Abstract
Importance
Postnatal cytomegalovirus (CMV) infection can cause serious morbidity and mortality in very low birth weight (VLBW) infants. The primary sources of postnatal CMV infection in this population are breast milk and blood transfusion. The current risks attributable to these vectors, and the efficacy of approaches to prevent CMV transmission, are poorly characterized.
Objectives
To estimate the risk of postnatal CMV transmission from 2 sources: 1) transfusion of CMV-seronegative and leukoreduced blood and 2) maternal breast milk.
Design
Prospective, multicenter birth-cohort study conducted from January 2010 to June 2013. CMV serologic testing of enrolled mothers was performed to determine their status. CMV nucleic acid testing (NAT) of transfused blood components and breast milk was performed to identify sources of CMV transmission. Enrolled VLBW infants underwent serum and urine CMV NAT testing at birth, to evaluate congenital infection, and surveillance CMV NAT testing at 5 additional intervals between birth and 90 days, discharge or death.
Setting
Three neonatal intensive care units (2 academically-affiliated and 1 private) in Atlanta, Georgia.
Participants
539 VLBW infants (birth weight ≤1500 grams) who had not received a blood transfusion were enrolled, with their mothers, within 5 days of birth.
Exposure
Blood transfusion and breast milk feeding
Main Outcomes and Measures
Cumulative incidence of postnatal CMV infection, detected by serum or urine NAT.
Results
CMV positive sero-prevalence among enrolled mothers was 76% (352/462). Among 539 enrolled VLBW infants, the cumulative incidence of postnatal CMV infection at 12 weeks was 6.9% (95% CI: 4.2%–9.2%); five infants with postnatal CMV infection developed symptomatic disease or died. Although 58% (310/539) of infants received 2061 transfusions, none of the CMV infections were linked to transfusion, resulting in a CMV infection incidence of 0.0% (95%CI: 0.0%–0.3%) per unit of CMV-seronegative and leukoreduced blood. Twenty-seven of 28 postnatal infections occurred among infants fed CMV-positive breast milk (12-week incidence: 15.3%; 95%CI: 9.3%–20.2%).
Conclusions and Relevance
Transfusion of CMV-seronegative and leukoreduced blood products effectively prevents transmission of CMV to VLBW infants. Among infants managed with this transfusion approach, maternal breast milk is the primary source of postnatal CMV infection.
Trial Registration
clinicaltrials.gov Identifier: NCT00907686
Introduction
Transfusion-transmitted cytomegalovirus (TT-CMV) and breast milk-transmitted CMV (BM-CMV) infections can cause serious morbidity and mortality in immunologically immature, very low birth weight (VLBW) infants (birthweight ≤1500 grams). Transfusion of CMV-seronegative and/or leukoreduced blood components are common strategies to prevent TT-CMV; however, prior studies to validate these approaches were small and yielded imprecise estimates of TT-CMV risk.1–3 Many of these studies did not address factors associated with breakthrough cases of TT-CMV, including leukoreduction quality control (linked to white blood cell (WBC) filter failures and CMV transmission) and donor window period infections (when immunologically-based assays may not detect CMV viremia).4 Additionally, studies of TT-CMV have not systematically evaluated BM-CMV, which may confound identification of the source of infection. The burden of BM-CMV in VLBW infants has not been well quantified.5 Other less common sources of CMV in this population are genital secretion from CMV-seropositive mothers and community-acquired transmission.6,7
We performed a multicenter prospective birth cohort study to quantify the risk of CMV infection from transfusion of CMV-seronegative and leukoreduced blood components. We also evaluated CMV transmission from maternal breast milk among breast milk-fed infants, and applied CMV nucleic acid testing (NAT) to transfused blood products and breast milk samples to determine the source in cases of postnatal CMV transmission.
Methods
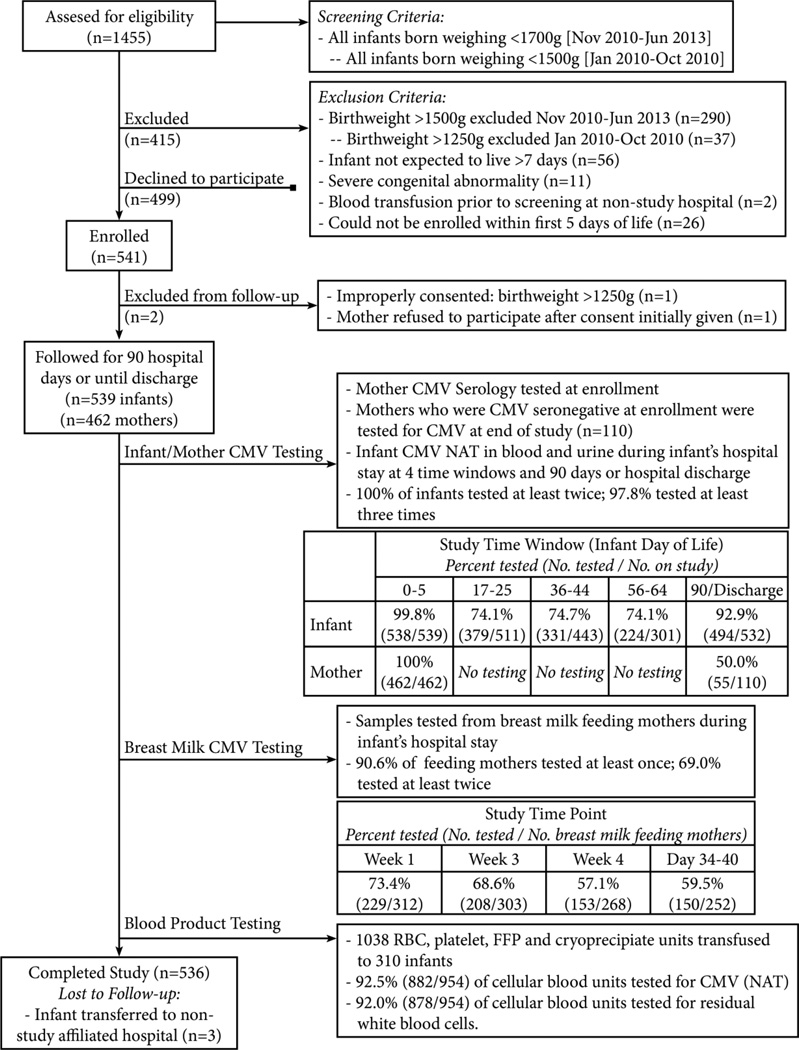
Infants born at three Atlanta-area hospitals (Emory University Hospital-Midtown, Grady Memorial Hospital and Northside Hospital) were screened (Figure 1). Infants meeting study criteria and whose parent or guardian gave written informed consent were enrolled, and followed from birth to 90 postnatal days, hospital discharge, or death. Infants transferred to Children's Healthcare of Atlanta Hospitals were followed at that hospital. The institutional review boards of all centers approved the study. Race and/or ethnicity, known to be associated with CMV infection, was determined by maternal report from options defined by federally funded study guidelines.8
Figure 1.
Study flow diagram and laboratory testing schematic.
*Weight inclusion criterion changed in November 2010 to increase recruitment.
CMV Surveillance in Mothers, Infants, Transfused Blood Products and Breast Milk
Maternal serum at study entry was tested with a CMV IgG/IgM assay. If serology was positive, the sample was re-tested by an IgM-specific assay. For seronegative mothers, CMV NAT was performed on maternal blood at study entry and conclusion to exclude infection during the study.
CMV infection was prospectively evaluated in all infants through CMV NAT of residual blood samples and urine. Congenital CMV infection was defined as positive CMV NAT (or positive viral culture obtained from clinician-ordered testing) in blood or urine within 2 weeks of life. Postnatally-acquired CMV infection was defined as positive CMV NAT or viral culture in blood or urine after 2 weeks of life with a previous negative result documented5. TT-CMV was defined as a positive CMV NAT test on blood products that were transfused to the infant combined with a positive blood or urine CMV NAT detected in the infant after transfusion. Blood was tested on the day of birth and at 4 time windows (+/− 4 days) around days of life 21, 40, 60, 90, and at discharge; urine was collected on day of birth and also at discharge, if blood was not available. In the event of clinical or laboratory suspicion of CMV infection, results from clinician-initiated CMV testing were included in study data. At least 2 times per week, screening for CMV disease, defined as pneumonitis, hepatitis, abnormal hematologic indices or fever in the setting of CMV infection, was performed by study personnel.
All transfused red blood cell (RBC) and apheresis platelet units were CMV seronegative, pre-storage leukoreduced, and irradiated; residual leukocyte quantitation and CMV NAT were performed on samples from these products (Figure 1). Breast milk samples were obtained from breast milk expressing mothers during weeks 1,3,4 and days 34–4010. If postnatal CMV infection was detected, CMV NAT was immediately performed on available milk samples. Otherwise, breast milk was stored and batch tested once an infant reached the study endpoint. Positive CMV tests were reviewed immediately by the study investigators and reported to the patient’s treating neonatologist who determined further evaluation and/or treatment.
Laboratory Methods
Presence of IgG/IgM polyspecific CMV antibodies in maternal blood were determined by an FDA approved commercial serology assay (Immucor, Norcross, GA). Serum samples were tested for CMV IgM by ELISA kit (Bio-Quant, San Diego, CA). Nucleic acid extraction for CMV NAT was performed using the EZ1 virus Mini kit version 2.0 (Qiagen, Inc, Valencia, CA) All assays were performed following manufacturer’s protocols. NAT was performed using the Artus CMV TM PCR kit (Qiagen, Inc, Valencia, CA) on the Qiagen Rotor Gene instrument.9 The PCR assay was validated on who blood, urine, and breast milk samples, calibrated to the 1st WHO International Standard.11 Newly diagnosed infants with viral load of > 300 IU/mL were verified by repeating the first extraction along with a new extraction. If there was insufficient specimen for repeat testing, it was diluted 1:2. Any specimen that tested positive with a viral load < 300 ml IU/mL was repeated in duplicate and reported as Low Positive < 300 IU/mL. Specimens discordant upon repeat testing were reported as Indeterminate. Prior to testing breast milk samples were stored at 4°C for up to 7 days and at −20°C for long term storage. Blood samples were stored at 4°C and tested within 7 days of collection.
To quantify residual WBCs in leukoreduced cellular blood products, a 100 µL volume of blood was added to 400 µL of propidium iodide/RNase reagent (LeukoCount; Becton-Dickinson, CA) and analyzed by flow cytometry.
Statistical Methods
Onset of CMV infection time was estimated as the midpoint between the last negative CMV NAT and the first positive NAT result in blood or urine. The incidence of first-time CMV infection and death was estimated by the cumulative incidence function.13 A competing risk analysis was done to estimate the cause-specific hazard ratio (CSHR) and the subdistribution hazard ratio (SHR) for CMV and mortality using a Cox regression model. The competing risk model for the CSHR was implemented with SAS PHReg (SAS Institute Inc., Cary, NC) using robust sandwich covariance estimates to account for within-mother correlation that may occur in outcomes of multiple-birth infants.12 Additional methods are contained in an online supplement.
Results
Baseline Characteristics
From January 2010 to June 2013, 539 VLBW infants born to 462 mothers were enrolled (Figure 1). Seventy-six percent (n=352) of mothers tested positive for the CMV IgG/IgM combination test and, of these mothers, 11 (3.1%) tested positive for CMV IgM antibody. Infants born to CMV seropositive or seronegative mothers did not differ in baseline characteristics except race and Apgar score (Table 1). The maternal groups did not differ except in receipt of prenatal care and isolated spontaneous labor as an indication for premature delivery.13 Eighty percent of mothers (371/462) fed breast milk to at least one of their infants and the median duration of breast feeding to those infants was 38 days (interquartile range[IQR] 19–56 days).
Table 1.
Baseline Characteristics.
| Infant | Overall | CMV Seronegative | CMV Seropositive | Pb |
|---|---|---|---|---|
| (n=539a) | (n=127) | (n=412) | ||
| Gestational age – weeks | 27.8±2.6 | 28.1±2.3 | 27.7±2.7 | 0.14 |
| Birth weight – grams | 1011±273 | 1032±264 | 1004±276 | 0.33 |
| Male gender | 263 (48.8) | 65 (51.2) | 198 (48.1) | 0.54 |
| Hispanic ethnicity | 44 (8.2) | 6 (4.7) | 38 (9.2) | 0.11 |
| Race | ||||
| Black | 313 (58.1) | 47 (37.0) | 266 (64.6) | <0.001 |
| White | 179 (33.2) | 69 (54.3) | 110 (26.7) | |
| Asian | 23 (4.3) | 4 (3.1) | 19 (4.6) | |
| More than one race | 20 (3.7) | 6 (4.7) | 14 (3.4) | |
| Otherc | 4 (0.7) | 1 (0.8) | 3 (0.7) | |
| Singleton births | 366 (68.0) | 89 (70.1) | 277 (67.2) | 0.55 |
| Small for gestational aged | 91 (16.9) | 26 (29.2) | 65 (23.5) | 0.28 |
| Outborn | 7 (1.3) | 2 (1.6) | 5 (1.2) | 0.75 |
| 1 Minute Apgar scoree.f | 5 (3, 7) | 6 (4, 8) | 5 (2, 7) | 0.005 |
| 5 Minute Apgar scoree.f | 8 (7, 9) | 9 (8, 9) | 8 (6, 9) | <0.001 |
| Score for Neonatal Acute Physiologyf | 11 (7, 14) | 12 (5, 14) | 11 (7, 14) | 0.60 |
| Time on study – daysf | 64 (46, 90) | 71 (50, 90) | 63 (46, 90) | 0.17 |
| Mother | n=462† | n=110 | n=352 | |
| Age – years | 29.4±6.5 | 29.3±5.8 | 29.4±6.7 | 0.97 |
| At least one prenatal visit | 429 (92.9) | 108 (98.2) | 321 (91.2) | 0.01 |
| Premature rupture of membranes | 173 (37.4) | 41 (37.3) | 132 (37.6) | 0.95 |
| Rupture of membranes (greater than 18 hours) | 99 (21.4) | 27 (24.5) | 72 (20.5) | 0.20 |
| Chorioamnionitis | 68 (14.7) | 10 (9.1) | 58 (16.5) | 0.06 |
| Caesarean delivery | 349 (75.5) | 88 (80.0) | 261 (74.1) | 0.21 |
| Receipt of antenatal steroids | 382 (82.7) | 92 (83.6) | 290 (82.4) | 0.76 |
| Indications for premature delivery | ||||
| Isolated spontaneous labor | 138 (29.8) | 24 (21.8) | 114 (32.4) | 0.03 |
| Premature rupture of membranes (<37 weeks) | 128 (27.7) | 34 (30.9) | 94 (26.7) | 0.39 |
| Pregnancy-associated hypertension | 109 (23.6) | 29 (26.4) | 80 (22.7) | 0.43 |
| Fetal distress / Poor biophysical profile | 67 (14.5) | 14 (12.7) | 53 (15.1) | 0.54 |
| Bleeding complication | 44 (9.5) | 10 (9.1) | 34 (9.7) | 0.86 |
Unless otherwise noted, continuous variables are reported as mean±SD and categorical variables are reported as no. (%).
Data are not included for two infant/mother pairs who were excluded from follow-up.
Groups are compared with a two-sample t-test for continuous variables and a Chi-square test for categorical variables.
Other race category includes American Indian and Alaska Native, Native Hawaiian or Other Pacific Islander, or other unidentified race.
Based on previously reported weight percentiles for small for gestational age15.
1 minute APGAR score is missing for four infants. 5 minute APGAR score is missing for the two infants.
Variable reported as median (interquartile range)
CMV Infection and Disease
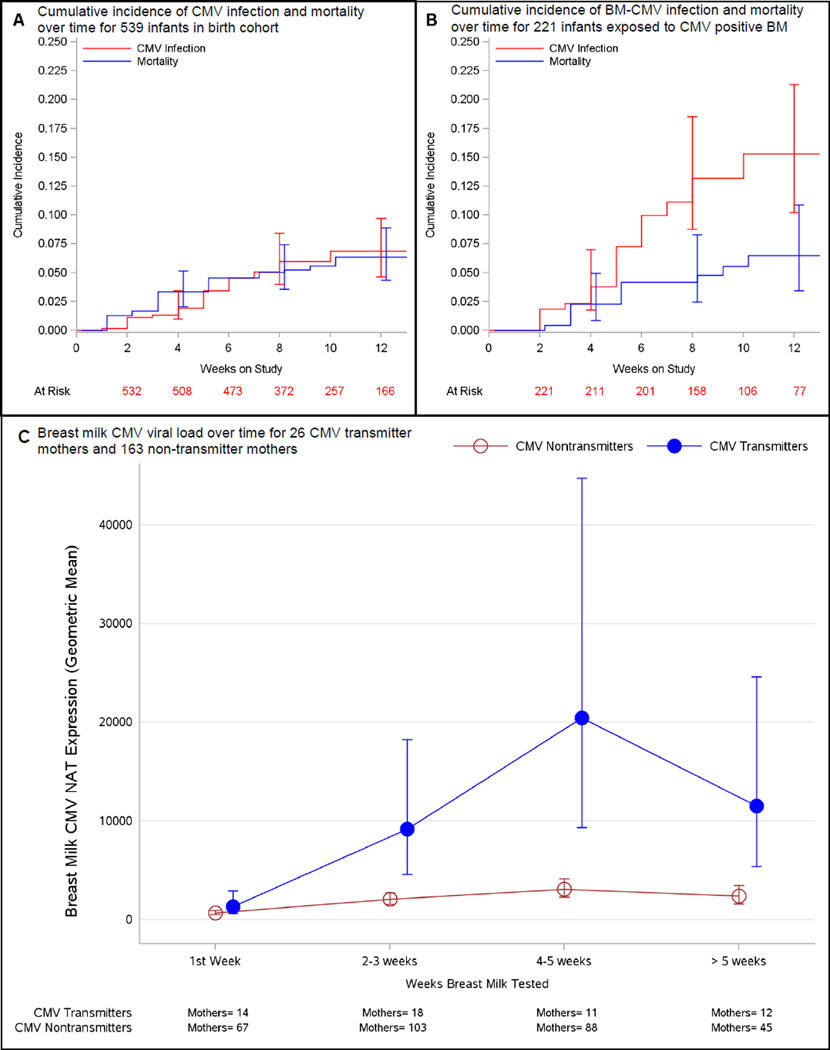
CMV infection was detected in 5.4% of the cohort (29 infants) (Table 2). The cumulative incidence of postnatal CMV infection at 12 weeks was 6.9% (95% CI: 4.2%–9.2%). Five of 29 (17.2%) CMV infected infants progressed to develop CMV disease and/or death (Figures 2a and 2b). All 29 infants with CMV infection had blood or urine CMV NAT within the first 5 days of life. 27 infants (93%) had CMV NAT in blood and 25 infants (86%) had CMV NAT in urine. One infant had positive blood and urine results, consistent with congenital infection, with all remaining infants testing CMV NAT negative on initial testing.
Table 2.
Study Outcomes
| Infants | Incidencea | 95% CI | |
|---|---|---|---|
| CMV infection | 5.4 (29/539) | 3.8 – 7.6 | |
| Cumulative incidence function | -- 4 weeks | 2.0 | 0.8 – 3.3 |
| -- 8 weeks | 6.0 | 3.7 – 8.2 | |
| -- 12 weeks | 6.9 | 4.2 – 9.2 | |
| - CMV infection in infants born to CMV seronegative mothers | 0.0 (0/127) | 0.0 – 2.1 | |
| - CMV infection in infants born to CMV seropositive mothers | 7.0 (29/412) | 4.9 – 9.9 | |
| Cumulative incidence function | -- 4 weeks | 2.6 | 1.0 – 4.3 |
| -- 8 weeks | 7.9 | 4.9 – 10.5 | |
| -- 12 weeks | 9.1 | 5.6 – 12.3 | |
| - CMV disease | 0.4 (2/539) | 0.1 – 1.3 | |
| - CMV infection-related mortality | 0.6 (3/539) | 0.2 – 1.6 | |
| All cause mortality | 5.6 (30/539) | 3.9 – 7.8 | |
| Source of CMV Infection: | |||
| - Breast milk transmittedb | 12.2 (27/221) | 8.5 – 17.1 | |
| Cumulative incidence function | -- 4 weeks | 3.8 | 1.4 – 4.5 |
| -- 8 weeks | 13.2 | 8.4 – 17.6 | |
| -- 12 weeks | 15.3 | 9.3 – 20.2 | |
| - Transfusion transmittedc | 0.0 (0/310) | 0.0 – 0.9 | |
| - Vertical transmission | 0.2 (1/539) | 0.0 – 1.0 | |
| - Unknown source | 0.2 (1/539) | 0.0 – 1.0 | |
| Breast milk | |||
| CMV DNA lactia in CMV-seropositive mothersd | 74.1 (189/255) | 69.7 – 80.3 | |
| CMV DNA lactia in CMV-seronegative motherse | 0.0 (0/81) | 0.0 – 4.5 | |
| Blood products | |||
| TT-CMV rate from CMV seronegative, leukoreduced cellular blood componentsf | 0.0 (0/880) | 0.0 – 0.3 | |
| Leukoreduction failure rateg | 0.11 (1/878) | 0.02 – 0.6 | |
Abbreviations: CMV, cytomegalovirus; CI, confidence interval; TT-CMV, transfusion-transmitted CMV; BM-CMV, breast-milk transmitted CMV.
Incidence is reported as relative frequency with number of cases over total in parentheses. Confidence intervals are calculated using the Wilson score method and are two-sided except in cases where incidence is zero wherein a one-sided upper limit confidence bound is given9. Cumulative incidence of CMV infection at given time points estimated from the CMV cumulative incidence function. Confidence intervals estimated using bootstrapping by mother as the clustering unit (1000 bootstrap samples).
BM-CMV reported in 221 infants who fed breast milk from 189 mothers (32 infants from multiple births) whose milk was CMV positive by NAT.
TT-CMV reported in transfused infants.
282 of 352 CMV-seropositive mothers fed breast milk to their infants (80.1%). Breast milk samples were obtained for testing from 255 feeding mothers (90.4%).
89 of 110 CMV-seronegative mothers fed breast milk to their infants (80.9%). Breast milk samples were obtained for testing from 81 feeding mothers (91.0%).
882 of 954 (92.4%) cellular blood components were tested with CMV NAT. 880 units were negative and 2 tests were indeterminate.
878 of 954 (92.0%) cellular blood components were tested for residual white blood cells.
Figure 2.
In Panels A and B, the cumulative incidnce of CMV infection and mortality are given over the study period, including 95% confidence intervals at weeks 4, 8, and 12 (vertical bars). In Panel C, Log 10 CMV viral load values were analyzed with a repeated measures model; mean estimates and their 95% confidence intervals were back transformed to the original scale and are reported as the geometric mean with 95% confidence intervals.
The percentage of longitudinal blood and urine samples from 539 VLBW infants with detectable CMV increased from 0.5% at 1–3 weeks to 3.2% at 4–6 weeks. By 10–12 weeks, 9.1% (95% CI: 4.9% – 16.8%) of the samples had detectable virus (eFigure 1). Of 29 infants with CMV infection, virus was detected in blood for 26 and in urine for 16 infants (eFigure 1). The geometric mean viral load detected in the infant was estimated to be 2,887 IU/ml (95% CI: 1,462–5,703) in blood and 133,783 IU/ml (95% CI: 23,922–748,170) in urine, using mixed linear models to account for multiple tests from each infant. Of the 27 mothers with infants who developed postnatal CMV infection, only 2 (7.4%) had a positive CMV IgM test. Further, of the 11 mothers who tested positive for IgM antibody, only 18.2% (2/11) had an infant with CMV infection.
Five of 29 infants with CMV infection had abnormal laboratory values at the time of initial detection of CMV (see eResults for details). Among the 24 infants determined to have asymptomatic CMV infection, including one infant with congenital infection, no laboratory abnormalities associated with CMV were detected up to 10 days prior to diagnosis of CMV infection (see eResults for details). Furthermore, no clinical suspicion of disease occurred for these 24 infants, and no further investigation or anti-viral treatment was pursued. However, five CMV-infected infants developed disease or died. Infants with CMV disease or associated mortality had similar viral loads to those infants with asymptomatic CMV infection. One infant died of pneumonia following necrotizing enterocolitis (NEC). This infant had a maximum CMV viral load of 13,000 IU/mL. Two other infants died of NEC with viral loads at death of 8,000 and 4,000 IU/mL. The two surviving infants that developed CMV disease, one with punctate densities in the basal ganglia consistent with early signs of CMV infection and the other with a sepsis-like syndrome, were the only infants treated with ganciclovir and valganciclovir. Both patients had clinical improvement with treatment. All infants with CMV disease or associated mortality received only frozen/thawed breast milk and had negative initial CMV testing in the first two weeks of life.
CMV and Blood Transfusions
Fifty-eight percent of the cohort (310 infants) received one or more transfusions. A total of 2,061 transfusions were administered from 1,038 cellular blood components during the study (1,545 RBC transfusions from 703 units; 379 platelet transfusions from 251 units). The overall TT-CMV incidence for infants was 0.0% (0/310; 95% CI: 0.0%–0.9%) and similarly the TT-CMV incidence from CMV seronegative and leukoreduced cellular blood components was 0.0% (0/880; 95% CI: 0.0%–0.3%; Table 2). One platelet unit had a leukoreduction failure (5.2 × 106 residual leukocytes), for an overall failure incidence of 0.11% (95% CI: 0.02%–0.6%). All blood components were CMV NAT-negative. The unit that failed leukoreduction was not associated with CMV transmission.
CMV and Maternal Breast Milk Feeding
All 28 infants with postnatal CMV infection were fed maternal breast milk from CMV-seropositive mothers. Twenty-seven of these infants (96%) received CMV NAT-positive maternal breast milk prior to BM-CMV from 26 mothers (one set of twins). The time from first detection of CMV in maternal breast milk to first detection of postnatal CMV infection in the infant was 36 ± 22 days (mean ± standard deviation). The source of CMV infection for the 28th infant, born to a CMV seropositive mother, could not be identified, This infant’s CMV infection was detected by NAT on day of life 25, prior to any blood transfusion and after receipt of CMV NAT negative maternal breast milk (tested in week 1). The 12 week incidence of CMV infection among infants fed CMV-positive breast milk was 15.3% (95% CI: 9.3–20.2%; N=221; Table 2).
Overall, 74.1% (95% CI: 69.7%–80.3%) of CMV-seropositive mothers had CMV DNA lactia in their expressed breast milk, as compared to 0% (95% CI: 0.0%–4.5%) of CMV-seronegative mothers (Table 2). Once CMV was initially detected in breast milk, all subsequent breast milk samples contained CMV DNA. Of 189 mothers with CMV-positive breast milk, 26 (13.7%) were CMV transmitters and 163 (86.2%) were CMV non-transmitters. Mean breast milk CMV NAT viral loads were similar for transmitting and non-transmitting mothers at week 1 (1306 versus 664 IU/mL; p=0.13) but became significantly higher in CMV transmitting mothers during post-partum weeks 2–3 (9,129 IU/mL versus 2,033 IU/mL; p<0.001) and in weeks 4–5 (20,421 IU/mL versus 3,064 IU/mL; p<0.001) (Figure 2c).
The majority of breast milk-fed infants received exclusively frozen/thawed milk (78.2%). The CMV transmission rate from breast milk for 221 infants fed CMV positive breast milk did not differ between infants who were fed some fresh breast milk versus those fed exclusively frozen/thawed milk (12-week CMV incidence: 17.6% versus 11.6%; hazard ratio: 0.55; 95% CI: 0.19–1.56; p=0.26).
Risk factors for CMV infection
Factors that increased the risk for postnatal CMV infection included a higher number of breast milk feeding days, higher breast milk CMV viral load, and premature rupture of membranes (PROM) (Table 3). The adjusted hazard of CMV infection increased as the breast milk CMV viral load increased and the hazard was over 3 times higher for infants born to mothers with PROM prior to delivery compared to infants born to mothers with other indications for preterm delivery (Table 4). PROM was also associated with an increase in the cumulative incidence of CMV infection (subdistribution hazard rate, SHR=3.07; 95% CI: 1.31–7.18; p=0.01).
Table 3.
Risk factors for CMV infection and mortality using univariable Cox regression model
| CMV | Mortality | ||||||
| CMV Risk Factor | N | CSHR | 95% CI | P | CSHR | 95% CI | P |
| Birth Weight (per 100g increase) | 539 | 0.97 | 0.85–1.11 | 0.62 | 0.58 | 0.48–0.69 | <0.0001 |
| Leukopenia at birth (WBC <5000/µl) | 539 | 1.54 | 0.62–3.84 | 0.35 | 1.34 | 0.50–3.53 | 0.56 |
| Late onset sepsis (>7 days)a | 539 | 0.58 | 0.15–2.21 | 0.42 | 2.57 | 0.79–8.36 | 0.12 |
| Breast milk feeding daysa (per 7 day increase) | 539 | 1.68 | 1.21–2.34 | 0.002 | 0.64 | 0.41–0.99 | 0.05 |
| Isolated spontaneous labor | 539 | 0.53 | 0.22–1.29 | 0.16 | 1.28 | 0.58–2.80 | 0.54 |
| Chorioamnionitis (clinical or histologic diagnosis) | 539 | 0.90 | 0.31–2.62 | 0.84 | 1.71 | 0.69–4.20 | 0.25 |
| Premature rupture of membranes | 538 | 3.14 | 1.46–6.73 | 0.003 | 1.30 | 0.61–2.78 | 0.50 |
| Rupture of membranes (greater than 18 hours) | 539 | 1.83 | 0.86–3.90 | 0.12 | 0.81 | 0.31–2.12 | 0.66 |
| Log10 CMV NAT expression in breast milka (per 1 log10 IU increase) | 505b | 2.71 | 2.20–3.35 | <0.0001 | 1.17 | 0.88–1.56 | 0.30 |
| CMV | Mortality | ||||||
| Additional Mortality Risk Factors | N | CSHR | 95% CI | P | CSHR | 95% CI | P |
| Female gender | 539 | 0.50 | 0.23–1.07 | 0.07 | 1.05 | 0.49–2.21 | 0.91 |
| SNAP (per 1 unit increase) | 539 | 1.02 | 0.95–1.09 | 0.58 | 1.15 | 1.07–1.23 | 0.0001 |
| Receipt of antenatal steroids | 539 | 0.96 | 0.37–2.46 | 0.93 | 0.47 | 0.21–1.08 | 0.07 |
Abbreviations: CMV, Cytomegalovirus; CSHR, cause-specific hazard ratio; CI, confidence interval; WBC, white blood cell count; NAT, nucleic acid testing; SNAP, score for neonatal acute physiology.
Competing risks: 29 infants with CMV infection and 30 deaths total; 3 infants with CMV infection died. 27 deaths used to estimate CSHR for mortality.
Time dependent covariate.
4 infants who fed breast milk from 31 mothers whose breast milk was not tested are not included. N=505 (431 mothers) with 28 infected infants (one CMV source unknown).
Table 4.
Risk factors for CMV infection and mortality using multivariable Cox regression model
| CMV | Mortality | |||||
|---|---|---|---|---|---|---|
| Risk Factor | CSHR | 95% CI | P (Reliabilityb) | CSHR | 95% CI | P (Reliabilityb) |
| Birth Weight (per 100g increase) | - | - | - | 0.58 | 0.47–0.71 | <0.0001 (100) |
| Breast milk feeding daysa (per 7 day increase) | - | - | - | 0.65 | 0.43–1.00 | 0.05 (54) |
| Premature rupture of membranes | 3.21 | 1.43–7.23 | 0.005 (83) | - | - | - |
| Log10 CMV NAT expression in breast milka (per 1 log10 IU increase) | 1.87 | 1.48–2.35 | <0.0001 (100) | - | - | - |
Abbreviations: CMV, Cytomegalovirus; CSHR, cause-specific hazard ratio; CI, confidence interval; NAT, nucleic acid testing.
Competing risks: 29 infants with CMV infection and 30 deaths total; 3 infants with CMV infection died. 27 deaths used to estimate CSHR for mortality.
Time dependent covariate.
Furthermore, PROM was an independent predictor of mother-to-infant CMV transmission among 189 CMV seropositive mothers, whereas mode of delivery was not associated with mother-to-infant transmission (eTable 1). In addition, maximum log10 CMV expression in breast milk was associated with maternal-infant CMV transmission among CMV seropositive mothers although the accuracy of CMV viral expression in breast milk to identify postnatal CMV infection was poor, as reflected by the receiver operating characteristic curve (eFigure 2 and eMethods).
Discussion
Our prospective, multi-center birth cohort study is the largest reported study to evaluate both blood transfusion and breast milk sources of postnatal CMV infection in VLBW infants. Prior to our study, the residual risks of TT-CMV with CMV-seronegative or leukoreduced transfusions were estimated at 1–3%.2,3,4,14 Furthermore, the efficacy of combining both approaches had not been rigorously examined.15 The current results demonstrate that the exclusive use of blood components that are both CMV-seronegative and leukoreduced is effective in preventing TT-CMV. We believe this approach should be adapted as a standard of care when transfusing VLBW infants until the comparative effectiveness of alternative transfusion strategies to prevent TT-CMV can be evaluated.
Historically, failure to prevent TT-CMV with CMV-seronegative units was attributed to donors in the window phase of an infection16, whereas leukoreduced units were believed to transmit CMV if the leukoreduction filters failed.17,18 In our study, only one unit had a filter failure and no donor window phase infections were identified. Thus, recent advances in serology and leukoreduction methods may account for the effectiveness of the combined approach to prevent TT-CMV.
Currently, The American Academy of Pediatrics states that, “The value of routinely feeding [fresh] human milk from [CMV] seropositive mothers to preterm infants outweighs the risks of clinical disease, especially because no long-term neurodevelopmental abnormalities have been reported”.19 Given the known benefits of breast feeding, new strategies to prevent BM-CMV are needed, as freezing/thawing breast milk did not completely prevent transmission in this study. Alternative approaches may include routine CMV-serology of pregnant mothers to enable counselling regarding risk of CMV infection and risk stratification of infants20. For breastfeeding VLBW infants born to seropositive mothers, pasteurization of breast milk until a corrected gestational age of 34 weeks as is recommended by the Austrian Society of Pediatrics21,22 and routine screening for postnatal CMV infection may be warranted. Given the toxicity of antiviral therapy,23 further research is needed to determine if antiviral treatment in infants with asymptomatic CMV infection is beneficial, especially as it is unclear which infants will progress to CMV disease. Although we found an association between CMV DNA levels in breast milk and BM-CMV, we could not identify a viral load cut-off below which BM-CMV did not occur. Thus, any level of CMV DNA in breast milk should be considered potentially infectious until more detailed investigations can be performed. We also found that PROM and the amount of CMV virus in breast milk were each independently associated with an increased risk of postnatal CMV infection. The role of PROM in postnatal CMV infection is unclear. Two studies have reported that intrauterine CMV infection is not associated with PROM.24,25 Further, vaginal delivery was not associated with postnatal infection in our study, making intrapartum acquisition an unlikely source of postnatal CMV infection.
Our study has several limitations. We did not compare the relative risk of TT-CMV between CMV-seronegative and leukoreduced units and blood components that were only leukoreduced from CMV untested donors. Therefore, we could not determine the relative safety of the latter approach in VLBW infants. Further, we were unable to test all breast milk for enrolled infants as samples were not available or mothers were not breastfeeding during the period of evaluation. Also, we were unable to ascertain with certainty if CMV infection caused NEC or was simply a co-occurrence in the three infants with CMV infection who died, although CMV infection is a reported cause of NEC26, We were also unable to test genital tract secretions at delivery to identify this potential source of CMV infection due the complexity of our enrollment at sites involving numerous different obstetrical practices. However, most infants in this study were delivered by cesarean and we did not detect an association between mode of delivery and mother-to-infant transmission of CMV infection. Further, all urine and blood CMV NAT testing of infants with the exception of 1 infant with congenital CMV were negative in the first 2 weeks of life. This makes the possibility that we misclassified infants with congenital CMV infection as having postnatal CMV infection unlikely. Finally, we did not perform systematic hearing assessments or long-term neurodevelopmental assessments. The impact of asymptomatic postnatal CMV infection on long-term neurodevelopmental outcomes is unclear, with some studies demonstrating an increased risk of adverse neurologic outcomes27 and others revealing no difference in long-term outcomes28 or suspected sensorineural hearing loss.28,29 The frequency of CMV infection in our cohort raises significant concern regarding the potential burden of CMV infection among VLBW infants and potential sequelae necessitating large, long-term follow-up studies of neurodevelopmental outcomes in infants with postnatal CMV infection.
Supplementary Material
ACKNOWLEDGEMENTS
Except Dr. Caliendo whose laboratory used Qiagen products during the study that were supported, in part, by the company and Dr. Roback whose instrument from 3Ti, a company he has partial ownership in, was used to count residual white blood cells for the donor blood products
Funding/Support: Supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health to Emory University School of Medicine (P01 HL086773).
Role of the Sponsors: The NHLBI program officers provided advice on the study design, but had no input on: 1) the conduct of the study; 2) data collection, management, analysis or interpretation; 3) preparation, review or approval of the manuscript; and 4) decision to submit the manuscript for publication
Additional Contributions
We would like to thank all of the families who volunteered to participate in this study and the nurses, laboratory technologists, and physicians for their dedication, critical thinking, and kindness. The following individuals helped make this study successful: Deborah Abdul-Ali, Jessica Ingersoll, Doris Igwe, Jane Skavarich, Katrina H. Grier, Janna M. Benston, Shieghla Barclay, Natia Saakadze, Martha Sola-Visner.
Footnotes
Conflict of Interest Disclosures: All authors have no conflicts of interest relevant to this paper
Author contributions: Dr, Josephson, the principal investigator had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Josephson, Caliendo, Easley, Knezevic, Shenvi, Hinkes, Hillyer, Roback
Acquisition of data: Josephson, Caliendo, Easley, Knezevic, Shenvi, Hinkes, Patel, Roback
Analysis and interpretation of data: Josephson, Caliendo, Easley, Knezevic, Shenvi, Hinkes, Patel, Roback
Drafting of the manuscript: Josephson, Easley, Knezevic, Shenvi, Hinkes, Patel, Roback
Critical revision of the manuscript for important intellectual content: Josephson, Caliendo, Easley, Knezevic, Shenvi, Hinkes, Patel, Hillyer, Roback
Statistical analysis: Josephson, Caliendo, Easley, Knezevic, Shenvi, Patel, Roback
Obtained funding: Josephson, Hillyer, Roback
Administrative, technical, or material support: Josephson, Easley, Knezevic, Shenvi, Patel, Roback
Study supervision: Josephson, Caliendo, Easley, Knezevic, Shenvi, Hinkes, Hillyer, Roback
References
- 1.Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert GL, Hayes IL, Hudson IL, James J. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leukocytes.: Neonatal Cytomegalovirus Infection study group. Lancet. 1989;1(8649):1228–1231. doi: 10.1016/s0140-6736(89)92330-1. [DOI] [PubMed] [Google Scholar]
- 3.Eisenfeld L, Silver H, McLaughlin J, Klevjer-Anderson Prevention of transfusion-associated cytomegalovirus infection in neonatal patients by the removal of white cells from blood. Transfusion. 1992;32(3):205–209. doi: 10.1046/j.1537-2995.1992.32392213801.x. [DOI] [PubMed] [Google Scholar]
- 4.Josephson CD, Roback JR. New insights for preventing transfusion-transmitted cytomegalovirus and other white blood cell-associated viral infections. Transfusion. 2013;53(10):2112–2116. doi: 10.1111/trf.12366. [DOI] [PubMed] [Google Scholar]
- 5.Lanzieri TM, Dollard SC, Josephson CD, Schmid S, Bialek SR. Breast Milk-Acquired Cytomegalovirus Infection and Disease in VLBW and Premature Infants. Pediatrics. 2013;131(6):e1937. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA. Maternal Cytomegalovirus Excretion and Perinatal Infection. N Engl J Med. 1973;289(1):1–5. doi: 10.1056/NEJM197307052890101. [DOI] [PubMed] [Google Scholar]
- 7.Cunha BA. Cytomegalovirus pneumonia: community-acquired pneumonia in immunocompetent hosts. Infect Dis Clin North Am. 2010;24(1):147–158. doi: 10.1016/j.idc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC infectious diseases. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul-Ali D, Kraft CS, Ingersoll J, Frempong M, Caliendo AM. Cytomegalovirus DNA stability in EDTA anti-coagulated whole blood and plasma. J Clin Virol. 2011;52(3):222–224. doi: 10.1016/j.jcv.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephson CD, Castillejo MI, Caliendo AM, et al. Prevention of transfusion-transmitted Cytomegalovirus in low-birth weight infants (≤ 1500 g) using Cytomegalovirus-Seronegative and leukoreduced transfusions. Transfus Med Rev. 2011;25(2):125–132. doi: 10.1016/j.tmrv.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannonen L, Loginov R, Helantera I, et al. Comparison of two quantitative real-time CMV-PCR tests calibrated against the 1st WHO international standard for viral load. doi: 10.1002/jmv.23733. [DOI] [PubMed]
- 12.Lee EW, Wei LJ, Amato D. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. 4. Vol. 221. Netherlands: Kluwer Academic; 1992. pp. 237–247. [Google Scholar]
- 13.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 14.Bowden R, Slichter S, Sayers M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86(9):3598–3603. [PubMed] [Google Scholar]
- 15.Laupacis A, Brown J, Costello B, et al. Prevention of posttransfusion CMV in the era of universal WBC reduction: A consensus statement. Transfusion. 2001;41(4):560–569. doi: 10.1046/j.1537-2995.2001.41040560.x. [DOI] [PubMed] [Google Scholar]
- 16.Drew WL, Tegtmeier G, Alter HJ, Laycock ME, Miner RC, Busch MP. Frequency and duration of plasma CMV viremia in seroconverting blood donors and recipients. Transfusion. 2003;43(3):309–313. doi: 10.1046/j.1537-2995.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Roback JD. CMV and blood transfusions. Rev Med Virol. 2002;12(4):211–219. doi: 10.1002/rmv.353. [DOI] [PubMed] [Google Scholar]
- 18.Dumont LJ, Luka J, VandenBroeke T, Whitley P, Ambruso DR, Elfath MD. The effect of leukocyte-reduction method on the amount of human cytomegalovirus in blood products: A comparison of apheresis and filtration methods. Blood. 2001;97(11):3640–3647. doi: 10.1182/blood.v97.11.3640. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 20.Walker SP, Palma-Dias R, Wood EM, Shekleton P, Giles ML. Cytomegalovirus in pregnancy: to screen or not to screen. BMC Pregnancy Childbirth. 2013;18(13):96. doi: 10.1186/1471-2393-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier J, Lienichke U, Tschirsch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-child transmission in preterm infants. J Clin Micro. 2005;43(3):1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goelz R, Hihn E, Hamprecht K, Dietz K, Jahn G, Poets C, Elmlinger M. Effects of different CMV-heat-inactivation-methods on growth factors in human breast milk. Pediatr Res. 2009;65(4):458–461. doi: 10.1203/PDR.0b013e3181991f18. [DOI] [PubMed] [Google Scholar]
- 23.Marshall BC, Koch WC. Antivirals for cytomegalovirus infection in neonates and infants: focus on pharmacokinetics, formulations, dosing, and adverse events. Paediatr Drugs. 2009;11(5):309–321. doi: 10.2165/11316080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Bopegamage S, Kacerovsky M, Tambor V, Musilova I, Sarmirova S, Snelders E, de Jong AS, Vari SG, Melchers WJ, Galama JM. Preterm prelabor rupture of membranes (PPROM) is not associated with presence of viral genomes in the amniotic fluid. J Clin Virol. 2013;58(3):559–563. doi: 10.1016/j.jcv.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Naresh A, Simhan H. Absence of viruses in amniotic fluid of women with PPROM: a case series. J Reprod Immunol. 2012;96(1–2):79–83. doi: 10.1016/j.jri.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Tengsupakul S, Birge ND, Bendel CM, Reed RC, Bloom B, Hernandez N, Schleiss MR. Asymptomatic DNAemia Heralds CMV-Associated NEC: Case Report, Review, and Raionale for Preemption. Pediatrics. 2013;132(5):e1428. doi: 10.1542/peds.2013-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonat Ed. 2013;98(5):F430–F433. F1–F4. doi: 10.1136/archdischild-2012-303384. [DOI] [PubMed] [Google Scholar]
- 28.Bevot A, Hamprecht K, Krageloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167–e172. doi: 10.1111/j.1651-2227.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 29.Turner KM, Lee HC, Boppana SB, Carlo WA, Randolph DA. Incidence and Impact of CMV Infection in Very Low Birth Weight Infants. Pediatrics. doi: 10.1542/peds.2013-2217. [Published online Feb 2, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.