Highlights
-
•
We described the first case of a primary ectopic fronto-temporal craniopharyngioma.
-
•
A primary ectopic craniopharyngioma is a tumor that arises for the first time without previous surgery in any compartment with no midline involvement from remnants of the Rathke’s pouch.
-
•
Keyhole endoscopic approach gives a better screen image of neurovascular elements, better illumination and less brain retraction.
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; KPS, karnofsky performance status scale; MRI, magnetic resonance imaging
Keywords: Ectopic, Craniopharyngioma, Endoscopic, Keyhole
Abstract
Introduction
Primary ectopic craniopharyngiomas have only rarely been reported. Craniopharyngiomas involve usually the sellar and suprasellar region, but can be originated from cell remnants of the obliterated craniopharyngeal duct or metaplastic change of andenohypophyseal cells. We present the first case of a primary ectopic frontotemporal craniopharyngioma.
Presentation of case
A 35-year old woman presented with a one-year history of headache and diplopia. MRI showed a large frontotemporal cystic lesion. Tumor resection was performed with a keyhole endoscopic frontal lateral approach. The pathological features showed an adamantinomatous craniopharyngioma with a cholesterol granuloma reaction.
Discussion
There have been reported different localizations for primary ectopic craniopharyngioma. Our case presented a lobulated frontotemporal cystic mass formed by a dense eosinophilic proteinaceous material dystrophic calcifications and cholesterol crystals, with epithelial remnants. No tumor regrowth was observed in the magnetic resonance image 27 months postoperatively.
Conclusion
Primary ectopic craniopharyngioma is a rare entity with a pathogenesis that remains uncertain. This is an unusual anatomic location associated with unique clinical findings.
1. Introduction
Craniopharyngioma is an epithelial tumor that arise from the remnants of Rathkés pouch, they often involve the sellar/suprasellar region; but primary ectopic [1–10] localizations had been reported since 1924 when Bock described an isolated infracranial tumor [11]. They arise from epithelial remnants anywhere along the obliterated craniopharyngeal tract from Rathkés cleft to the floor of the third ventricle. Two common patterns of ectopia have been described: contamination with tumor cells along the surgical tract and spreading via CSF and the subarachnoid space [12]. Retrostalk growth may extend to the posterior fossa and mismigrated cleft cells may originate tumor growth in other compartments [4].
2. Case report
2.1. Clinical findings and evaluation
A 35-year old woman presented with a one-year history of intermittent bilateral frontal headache of moderate severity, worsened with bending and improved with NSAID́s. Fifteen days before admission the severity increased associated with horizontal binocular diplopia and transient metamorphopsias. On examination she had papilledema, no visual field defects and left lateral rectus paresis. The pituitary hormonal test was normal.
MRI revealed a large frontotemporal cystic lesion with a rostral small solid nodule, isointense in T1, hypointense in T2, with heterogeneous contrast enhancement of the solid nodule (Fig. 1A and B). CT showed a frontotemporal cystic lesion with an irregular heterogenous calcified rostral nodule. The sellar and suprasellar region appeared intact without invasion.
Fig. 1.
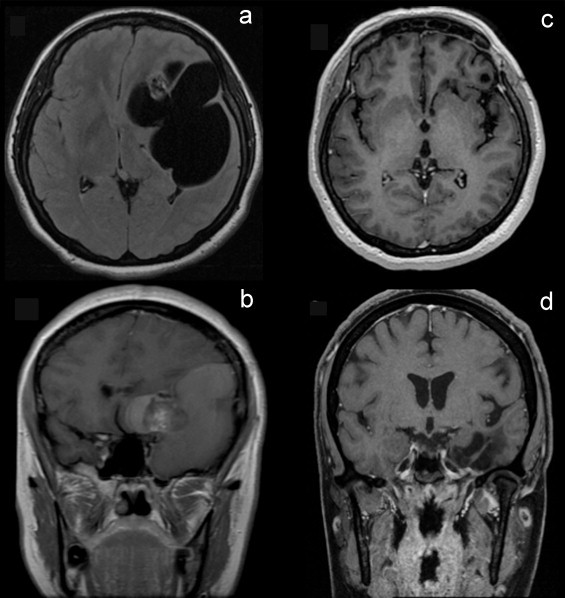
Preoperative MRI (A and B) notice that midline structures and sellar compartment is not involved. Postoperative MRI (C and D) after 27 months revealed no residual tumor.
2.2. Surgical technique
The patient was positioned supine with the head tilted to the contralateral side. We started with a 4 cm frontal curvilinear skin incision. The temporal muscle was transected and the fibers were retracted. A keyhole craniotomy of 3 × 2.5 cm was performed and the dura was opened in a curved fashion. A 7 mm 0° Karl Storz endoscope was introduced into the cystic cavity. A brownish fluid was drained and the natural cystic cavity was used for endoscopic navigation. Tumor resection was performed with endoscopic visualization with two-handed microsurgical technique with microsurgical dissectors, microscissors, aspirator and bipolar coagulation (see video).
2.3. Postoperative course
Left rectus paresis improved immediately after surgery. No diabetes insipidus, electrolyte imbalances or hormone deficiencies were documented during her hospital stay. KPS was 100 after surgery and during the follow up. MRI 27 months after surgery was negative to any residual tumor (Fig. 1C and D).
2.4. Pathology
The gross aspect of the tumor resected was a lobulated mass with spongy consistency and a cystic component of brownish thick liquid. Histologically we found a stratified epithelium remnant (Fig. 2D), immersed in dense, necrotic, eosinophilic and proteinaceous material (Fig. 2B), which formed cleft cholesterol (Fig. 2A) and dystrophic calcifications (Fig. 2C). No stellate reticulum was observed. Histopathology was suggestive of adamantinomatous variety of craniopharyngioma with a cholesterol granuloma reaction.
Fig. 2.
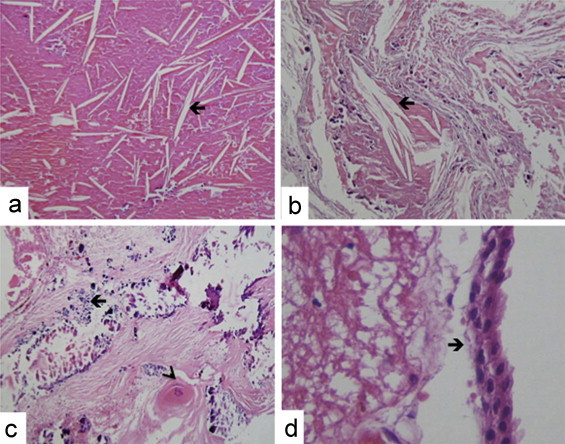
Histopathologic features of the tumor: (A and B) Most of the tumor was formed by cholesterol granuloma (arrow) (H&E, 200×). (C) Multiple calcifications (arrow), necrosis and wet keratin (arrowhead) (H&E, 200×). (D) Cuboidal epithelial cells remnants (arrow) (H&E, 400×).
3. Discussion
3.1. Pathogenesis
The first tumor description of a craniopharyngioma was published by Mott and Barratt [13], in which they proposed that craniopharyngiomas might arise from the hypophyseal duct. In 1904, the pathologist Jakob Erdheim [14] was surprised to find small clusters of squamous epithelium above the cyst, at the junction of the infundibulum and pituitary. He proposed that craniopharyngioma originated from the embryonic squamous cell remnants of the obliterated craniopharyngeal duct. This theory explains the embryological reason for most of the adult craniopharyngiomas including the extracranial nasopharyngeal craniopharyngioma, unless they lacked of dural covering, intracranial extension or deformation on the sella; supporting the theory of ectopic infrasellar tissue [7]. The anterior, posterior and lateral extension has been reported extensively, but an ectopic craniopharyngioma does not have an intrasellar or suprasellar involvement. A primary ectopic craniopharyngioma is a tumor that arises for the first time without any previous surgery in any compartment with no midline involvement from remnants of the Rathke’s pouch within the vestigial craniopharyngeal duct. Secondary ectopic craniopharyngioma is an ectopic recurrence of a previously operated tumor, that arises from tumor cell spillage along the resection corridor or via the cerebrospinal fluid, which is a rare but more common phenomenon [12].
Craniopharyngiomas are rare epithelial tumors that account for approximately 1–4% of all primary intracranial neoplasms in adults [8,15]. In our hospital The National Institute of Neurology and Neurosurgery, 153 adult patients underwent transcranial and transsphenoidal surgery in a period of 25 years [16], our neuropathology department reported that 86% were adamantinomatous and no primary ectopic case had been documented before [17].
There have been reported different localizations for primary ectopic craniopharyngioma including the cerebellopontine angle [5,6], extracranial infrasellar without dural covering,[7] petroclival [3], ethmoid sinus [10], third ventricle [9], pineal gland [8] and temporal lobe [1]. To our knowledge, this is the first case of a primary ectopic frontotemporal craniopharyngioma. Two hypotheses have been proposed for the development of a craniopharyngioma. The first proposed by Erdheim, in which they may arise from any location along the craniopharyngeal duct [7]. The second supports the squamous cell nests may derive from metaplastic change of andenohypophyseal cells. The close morphologic similarity between craniopharyngioma and ameloblastoma of the jaw may also point to a common cellular origin such as embryonic misplacement of the stomodeal cells [18]. For secondary ectopic craniopharyngiomas the literature suggests that they can seed the surgical corridor or there may be originated from spillage of tumor cells into the subarachnoid space including either the cisterns or the Virchow–Robin spaces [12]. For a primary ectopic craniopharyngioma there is no hypothetical consensus, we believed that is a multifactorial process that starts with an embryological error from mismigrated cells in addition to mutations of oncogenes and tumor suppressor genes in which different endocrinological, growth and vascular factors produce an ideal environment.
Our case presented a lobulated cystic mass formed by a dense eosinophilic proteinaceous material dystrophic calcifications and cholesterol crystals, with epithelial remnants. Xanthogranulomatous change of craniopharyngioma, consisting of cholesterol clefts, macrophages, chronic inflammatory infiltrates, necrotic debris and hemosiderin deposits, has been traditionally considered a hallmark of the adamantinomatous variant, even in the absence of epithelium [19]. We believed that the patient did a cholesterol granuloma reaction, depending on the characteristic histological feature, cholesterol granuloma reaction was classified into 3 groups according Shirataki et al, [20] each of which also represented a step of process of organization. Group I (cholesterol clefts predominates in hemorrhagic and necrotic foci), group II (foreign body giant cells, macrophages, round cell infiltrations are present other than cholesterol clefts, signifying active organization) and group III (numerous cholesterol clefts predominates in fibrous scar tissue). Our tumor had a cholesterol granuloma reaction Grade III and only remnants of the epithelium were observed.
3.2. Endoscopic approach.
Although endoscopic techniques have been used since the beginning of the last century it is not always the preferred technique for craniopharyngioma depending on the origin of the tumor in relation to the diaphragm sella and the floor of the third ventricle. In our case the tumor was not in relation with the midline so the hypothalamus and pituitary were not at risk. A pterional approach would be reasonable for our frontotemporal cystic lesion, but we rather preferred a keyhole frontolateral approach. We thought about the same principle that is used for middle fossa arachnoid cysts in which the natural cavity allows better visualization and better angles for adequate dissection. After draining the giant cyst we introduced the endoscope and used a two-handed microsurgical technique with a better screen image of neurovascular elements, better illumination and less brain retraction. Endoscope intradural dissection is controversial because carries risks of neurovascular injury proximal to the endoscopic tip, the manipulation of the instruments through the corridor has to be made with precaution and direct vision. This is a unique cystic tumor that was proper for endoscopic resection. There is oncological control after two years without adjuvant radiation therapy and no endocrinological or neurological deficit.
4. Conclusion
Primary ectopic craniopharyngioma is a rare entity with a pathogenesis that remains uncertain. The primary treatment of craniopharyngioma remains surgical and the approach depends on the origin of the tumor. Pure endoscopic resection of the solid tumor was achieved in this case because the cavity allowed us proper navigation and safe dissection.
Conflicts of interest
The content of this manuscript, in part or in full, has not been published elsewhere in any form.
The authors contributed actively and originally for the conception, design and analyses of the manuscript under ethical adherence.
The authors have no personal, financial or institutional interest in any of the materials and devices described in this article. No conflict of interest and no financial disclosure.
Funding
None
Author contribution
Conception and design: Ortega-Porcayo, Gómez-Amador.
Acquisition of data: Ortega-Porcayo, Ponce-Gómez, Martínez-Moreno.
Analysis and interpretation of data: Ortega-Porcayo, Ponce-Gómez, Martínez-Moreno, Gómez-Amador, Protocarrero-Ortíz, Tena-Suck.
Drafting the article: Ortega-Porcayo.
Critically revising the article: Gómez-Amador, Protocarrero-Ortíz, Tena-Suck.
Reviewed submitted version of manuscript: Ortega-Porcayo, Ponce-Gómez, Martínez-Moreno, Gómez-Amador, Protocarrero-Ortíz, Tena-Suck.
Approved the final version of the manuscript on behalf of all authors: Gómez-Amador.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Aknowledgements
Authors are in debt to the whole team of surgical and nursing staff of the National Institute of Neurology and Neurosurgery Manuel Velasco Suárez. Thanks to Karla Ortega for proof reading the article.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Sohn C.H., Baik S.K., Kim S.P., Kim I.M., Sevick R.J. Craniopharyngioma in the temporal lobe: a case report. Korean J. Radiol. 2004;5(1):72–74. doi: 10.3348/kjr.2004.5.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nourbakhsh A., Brown B., Vannemreddy P., Lian T., Nanda A., Guthikonda B. Extracranial infrasellar ectopic craniopharyngioma: a case report and review of the literature. Skull base. 2010;20(6):475–480. doi: 10.1055/s-0030-1261269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y.H., Kim S.D., Lim D.J., Park J.Y., Chung Y.G., Kim Y.S. Isolated petroclival craniopharyngioma with aggressive skull base destruction. Yonsei Med. J. 2009;50(5):729–731. doi: 10.3349/ymj.2009.50.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordes U., Flitsch J., Hagel C. Ectopic craniopharyngioma. Klinische Padiatrie. 2011;223(3):176–177. doi: 10.1055/s-0031-1273743. [DOI] [PubMed] [Google Scholar]
- 5.Altinors N., Senveli E., Erdogan A., Arda N., Pak I. Craniopharyngioma of the cerebellopontine angle. Case report. J. Neurosurg. 1984;4:842–844. doi: 10.3171/jns.1984.60.4.0842. [DOI] [PubMed] [Google Scholar]
- 6.Link M.J., Driscoll C.L., Giannini C. Isolated, giant cerebellopontine angle craniopharyngioma in a patient with Gardner syndrome: case report. Neurosurgery. 2002;51(1):221–225. doi: 10.1097/00006123-200207000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Shuman A.G., Heth J.A., Marentette L.J., Blaivas M., Muraszko K.M. Extracranial nasopharyngeal craniopharyngioma: case report. Neurosurgery. 2007;60(4):E780–E781. doi: 10.1227/01.NEU.0000255411.69022.A1. [DOI] [PubMed] [Google Scholar]
- 8.Solarski A., Panke E.S., Panke T.W. Craniopharyngioma in the pineal gland. Arch. Pathol. Lab. Med. 1978;102(9):490–491. [PubMed] [Google Scholar]
- 9.Cashion E.L., Young J.M. Intraventricular craniopharyngioma. Report of two cases. J. Neurosurg. 1971;34(1):84–87. doi: 10.3171/jns.1971.34.1.0084. [DOI] [PubMed] [Google Scholar]
- 10.Jiang R.S., Wu C.Y., Jan Y.J., Hsu C.Y. Primary ethmoid sinus craniopharyngioma: a case report. J. Laryngol. Otol. 1998;112(4):403–405. doi: 10.1017/s0022215100140599. [DOI] [PubMed] [Google Scholar]
- 11.Bock E. Beitrag zur pathologie der hypophyse, Virchow. Arch. Pathol. Anat. 1924;252:98–112. [Google Scholar]
- 12.Elliott R.E., Moshel Y.A., Wisoff J.H. Surgical treatment of ectopic recurrence of craniopharyngioma. Report of 4 cases. J. neurosurg. Pediatr. 2009;4(2):105–112. doi: 10.3171/2009.3.PEDS0948. [DOI] [PubMed] [Google Scholar]
- 13.Mott F.W., Barratt J. Three cases of tumor of the third ventricule. Arch. Neurol. (London) 1899;1:417–440. [Google Scholar]
- 14.Erdheim J. About hypophyseal adenomas and brain cholesteatomas. Sitzungsber. Finn. Akad. Wiss. 1904;113:537–726. [Google Scholar]
- 15.Pekmezci M., Louie J., Gupta N., Bloomer M.M., Tihan T. Clinicopathological characteristics of adamantinomatous and papillary craniopharyngiomas: University of California, San Francisco experience 1985–2005. Neurosurgery. 2010;67(5):1341–1349. doi: 10.1227/NEU.0b013e3181f2b583. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Serna R., Gomez-Amador J.L., Barges-Coll J. Treatment of craniopharyngioma in adults: systematic analysis of a 25-year experience. Arch. Med. Res. 2012;43(5):347–355. doi: 10.1016/j.arcmed.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Tena-Suck M.L., Moreno-Reyes I., Rembao D. Craneofaringioma, estudio clínico-patológico. Quince años del Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suárez”. Gac. Méd. Méx. 2009;145(5):361–368. [PubMed] [Google Scholar]
- 18.Yamada H., Haratake J., Narasaki T., Oda T. Embryonal craniopharyngioma. Case report of the morphogenesis of a craniopharyngioma. Cancer. 1995;75(12):2971–2977. doi: 10.1002/1097-0142(19950615)75:12<2971::aid-cncr2820751227>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Paulus W., Honegger J., Keyvani K., Fahlbusch R. Xanthogranuloma of the sellar region: a clinicopathological entity different from adamantinomatous craniopharyngioma. Acta Neuropathol. 1999;97(4):377–382. doi: 10.1007/s004010051001. [DOI] [PubMed] [Google Scholar]
- 20.Shirataki K., Okada S., Matsumoto S. Histopathological study of the “cholesterol granuloma reaction” in the sellar and juxta-sellar tumors. Brain Nerve. 1988;40(2):133–139. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


