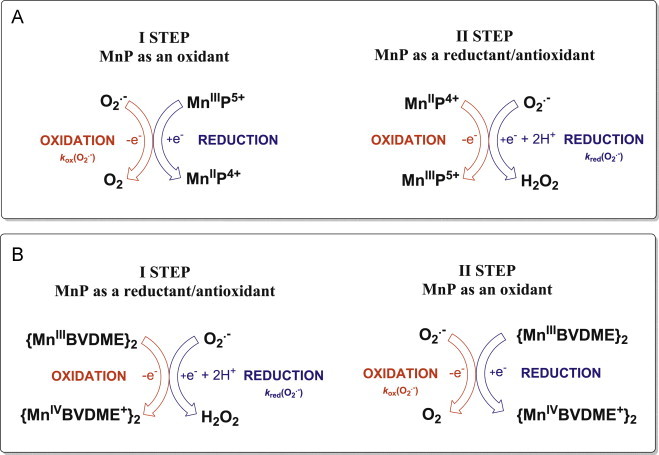
The O2•− dismutation process catalyzed by pentacationic Mn porphyrins (A) and Mn biliverdin (and its derivatives) (B). A. Dismutation of O2•− by pentacationic Mn complexes involves MnIII/MnII redox couple. The dismutation process shown here communicates important information that SOD mimics exhibit PRO- and ANTI-oxidative actions during O2•− dismutation, i.e. they can accept (1st step) electron from O2•− producing O2, and give away electron to another molecule of O2•− (2nd step) producing H2O2 if their metal-centered reduction potential, E1/2 for MnIIIP/MnIIP redox couple is within the range of +100 to +500 mV vs NHE. Such E1/2 values indicate that SOD mimics are mild oxidants and reductants/antioxidants and that their reduction potentials are biologically compatible and allow them to couple with numerous other biological targets. Yet, there is another side of dismutation process. The 2nd step (while antioxidative in its nature with regards to O2•−) produces an oxidant, H2O2. The SOD enzymes and their mimics may in turn be only viewed as anti-oxidative defense when (under physiological conditions) are coupled with multiple H2O2 removing systems. It explains why in numerous in vivo reactions (oxidation of thiols, ascorbate, lipids, NADPH, NADH, see below) MnP acts as pro-oxidant, while still GENERATING therapeutic EFFECTS. Thus the additional distinction must be made here between the actions of redox-active compounds and therapeutic effects observed. Please note that MnPs with very negative E1/2<0 mV vs NHE (very electron rich such as MnTBAP3− or MnTSPP3−) are fairly redox-inert and cannot be reduced in a 1st step with O2•−, but can act as anti-oxidants (reductants) during oxidation with strong oxidants (such as ONOO−, ClO−, lipid reactive species). Since they are electron-rich, and oxidation with those species involves their binding to Mn site, such reactions are less favored than those with electron-deficient Mn porphyrins (such as MnTE-2-PyP5+) with E1/2 in between +100 and +500 mV vs NHE [7]. Note, also that MnPs with E1/2>+450 (very electron-deficient, MnIIBr8TM-3(or 4)-PyP4+ and Cl5MnIITE-2-PyP4+), are stabilized in +2 oxidation state and lose Mn readily at pH 7.8. For comparison those Mn(III) N-alkylpyridylporphyrins with Mn in +3 oxidation state (indicated with III) resist even 98% sulfuric and 36% hydrochloric acids. Such compounds therefore start dismutation by reducing O2•− while oxidizing Mn in a 1st step, and get reduced to Mn(II) while oxidizing O2•− in a 2nd step, reversal of Scheme A. B. Dismutation of O2•− by Mn(III) biliverdin and its analogs employ MnIV/MnIII redox couple, shown in Scheme for Mn(III) biliverdin dimethylester (BVDME). Mn(III)- and Fe(III) corroles employ metal +3/+4 redox couple also [8]. In turn, in a 1st step those metal sites reduce O2•− while undergoing oxidation to M(IV) complex, whereas in backward reaction they get reduced with O2•− (and restored as catalysts) (see [3] for details). Regardless of which metal redox couple is used (+3/+2 or +3/+4), O2•− only cares if the metal easily accepts electron from and donates the electron to it, thus operating at E1/2 value that is around the midway potential between the oxidation and reduction of O2•−; consequently both steps of dismutation are facilitated to a similar extent. That indeed is true, the E1/2 ranges between +440 and +470 mV vs NHE for Mn(III) biliverdin analogs, while E1/2=+468 and +480 mV vs NHE for Br8MnIITM-3-PyP4+ for Br8MnIITM-4-PyP4+[9].
Scheme II.
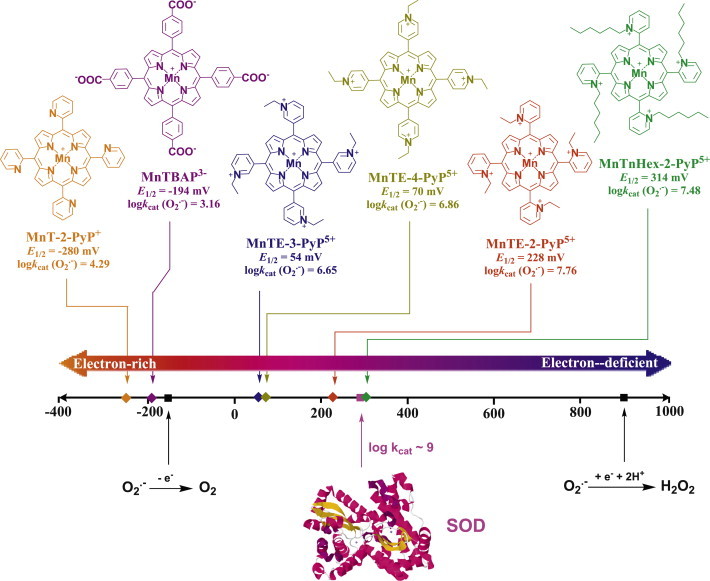
SOD mimics were designed in a process where the kinetics and thermodynamics of O2•− dismutation catalyzed by superoxide dismutases were mimicked. The kinetics of the catalysis O2•− dismutation relates to the electrostatic facilitation of the approach of anionic O2•− towards the metal site. In the case of enzyme the anionic superoxide is pulled towards the metal site through the tunnel of amino acids with cationic residues. Those MnPs which have cationic charges on periphery are ~2 or 2.5 orders of magnitude faster catalysts than those which are either neutral or anionic at the periphery, respectively. The thermodynamics relates to the redox properties of the metal site (described with metal-centered reduction potential, E1/2) which would allow Mn to equally easily accept electron from one O2•− and give it to another O2•−. Cationic compounds with E1/2 for MnIIIP/MnIIP redox couple around the E1/2 of SOD enzymes (~+300 mV vs NHE) were synthesized and demonstrated to efficiently catalyze O2•− dismutation in aqueous solution with high rate constants. In addition, they are extremely stable complexes towards the loss of Mn. Such E1/2 of SOD enzyme and its mimics sits around the midway potential between the E1/2 for O2•− reduction at +890 mV vs NHE and its oxidation at −180 mV vs NHE. In turn, it allows equal facilitation for both steps of dismutation process [3,9]. Indeed both steps occur with the same rate constants with SOD enzyme as was demonstrated for its mimic, MnTE-2-PyP5+[10]. The most powerful SOD mimics belong to the class of Mn(III) ortho (N-substituted pyridinium-2-yl)porphyrins. They possess both favorable electrostatics, as they bear 4 positive charges at the periphery to attract O2•−, and favorable thermodynamics, E1/2 ~+200 to +450 mV vs NHE. Among them the ortho, meta and para pyridyl MnPs which are β-halogenated (with chlorines at β positions of pyrrole rings, see Scheme IV) possess the highest kcat(O2•−), close to that of SOD enzymes; yet they are the least stable. Adapted from [3].
Scheme VI.
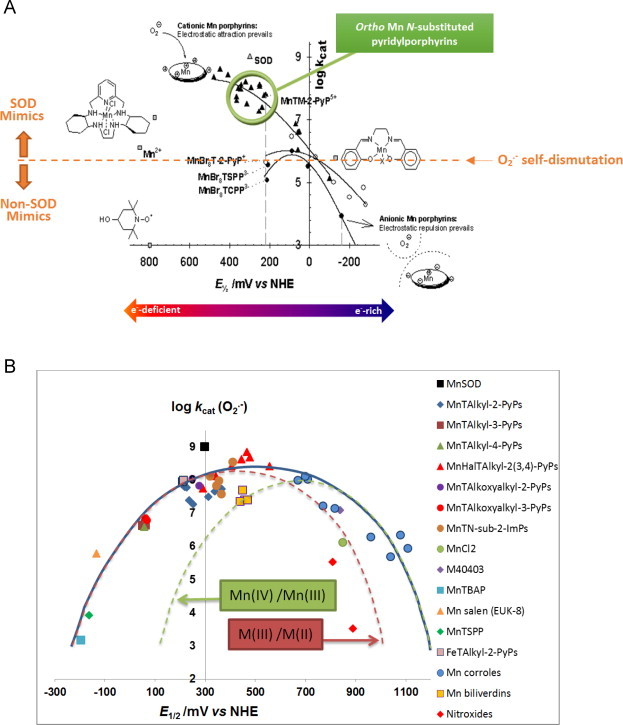
(A) Structure–activity relationships, SARs, relate E1/2 to log kcat(O2•−) for cationic, anionic and compounds which are neutral on periphery. All MnIIIPs carry one+charge on Mn center. SARs have been initially established for Mn porphyrins and later proven to have wider applicability to any redox active metal complex (Scheme VIB). As compared to cationic MnPs, the ~2–2.5 orders of magnitude lower kcat(O2•−) was determined with neutral or anionic compounds than with cationic MnPs, where either no attraction exists or anionic O2•− is repulsed from anionic porphyrin. The additional importance of such SARs lies in the fact that the magnitude of the interactions of SOD mimics with other reactive species (thus far explored, Schemes IX–XIV), and their therapeutic effects are directly proportional to their SOD-like activity; in turn SAR established for SOD-like activity, can offer best guidance for the development of powerful therapeutics. Compounds which dismute O2•− with rate constants lower than that for self-dismutation, log kcat(O2•−)<5.7, are not mimics of SOD. A number of Mn porphyrins with no SOD-like activities reportedly demonstrate beneficial therapeutic effects in vitro and in vivo models of diseases (Scheme XXV). Such data suggest that mechanism of action, other than SOD-like, may be operative [4]. Adapted from [16]. (B) Structure activity relationships, SARs, relates E1/2 to log kcat(O2•−) for all compounds irrespectively of their electrostatics, their size and shape and type of redox couple involved. The blue curve relates to all compounds irrespective of the type of redox couple involved: either Mn(III)/Mn(II) or Mn(IV)/Mn(III). The O2•− does not care which type of redox couple is involved, as long as the magnitude of E1/2 is adequate. The red dashed curve relates to the compounds which operate via Mn(III)/Mn(II) redox couple, while the green one relates to compounds that utilize Mn(IV)/Mn(III) redox couple for O2•− dismutation. While we have not explored it further, the maximum seems to be shifted right for compounds which use Mn(IV)/Mn(III) redox couple; therefore the dashed curves are suggested. The larger deviation from the plot is demonstrated with compounds which have unfavorable electrostatics (anionic, bulky compounds). The largest deviation is seen with non-metal-based SOD mimics such as nitroxides. When the E1/2 is very negative, left arm of bell-shape curve, the reduction of MnIIIP to MnIIP is a rate limiting step in O2•− dismutation. As the E1/2 increases, reaching maximal log kcat values, both steps of dismutation become similar and offer similar thermodynamics for the dismutation process, such conditions favor high kcat(O2•−). Once the electron-density of Mn center gets overly reduced, E1/2 of Mn(III)/Mn(II) redox couple >+450 mV vs NHE, the compound becomes stabilized in Mn +2 oxidation state (the right arm of the bell-shape curve). The consequence of this is that Mn(II) porphyrins are unstable (Mn gets easily lost) and are therefore not prospective therapeutics. Under such conditions the 1st and 2nd steps of dismutation process are reversed. With Mn(II) porphyrins, as the E1/2 becomes too positive, the 1st step of dismutation (oxidation of MnIIP to MnIIIP and reduction of O2•− to H2O2) becomes unfavorable and rate limiting; in turn the kcat(O2•−) decreases again (the right arm of bell-shape curve). Adapted from [3].
Over years diverse compounds were synthesized and/or obtained from commercial sources of either high, modest or no SOD-like activities. Mn porphyrins with high (log kcat(O2•−)>7), modest log kcat(O2•−)<7 and no SOD-like activity (log kcat(O2•−)≤5.6) are listed in Schemes IV, V and VII. Those of no SOD-like activities have been often incorrectly used to support O2•−-related pathways.
Scheme IV.
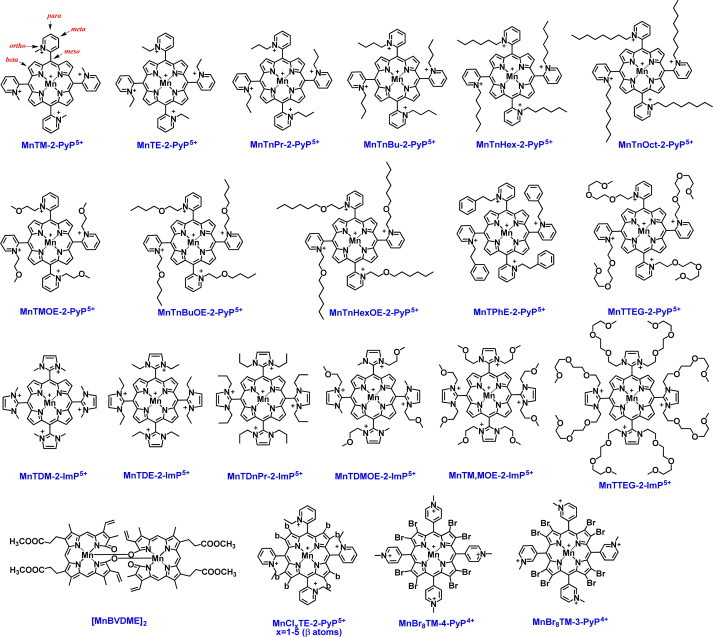
Powerful Mn porphyrin-based SOD mimics (log kcat(O2•−)>7). The most powerful compounds bear ortho cationic pyridyl- or di-ortho imidazolyl nitrogens which carry positive charges to guide anionic O2•− to the metal site and tune E1/2 to be in between +200 and +500 mV vs NHE. For numerical values of kcat(O2•−) please see [3,9,15].
Scheme V.
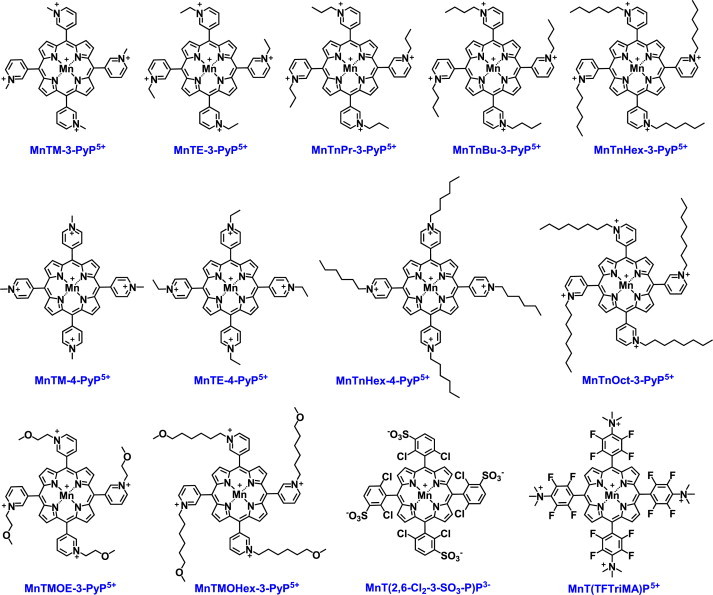
Modest mimics of SOD enzymes (log kcat(O2•−)= 5.7–7). Some of those compounds have increased lipophilicity which allows higher intracellular accumulation and compensates largely for their inferior SOD-like activity. That has been most strikingly demonstrated with ortho MnTE-2-PyP5+ and its meta isomer, MnTE-3-PyP5+. With 10-fold lower kcat(O2•−), but with 10 fold higher cellular accumulation, MnTE-3-PyP5+ protected SOD-deficient E. coli to the same extent as did MnTE-2-PyP5+. For values of kcat(O2•−) please see [3,9,15].
Scheme VII.
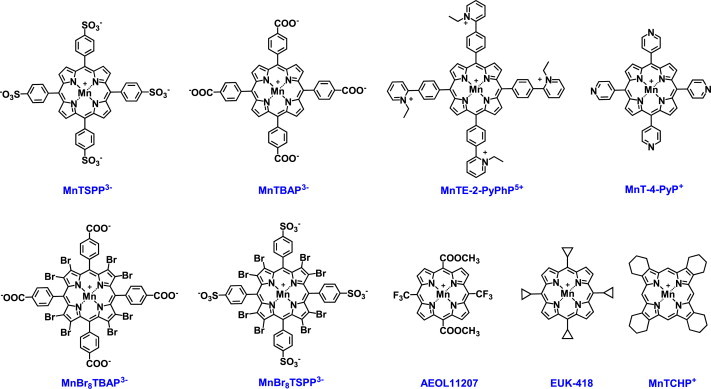
Compounds with kcat(O2•−) lower than the value for O2•− self-dismutation, i.e. below 5.7 are not SOD mimics. Can non-SOD mimic be a good therapeutic?A simple answer is: yes. In addition to other groups, our group has also reported therapeutic effects of MnTBAP3− which has a very low log kcat=3.16 and is thus not an SOD mimic. The nature of the actions of this compound resulting in therapeutic effects is not yet understood. Some of the reactive species, such as ONOO− (see Scheme X), are highly oxidizing and reportedly oxidize even fairly inert compounds such as MnTBAP3−, AEOL11207 or MnTCHP+. The resulting O=MnIVP species may be reduced back to MnIIIP with O2•− affecting in turn the in vivo levels of O2•− and restoring physiological redox environment. Reduction of O=MnIVP species to MnIIIP may occur by cellular reductants also (Schemes XI and XII). This being said, the accurate assignment of the effects observed in vivo is needed as that would ultimately help us in understanding the relations between the redox reactions of those compounds and redox-based pathways of a cell. This will in turn positively affect drug development helping us understand what kind of drugs may be the best therapeutics.
Structure–activity relationships, SAR, for mimicking SOD enzymes
See Scheme VI.
Mn porphyrin-based non-SOD mimics
See Scheme VII.
Fe porphyrin-based SOD mimics
See Scheme VIII.
Scheme VIII.
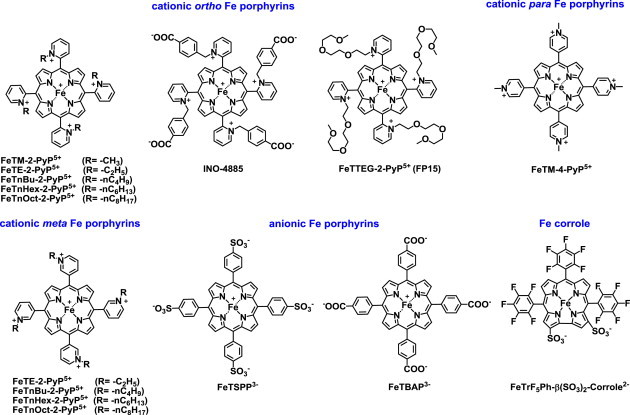
Fe porphyrin-based SOD mimics. Fe porphyrins and Fe complex with shrunk porphyrin, corrole, which is missing a methine group at one meso position, are listed here. Cationic Fe(III) N-substituted pyridylporphyrins (FePs) have different chemistry from Mn analogs. Both have same conformation with two axially ligated aqua ligands, but under different conditions. Such conformation of FeP exists at pH~2, but not at pH=7.8. The E1/2 of (H2O)2FeTE-2-PyP5+ is ~160 mV more positive than E1/2 of (H2O)2MnTE-2-PyP5+. Thus, at pH=7.8, one aqua ligand of FeP gets deprotonated: (OH)(H2O)FeTE-2-PyP4+ is formed. MnP however has two aqua ligands at pH=7.8. Aqua ligands are not indicated in formulas in this review (unless involved in reactions) though all Mn(III) porphyrins bear 2 aqua ligands at pH=7.8, while all FePs discussed here have one hydroxo and one aqua ligands. The presence of electron-donating hydroxo ligand stabilizes Fe in +3 oxidation state. This results in a shift of E1/2 from +380 to +215 mV vs NHE. Consequently, the E1/2 becomes identical to the one of MnTE-2-PyP5+ (+228 mV vs NHE); thus MnPs and FePs have similar SOD-like activities [17,18]. Another difference, FePs undergo faster degradation in vivo than MnPs. At ≤1 µM concentration, FeTE-2-PyP5+ is as efficacious as ≤1 µM Fe(II) citrate in protecting aerobic growth of SOD-deficient E. coli. That is due to the release of Fe from Fe porphyrin which subsequently restores Fe-bearing proteins which have lost Fe due to the superoxide-mediated oxidative damage. At 20 µM, though, where MnP is efficacious, FeP is toxic. FePs have been successfully used in many animal models even at fairly high concentrations similar to those of MnPs [3,18–20]. One of them, INO-4885 is in clinical trials. Another porphyrin-like class of compounds with therapeutic potential – Fe corroles – has been explored by Gross’s group [8,21]. Some members of corrole class, including FeTrF5Ph-β(SO3)-corrole2−, are advancing towards clinic.
Reactivities of MnPs towards diverse low-molecular weight reactive species
The reactivity of ortho Mn(III) N-substituted pyridylporphyrin towards a whole spectrum of reactive species with corresponding rate constants (estimated based on reactions of MnTE-2-PyP5+) is summarized in Scheme IX. The related reactions are described by equations in Scheme X. These reactions may dominate their in vivo actions and may not necessarily be related to SOD mimicking. Most of those reactions are not catalytic, and their rate constants refer to either reduction or oxidation of MnP, i.e. or oxidation or reduction of respective reactive species. The reactivities of MnPs could become catalytic in vivo, i.e. MnP as a catalyst could be recovered. This could happen when the reduction or oxidation of MnP is coupled with other reactive species or cellular reductant. In addition to direct reactions with small reactive species listed in Schemes IX and X, MnPs react directly with small thiols such as GSH and cysteine and with protein thiols of signaling proteins/transcription factors (Schemes XI and IV) as well as with ascorbate (Scheme XII) and tetrahydrobiopterin (Scheme XIII). MnPs interactions with thiols may or may not involve H2O2. If driven by H2O2, various MnP redox couple will be involved (Schemes XI, XII and XIV). MnP/H2O2-mediated oxidation of NADH and NADPH was demonstrated also (Scheme XIV).
Scheme IX.
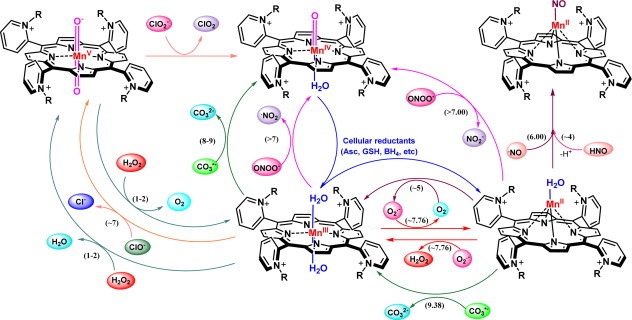
Reactivity of Mn(III) ortho N-substituted pyridylporphyrins towards different low-molecular weight reactive species. In late 1990s, along with growing awareness of highly oxidizing species, ONOO–, it became obvious that all potent SOD mimics can effectively reduce ONOO− either one- (if starting from MnIIIP) or two-electronically (if starting from MnIIP), producing either highly oxidizing •NO2 or benign NO2−, respectively. The ONOO− reduction by MnP is only few-fold slower than the catalysis of O2•− dismutation, demonstrating that all potent SOD mimics are potent peroxynitrite scavengers. Compounds which cannot dismute O2•−, such as MnTBAP3−, could be oxidized with strong oxidant, such as ONOO−, in a 1st step and reduced with either ascorbate or thiol or O2•− in a 2nd step. If O2•− is involved in a 2nd step, such compounds can affect in vivo levels of O2•−. Yet, the lower the SOD-like activity, the lower is the ability to reduce ONOO−. Indeed, MnTBAP3− is ~2 orders of magnitude less able reductant of ONOO− than is MnTE-2-PyP5+. Coupling of ONOO− reduction to O2•− oxidation, has been explored with MnTM-4-PyP5+[25], and may be operative with other compounds. Coupling of ONOO− reduction to cellular reductants is likely operative with potent SOD mimics also (Scheme X). Due to high levels of carbonate in vivo, ONOO− forms an adduct with CO2. The ONOOCO2− adduct degrades to CO3•− and •NO2 radicals. Due to its radical nature, all potent SOD mimics react rapidly with CO3•− in a pH-dependent manner, log kred(CO3•-) ranging between 8 and 9 (Scheme X, Eqs. 5 and 6). Most of the rate constants provided here were based on those determined for MnTE-2-PyP5+. The linear relationship between log kcat(O2•−) and log kred(ONOO−) allows for calculations of other kred(ONOO−) (see Scheme XIV).
Scheme X.

Reactions of Mn(III) ortho N-substituted pyridylporhyrins with different reactive species presented by equations. A number of reactions whose equations are listed have been studied and rate constants determined [3]. Most of those have been determined with frequently explored MnTE-2-PyP5+. It is very likely that many other reactions are possible in vivo and await exploration. Peroxynitrite reduction with MnP: Are Mn(III) and Fe(III) porphyrins specific peroxynitrite decomposition catalysts? Simple answer – they are not. Firstly, to the best of our knowledge none of them are specific to ONOO−[20]. Most compounds reported as peroxynitrite decomposition catalysts are potent SOD mimics also (see Scheme XV also) [20]. Secondly, the reported rate constants for Mn porphyrins relate to the reduction of ONOO− only (Eqs. (1) and (4)), and are not of catalytic nature and cannot be described as log kcat(ONOO−). What happens in vivo is another story and type of process involved (catalytic with FeTM-4-PyP5+ or non-catalytic with MnTE-2-PyP5+) may be irrelevant in elimination of ONOO−; very likely MnP will be reduced with abundant cellular reductants (Eq. (3)), most so with ascorbate, and will then reduce ONOO− in a 2nd step (Eq. (4)), closing the catalytic cycle. This would happen only if MnP encounters ONOO− in its immediate neighborhood and at high enough concentration. The second re-oxidation step of MnIIP4+, given the fairly high kred(O2) and the higher in vivo levels of oxygen than ONOO−, could occur with oxygen, O2 and will give rise to O2•− and subsequently H2O2. For FeTM-4-PyP5+, however, the catalytic rate constant was reported; same may be valid for other similar FePs [26]. Oxidation of MnP with oxygen. Reactivity towards oxygen, O2 is much lower than towards O2•− ONOO− or ClO−. However, given the orders of magnitude higher in vivo levels of O2, such reactivity may play a role and be a critical step in the interactions of MnPs with cellular reductants, most so with ascorbate (see Scheme XII). Oxidation of •NO with MnP – nitrosylation of MnP. The report by Meyer et al. tempted us to study the reactions of MnIIIP5+ and MnIIP4+ with •NO (Eqs 8 and 9) [27]. MnIIIP may be reduced in a slow reaction with •NO (Eq. (8)). Yet, in vivo MnIIIP5+ would rather be reduced rapidly with ascorbate (Eq. (3)) in a 1st step followed by nitrosylation (Eq. (9)) of MnIIP4+ to give rise to (NO)MnIIP4+ in a 2nd step. Given the electron deficiency of Mn center, the distribution of electron in a complex of •NO with MnIIP may be better described as [(NO+)MnIP3+]4+. The (NO)MnIIP4+ species is not very stable and under aerobic conditions releases •NO slowly which subsequently gets oxidized to nitrite and/or nitrate [28]. Possibly (. NO)MnIIP4+, formed rapidly, may serve as a depot for slow release of •NO. HNO oxidation with MnP − nitroxylation of MnP: reported by Doctorovich’s group, the reaction of MnP with HNO gives rise to the same product as reaction of MnP with •NO [29]. Catalase-like activity of MnP:A poor ability of 13 Mn porphyrins to act in a catalase-like fashion (2-electron reduction and oxidation of H2O2, Eq. 11) has been demonstrated. MnTE-2-PyP5+ has the highest kcat(H2O2)=63 M−1 s−1. The lowest kcat(H2O2) is 5.6 M−1 s−1 for MnTBAP3−. The kcat(H2O2) for FeTE-2-PyP5+ is 803 M−1 s−1. The most potent MnPs and FePs have only ~0.006% and 0.06% of catalase enzyme activity [30–32]. Both MnPs and FePs could undergo similar turnovers: slower oxidative degradation of MnPs compensates for their lower kcat(H2O2). The catalysis of dismutation by MnPs, is unlikely to happen in vivo due to high levels of cellular reductants; the catalytic cycle may be closed by the reduction of oxo species in a 2nd step with cellular reductants, [35] Eq. (2); in such scenario H2O2 would still be eliminated/reduced to water in a 1st step. The dismutation may also involve O=MnIVP/MnIIIP via Eq. 12. Fe corrole is a complex with shrunk porphyrin ligand [8,33]. It has ~10-fold higher kcat(H2O2) than FeTE-2-PyP5+[33,34]. It also has higher turn-over number, due to higher stability resulting from tri-anionic nature of corrole ligand vs di-anionic nature of porphyrin ligand. In turn, Fe(III) corrole can dismute larger quantities of peroxide relative to either MnPs or FePs. The overall behavior of Fe corrole in vivo may be different than that of either FeP or MnP. For example, Fe(III) corrole, due to the stabilization of higher oxidation state, cannot be reduced in vivo to Fe(II) corrole with cellular reductants. The coupling with cellular reductants could happen only in a 2nd reduction step once Fe(III) corrole is in a first step oxidized to higher (+4 or +5) oxidation state with either O2•− or ONOO−. The relevance of such coupling and the biological relevance of catalysis of H2O2 by metallocoroles, and the possible advantage over MnPs (claimed by Gross’s group, [35]) needs to be carefully explored. Most so in the light of recent data which indicate that MnPs, rather than removing H2O2, take advantage of it. Thus MnPs couple with H2O2 in a pro-oxidative and catalytic manner (see below), presumably in glutathione peroxidase or thiol oxidase fashion while imparting therapeutic effects (see Schemes XI and XIV). Note that in a first step H2O2 is reduced with MnP which in essence represents elimination of H2O2 (Scheme XIA). Such interactions vs H2O2 dismutation may be major or at least one of the major actions of metalloporphyrins or metallocorroles in vivo. Further, none of such compounds would experience H2O2 concentrations high enough to degrade them to any significant extent. Further studies are needed to address the issue of the advantage of higher kcat(H2O2) of Fe corrole (Scheme VIII) over MnPs, which has been used by Gross’s group to discuss the therapeutic advantage of Fe corroles over Mn porphyrins [35]. Reduction of lipid reactive species with MnP: while those reactions have not been quantified, the indirect evidence suggests that MnIIIP scavenges lipid reactive species whereby preventing lipid peroxidation. However, that happens only in the presence of cellular reductants. When the low-density lipoproteins were exposed to low flux of ONOO−, the 5 µM MnTE-2-PyP5+, MnTnOct-2-PyP5+ and MnTBAP3- prevented lipid peroxidation, i.e. the formation of cholesteryl ester hydroperoxide in the presence of uric acid. The most lipophilic MnTnOct-2-PyP5+ was the most efficacious and ~2-fold better than MnTE-2-PyP5+. The MnTBAP3− was ~2-fold less efficacious than MnTE-2-PyP5+. Also α and γ tocopherol were spared. Yet in the absence of uric acid MnPs acted as a pro-oxidant; the consumption of tocopherols was potentiated and more so with more redox-active cationic MnPs than with MnTBAP3−[36]. Similar impact of MnTE-2-PyP5+ on lipid reactive species has been suggested in another study also [37]. Hypochlorite reduction with MnP. While only preliminary data are collected on MnTalkyl-2-PyP5+, the reactivity towards hypochlorite seems to be of the same order of magnitude as towards ONOO−[38,39]. The reaction described by Eq. (13) is based on the Umile et al. data on imidazolyl analog, MnTDM-2-PyP5+[38].
Scheme XI.
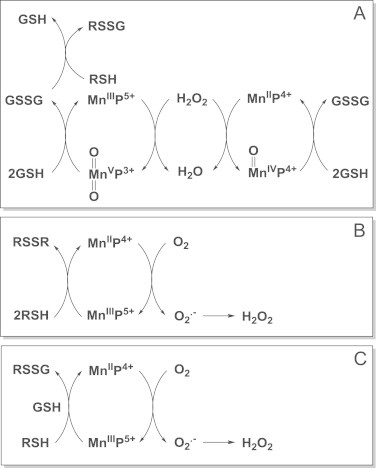
Interaction of MnPs with thiols/H2O2 in thiol oxidase or GPx fashion. While catalase-like action may be of no in vivo relevance due to low kcat(H2O2), the key role of H2O2 in the actions of MnPs, other than catalase-like, has already been established and occur in reactions of MnPs with cellular reductants (see also Schemes XIII and XIX). Note that H2O2 is reduced to water (H2O2 is eliminated) in Scheme A, while it is generated in Schemes B and C. The GSSG species can exchange thiol spontaneously with RSH whereby glutathionylated protein is formed. Reactivity towards thiols may predominate in therapeutic effects of MnPs. Studies on CNS injuries, diabetes and cancer show that oxidation of cysteines of p50 and p65 subunits by MnP (enhanced with dexamethasone as a producer of H2O2) suppresses/precludes the NF-κB activation and in turn suppresses inflammation rescuing the diseased cell and/or precluding the survival of cancer cell [45–49]. In addition S-glutathionylation of cysteines of complexes I, III and IV and subsequent inactivation of complexes I and III of mitochondrial respiration may play key role in reducing ATP levels in tumor cell whereby suppressing tumor growth (see Scheme XX) [46]. The impact of MnP on ATP levels via glycolysis has been demonstrated also [50] (Scheme XVII) MnIIIP5+ can either oxidize thiols directly (B and C), undergoing one-electron reduction to MnIIP4+ whereby thiyl radical is formed in a thiol oxidase fashion; or it can act in GPx fashion while coupling with H2O2 to S-glutathionylate thiols (A and C). Indeed, studies on lymphoma cells showed clearly that S-glutathionylation happens only in the presence of H2O2 and GSH [45,47]. The H2O2 may originate either from: (i) already increased oxidative stress of a cancer cell, or (ii) additional generation via radio- or chemotherapy, or (iii) catalytic cycling of MnP with ascorbate or thiols.
Scheme XII.
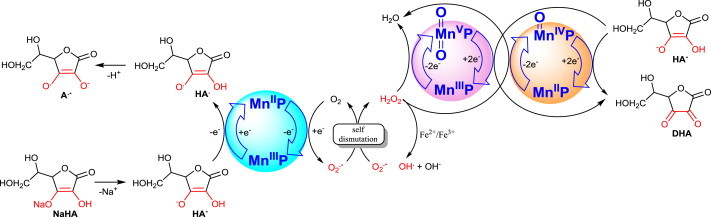
Interaction of MnPs with ascorbate (NaHA–sodium ascorbate, HA- - monodeprotonated ascorbate, major species at pH 7.8). Due to high intracellular levels of ascorbate (up to 0.1 mM), its interaction with MnP has been of our continuous interest since 1998 [3,6,17,41,43,44,51,52]. Due to its catalytic nature, such cycling generates high levels of H2O2, which are limited by levels of either ascorbate or oxygen. H2O2 formed can alternatively oxidize MnIIP and MnIIIP to (O)MnIVP and (O)2MnVP, respectively (Eqs. 11a and 12a), with concomitant reduction to H2O. The oxidized MnPs can be reduced back to two-electronically with HA− producing dehydroascorbate (DHA). MnP/ascorbate system carries the therapeutic potential as anticancer therapeutic strategy [3,43]. MnP will in vivo likely couple with endogenous ascorbate due to its high levels. Yet therapeutic effects may be enhanced with exogenous ascorbate [42,44]. The system MnP + ascorbate, with regards to its mechanism of action, resembles to other major anticancer therapies which generate H2O2 (radiation and chemotherapy) where MnPs act as radio- and chemosensitizers [3,47,53] See also Schemes XVI–XVIII.
Scheme XIII.
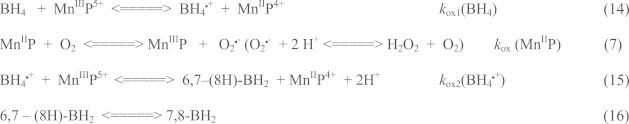
Interaction of MnP with tetrahydrobiopterin. Tetrahydrobiopterin is a cofactor of nitric oxide synthase, NOS. The reaction with tetrahydrobiopterin, BH4, would result in a loss of reducing equivalents for NOS and could in turn result in NOS inhibition. Under such conditions NOS would make O2•− instead of •NO. The kox1(BH4) was found to be 1.0×104 M−1 s−1 for one-electron oxidation of BH4 to BH4•+ and kox2(BH4•+) 1.5×103 M−1 s−1 for one-electron oxidation of BH4•+ to 6,7-(8H)-BH2[54]. Cycling with BH4 could result in tumor growth suppression via inhibition of its angiogenesis [54]. Recovery of MnP catalyst may lead to H2O2 production via Eq. (7). Alternatively, BH4 may be oxidized with high-valent, highly oxidizing O=MnIVP4+ species formed in reactions with ONOO− or H2O2 (Eqs. (1) and (12a)).
Scheme XIV.
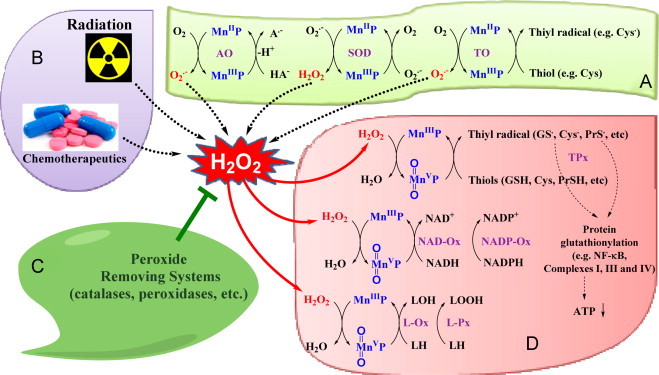
Role of H2O2 in the actions of MnPs. Over years, our data have contributed to our understanding that H2O2 plays a key role in the redox biology of MnPs. The Scheme summarizes all aspects of MnP/H2O2 interactions addressed thus far ( [3,30,31,45,47]. The direct evidence was provided that H2O2 is essential for the impact of MnP/dexamethasone on cellular transcription and cell viability in lymphoma cellular model[45]. In such model MnP/dexamethasone system produces H2O2 which MnP employs to S-glutathionylate NF-κB which in turn causes cellular apoptosis and reduces number of viable cells. When catalase over-expressor CAT2 and CAT38 cells were treated with MnP/dexamethasone the identical number of viable cells was obtained as with MnP alone. Further, depletion of GSH with buthionine sulfoximine inhibitor of GSH synthesis suppressed S-glutathionylation of NF-κB. The same effect on NF-κB inhibition, demonstrated with MnP/dexamethasone, was replicated with NF-κB inhibitor, SN50 [3,31,45,47]. The oxidation of NADH and NADPH in the presence (but not absence) of H2O2 was demonstrated in aqueous system [31]. Hydroxylation of cyclophosphamide with MnP/asc – H2O2 generator system – was reported (see Scheme XXI) [52,55]. Adapted from [55] TPx–thiol peroxidation; TO–thiol oxidation; L-Ox–lipid oxidation; L-Px–lipid peroxidation.
Researchers need to pay attention to the type of rate constants they refer to. Immediate translation of data from aqueous solutions to cellular and animal models is not straightforward and often requires substantial insight into extremely complex chemistry of those redox active drugs, their biodistribution (affected by their lipophilicities, polarities, charge, size and bulkiness) at sufficient concentrations and at targeted location [3,5,9,15,22–24].
Reactivity of MnPs towards cellular reductants – thiols, ascorbate and tetrahydrobiopterin
The interactions of Mn(III) ortho N-substituted pyridylporhyrins with cellular reductants seems to play critical role in the biology of MnPs, in great part due to high levels of cellular reductants. Such interactions have been explored in aqueous solutions, in vitro and in vivo and are depicted in Schemes IX–XIV [3,18,30,40–44]. Such interactions also play major role in MnP/H2O2 biology depicted in Schemes XIV, XVII and XIX.
Scheme XVII.
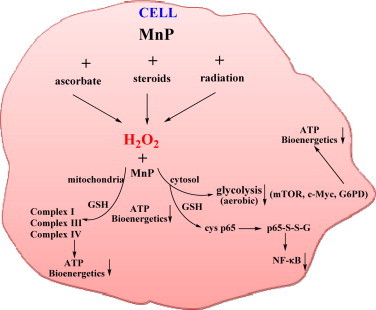
Differential effects of MnPs on normal vs cancer cell are controlled by their differential redox environments. Differential redox environment of normal and cancer cells promotes differential actions of MnPs. The rising data indicate that many antioxidant systems are differently regulated in cancer cell. Thus catalases, GPx, peroxiredoxins may be down regulated and often inactivated, while MnSOD may be upregulated which results in increased levels of H2O2[4–6,60]. Reactions involved in the actions of MnPs are indicated in this Scheme and Scheme XIV and occur with different yield/magnitude in normal vs cancer due to their different levels of H2O2. Thus much larger levels of H2O2 in cancer vs normal cell would promote MnP/H2O2-driven oxidations to such extent that cancer cell would be forced to undergo apoptosis. Oxidations and/or S-glutathionylation (in GPx fashion) of thiols of NF-κB and complexes I and III of electron transport chain by MnPs/H2O2 may predominate in vivo [45,47–49]. MnP/H2O2-driven oxidations in normal cell would be of lesser extent that in cancer cell and thus would only suppress excessive inflammation which would result in healing of a normal cell [2,34,3,24]. Note: the beneficial therapeutic effects we observe may be different in nature. With normal cells, healing is demonstrated and thus the effects observed are of antioxidative nature. With cancer cells the actions of MnP/H2O2 are of magnitude high enough to cause cancer cell death: the therapeutic effects observed are pro-oxidative. Note that the actions behind such effects are pro-oxidative with both normal and cancer cell (oxidative modification of thiols), but due to differences in their magnitudes the therapeutic effects observed are of different type: transient suppression of NF-κB in a normal cell similar to what is seen with aspirin and ibuprofen, but excessive NF-κB inhibition in a cancer cell similar to effects induced with steroid therapy leading to cell death[64].
Scheme XIX.
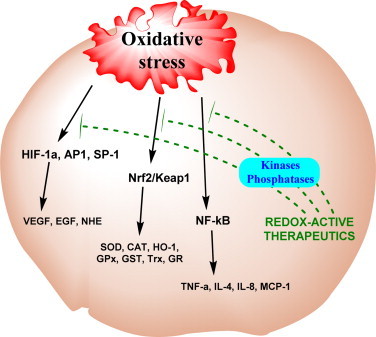
Impact of MnPs on cellular transcription. The reactions of MnPs were thus far explored with respect to several transcription factors, NF-κB, AP-1, SP-1, HIF-1α (see also Scheme XIV) [48]. MAPK/ERK pathway has been implicated also [41]. Oxidation of cysteine residues of p50 and p65 subunits of NF-κB by MnPs/H2O2 (the pro-oxidative actions of MnPs) prevents the activation of NF-κB, and in turn suppresses excessive inflammation and thus produces therapeutic effects which we identify as anti-oxidative [3]. Data obtained by us on MnPs (Scheme XXI)[66,67] and others on a member of other class of SOD mimics, Mn(II) cyclic polyamines, M40403 [68], suggest the impact of MnPs on the activation of Nrf2. Upon oxidation of redox sensitive cysteines of Keap1, Nrf2 transcription factor is activated, which results in upregulation of endogenous antioxidative defenses [67,68]. Nitroxides were shown to activate that pathway also [69–71]. It may be mechanism of actions of flavonoids also [70,71] (see also Scheme XX).
Role of H2O2 in the actions of MnPs
While MnPs are not functional mimics of catalase [30], H2O2 seems to be critical in the redox biology of MnPs. MnP produces H2O2 in a reaction of reduced MnIIP with oxygen, O2 (Eq. (7) in Scheme X) and superoxide, O2•− during cycling with either thiol or ascorbate or tetrahydrobiopterin (Schemes XI, XII, XIII). Subsequently it can use it to oxidize biological targets listed in Scheme XIV. Such scenario may allow for maintenance of the (unchanged) levels of H2O2; this was indeed demonstrated by Tome's group in lymphoma studies when lymphoma cells were treated with MnP/dexamethasone [43]. Many aspects of H2O2 in the biology of MnPs are listed in Scheme XIV; there may still be reactions which we have not yet explored. Present data point to a complex MnP redox-biology.
SOD-like activity parallels all other reactivities and in turn controls therapeutic effects of MnPs
We came a long way-from SOD mimicking to H2O2-related pathways
We initially believed that MnPs are specific SOD mimics. In 2006, Dr. Radi approached us at the 13th Annual Meeting of the SFRBM suggesting that MnPs may react with ONOO− also. Peroxynitrite was an emerging species at that time. Intuitive reasoning would say that nature would not make a more dangerous species from two less dangerous and physiologically important radicals, O2•− and •NO. Moreover, it seems that nature has not developed an enzymatic system which could take care of ONOO− specifically, unless peroxiredoxins serve that purpose [56,57]. The studies on ONOO− in collaboration with Radi's group reveal that reactivities towards ONOO− and O2•− are similar, only few fold away from each other [7,58]. That has opened the door to considerations that MnPs may play crucial role in in vivo destiny of reactive species other than O2•−. Along with the growing awareness of H2O2 as a central modulator in cellular redox processes, the role of ONOO− in the chemistry and biology of MnPs has been increasingly appreciated (Scheme XV).
Scheme XV.
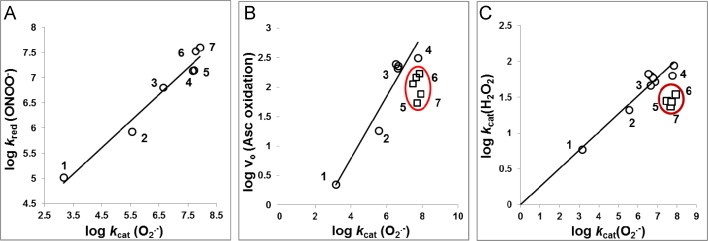
SOD-like activity parallels other reactions of MnPs thus far studied as exemplified here with reduction of ONOO−, oxidation of ascorbate and catalysis of H2O2 dismutation by MnP. Why potent SOD mimics are effective scavengers of other species and why in turn they may still be the most prospective therapeutic agents (assuming they are optimized for bioavailability and toxicity)? (1) MnTBAP3−, (2) MnTE-2-PyPhP5+, (3) MnTE-3-PyP5+, (4) MnTE-2-PyP5+, (5) MnTPhE-2-PyP5+, (6) MnTnHexOE-2-PyP5+, and (7) MnTnOct-2-PyP5+. In addition to those MnPs numerated, encircled in B are MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ and in C is MnTnHex-2-PyP5+. The maximal kcat(O2•−) has been obtained at E1/2 for MnIIIP/MnIIP of ~+200 to +450 mV. The values of E1/2≥+400 mV stabilize Mn in + 2 oxidation state and either MnIIP or a very weak MnIIIP complex is formed. At values of E1/2≤+100 mV Mn is stabilized in +3 oxidation state to such extent that it does not favor reduction to MnIIP complex in a 1st step in order to start the O2•− dismutation. The E1/2 of around +200 to +400 mV vs NHE is around the E1/2 of SOD enzymes where both steps of dismutation process occur with similar rate constants and are thus similarly thermodynamically favored. The E1/2 describes the magnitude of metal electron density which in turn reflects electron density of porphyrin ligand. E1/2 of metal center thus relates to the protonation equilibria at all porphyrin sites (at pyrrolic nitrogens of porphyrin ligand and at waters axially ligated to metal site). Two such equilibria have been quantified at: (i) 3rd inner pyrrolic nitrogen of metal-free porphyrin, pKa3 [H3P+↔H2P+H+], and (ii) at 1st axially bound waters, pKa(ax) [(H2O)2MnP5+↔(OH)(H2O)MnP4++H+]. The inversed linear relationship exists between E1/2 and pKa(ax) and pKa3 each: the higher the propensity of MnP to be reduced (the higher the E1/2), the more-electron-deficient MnP is and less strongly it keeps its axial waters, thus easier (at lower pH) the proton is released and in turn the lower is the pKa(ax). The same is true for pKa3. The more pyrrolic nitrogen favors the proton association (the higher the pKa3), the more electron-rich nitrogen is. In turn, such electron rich porphyrin ligand favors the formation of a stable complex with Mn. That means the higher the pKa3 the less positive is the E1/2 for MnIIIP/MnIIP redox couple. The consequence of such relationships is that the more electron-deficient the Mn complex is (lower the pKa3), in addition to aqua ligand, the more it would favor binding different ligands, such as ONOO−, ClO−, CO3. − and H2O2. The binding of these species comprises the 1st step in their reductions. This further explains why the rate constants for the reduction of ONOO− or ClO− and H2O2 dismutation are related to the E1/2 of the MnIIIP/MnIIP redox couple though it is not involved in such reactions (Scheme XII). As E1/2 is proportional to log kcat(O2•−), the logkcat(O2•−) is proportional to both log kred(ONOO−) and log kcat(H2O2) as shown in plots A and C. Importantly, the E1/2 of electron transfer which follows the binding of those species, and involves Mn + 3/+4 oxidation states (Eq. (1)), appears to be nearly identical for all MnPs [6,59]. In vivo, the 2nd step may preferentially involve two-electron electron transfer between Mn +2 and Mn +4 oxidation states (Eq. (4)). Under such conditions, the differences in E1/2 for the MnIIIP/MnIIP redox couple will affect the E1/2 of O=MnIVP/MnIIP redox couple. The ability of MnP to couple with ascorbate (plot B), catalyzing its oxidation follows the same trend as described for ONOO− and H2O2, as binding of ascorbate is involved in a 1st step of ascorbate oxidation. The deviation from the linearity has been demonstrated with compounds which have bulky pyridyl substituents (encircled in plots B and C). While not showed in plot A, the deviation from linearity has been seen also [7,10].
Differential (therapeutic) effects of MnPs on cancer vs normal cell
Differential effects of MnPs in cancer vs normal cell have been shown and reported in a number of cellular models (Schemes XVI and XVII).
Scheme XVI.
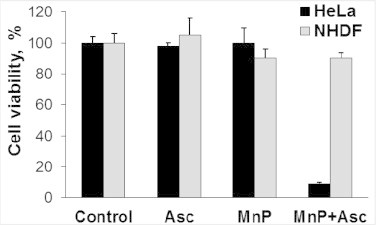
Differential therapeutic effects of MnP/ascorbate system on cancer (cervix cancer HeLa) vs normal cell line (human dermal fibroblasts, NHDF). MnP stands here for MnTE-2-PyP5+. The differential therapeutic effect is based on differential redox environment of cancer vs normal cell[5,60]. Growing insight into the biology of cell and redox chemistry of MnPs taught us that we can take advantage of the rapid coupling of MnP with abundant cellular reductants [3,6,17,41,44,51,52]. Thus far we have shown that the catalytic oxidation of ascorbate with MnP (coupled to oxygen, Scheme XII) would enhance peroxide levels and could kill cancer without affecting much normal tissue [3,46]. The reason lies in fact that cancer is frequently low in H2O2-removing systems (catalases, GPx, peroxiredoxins) and cannot cope with additional increase in H2O2 levels [4–6,60]. This reasoning is the same as the one behind the use of other H2O2-producing strategies: radio- and chemotherapy. Oxidation of ascorbate at the expense of endogenous metalloproteins has already been employed in clinical trials in patients with pancreatic cancer [61,62]. The use of Mn porphyrin, MnTE-2-PyP5+ and analogs of similar redox properties (MnTnHex-2-PyP5+, MnTnBuOE-2-PyP5+), optimized for ascorbate oxidation, has been reported by us [3,6,41,43,44] and subsequently by others to bear therapeutic potential [40,42,63]. The cytotoxicity of MnP/ascorbate has been shown with several other cancer cell lines, such as CaCo-2 (colorectal cancer), HCT116 (human colon cancer) MCF-7 (breast cancer), SUM149 (inflammatory breast cancer), 4T1 (mammary breast cancer), and 3 different gliomas (deltaGli36, U87MG, D-245MG) ([41,43] Batinic-Haberle I, Lam PYP, Roberts ERH et al., unpublished). Such system has been further shown by us and others not to be deleterious to normal cells [3,40,43,46]. Adapted from [3].
Differential (therapeutic) effects of MnPs on cancer vs normal tissue in animal models
Differential effects of MnPs in cancer vs normal cell have also been shown and reported in a number of animal models. Radioprotection of salivary glands and mouth mucosis in non-tumor bearing mice receiving daily MnTnBuOE-2-PyP5+ treatment was recently demonstrated. At the same time, radiosensitization of head and neck tumor in a sc mouse tumor xenograft model was demonstrated [Ashcraft et al., unpublished]. The radioprotective effects of MnPs have been demonstrated in numerous other models as well. Most striking data were obtained with mouse prostate radiation with complete reversal of radiation-induced erectile dysfunction (ED) ([65], Koontz, Batinic-Haberle, Tovmasyan et al., unpublished). Such treatment has a chance to reduce prostate radiation-induced ED which would encourage the men with prostate cancer, who have thus far been reluctant, to undergo radiation therapy. Scheme XVII describes the data on the radioprotection of a normal brain in non-tumor bearing mice and radiosensitization of brain tumor in a mouse sc glioma xenograft model [53] (Scheme XVIII).
Scheme XVIII.
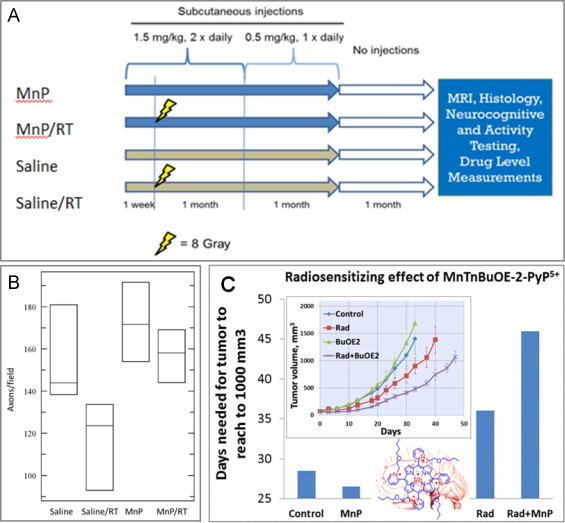
Radioprotection of normal brain and radiosensitization of brain tumor by MnTnBuOE-2-PyP5+. (A) The treatment strategy for brain radioprotection with MnTnBuOE-2-PyP5+(MnP) [53]. (B) Histological evaluation of myelinated axons in the corpus callosum. Representative images of toluidine blue-stained mid-sagittal corpus callosum tissue were imaged by brightfield microscopy (n=3 mice/group). Groups studied were (1) Saline, (2) MnP (3) Saline / radiation (RT), and (4) MnP/RT. Toluidine blue-stained mid-sagittal corpus callosum tissue were imaged by brightfield microscopy (n=3 mice/group). Three images from each mouse underwent automated axon counting that is quantitated in the box graph with 95% confidence intervals. ANOVA analysis of the group resulted in significant differences among the groups (p=0.040). (C) The impact of MnTnBuOE-2-PyP5+-based radiosensitization on tumor growth delay in a sc mouse xenograft D-245MG glioblastoma multiforme study. Patients-derived glioblastoma cells (kindly provided by The Preston Robert Tisch Brain Tumor Center at Duke University) were inoculated subcutaneously in the right flank of BALB/c nu/nu mice. Once tumors reached approximately 65 mm3 (Day 0), mice were randomly assigned to 4 groups with 8 mice per group: saline, saline/RT, MnP, and MnP/RT. Mice were injected subcutaneously with 1.6 mg kg−1 MnP twice daily (beginning at 24 h before RT and continuing for the duration of the study). Tumors were irradiated for 3 days with 1 Gy day−1 on day 2–4 (XRAD-320, Precision X-ray, North Branford, CT) [53]. The impact of similar magnitude was achieved with MnP/temozolomide treatment, where MnP acted as a chemosensitizer; the action is similar to MnP-driven sensitization of lymphoma cells to dexamethasone [45–47]. Both MnTnHex-2-PyP5+[2] and MnTnBuOE-2-PyP5+ suppressed tumor growth of D-245MG to similar magnitude under identical experimental conditions. Adapted from [53].
Impact of MnPs on cellular transcription
At present stage of our knowledge, the direct reactions of MnPs with thiols of transcription factors, such as NF-κB or Keap1/Nrf2, and/or coupled to other species, may present major contributions to the therapeutic efficacy of MnPs. In addition to direct reactions, the indirect impact of MnP on NF-κB via scavenging reactive species, which would have otherwise signaled its activation, cannot be ruled out [53] (Scheme XIX).
Suppression of cellular energetics of a cancer cell with MnPs
S-glutathionylation of complexes I, III and IV of mitochondrial electron transport chain with subsequent inactivation of complexes I and III and suppression of ATP production of lymphoma cells when those were treated with MnP/dexamethasone was reported by Tome’s group [45]. In addition to the radiosensitizing actions of MnPs, such chemosensitization may further contribute to their anticancer effects [3] (Scheme XX).
Scheme XX.
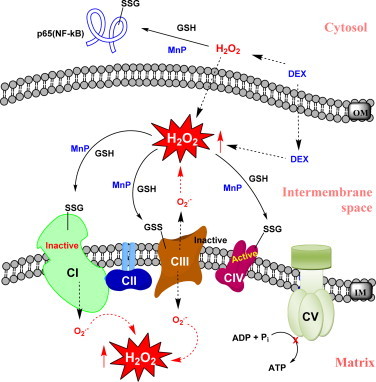
MnP inactivates complexes I and III via their S-glutathionylation when combined with dexamethasone in lymphoma cellular model. MnTE-2-PyP5+ was tested in lymphoma cellular model where it enhanced cell killing in the presence of dexamethasone via enhancing H2O2 production [45,46]. H2O2 in turn was employed by MnP to catalyze S-glutathionylation of protein thiols. Such action, depicted in Scheme XI, is in essence either GPx- or thiol oxidase-like activity of MnP. Such oxidative modification has been demonstrated on both cytosolic and mitochondrial levels. In cytosol cysteine units of p50 and most so p65 of NF-kB seem to be modified, while complexes I, III and IV were S-glutathionylated in mitochondria. Also oxidation of p50 by MnP in nucleus has been suggested by Piganelli's group [48]. All proteins except complex IV (though S-glutathionylated) were inactivated by such oxidative modifications. That indicates that MnP under conditions of high oxidative stress, i.e. high H2O2 levels, which may be produced by different treatment modalities such as radiation and chemotherapy (dexamethasone or ascorbate or temozolomide, [3,41,42,44,45,47,53], would suppress anti-apoptotic, survival pathways and cellular energetics, killing cancer cell on two metabolic fronts. Adapted from [45,46].
MnPs as inducers of adaptive responses via (pro)oxidative mechanism(s)
Oxidative modification of Keap1 thiols with subsequent activation of Nrf2 and upregulation of endogenous antioxidative defenses was shown with another class of SOD mimics, M40403 [72]. Such data along with results shown in Scheme XXI strongly suggest that MnP could act in a similar manner as inducer of endogenous antioxidative defenses. The upregulation of different SOD enzymes in rat kidney ischemia/reperfusion injury strongly suggests that MnP does not act as SOD mimic. A lipophilic analog, MnTnHex-2-PyP5+ was tested.
Scheme XXI.
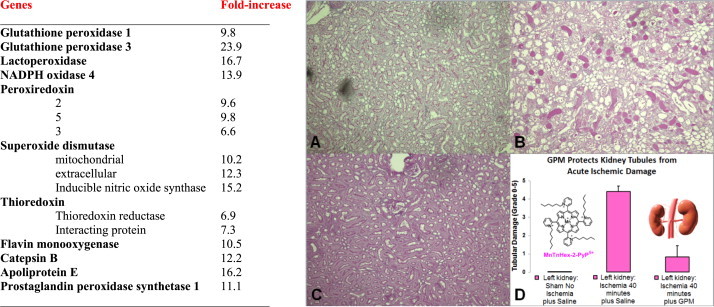
Increase in antioxidative genes due to MnP-based treatment of rats which underwent kidney ischemia–reperfusion I/R. The GPM mixture treatment contained: bFGF, recombinant EPO, SDF-1 and BMP-7, and a mixture of mitochondria protective amino acids, including α-ketoglutarate and l-aspartic acid, and MnTnHex-2-PyP5+[67]. Note that major endogenous antioxidative defenses are up-regulated indicating that MnP under given conditions could not have acted as SOD mimic. During I/R the H2O2 is produced which MnP supposedly employs for oxidative modifications of protein thiols as indicated in other studies (Schemes XI, XIV and XX). We hypothesized that in this model MnP oxidizes Keap1 cysteine and in turn activates Nrf2 which then upregulates endogenous antioxidative defenses. Indeed the Keap1 oxidation and Nrf2 activation were implicated with another class of SOD mimic, M40403 [68], nitroxides, curcumin [73] and flavonoids [70,71]. The following groups, with 10 male Sprague–Dawley rats each, were studied: (a) sham-operated; (b) I/R with saline only; (c) I/R with growth factors only (G); (d) I/R with MnP only (P); (e) I/R a mixture of α-keto-glutarate and l-aspartic acid (M), and (f) GPM mixture. GPM was given 24 h before 40-min ischemia and 24 h post-reperfusion. The study was terminated at 48 h post-reperfusion. Periodic acid –Schiff (PAS) stains and tubular damage grading was assessed at 48 h post-I/R. All individual components of GPM mixture produced beneficial effects which were lower than those produced with whole GPM mixture. (A) Normal PAS staining of non-ischemic sham operated left kidney; (B) dramatically increased PAS positive necrosis in ischemic left kidney; (C) reversal of necrosis in ischemic+GPM treated left kidney; (D) significant decrease in the acute tubular necrosis grading in ischemic left kidney treated with the GPM mixture (p<0.0003). In next kidney I/R study, the authors introduce N-acetyl cysteine into the GPM mixture; all effects were enhanced; note that MnP cycles with thiols producing H2O2 (Scheme XI) [66,67]. Adapted from [67].
MnTBAP3- – the actions behind its therapeutic effects are still largely unknown
See Scheme XXII.
Scheme XXII.
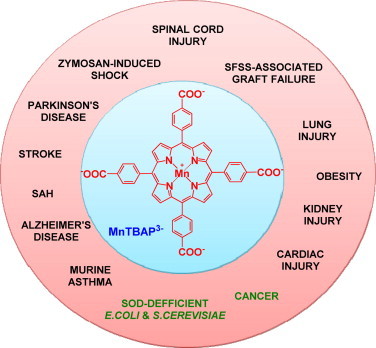
MnTBAP3−, while not an SOD mimic, produces therapeutic effects. The therapeutic effects have been reported in all cases listed in the Scheme, other than in cancer and in O2•−-specific aerobic growth of E. coli and S. cerevisiae. The impure MnTBAP3− obtained from Alexis or Calbiochem has often been used in past [74]. It contained SOD-active Mn oxo/hydroxo/acetato complexes; in turn the origin of effects has been dubious [75]. Still even when pure MnTBAP3− was used the therapeutic effects have been observed, though of lesser magnitude relative to cationic Mn(III) N-substituted pyridylporphyrins [4,76,77]. 50 nM MnTE-2-PyP5+, 1 µM FeTM-4-PyP5+ while 100 µM MnTBAP3– were able to attenuate ATN-224-induced peroxynitrite-dependent death of lymphoma cells which are oxidative stress-resistant and are overexpressing Bcl-2. ATN-224 is a Cu chelator, choline tetrathiomolybdate, that reduces the activity of Cu,ZnSOD enzyme. While not an SOD mimic, MnTBAP3− reduces ONOO−, thus exhibiting some specificity towards elimination of ONOO− over O2•−[77]. This suggests that much about its in vivo actions awaits further clarification. In a rat spinal cord ischemia/reperfusion model, under same dosing regime, MnTBAP3− suppressed NF-κB expression as does a powerful SOD mimic, MnTnHex-2-PyP5+. Such action prevented excessive NF-κB-driven inflammation [4]. The dose-dependence and the mechanism behind NF-κB inactivation by those MnPs are under further investigation. Adapted from [4].
Bioavailability – pharmacokinetic studies
Scheme XXIII.
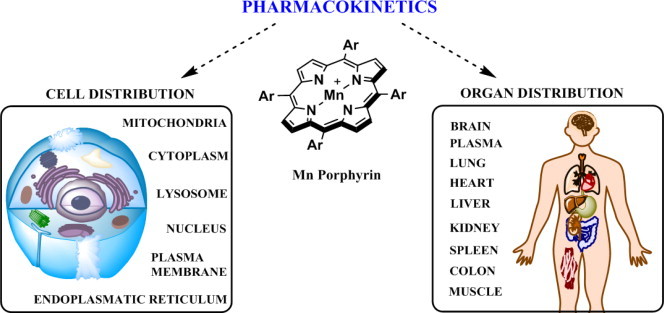
Pharmacokinetic and bioavailability studies with MnPs. Bioavailibilities of MnPs depend upon their lipophilicities, polarities, charge, size, shape, and bulkiness. Comprehensive studies have been reported on MnPs that bear similar redox properties (MnTE-2-PyP5+, MnTM-2-PyP5+, MnTnBu-2-PyP5+, MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ in different organisms via various administration routes. The PK studies involved plasma and different organs such as liver, kidney, lungs, spinal cord, brain, spleen, heart, colon, salivary glands and tongue. Also intracellular accumulation has been addressed on ZnPs and MnPs and data published on membrane, cytosolic, nuclear, mitochondrial, endoplasmatic reticular and lysosomal accumulation [78–83]. Largest body exposure of MnPs, expressed as AUC (area under pharmacokinetic curve) has been obtained via subcutaneous route; this route also has another advantage as it imposes the lowest acute side effects in MnP administration − blood pressure drop. Highest levels of MnPs are found in liver and kidneys (at µM levels), while the lowest were detected in brain (at nM levels). Lipophilic and cationic, MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ accumulate several fold more in brain (driven there by the anionic phospholipids) than does hydrophilic MnTE-2-PyP5+ [Spasojevic, Tovmasyan, Weitner et al., unpublished]. Given the highly positive charge, MnTE-2-PyP5+ accumulates ~3-fold more in nucleus than in cytosol of macrophages and LPS stimulated macrophages [48]. Such accumulation seems to be important given the suggestion by Tse and Piganelli that MnP oxidizes p50 in nucleus [48,49]. Cationic MnPs were also found to favor accumulation in cell wall of E. coli due to abundance of negatively charged phospholipids [84]. Extensive distribution studies of fluorescent Zn analogs using confocal fluorescent microscopy indicate their accumulation in membranes, lysosomes and endoplasmatic reticulum also [78,85]. The study was carried out on adenocarcinoma cells where lipophilic ZnTnHex-2-PyP5+ was also found in mitochondria in the vicinity of cytochrome c oxidase [79]. The only difference between Zn and Mn analogs is the lack of single charge on Zn center. Yet, with longer alkyl analogs, such as MnTnHex-2-PyP5+ no difference in their lipophilicities exists as single cationic charge on Mn is shielded from the solvent by long hexyl chains. Moreover, in vivo MnPs get readily reduced with cellular reductants, lose single charge on metal site and become similarly hydrophilic as ZnPs [86]. Thus, accumulation data on ZnPs (in particular those with long alkylpyridyl substituents) can be readily applied to MnPs.
Targeting mitochondria
Due to the critical role mitochondria has in all organisms, the compounds that are able to cross two mitochondrial membranes and preferentially target its matrix have been sought. Excellent work has been done on the basics of mitochondrial transport by Skulachev's and Murphy's groups [87,88]. Based on their research, the essential properties of mitochondrially-targeted drug are identified to be cationic charge and lipophilicity. Most efficacious MnPs bear penta-cationic charge. Moreover the lipophilic analogs bear long hydrophobic substituents. Such facts justified extensive studies of mitochondrial accumulation [80–82,89]. We have demonstrated that MnPs accumulate in mouse heart mitochondria, and those that are more lipophilic do so to a higher extent than the hydrophilic compounds, such as MnTE-2-PyP5+. Lipophilic MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ were found in mouse brain mitochondria also. We were not able to measure the hydrophilic MnTE-2-PyP5+ in brain mitochondria as its levels were below LCMS/MS detection limit [90]. The mitochondrial/cytosolic ratios of MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ in heart and brain mitochondria are similar [68]. The remarkable impact of lipophilicity on the accumulation of cationic MnPs in mitochondria relative to cytosol of Saccharomyces cerevisae is shown in Scheme XXIV.
Scheme XXIV.
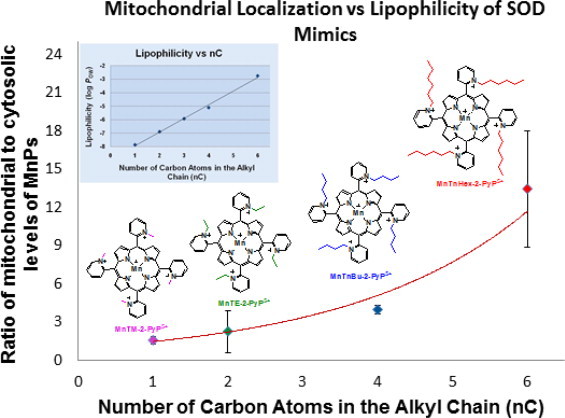
Impact of lipophilicity on mitochondrial vs cytosolic accumulation of cationic MnPs in S. cerevisiae. Impressive levels of otherwise hydrophilic compounds were found in mitochondria relative to cytosol and are presumably due to their high penta-cationic charge. The striking impact of lipophilicity on their mitochondrial accumulation was demonstrated [91]. Even the most hydrophilic MnTM-2-PyP5+ and MnTE-2-PyP5+ prefer mitochondria to cytosol. Such data suggest that pentacationic charge, rather than lipophilicity of pyridyl alkyl chains, controls mitochondrial accumulation of MnPs.
Diminishing toxicity
While most of the requirements for a good MnP-based redox-active drug are met, lowering MnP toxicity remains a challenge. Since, most but not all MnPs cause acute toxicity due to the blood pressure drop (BPD), we are far away from understanding that phenomenon. MnPs and FePs of similar redox properties but different lipophilicities and polarities, and with differently hindered cationic charges, that may affect their interactions with biomolecules, induce BPD of different magnitude. Some, such as (OH)FeTnHex-2-PyP4+ and (OH)FeTnBuOE-2-PyP4+, for reasons not yet understood, do not cause BPD. However, their Mn analogs, with similar kcat (O2•−), cause BPD [18]. MnPs that are of relatively high lipophilicity, such as MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+, cross blood brain barrier to significant extent and tend to damage membranes and become toxic at higher levels. Therapeutic window needs to be identified for each MnP. The most frequently studied compound with excellent safety/toxicity profile, MnTE-2-PyP5+, [92] has two “weaknesses”: (i) low transport across the blood brain barrier which reduces its chances to be used for treatment of CNS injuries, and (ii) short patent life of only 4 years which precludes Pharma’s investment into its development.
Therapeutic effects
What controls the magnitude of MnPs therapeutic effects?
Bioavailibility and redox properties control therapeutic effects. Redox properties are best described with E1/2 for MnIIIP/MnIIP redox couple which controls log kcat(O2•−), i.e. SOD-like activities of MnPs (Scheme XV, plot A). E1/2 describes best the electron-density of metal site. Electron-density of MnP in turn controls (i) predominantly outer-sphere electron transfer in O2•− dismutation and (ii) propensity of Mn center to bind other stronger oxidants (ONOO−, ClO−, H2O2, CO3.−) in a 1st step which is followed by electron transfer in a 2nd step. Thermodynamics of electron transfer, which involves either Mn +3/+4 or Mn +3/+5 oxidation states, seems to be similar for all MnPs. In turn, E1/2 of MnIIIP/MnIIP redox couple (which involves Mn +3/+2 oxidation states) parallels SOD-like and all other activities of MnPs thus far explored (see Schemes X and XV). Bioavailability describes levels (concentrations) of MnPs at targeted sites (organs and cellular organelles) and is in turn controlled by lipophilicity, polarity, charge, size, shape and bulkiness of a molecule. The reactivities and in turn therapeutic effects are further controlled by the levels or reactive species in immediate neighborhood of MnPs. Based on all said, the best strategy in the design of an excellent therapeutic able to restore normal physiological redox environment may be to: (i) assure high metal/ligand stability, and in turn provide integrity of metal site; (ii) target as high as possible SOD-like activity; (iii) maintain positive charges to enhance electrostatically the reactions of MnPs with predominantly anionic reactive species, while enhancing lipophilicity. Enhanced lipophilicity would result in (iv) enhanced accumulation of MnPs at all sites abundant with anionic phosphates such as mitochondria and brain. Importantly, the efforts need to be continuously invested in (v) minimizing toxicity of MnPs (Scheme XXV).
Scheme XXV.
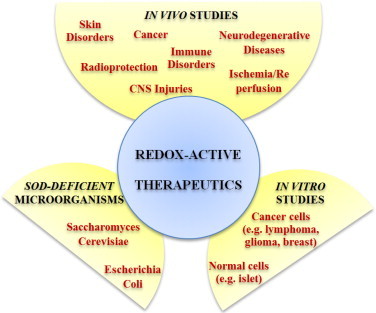
Therapeutic effects of MnPs. The O2•− specific models of SOD-deficient single-cell pro- and eukaryotic organisms, E. coli and S. cerevisiae, are the first and major steps in evaluating therapeutic potential of MnPs. Once they are successfully tested in unicellular organisms, MnPs are forwarded to various cellular and animal models. During the last two decades numerous cellular and animal studies (on mice, rats, rabbits and monkeys), conducted on models of diverse diseases, support remarkable therapeutic potential of metalloporphyrins; thus we hope that some will successfully Clinical Trials. Therapeutic effects demonstrated in models of CNS injuries, immune disorders, cancer and radioprotections were summarized in several manuscripts of Forum Issue “ SOD therapeutics” of Antioxidants & Redox Signaling 2014.
Distinction must be made between anti- and pro-oxidative reactions and anti- and pro-oxidative therapeutic effects
See Scheme XXVI.
Scheme XXVI.
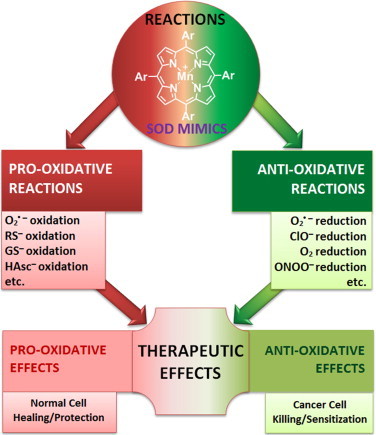
MnPs undergo anti- and pro-oxidative reactions which may be demonstrated either as anti- or pro-oxidative therapeutic effects. While such reasoning and experimental data behind it have been a main theme across this review, it is important to stress it here again: researchers need to differentiate between the type of reactions of MnPs and nature of therapeutic effects they observe. For example, oxidation of cysteines of NF-kB by MnP suppresses its activation which in turn suppresses secondary oxidative stress. This is demonstrated as reduced inflammation – commonly accepted as anti-oxidative therapeutic effects. Further, the oxidation of cysteine of Keap1 by MnP would activate Nrf2 which would upregulate endogenous antioxidative defenses (catalases, SODs, peroxidases, etc) in an adaptive response. This would result in suppression of inflammation –clear case of antioxidative effect. Again pro-oxidative reaction of MnP results in anti-oxidative therapeutic effects. Please note that not all reactions thus far studied are listed in this Scheme, such as reactions with tetrahydrobiopterin, H2O2 and CO3•− (for further information see other chapters). Many more in vivo reactions of MnPs are likely yet to be identified.
SOD mimics – the story continues
The perfect therapeutic should promote adaptive responses and help our body with upregulation of our own endogenous antioxidative defenses. The most obvious case of such adaptive response is demonstrated during regular physical exercising. Increasing number of reports support pro-oxidative actions of MnPs, nitroxides and flavonoids which result in adaptive responses. During 2 decades of research, we have gone a long way from initially viewing MnPs as antioxidants exclusively (SOD mimics) to considering them (pro)oxidants. Interestingly enough, with data right in front of our noses, it took us a long time to consider all aspects of O2•− dismutation process. The incorrect viewing of SOD enzyme and its mimic as antioxidants lies in 2 facts. Firstly, the two reactions involved in a dismutation process require a compound to be equally good anti- and pro-oxidant (Scheme I). Secondly, the dismutation process produces an oxidant, H2O2. In turn, the SODs and their mimics can only be considered antioxidative defenses if H2O2 is removed rapidly in conjunction with catalases or peroxidases. Such considerations help us understand that, based on identical thermodynamics of metal centers and depending upon the cellular redox environment (levels of oxidative stress) plethora of reactions are possible with SOD enzymes and MnPs. Yet, due to steric hindrance imposed by large protein structure such reactions of SODs occur, if at all, with orders of magnitude lower rate constants as compared to their small molecular-weight mimics.
A number of other issues need to be addressed as the MnPs advance towards Clinic. How long the treatment with MnP, following injury, should last? Will we be able to ever restore the physiological redox status, or as soon as the therapy is discontinued some level of inflammation will be re-established. Studies on CNS indicate that the longer the treatment lasts, the longer the reduction in stroke volume post-injury was demonstrated [93]. What are the therapeutic concentrations of MnPs at the sites of injury? We have only marginal knowledge how much of MnP we need in target tissues. It seems that ~30 nM brain levels afford protection with MnTnHex-2-PyP5+ in a stroke model [94]. Yet, we do not know if lower levels would produce such effects also. Clarification of such issues is costly but eventually will be needed.
Several metalloporphyrins are progressing towards or are in Clinical Trials. MnTDE-2-ImP5+ passed Safety/toxicity Phase I Clinical Trials on ALS patients [95]. MnTE-2-PyP5+ is in ongoing Clinical Trials, whereas STTR/NIH support has been granted for the Phase I/II Clinical trials on Head and Neck cancer patients on radioprotection of salivary glands and mouth mucosis. Another STTR/NIH funding for Phase I/II Clinical Trials on radioprotection of normal brain (with brain tumor patients) is pending. Another type of SOD mimics, M40403, is in Clinical Trials for the radioprotection of mouth mucosis as well. Fe porphyrin INO-4885 is in Clinical Trials for the use in contrast-induced nephropathy [20].
Summary. We may thus conclude our story with our most recent understanding that cationic Mn(III) substituted pyridyl- or imidazolyl porphyrins suppress inflammatory processes in normal cell and cancer cell to a different extent but through identical actions – modifications of thiol-based signaling pathways. As SOD potency relates to the therapeutic effects of Mn porphyrins we alternate between describing them as SOD mimics or redox active therapeutics.
Scheme III.
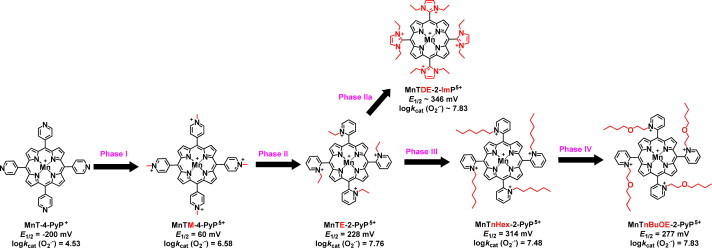
The design of SOD mimics occurred through phases I to IV. The unsubstituted electron-rich MnT-4-PyP+ has E1/2 value for MnIIIP/MnIIP redox couple of −200 mV vs NHE which is too negative (more negative than E1/2=−180 mV vs NHE for O2•− oxidation) and precludes its reduction (along with O2•− oxidation) to start the 1st step of dismutation process. In addition, there is no electrostatic facilitation; subsequently it has no SOD like activity, log kcat(O2•−)=4.53. In order to decrease metal center electron density to enable its acceptance of electron and reduction with O2•−, the alkyl groups were added to pyridyl nitrogens in Phase I; subsequently nitrogens became cationic and withdrew the electron density from Mn site. In turn, Mn becomes electron-deficient and prone to be reduced at biologically feasible Ε1/2 of +60 mV vs NHE, which results in a modest log kcat(O2•−)=6.58. Such rate constant is still ~3.5 orders of magnitude lower than that of SOD enzyme. Shifting nitrogens from para (4) positions to ortho(2) positions in Phase II moves positive charges closer to Mn site, which enhances both the electron-withdrawing effects and the attraction of anionic superoxide to cationic Mn porphyrin. In turn, E1/2 of MnTM-2-PyP5+ is increased for another order of magnitude, log kcat(O2•−)=7.79. Very importantly alkyl chains in ortho positions are stuck above or below the porphyrin plane and suppress the interaction of MnP with nucleic acid [11]. Such interaction demonstrated with planar para MnTM-4-PyP5+ prevent its SOD-like activity and may impose toxicity. Ortho isomers exist as 4 atropoisomers which all have the same E1/2 and same log kcat(O2•−), but likely have different biodistribution [12]. Once the knowledge on key ortho impact has been gained, ortho N-ethylpyridyl analog MnTE-2-PyP5+ and its di-orthoN,N′-diethylimidazolyl derivative, MnTDE-2-ImP5+, were synthesized in Phase II and Phase IIa and are among the most frequently studied compounds both in vitro and in vivo. Both compounds are pentactionic and thus highly hydrophilic. Initial studies suggested that, by increasing lipophilicity, we should be able to enhance their accumulation within organs and cells and mostly so brain. While cationic charge is a driving force, lipophilicity is another major factor that affects their transport across the blood brain barrier and mitochondrial accumulation and in turn their therapeutic efficacy [80–82,89]. The lipophilicity was addressed in Phase III. Several lipophilic compounds have been synthesized. MnTnHex-2-PyP5+ (~6,500-fold more lipophilic than MnTE-2-PyP5+) has been the most frequently studied in vitro and in vivo. The longer the hydrophobic alkyl chains the higher their micellar properties and the higher ability to damage lipid membranes. This is however outbalanced with the much higher accumulation of MnTnHex-2-PyP5+ within cell; in turn, up to 120-fold lower doses are needed for the efficacies identical to those produced by hydrophilic MnTE-2-PyP5+. This further allowed for a wider therapeutic window for the hexyl than for ethyl analog [13]. The micellar property and in turn toxicity was suppressed by ~4–5-fold relative to MnTnHex-2-PyP5+ when polar oxygen atoms were introduced into alkyl chains: MnTnBuOE-2-yP5+ was synthesized in Phase IV[14] and has been aggressively developed towards clinic.
Acknowledgment
Authors acknowledge financial help from NIH U19AI067798 (IBH, AT, IS), NIH/NCI, USA, Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29) (IS) and IBH General Research Funds (AT, IBH). IBH and IS are consultants with BioMimetix JVLLC (USA) and hold equities in BioMimetix JVLLC. IBH and IS and Duke University have patent rights and have licensed technologies to BioMimetix JVLLC.
References
- 1.Abreu I.A., Cabelli D.E. Superoxide dismutases – a review of the metal-associated mechanistic variations. Biochimica et Biophysica Acta. 2010;1804(2):263–274. doi: 10.1016/j.bbapap.2009.11.005. 19914406 [DOI] [PubMed] [Google Scholar]
- 2.Holley A.K., Miao L., St Clair D.K., St Clair W.H. Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxidants and Redox Signaling. 2014;20(10):1567–1589. doi: 10.1089/ars.2012.5000. 24094070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batinic-Haberle I., Tovmasyan A., Roberts E.R., Vujaskovic Z., Leong K.W., Spasojevic I. SOD therapeutics: latest insights into their structure–activity relationships and impact on the cellular redox-based signaling pathways. Antioxidants and Redox Signaling. 2014;20(15):2372–2415. doi: 10.1089/ars.2012.5147. 23875805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celic T., Španjol J., Bobinac M., Tovmasyan A., Vukelic I., Reboucas J.S., Batinic-Haberle I., Bobinac D. Mn porphyrin-based SOD mimic, MnTnHex-2-PyP(5+), and non-SOD mimic, MnTBAP(3-), suppressed rat spinal cord ischemia/reperfusion injury via NF-κB pathways. Free Radical Research. 2014;48:1426–1442. doi: 10.3109/10715762.2014.960865. 25185063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miriyala S., Spasojevic I., Tovmasyan A., Salvemini D., Vujaskovic Z., St Clair D., Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochimica et Biophysica Acta. 2012;1822(5):794–814. doi: 10.1016/j.bbadis.2011.12.002. 22198225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tovmasyan A., Carballal S., Ghazaryan R., Melikyan L., Weitner T., Maia C.G., Reboucas J.S., Radi R., Spasojevic I., Benov L., Batinic-Haberle I. Rational design of superoxide dismutase (SOD) mimics: the evaluation of the therapeutic potential of New cationic Mn porphyrins with linear and cyclic substituents. Inorganic Chemistry. 2014;53(21):11467–11483. doi: 10.1021/ic501329p. 25333724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer-Sueta G., Vitturi D., Batinic-Haberle I., Fridovich I., Goldstein S., Czapski G., Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. Journal of Biological Chemistry. 2003;278(30):27432–27438. doi: 10.1074/jbc.M213302200. 12700236 [DOI] [PubMed] [Google Scholar]
- 8.Eckshtain M., Zilbermann I., Mahammed A., Saltsman I., Okun Z., Maimon E., Cohen H., Meyerstein D., Gross Z. Superoxide dismutase activity of corrole metal complexes. Dalton Transactions. 2009;7879–7882(38):7879–7882. doi: 10.1039/b911278b. 19771348 [DOI] [PubMed] [Google Scholar]
- 9.Batinić-Haberle I., Rebouças J.S., Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxidants and Redox Signaling. 2010;13(6):877–918. doi: 10.1089/ars.2009.2876. 20095865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batinić-Haberle I., Spasojević I., Stevens R.D., Hambright P., Neta P., Okado-Matsumoto A., Fridovich I. New class of potent catalysts of O2.-dismutation. Mn(III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. Dalton Transactions. 2004:1696–1702. doi: 10.1039/b400818a. 15252564 [DOI] [PubMed] [Google Scholar]
- 11.Batinić-Haberle I., Benov L., Spasojević I., Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. Journal of Biological Chemistry. 1998;273(38):24521–24528. doi: 10.1074/jbc.273.38.24521. 9733746 [DOI] [PubMed] [Google Scholar]
- 12.Spasojević I., Menzeleev R., White P.S., Fridovich I. Rotational isomers of N-alkylpyridylporphyrins and their metal complexes. HPLC separation, (1)H NMR and X-ray structural characterization, electrochemistry, and catalysis of O(2)(.-) disproportionation. Inorganic Chemistry. 2002;41(22):5874–5881. doi: 10.1021/ic025556x. 12401096 [DOI] [PubMed] [Google Scholar]
- 13.Pollard J.M., Reboucas J.S., Durazo A., Kos I., Fike F., Panni M., Gralla E.B., Valentine J.S., Batinic-Haberle I., Gatti R.A. Radioprotective effects of manganese-containing superoxide dismutase mimics on ataxia-telangiectasia cells. Free Radical Biology and Medicine. 2009;47(3):250–260. doi: 10.1016/j.freeradbiomed.2009.04.018. 19389472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajic Z., Tovmasyan A., Spasojevic I., Sheng H., Lu M., Li A.M., Gralla E.B., Warner D.S., Benov L., Batinic-Haberle I. A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity. Free Radical Biology and Medicine. 2012;52(9):1828–1834. doi: 10.1016/j.freeradbiomed.2012.02.006. 22336516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batinić-Haberle I., Rebouças J.S., Benov L., Spasojević I. 52 Chemistry, biology and medical effects of water-soluble metalloporphyrins. In: Kadish K.M., Smith K.M., Guillard R., editors. Handbook of Porphyrin Science. World Scientific; Singapore: 2011. pp. 291–393. [Google Scholar]
- 16.Rebouças J.S., DeFreitas-Silva G., Spasojević I., Idemori Y.M., Benov L., Batinić-Haberle I. Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier. Free Radical Biology and Medicine. 2008;45(2):201–210. doi: 10.1016/j.freeradbiomed.2008.04.009. 18457677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batinić-Haberle I., Spasojević I., Hambright P., Benov L., Crumbliss A.L., Fridovich I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese(III) and iron(III) water-soluble porphyrins. Inorganic Chemistry. 1999;38(18):4011–4022. [Google Scholar]
- 18.Tovmasyan A., Weitner T., Sheng H., Lu M., Rajic Z., Warner D.S., Spasojevic I., Reboucas J.S., Benov L., Batinic-Haberle I. Differential coordination demands in Fe versus Mn water-soluble cationic metalloporphyrins translate into remarkably different aqueous redox chemistry and biology. Inorganic Chemistry. 2013;52(10):5677–5691. doi: 10.1021/ic3012519. 23646875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovmasyan A., Reboucas J.S., Benov L. Simple biological systems for assessing the activity of superoxide dismutase mimics. Antioxidants and Redox Signaling. 2014;20(15):2416–2436. doi: 10.1089/ars.2013.5576. 23964890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slosky L.M., Vanderah T.W. Therapeutic potential of peroxynitrite decomposition catalysts: a patent review. Expert Opinion on Therapeutics Patents. 2015:1–24. doi: 10.1517/13543776.2014.1000862. [DOI] [PubMed] [Google Scholar]
- 21.Haber A., Mahammed A., Fuhrman B., Volkova N., Coleman R., Hayek T., Aviram M., Gross Z. Amphiphilic/bipolar metallocorroles that catalyze the decomposition of reactive oxygen and nitrogen species, rescue lipoproteins from oxidative damage, and attenuate atherosclerosis in mice. Angewandte Chemie International Edition (England) 2008;47(41):7896–7900. doi: 10.1002/anie.200801149. 18798207 [DOI] [PubMed] [Google Scholar]
- 22.Batinic-Haberle I., Tovmasyan A., Spasojevic I. The complex mechanistic aspects of redox-active compounds, commonly regarded as SOD mimics. BioInorganic Reaction Mechanisms. 2013;9(1–4):35–58. [Google Scholar]
- 23.Batinic-Haberle I., Rajic Z., Tovmasyan A., Reboucas J.S., Ye X., Leong K.W., Dewhirst M.W., Vujaskovic Z., Benov L., Spasojevic I. Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radical Biology and Medicine. 2011;51(5):1035–1053. doi: 10.1016/j.freeradbiomed.2011.04.046. 21616142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tovmasyan A., Sheng H., Weitner T., Arulpragasam A., Lu M., Warner D.S., Vujaskovic Z., Spasojevic I., Batinic-Haberle I. Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators. Medical Principles and Practice. 2013;22(2):103–130. doi: 10.1159/000341715. 000341715] [Pubmed: 23075911] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J., Hunt J.A., Groves J.T. Manganese porphyrins as redox-coupled peroxynitrite reductases. Journal of the American Chemical Society. 1998;120(24):6053–6061. [Google Scholar]
- 26.Lee J., Hunt J.A., Groves J.T. Mechanisms of iron porphyrin reactions with peroxynitrite. Journal of the American Chemical Society. 1998;120(30):7493–7501. [Google Scholar]
- 27.Pfeiffer S., Schrammel A., Koesling D., Schmidt K., Mayer B. Molecular actions of a Mn(III)porphyrin superoxide dismutase mimetic and peroxynitrite scavenger: reaction with nitric oxide and direct inhibition of NO synthase and soluble guanylyl cyclase. Molecular Pharmacology. 1998;53(4):795–800. doi: 10.1124/mol.53.4.795. 9547373 [DOI] [PubMed] [Google Scholar]
- 28.Ford P.C., Wink D.A., Stanbury D.M. Autoxidation kinetics of aqueous nitric oxide. FEBS Letters. 1993;326(1–3):1–3. doi: 10.1016/0014-5793(93)81748-o. 8325356 [DOI] [PubMed] [Google Scholar]
- 29.Alvarez L., Suarez S.A., Bikiel D.E., Reboucas J.S., Batinić-Haberle I., Martí M.A., Doctorovich F. Redox potential determines the reaction mechanism of HNO donors with Mn and Fe porphyrins: defining the better traps. Inorganic Chemistry. 2014;53(14):7351–7360. doi: 10.1021/ic5007082. 25001488 [DOI] [PubMed] [Google Scholar]
- 30.A. Tovmasyan, C.G.C. Maia, T. Weitner, S.R. Sampaio, D. Lieb, R. Ghazaryan, I. Ivanovic-Burmazovic, J.S. Reboucas, L. Benov, I. Batinic Haberle, A comprehensive evaluation of the catalase-like activity of different classes of redox-active therapeutics, 2015 (in revision). [DOI] [PMC free article] [PubMed]
- 31.Tovmasyan A., Weitner T., Jaramillo M., Wedmann R., Roberts E.R.H., Leong K.W., Filipovic M., Ivanovic-Burmazovic I., Benov L., Tome M.E., Batinic-Haberle I. We have come a long way with Mn porphyrins: from superoxide dismutation to H2O2-driven pathways. Free Radical Biology and Medicine. 2013;65:S133. [Google Scholar]
- 32.Maia C.G.C., Tovmasyan A., Weitner T., Lieb D., Ivanovic-Burmazovic I., Reboucas J.S., Batinic-Haberle I. A comprehensive study of the catalase activity of different classes of experimental therapeutics commonly used as redox modulators. Free Radical Biology and Medicine. 2014;76:S85–S86. [Google Scholar]
- 33.Mahammed A., Gross Z. Highly efficient catalase activity of metallocorroles. Chemical Communications (Cambridge) 2010;46(37):7040–7042. doi: 10.1039/c0cc01989e. 20730224 [DOI] [PubMed] [Google Scholar]
- 34.Mahammed A., Gross Z. The importance of developing metal complexes with pronounced catalase-like activity. Catalysis Science and Technology. 2011;1(4):535–540. [Google Scholar]
- 35.Haber A., Gross Z. Catalytic antioxidant therapy by Metallodrugs: lessons from Metallocorroles. Chemical Communications. 2015 doi: 10.1039/c4cc08715a. [DOI] [PubMed] [Google Scholar]
- 36.Trostchansky A., Ferrer-Sueta G., Batthyány C., Botti H., Batinić-Haberle I., Radi R., Rubbo H. Peroxynitrite flux-mediated LDL oxidation is inhibited by manganese porphyrins in the presence of uric acid. Free Radical Biology and Medicine. 2003;35(10):1293–1300. doi: 10.1016/j.freeradbiomed.2003.07.004. 14607528 [DOI] [PubMed] [Google Scholar]
- 37.Bloodsworth A., O’Donnell V.B., Batinic-Haberle I., Chumley P.H., Hurt J.B., Day B.J., Crow J.P., Freeman B.A. Manganese-porphyrin reactions with lipids and lipoproteins. Free Radical Biology and Medicine. 2000;28(7):1017–1029. doi: 10.1016/s0891-5849(00)00194-5. 10832063 [DOI] [PubMed] [Google Scholar]
- 38.Umile T.P., Groves J.T. Catalytic generation of chlorine dioxide from chlorite using a water-soluble manganese porphyrin. Angewandte Chemie International Edition (England) 2011;50(3):695–698. doi: 10.1002/anie.201004482. 21226156 [DOI] [PubMed] [Google Scholar]
- 39.Carballal S., Valez V., Batinic-Haberle I., Ferrer-Sueta G., Radi R. Reactivity and cytoprotective capacity of the synthetic catalytic antioxidants Mnporphyrins towards peroxynitrite and hypochlorite. Free Radical Biology and Medicine. 2013;65(Suppl. 2):S121–S122. [Google Scholar]
- 40.Cieslak J.A., iii, Strother R., Du J., Rawal M., Doskey C.M., Schroeder S.R., Wagner B.A., Buettner G.R., Cullen J.J. 287 − the addition of Manganoporphyrins and ascorbate to Standard of Care chemotherapy enhances tumor-specific cytotoxicity in pancreatic cancer. Free Radical Biology and Medicine. 2014;76(Suppl. 1):S122–S123. doi: 10.1016/j.freeradbiomed.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans M.K., Tovmasyan A., Batinic-Haberle I., Devi G.R. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radical Biology and Medicine. 2014;68:302–314. doi: 10.1016/j.freeradbiomed.2013.11.031. 24334253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawal M., Schroeder S.R., Wagner B.A., Cushing C.M., Welsh J.L., Button A.M., Du J., Sibenaller Z.A., Buettner G.R., Cullen J.J. Manganoporphyrins increase ascorbate-induced cytotoxicity by enhancing H2O2 generation. Cancer Res. 2013;73(16):5232–5241. doi: 10.1158/0008-5472.CAN-13-0470. 23764544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovmasyan A., Roberts E.R.H., Yuliana Y., Haberle S., Boss M., Venkatraman T.N., Lascola C., Dewhirst M.W., Lam P.Y.P., Benov L., Leong K.W., Batinic-Haberle I. The role of ascorbate in therapeutic actions of cationic Mn porphyrin-based SOD mimics. Free Radical Biology and Medicine. 2014;76:S94–S95. [Google Scholar]
- 44.Ye X., Fels D., Tovmasyan A., Aird K.M., Dedeugd C., Allensworth J.L., Kos I., Park W., Spasojevic I., Devi G.R., Dewhirst M.W., Leong K.W., Batinic-Haberle I. Cytotoxic effects of Mn(III) N-alkylpyridylporphyrins in the presence of cellular reductant, ascorbate. Free Radical Research. 2011;45(11–12):1289–1306. doi: 10.3109/10715762.2011.616199. 21859376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaramillo M.C., Briehl M.M., Batinic-Haberle I., Tome M.E. Inhibition of the Electron transport chain via the pro-oxidative activity of manganese porphyrin-based SOD mimetics modulates bioenergetics and enhances the response to chemotherapy. Free Radical Biology and Medicine. 2013;65:S25. doi: 10.1016/j.freeradbiomed.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.M.C. Jaramillo, M.M. Briehl, I. Batinic-Haberle, M.E. Tome, MnTE-2-PyP5+ acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics and enhance the response to chemotherapy in lymphoma cells. Free Radical Biology and Medicine, 2015 (http://dx.doi.org/10.1016/j.freeradbiomed.2015.01.031, in press). [DOI] [PMC free article] [PubMed]
- 47.Jaramillo M.C., Briehl M.M., Crapo J.D., Batinic-Haberle I., Tome M.E. Manganese porphyrin, MnTE-2-PyP5+, acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radical Biology and Medicine. 2012;52(8):1272–1284. doi: 10.1016/j.freeradbiomed.2012.02.001. 22330065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batinic-Haberle I., Spasojevic I., Tse H.M., Tovmasyan A., Rajic Z., St Clair D.K., Vujaskovic Z., Dewhirst M.W., Piganelli J.D. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2012;42(1):95–113. doi: 10.1007/s00726-010-0603-6. 20473774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tse H.M., Milton M.J., Piganelli J.D. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radical Biology and Medicine. 2004;36(2):233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. 14744635 [DOI] [PubMed] [Google Scholar]
- 50.Delmastro-Greenwood M.M., Votyakova T., Goetzman E., Marre M.L., Previte D.M., Tovmasyan A., Batinic-Haberle I., Trucco M.M., Piganelli J.D. Mn porphyrin regulation of aerobic glycolysis: implications on the activation of diabetogenic immune cells. Antioxidants and Redox Signaling. 2013;19(16):1902–1915. doi: 10.1089/ars.2012.5167. 23682840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batinic-Haberle I., Rajic Z., Benov L. A combination of two antioxidants (an SOD mimic and ascorbate) produces a pro-oxidative effect forcing Escherichia coli to adapt via induction of oxyR regulon. Anti Cancer Agents in Medicinal Chemistry. 2011;11(4):329–340. doi: 10.2174/187152011795677562. 21355843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spasojević I., Colvin O.M., Warshany K.R., Batinić-Haberle I. New approach to the activation of anti-cancer pro-drugs by metalloporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. Journal of Inorganic Biochemistry. 2006;100(11):1897–1902. doi: 10.1016/j.jinorgbio.2006.07.013. 16965820 [DOI] [PubMed] [Google Scholar]
- 53.Weitzel D.H., Tovmasyan A., Ashcraft K.A., Rajic Z., Weitner T., Liu C., Li W., Buckley A.F., Prasad M.R., Young K.H., Rodriguiz R.M., Wetsel W.C., Peters K.B., Spasojevic I., Herndon J.E., 2nd, Batinic-Haberle I., Dewhirst M.W. Radioprotection of the brain White matter by Mn(III) N-Butoxyethylpyridylporphyrin-Based superoxide dismutase mimic MnTnBuOE-2-PyP5+ Molecular Cancer Therapeutics. 2015;14(1):70–79. doi: 10.1158/1535-7163.MCT-14-0343. 25319393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batinić-Haberle I., Spasojević I., Fridovich I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn(III) ortho-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radical Biology and Medicine. 2004;37(3):367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. 15223070 [DOI] [PubMed] [Google Scholar]
- 55.Ali D.K., Oriowo M., Tovmasyan A., Batinic-Haberle I., Benov L. Late administration of Mn porphyrin-based SOD mimic enhances diabetic complications. Redox Biology. 2013;1:457–466. doi: 10.1016/j.redox.2013.09.005. 24191241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trujillo M., Ferrer-Sueta G., Radi R. Kinetic studies on peroxynitrite reduction by peroxiredoxins. Methods Enzymology. 2008;441:173–196. doi: 10.1016/S0076-6879(08)01210-X. 18554535 [DOI] [PubMed] [Google Scholar]
- 57.Trujillo M., Ferrer-Sueta G., Radi R. Peroxynitrite detoxification and its biologic implications. Antioxidants and Redox Signaling. 2008;10(9):1607–1620. doi: 10.1089/ars.2008.2060. 18500925 [DOI] [PubMed] [Google Scholar]
- 58.Ferrer-Sueta G., Batinić-Haberle I., Spasojević I., Fridovich I., Radi R. Catalytic scavenging of peroxynitrite by isomeric Mn(III) N-methylpyridylporphyrins in the presence of reductants. Chemical Research in Toxicology. 1999;12(5):442–449. doi: 10.1021/tx980245d. 10328755 [DOI] [PubMed] [Google Scholar]
- 59.Weitner T., Kos I., Mandić Z., Batinić-Haberle I., Biruš M. Acid–base and electrochemical properties of manganese meso(ortho- and meta-N-ethylpyridyl)porphyrins: voltammetric and chronocoulometric study of protolytic and redox equilibria. Dalton Transactions. 2013;42(41):14757–14765. doi: 10.1039/c3dt50767j. 23933742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hempel N., Carrico P.M., Melendez J.A. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anti Cancer Agents in Medicinal Chemistry. 2011;11(2):191–201. doi: 10.2174/187152011795255911. 21434856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen B.G., Sibenaller Z.A., Cullen J.J., Buettner G.R., Welsh J.L., Wagner B.A., van’t Erve T.J., Buatti J.M., Carlisle T.L., Smith M.C., Walsh S.A., Bayouth J.E., TenNapel M., Spitz D.R. Pharmacological ascorbate enhances chemo-radio-sensitization in brain and lung cancer. Free Radical Biology and Medicine. 2012;53:S39. [Google Scholar]
- 62.Welsh J.L., Du J., Sibenaller Z.A., Kalen A.L., Wagner B.A., Allen B.G., Spitz D.R., Goswami P.C., Buettner G.R., Cullen J.J. Ascorbate Is a Radiosensitizer in Pancreatic Cancer. Free Radical Biology and Medicine. 2012;53:S52. [Google Scholar]
- 63.Tian J., Peehl D.M., Knox S.J. Metalloporphyrin synergizes with ascorbic acid to inhibit cancer cell growth through Fenton chemistry. Cancer Biotherapy and Radiopharmaceuticals. 2010;25(4):439–448. doi: 10.1089/cbr.2009.0756. 20735206 [DOI] [PubMed] [Google Scholar]
- 64.Takada Y., Bhardwaj A., Potdar P., Aggarwal B.B. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004;23(57):9247–9258. doi: 10.1038/sj.onc.1208169. 15489888 [DOI] [PubMed] [Google Scholar]
- 65.Oberley-Deegan R.E., Steffan J.J., Rove K.O., Pate K.M., Weaver M.W., Spasojevic I., Frederick B., Raben D., Meacham R.B., Crapo J.D., Koul H.K. The antioxidant, MnTE-2-PyP, prevents side-effects incurred by prostate cancer irradiation. PLoS One. 2012;7(9):e44178. doi: 10.1371/journal.pone.0044178. 22984473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen J., Dorai T., Ding C., Batinic-Haberle I., Grasso M. The administration of renoprotective agents extends warm ischemia in a rat model. Journal of Endourology. 2013;27(3):343–348. doi: 10.1089/end.2012.0194. 23102208 [DOI] [PubMed] [Google Scholar]
- 67.Dorai T., Fishman A.I., Ding C., Batinic-Haberle I., Goldfarb D.S., Grasso M. Amelioration of renal ischemia-reperfusion injury with a novel protective cocktail. Journal of Urology. 2011;186(6):2448–2454. doi: 10.1016/j.juro.2011.08.010. 22019164 [DOI] [PubMed] [Google Scholar]
- 68.Filipović M.R., Duerr K., Mojović M., Simeunović V., Zimmermann R., Niketić V., Ivanović-Burmazović I. NO dismutase activity of seven-coordinate manganese(II) Pentaazamacrocyclic complexes. Angewandte Chemie International Edition (England) 2008;47(45):8735–8739. doi: 10.1002/anie.200801325. 18924192 [DOI] [PubMed] [Google Scholar]
- 69.Greenwald M.B., Anzi S., Ben Sasson S., Bianco-Peled H., Kohen R. Can nitroxides evoke the Keap1-Nrf2-ARE pathway in skin? Free Radical Biology and Medicine. 2014;77:258–269. doi: 10.1016/j.freeradbiomed.2014.08.021. 25236737 [DOI] [PubMed] [Google Scholar]
- 70.Cui G., Chui Wah Luk S., Li R.A., Chan K.K., Lei S.W., Wang L., Shen H., Leung G.P., Lee S.M. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. Journal of Cardiovascular Pharmacology. 2015;65(1):39–46. doi: 10.1097/FJC.0000000000000161. 25343567 [DOI] [PubMed] [Google Scholar]
- 71.Karthik D., Majumder P., Palanisamy S., Khairunnisa K., Venugopal V. Targeting cysteine rich C1 domain of scaffold protein kinase suppressor of Ras (KSR) with anthocyanidins and flavonoids − a binding affinity characterization study. Bioinformation. 2014;10(9):580–585. doi: 10.6026/97320630010580. 25352726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miljkovic J., Ivanovic-Burmazovic I., Filipovic M. H2S Generates HNO and HSNO from Nitrite by a Heme Iron-Catalyzed Metabolism in Mitochondria. Free Radical Biology and Medicine. 2013;65 doi: 10.1002/anie.201305669. [DOI] [PubMed] [Google Scholar]
- 73.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radical Biology and Medicine. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. 23747930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rebouças J.S., Spasojević I., Batinić-Haberle I. Quality of potent Mn porphyrin-based SOD mimics and peroxynitrite scavengers for pre-clinical mechanistic/therapeutic purposes. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(3):1046–1049. doi: 10.1016/j.jpba.2008.08.005. 18804338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rebouças J.S., Spasojević I., Batinić-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. Journal of Biological Inorganic Chemistry. 2008;13(2):289–302. doi: 10.1007/s00775-007-0324-9. 18046586 [DOI] [PubMed] [Google Scholar]
- 76.Batinić-Haberle I., Cuzzocrea S., Rebouças J.S., Ferrer-Sueta G., Mazzon E., Di Paola R., Radi R., Spasojević I., Benov L., Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radical Biology and Medicine. 2009;46(2):192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. 19007878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee K., Briehl M.M., Mazar A.P., Batinic-Haberle I., Reboucas J.S., Glinsmann-Gibson B., Rimsza L.M., Tome M.E. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free Radical Biology and Medicine. 2013;60:157–167. doi: 10.1016/j.freeradbiomed.2013.02.003. 23416365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ezzeddine R., Al-Banaw A., Tovmasyan A., Craik J.D., Batinic-Haberle I., Benov L.T. Effect of molecular characteristics on cellular uptake, subcellular localization, and phototoxicity of Zn(II) N-alkylpyridylporphyrins. Journal of Biological Chemistry. 2013;288(51):36579–36588. doi: 10.1074/jbc.M113.511642. 24214973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Odeh A.M., Craik J.D., Ezzeddine R., Tovmasyan A., Batinic-Haberle I., Benov L.T. Targeting mitochondria by Zn(II)N-Alkylpyridylporphyrins: the impact of Compound sub-mitochondrial partition on cell respiration and overall photodynamic efficacy. PLoS One. 2014;9(9):e108238. doi: 10.1371/journal.pone.0108238. 25250732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spasojević I., Chen Y., Noel T.J., Yu Y., Cole M.P., Zhang L., Zhao Y., St Clair D.K., Batinić-Haberle I. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radical Biology and Medicine. 2007;42(8):1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. 17382200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spasojevic I., Miriyala S., Tovmasyan A., Salvemini D., Fan P., Vujaskovic Z., Batinic-Haberle I., Clair D.K.S. Lipophilicity of Mn(III) N-Alkylpyridylporphyrins Dominates Their Accumulation Within Mitochondria and Therefore in Vivo Efficacy: a Mouse Study. Free Radical Biology and Medicine. 2011;51:S98–S99. [Google Scholar]
- 82.Spasojevic I., Weitner T., Tovmasyan A., Sheng H., Miriyala S., Leu D., Rajic Z., Warner D.S., Clair D.S., Huang T., Batinic-Haberle I. Pharmacokinetics, brain hippocampus and cortex, and mitochondrial accumulation of a New generation of lipophilic redox-active therapeutic, Mn(III) meso tetrakis(N-n-butoxyethylpyridinium-2-yl)porphyrin, MnTnBuOE-2-PyP5+, in comparison with its ethyl and N-hexyl analogs, MnTE-2-PyP5+ and MnTnHex-2-PyP5+ Free Radical Biology and Medicine. 2013;65:S132. [Google Scholar]
- 83.Weitner T., Kos I., Sheng H., Tovmasyan A., Reboucas J.S., Fan P., Warner D.S., Vujaskovic Z., Batinic-Haberle I., Spasojevic I. Comprehensive pharmacokinetic studies and oral bioavailability of two Mn porphyrin-based SOD mimics, MnTE-2-PyP5+ and MnTnHex-2-PyP5+ Free Radical Biology and Medicine. 2013;58:73–80. doi: 10.1016/j.freeradbiomed.2013.01.006. 23328731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kos I., Benov L., Spasojević I., Rebouças J.S., Batinić-Haberle I. High lipophilicity of meta Mn(III) N-alkylpyridylporphyrin-based superoxide dismutase mimics compensates for their lower antioxidant potency and makes them as effective as ortho analogues in protecting superoxide dismutase-deficient Escherichia coli. Journal of Medicinal Chemistry. 2009;52(23):7868–7872. doi: 10.1021/jm900576g. 19954250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.M. Thomas, J. Craik, A. Tovmasyan, I. Batinic-Haberle, L. Benov, Amphiphilic cationic Zn-porphyrins with high photodynamic antimicrobial activity. Future Microbiology, 2015 (accepted). [DOI] [PubMed]
- 86.Spasojevic I., Kos I., Benov L.T., Rajic Z., Fels D., Dedeugd C., Ye X., Vujaskovic Z., Reboucas J.S., Leong K.W., Dewhirst M.W., Batinic-Haberle I. Bioavailability of metalloporphyrin-based SOD mimics is greatly influenced by a single charge residing on a Mn site. Free Radical Research. 2011;45(2):188–200. doi: 10.3109/10715762.2010.522575. 20942564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liberman E.A., Topaly V.P., Tsofina L.M., Jasaitis A.A., Skulachev V.P. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222(5198):1076–1078. doi: 10.1038/2221076a0. 5787094 [DOI] [PubMed] [Google Scholar]
- 88.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochimica et Biophysica Acta. 2008;1777(7–8):1028–1031. doi: 10.1016/j.bbabio.2008.03.029. 18439417 [DOI] [PubMed] [Google Scholar]
- 89.Spasojevic I., Li A., Tovmasyan A., Rajic Z., Salvemini D., St. Clair D., Valentine J.S., Vujaskovic Z., Gralla E.B., Batinic-Haberle I. Accumulation of porphyrin-based SOD mimics in mitochondria is proportional to their lipophilicity: S. cerevisiae study of ortho Mn(III) N-alkylpyridylporphyrins. Free Radical Biology and Medicine. 2010;49:S199. [Google Scholar]
- 90.Weitner T., Sheng H., Miriyala S., Leu D., Tovmasyan A., Kos I., Reboucas J.S., Fan P., Vujaskovic Z., Batinic-Haberle I., Huang T.T., Clair D.K., Warner D.S., Spasojevic I. Comprehensive pharmocokinetic studies and biodistribution of two cationic Mn porphyrins-based catalysts, MnTE-2-PyP5+ and MnTnHex-2-PyP5+: plasma and organ oral availability, mitochondrial, cytosolic, whole brain, hippocampus and cortex distribution. Free Radical Biology and Medicine. 2012;53:S118. [Google Scholar]
- 91.Li A.M., Martins J., Tovmasyan A., Valentine J.S., Batinic-Haberle I., Spasojevic I., Gralla E.B. Differential localization and potency of manganese porphyrin superoxide dismutase-mimicking compounds in Saccharomyces cerevisiae. Redox Biology. 2014;3:1–6. doi: 10.1016/j.redox.2014.09.003. 25462059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gad S.C., Sullivan D.W., Jr., Crapo J.D., Spainhour C.B. A nonclinical safety assessment of MnTE-2-PyP, a manganese porphyrin. International Journal of Toxicology. 2013;32(4):274–287. doi: 10.1177/1091581813490203. 23704100 [DOI] [PubMed] [Google Scholar]
- 93.Sheng H., Yang W., Fukuda S., Tse H.M., Paschen W., Johnson K., Batinic-Haberle I., Crapo J.D., Pearlstein R.D., Piganelli J., Warner D.S. Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radical Biology and Medicine. 2009;47(7):917–923. doi: 10.1016/j.freeradbiomed.2009.05.039. 19631268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheng H., Spasojevic I., Tse H.M., Jung J.Y., Hong J., Zhang Z., Piganelli J.D., Batinic-Haberle I., Warner D.S. Neuroprotective efficacy from a lipophilic redox-modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: rodent models of ischemic stroke and subarachnoid hemorrhage. Journal of Pharmacology and Experimental Therapeutics. 2011;338(3):906–916. doi: 10.1124/jpet.110.176701. 21652782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orrell R.W. AEOL-10150 (Aeolus) Current Opinion in Investigational Drugs. 2006;7(1):70–80. 16425674 [PubMed] [Google Scholar]


