Highlights
-
•
Thymidine kinase 1 (TK1) is a tumor marker for the diagnosis of hepatocellular carcinoma (HCC).
-
•
Alpha-fetoprotein (AFP) was not detected in 25% of the HCC patients in our study.
-
•
The combined analysis of AFP, TKI and alpha-l-fucosidase could improve the sensitivity of HCC diagnosis.
Abbreviations: AFP, alpha-fetoprotein; AFU, alpha-l-fucosidase; AUC, area under receiver operating characteristic curve; CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; HIV, human immunodeficiency virus; ROC, receiver operating characteristic curve; RPM, rotation per minute; SE, standard error; TK1, thymidine kinase 1
Keywords: α-l-Fucosidase, Alpha-fetoprotein, Thymidine kinase 1, Hepatocellular carcinoma, ROC curve
Abstract
The purpose of this study was to evaluate the diagnostic efficiency for hepatocellular carcinoma (HCC) with the combined analysis of alpha-l-fucosidase (AFU), alpha-fetoprotein (AFP) and thymidine kinase 1 (TK1). Serum levels of AFU, AFP and TK1 were measured in: 116 patients with HCC, 109 patients with benign hepatic diseases, and 104 normal subjects. The diagnostic value was analyzed using the logistic regression equation and receiver operating characteristic curves (ROC). Statistical distribution of the three tested tumor markers in every group was non-normally distributed (Kolmogorov–Sminov test, Z = 0.156–0.517, P < 0.001). The serum levels of AFP and TK1 in patients with HCC were significantly higher than those in patients with benign hepatic diseases (Mann–Whitney U test, Z = −8.570 to –5.943, all P < 0.001). However, there was no statistically significant difference of AFU between these two groups (Mann–Whitney U test, Z = −1.820, P = 0.069). The levels of AFU were significantly higher in patients with benign hepatic diseases than in normal subjects (Mann–Whitney U test, Z = −7.984, P < 0.001). Receiver operating characteristic curves (ROC) in patients with HCC versus those without HCC indicated the optimal cut-off value was 40.80 U/L for AFU, 10.86 μg/L for AFP and 1.92 pmol/L for TK1, respectively. The area under ROC curve (AUC) was 0.718 for AFU, 0.832 for AFP, 0.773 for TK1 and 0.900 for the combination of the three tumor markers. The combination resulted in a higher Youden index and a sensitivity of 85.3%. The combined detection of serum AFU, AFP and TK1 could play a complementary role in the diagnosis of HCC, and could significantly improve the sensitivity for the diagnosis of HCC.
1. Introduction
Despite progress made being during the past few decades, HCC is still one of the most frequent and deadly cancers worldwide in both men and women. In the United States, HCC ranks as having the fifth highest mortality in males, with an estimated 14,890 death cases and ranked ninth in females, with an estimated 6780 death cases, according to the 2013 report by the United States cancer society [1]. Globally, there are approximately 750,000 new cases of liver cancer reported each year, 70–85% of which are HCC [2,3]. Due to the asymptomatic nature of an early case of HCC and lack of effective diagnostic and screening strategies, most patients (>80%) are presented with the apparent advanced stage of HCC [4]. It is well known that the prognosis of HCC is poor. Therefore, the prevention of HCC is a significant public health issue. Early detection for HCC is of the utmost importance [5]. Detection of a tumor biomarker is effective and a common approach to screen HCC because it is convenient, non-invasive and inexpensive with a high degree of accuracy.
Alpha-l-fucosidase (AFU) is a liposomal enzyme widely present in all mammalian cells, blood and body fluid. It can be found in the serum of healthy adults. The activity of this liposomal enzyme is detectable and elevated activities are observed in the sera of HCC patients compared with chronic liver disease and healthy individuals [6,7].
Alpha-fetoprotein (AFP) is a glycoprotein secreted by the fetal liver and yolk sac during prenatal development. The fetal liver becomes the main site of AFP synthesis as the yolk sac degenerates after 12 weeks of gestation [8]. AFP levels are usually high at birth and decrease to adult levels within the first year of life. In healthy pregnant women AFP may reach concentrations of 250 mg/L. After birth, these concentrations fall rapidly [9]. AFP is widely used as a tumor biomarker in the early diagnosis of HCC.
TK1 is a diagnostic biomarker for a variety of tumor types, which is involved in DNA repair and is primarily elevated during S phase. TK1 is associated with proliferating cells [10]. A clinical investigation [11] performed on 11,880 people within China during 2005 to 2007 showed that serum level of TK1 with values >2.0 pmol/L may indicate an early risk for the development of malignancies later in life.
Today, lots of tumor biomarkers have been conducted as a complement or substitute for AFP in order to improve sensitivity and specificity in diagnosing HCC. The aim of the present study is to assess the diagnostic value of joint detection of AFU, AFP and TK1 in the diagnosis for HCC.
2. Material and methods
We retrospectively analyzed the clinic pathologic data of patients with HCC and benign liver disorders at the Department of Oncology in Affiliated Fuding Hospital, Fujian University of Traditional Chinese Medicine in China between January 2012 and November 2014. They included a group of 116 patients with HCC and a group of 109 patients with benign liver diseases. Additionally, a group of 104 healthy blood specimens from routine check-ups within the same hospital were used as controls at the same time. There were 75 males and 41 females with a mean age of 57.8 ± 11.3 years (range, 27–79 years) in HCC group, 68 males and 41 females with a mean age of 54.1 ± 11.9 years (range, 32–84 years) in benign liver disease group and 70 males and 34 females with a mean age of 56.9 ± 16.0 years (range, 22–85 years) in normal control group, respectively.
Diagnosis for HCC was based on clinical investigations, which included laboratory tests, along with radiological imaging in selected cases. After hepatic resection, two pathologists confirmed the diagnosis of HCC conducted histopathological examination of the tissue specimens. Diagnosis of benign liver disease was based on liver histology as well as laboratory and imaging evidence of hepatic decomposition or portal hypertension. The benign liver disease included liver cirrhosis and chronic hepatitis. None of 104 healthy control subjects were positive for the biomarkers of hepatitis viruses A, B, C, D, and E; HIV antibodies; or had liver; gallbladder; or kidney disease.
All blood samples were collected from subjects before treatment. The local Ethics Committees approved this study.
2.1. Methods
Peripheral blood samples were extracted from cases and controls prior to treatment. Fresh serum samples were shipped to clot for a period of thirty minutes at 37 °C. Samples were then separated by 3000 RPM centrifugal 10 min. The supernatant sera were collected and stored at −20 °C until testing. Although, AFU activity was assayed within 30 days after collection [12]. The serum AFU activities were assayed using a biochemistry analyzer. The serum levels of AFP were assayed by an electrochemiluminescence analyzer Eleusis 2010 with supporting reagents (Roche, German). The concentrations of TK1 were measured by using a commercial Kit based on an enhanced chemiluminescent (ECL) dot blot assay in accordance with instructions by the manufacturer (SSTK Ltd., Shenzhen, China). Normal reference values of AFU, AFP and TK1 were <40.0 U/L, <10.0 μg/L and <2.0 pmol/L, respectively.
2.2. Statistical analysis
Statistical analysis was performed using the SPSS 22.0 software package. Markers concentration distribution of patients in HCC, benign liver disorder and normal control groups were carried out by means of Kolmogorov–Sminov test, calculating the tumor marker concentration and Median (P25 and P75).
Kruskal–Wallis and Chi-square tests were conducted for a comparison of tumor marker concentration levels among the different groups of subjects included. Comparison of continuous variables between the two groups was performed using the Mann–Whitney U-test. The receiver operating characteristics (ROC) curves, which correlated true-positive and false-positive rates (sensitivity and 1-specificity), were displayed. The areas under the ROC curve (AUC) were calculated for each biomarker as well. The statistical significance of differences after logistic regression between the two AUCs was also determined. Statistical analysis was conducted by logistic regression, analyzing diagnosis value of single and combined detection of the three tested tumor markers for HCC. The ROC curve was displayed with SPSS 22.0. In all tests, statistical significance was considered at P value less than 0.05.
3. Results
3.1. Study population
The present study was performed on a total of 225 patients with different liver diseases and 104 healthy subjects. Table 1 shows the major attributes of the subjects enrolled.
Table 1.
Clinical parameters of HCC group, benign liver disease group and normal control group [case (%)].
| Items | HCC group (n = 116) | Benign disease group (n = 109) | Normal group (104) | F/χ2 | P value |
|---|---|---|---|---|---|
| Mean age (year) | 57.8 ± 11.3 | 54.1 ± 11.9 | 56.9 ± 16.0 | 2.431 | 0.090 |
| Gender | 0.173 | 0.917 | |||
| Male | 75 (63.0) | 72 (67.9) | 70 (67.3) | ||
| Female | 41 (37.0) | 37 (32.1) | 34 (32.7) | ||
| Etiology | 0.551 | 0.458⁎ | |||
| HBV | 90 (77.6) | 83 (76.1) | |||
| HCV | 18 (15.5) | 21 (19.3) | |||
| Others# | 8 (6.9) | 5 (4.6) |
HCV versus HBV and others.
Including HAV, HDV, HEV and HIV.
3.2. Tumor markers Kolmogorov–Sminov test
Statistical distribution of the three tested tumor markers in every group was Kolmogorov–Sminov test with Z = 0.156–0.517, P < 0.001, which was non-normally distributed, and non-parametric tests were used in the following statistical analysis.
3.3. Tumor marker concentrations in cases and controls
The concentrations of AFU, AFP and TK1 were statistically different among the three groups (χ2 = 70.311–104.425, all P < 0.001). AFP and TK1 levels were higher in HCC group than those in benign liver disorder group with the exception of AFU; AFU levels were higher in benign liver disorder group than in normal control group, except AFP and TK1 (Table 2).
Table 2.
The mean concentrations of AFU, AFP and TK1 in cases and controls [Median (P25–P75)].
| Group | n | AFU (U/L) | AFP (μg/L) | TK1 (pmol/L) |
|---|---|---|---|---|
| HCC | 116 | 51.0 (37.5–79.2) | 306.0 (9.7–3020.0) | 2.34 (1.4–4.1) |
| Benign liver disorder | 109 | 46.1 (35.9–67.2) | 5.1 (2.6–8.1) | 1.3 (0.8–2.0) |
| Control | 104 | 28.0 (25.0–34.0) | 6.0 (4.0–8.0) | 1.3 (1.0–1.6) |
Kruskal–Wallis test was carried out among the three groups with three tumor markers: χ2 = 70.311–104.425, all P < 0.001. Mann–Whitney U test was carried out between the following two groups: (1), HCC group versus benign liver disease group: AFU (Z = −1.820, P = 0.069), AFP (Z = −8.570, P < 0.001), TK1 (Z = −5.943, P < 0.001); (2), benign liver disease group versus control group: AFU (Z = −7.984, P < 0.001), AFP (Z = −0.527, P = 0.598), TK1 (Z = −1.575, P = 0.115).
3.4. The positive rate of tumor markers in all groups
According to the reference values, the three tested tumor markers in patients with HCC were statistically different compared with those in benign liver disease group and control group (P < 0.001, Table 3).
Table 3.
Positive rates of AFU, AFP and TK1 in each group [case (%)].
| Groups | Case (n) | AFU [n(%)] | AFP [n(%)] | TK1 [n(%)] |
|---|---|---|---|---|
| HCC | 116 | 84 (70.6) | 87 (73.1) | 67 (56.3) |
| Benign disorder | 109 | 72 (67.9) | 22 (20.8) | 29 (27.4) |
| Healthy control | 104 | 8 (7.7) | 0 (0.0) | 2 (1.9) |
Chi-square test or Fischer’s exact test was performed when appropriate. HCC group versus benign disorder group: AFU (χ2 = 6.270, P = 0.012), AFP (χ2 = 18.538, P < 0.001), and TK1 (χ2 = 13.115, P = 0.003); benign liver disorder group versus control group: AFU (χ2 = 28.586, P < 0.001), AFP (χ2 = 16.958, P < 0.001), and TK1 (χ2 = 4.449, P < 0.001).
3.5. Logistic regression and ROC curve in HCC group versus non-HCC group
The receiver operating characteristic curve (ROC) for analysis of single AFU, AFP, TK1 and the combination in patients with HCC in comparison with patients without HCC were plotted. The areas under the curves of the three tested tumor markers were: AFU: 0.718 (95% CI: 0.662–0.774, P < 0.001); AFP: 0.832 (95% CI: 0.778–0.887, P < 0.001); TK1: 0.773 (95% CI: 0.719–0.823, P < 0.001); the combination: 0.900 (95% CI: 0.863–0.937). Logistic regression was used of calculating the logistic regression equation of the serum concentrations of APU, AFP and TK1. Logit (P) = −3.331 + 0.013 × AFU + 0.05 × AFP + 0.680 × TK1. The model was founded with logistic regression, and the ROC curve was fitted through the PRE of the model (Fig. 1). Among the AUCs of the three tumor markers with predicted probability connected with single tumor marker and logistic regression curve, when three tumor markers were tested separately, the AUC of AFP was the highest, followed by that of TK1, and the AUC of AFU was the lowest. Yet, the three tumor markers combined in the logistic regression model was higher than that of every tumor marker tested separately.
Fig. 1.
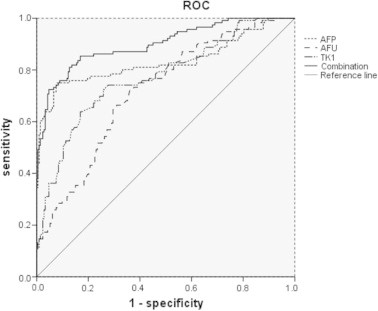
ROC curve comparing AFU, AFP and TK1 levels in patients with HCC versus patients without HCC (including patients with benign disease and normal subjects). The curves show optimal cut-off value for AFU of 40.80 U/L, for AFP of 10.86 μg/L and for TK1 of 1.92 pmol/L. The area under the ROC curve was 0.718 for AFU, 0.832 for AFP, 0.773 for TK1 and 0.900 for the combination of AFU, AFP and TK1.
3.6. Tumor marker evaluation parameters
Sensitivity, specificity, Youden’s index (sensitivity + specificity − 1) and diagnostic accuracy (Table 4) of AFU, AFP and TK1 were calculated according to the ROC curves and the logistic regression. Conjoint analysis: regarding AFP as the basic indicator, on the basis of which the indicators of AFU, AFP and TK1 were analyzed jointly. The sensitivity of the combination of the rest indicators was increased while the rest indicators, such as specificity and so on, were decreased evidently.
Table 4.
The diagnosis efficiency of the conjoint analysis of tumor markers (HCC group versus non-HCC group).
| Items | AFU | AFP | TK1 | Combination |
|---|---|---|---|---|
| Optimal cut-off | 40.80 U/L | 10.86 μg/L | 1.92 pmol/L | – |
| Sensitivity (%) | 72.4 | 75.0 | 63.8 | 85.3 |
| Specificity (%) | 63.8 | 92.0 | 83.1 | 83.1 |
| Diagnostic accuracy (%) | 66.9 | 85.9 | 76.1 | 83.9 |
| Younden’s index (%) | 36.2 | 67.0 | 46.9 | 68.4 |
| SE | 0.029 | 0.028 | 0.028 | 0.019 |
| AUC (95% CI) | 0.718 (0.662–0.774) | 0.832 (0.778–0.887) | 0.773 (0.719–0.827) | 0.900 (0.863–0.937) |
| Z-value# | 5.379 | 2.010 | 3.753 | – |
| P-value# | <0.01 | <0.05 | <0.01 | – |
Comparing the area under ROC curve of combination to single tumor marker in HCC versus non-HCC. SE, standard error. AUC, area under the receiver operating characteristic.
4. Discussion
The serum level of AFP has been commonly used as a traditional method for early diagnosis of HCC in China [13]. Be that as that may, an elevated AFP level is also obtained in certain benign liver disorders, such as viral hepatitis, liver cirrhosis and exacerbations of chronic hepatitis, and other cancers, which are the most prevalent cancers of the digestive tract (pancreas ∼ 24%, stomach ∼ 15%, large intestine ∼ 3%, and gallbladder) [14,15]. Moreover, false-negative or false–positive results can often be detected for some reason – including geographical and ethnic variations and different techniques being performed [7]. For these reason, it is necessary to perform a conjoint combination of various of biomarkers in the diagnosis of HCC [7].
The present study shows that the concentration of AFP is significantly higher in patients with HCC than patients with benign liver diseases and control subjects, a finding that came in agreement with the work of El-Tayeh et al. [16]. The ROC curve comparing HCC versus non-HCC shows its sensitivity of 75.0%, high specificity of 92.0% and diagnostic accuracy of 85.9% at the optimal cut-off of 10.86 μg/L. This means that 25% of the studied patients with HCC are negative for AFP. The result is similar to the study by Marrero et al. [17], which showed that AFP at a cutoff of 10.9 μg/L is currently the best serum biomarker for the diagnosis of early stage HCC.
AFU is also a useful biomarker of the diagnosis for HCC. AFU has been recommended as a serum biomarker for HCC in some studies. In the present study, the sensitivity of AFU was 72.4% and the specificity was 63.8%. Our finding came in agreement with a meta-analysis about the diagnosis of AFU for HCC by Gan et al. [18], which showed that the pooled sensitivity of AFU for HCC was 0.72 [95% confidence interval (CI):0.69–0.76], while the pooled specificity was 0.78 (95% CI: 0.74–0.81). Moreover, AFU in the current study recorded an AUC of 0.718 indicating its validity as a diagnostic biomarker of HCC. Therefore, AFU activity may be regarded as a valuable biomarker for the diagnosis of HCC.
Some clinical investigation [19] showed that elevated TK1 values indicated active tumor growth. Serological TK1 could be a useful marker for an early risk of development of any type of malignancy [20]. In the current study, the serum TK1 was dramatically elevated in patients with HCC comparing to patients with benign liver diseases and healthy subjects, which came in agreement with the study by Zhi et al. [11]. The latter study [11] showed a sensitivity of 85.7% and a specificity of 99.7% in the diagnosis of liver tumor versus normal control, whereas our present investigation had a sensitivity of 63.8% and a specificity of 83.1% in the diagnosis of liver tumor in comparison with benign liver diseases and normal subjects.
The serum tumor markers are widely used for diagnosis of HCC. Of all the serum tumor markers, AFP is the most commonly one and serve as an important tool in the monitoring of HCC patients [21]. However, the low sensitivity and specificity of AFP for HCC limits its clinical application. Consequently, in order to improve the diagnostic performance for HCC, the combination of AFP with other serum tumor markers is recommended in the diagnosis of HCC [2,22,23]. Previous studies have proven that a combination of serum tumor markers had a better diagnostic performance for HCC [2,22–25]. The results of these studies were in agreement with the current study concerning the combination of tumor markers for diagnosis of HCC. The simultaneous determination of the tested tumor markers (AFU, AFP and TK1) showed the largest AUC [0.900 (95% CI: 0.863–0.937)] and increased the sensitivity gradually up to 85.3%. The simultaneous sensitivity of 85.3% was highest in the three tumor markers. This suggests that several markers should be conducted in order to obtain a reliable detection of HCC [23].
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We like to thank Dr. Andrew Edward Wiles and Dr. Hou-Qun Ying for a critical reading of the manuscript.
Author’s contribution Shi-Yan Zhang, conceived and designed the project, analyzed and interpreted the data, and wrote the paper. Bi-Ding Lin, designed the project and interpreted the data. Bu-Ren Li, acquired and analyzed the data.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ge T., Shen Q., Wang N., Zhang Y., Ge Z., Chu W. Diagnostic values of alpha-fetoprotein, dickkopf-1, and osteopontin for hepatocellular carcinoma. Med. Oncol. 2015;32:59. doi: 10.1007/s12032-014-0367-z. [DOI] [PubMed] [Google Scholar]
- 3.Maluccio M., Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim A.M., Hashem M.E., Mostafa E.F., Refaey M.M., Hamed E.F., Ibrahim I. Annexin A2 versus Afp as an efficient diagnostic serum marker for hepatocellular carcinoma. J. Gastroenterol. Hepatol. Res. 2013;212:780–785. [Google Scholar]
- 5.Block T.M., Marrero J., Gish R.G., Sherman M., London W.T., Srivastava S. The degree of readiness of selected biomarkers for the early detection of hepatocellular carcinoma: notes from a recent workshop. Cancer Biomark. 2008;4:19–33. doi: 10.3233/cbm-2008-4103. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y.J., Ju Q., Li G.C. Tumor markers for hepatocellular carcinoma. Mol. Clin. Oncol. 2013;1:593–598. doi: 10.3892/mco.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J., Jiang F., Ni H.B., Xiao M.B., Chen B.Y., Ni W.K. Combined analysis of serum gamma-glutamyl transferase isoenzyme II, alpha-l-fucosidase and alpha-fetoprotein detected using a commercial kit in the diagnosis of hepatocellular carcinoma. Exp. Ther. Med. 2013;5:89–94. doi: 10.3892/etm.2012.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang O.D., Korff T., Eckhardt J., Rifaat J., Baal N., Herr F. Oncodevelopmental alpha-fetoprotein acts as a selective proangiogenic factor on endothelial cell from the fetomaternal unit. J. Clin. Endocrinol. Metab. 2004;89:1415–1422. doi: 10.1210/jc.2003-031721. [DOI] [PubMed] [Google Scholar]
- 9.Bredow M., Goldie D. Rapid falls in maternal serum a-fetoprotein concentrations. J. Clin. Pathol. 1985;38:473–474. doi: 10.1136/jcp.38.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alegre M.M., Robison R.A., O’Neill K.L. Thymidine kinase 1 upregulation is an early event in breast tumor formation. J. Oncol. 2012;2012:575647. doi: 10.1155/2012/575647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z.H., Huang S.Q., Wang Y., Yang A.Z., Wen J., Xu X.H. Serological thymidine kinase 1 is a biomarker for early detection of tumours – a health screening study on 35,365 people, using a sensitive chemiluminescent dot blot assay. Sensors (Basel) 2011;11:11064–11080. doi: 10.3390/s111211064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giardina M.G., Matarazzo M., AV M.D., RM M.D., Napoli A., Martino R. Serum alpha-l-fucosidase. A useful marker in the diagnosis of hepatocellular carcinoma. Cancer. 1992;70:1044–1048. doi: 10.1002/1097-0142(19920901)70:5<1044::aid-cncr2820700506>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Song P., Feng X., Zhang K., Song T., Ma K., Kokudo N. Perspectives on using des-gamma-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg. Nutr. 2013;2:227–231. doi: 10.3978/j.issn.2304-3881.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu El Makarem M. An overview of biomarkers for the diagnosis of hepatocellular carcinoma. Hepat. Mon. 2012;12:6122. doi: 10.5812/hepatmon.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ba M.C., Long H., Tang Y.Q., Cui S.Z. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int. J. Clin. Exp. Pathol. 2012;5:874–881. [PMC free article] [PubMed] [Google Scholar]
- 16.El-Tayeh S.F., Hussein T.D., El-Houseini M.E., Amer M.A., El-Sherbini M., Elshemey W.M. Serological biomarkers of hepatocellular carcinoma in Egyptian patients. Dis. Markers. 2012;32:255–263. doi: 10.3233/DMA-2011-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero J.A., Feng Z., Wang Y., Nguyen M.H., Befeler A.S., Roberts L.R. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan Y., Liang Q., Song X. Diagnostic value of alpha-l-fucosidase for hepatocellular carcinoma: a meta-analysis. Tumour Biol. 2014;35:3953–3960. doi: 10.1007/s13277-013-1563-8. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson L., Larsson A., Lindman H. Elevated levels of thymidine kinase 1 peptide in serum from patients with breast cancer. Ups. J. Med. Sci. 2009;114:116–120. doi: 10.1080/03009730802688835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z., Zhou H., Li S., He E., Hu J., Zhou J. Serological thymidine kinase 1 (STK1) indicates an elevated risk for the development of malignant tumours. Anticancer Res. 2008;28:3897–3907. [PubMed] [Google Scholar]
- 21.Wang L., Yao M., Dong Z., Zhang Y., Yao D. Circulating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol. 2014;35:9–20. doi: 10.1007/s13277-013-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ertle J.M., Heider D., Wichert M., Keller B., Kueper R., Hilgard P. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121–131. doi: 10.1159/000346080. [DOI] [PubMed] [Google Scholar]
- 23.E-H M., M M.S., Elshemey W.M., H T.D., D O.S., E A.A. Enhanced detection of hepatocellular carcinoma. Cancer Control. 2005;12:355–361. doi: 10.1177/107327480501200407. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.J., Yeon J.E., Suh S.J., Lee S.J., Yoon E.L., Kang K. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8:177–185. doi: 10.5009/gnl.2014.8.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G., Ha S.A., Kim H.K., Yoo J., Kim S., Lee Y.S. Combined analysis of AFP and HCCR-1 as an useful serological marker for small hepatocellular carcinoma: a prospective cohort study. Dis. Markers. 2012;32:265–271. doi: 10.3233/DMA-2011-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]


