Abstract
A multi-site, masked, randomised parallel group study employing a double dummy treatment design was performed in canine veterinary patients to determine the comparative efficacy and safety of mavacoxib and carprofen in the treatment of pain and inflammation associated with osteoarthritis for a period of 134 days. Treatments were administered according to their respective summaries of product characteristics. Of 139 dogs screened, 124 were suitable for study participation: 62 of which were dosed with mavacoxib and 62 with carprofen. Both treatments resulted in a very similar pattern of considerable improvement as indicated in all parameters assessed by both owner and veterinarian. The primary efficacy endpoint ‘overall improvement’ was a composite score of owner assessments after approximately six weeks of treatment. Both drugs were remarkably effective, with 57/61 (93.4 per cent) of mavacoxib-treated dogs and 49/55 (89.1 per cent) of carprofen-treated dogs demonstrating overall improvement and with mavacoxib's efficacy being non-inferior to carprofen. The treatments had a similar safety profile as evidenced by documented adverse events and summaries of clinical pathology parameters. The positive clinical response to treatment along with the safety and dosing regimen of mavacoxib makes it an attractive therapy for canine osteoarthritis.
Keywords: Dogs, Osteoarthritis, Pain, Non-steroidal anti-inflammatory drugs (NSAIDs)
Introduction
Osteoarthritis (OA) is characterised by a degenerative, progressive and irreversible deterioration of joints resulting in a decreased range of motion, pain, joint swelling and crepitus. This disease is estimated to affect 20 per cent of dogs over one year of age (Johnston 1997). Chronic locomotor system diseases such as OA result in a 20 per cent reduction of longevity in dogs (Moreau and others 2003). The majority of OA cases result from developmental (e.g. hip dysplasia, elbow dysplasia, shoulder osteochondrosis) and acquired conditions (e.g. articular fractures, cranial cruciate rupture). Until treatment modalities that reverse the disease process are readily available, the goals of therapy are to ameliorate joint pain, delay progression of the disease and restore joints to normal function with an overall increase in the quality of life (Singh 2003). While controlled exercise and physical therapy may have their benefits, a major weapon in the armoury of pharmacological agents is the NSAID (Sanderson and others 2009). Such products exert their anti-inflammatory activity through inhibition of cyclo-oxygenase (COX) enzymes of which there are two isoforms, COX-1 and COX-2. These convert arachidonic acid into prostaglandin H2, from which a series of prostanoids is derived; this can result in sensitisation of nociceptive neurone terminals and with repeated stimulation can lead to hyperalgesia and allodynia.
Central sensitisation amplifies signals coming from inflamed joints (Neugebauer and Schaible 1990) resulting in greater perceived pain; COX-2 contributes to this pain hypersensitivity (Samad and others 2001) whereas COX inhibitors can inhibit this process (Veiga and others 2004). A recent review from Innes and others (2010) indicates that long-term NSAID treatment in dogs with OA is more efficacious than short-term treatment with no evidence of any increase in side effects. Compliance with long term daily administration of medicines in routine veterinary clinical practice is known to be relatively poor, with daily doses being missed even during a relatively short 10-day treatment course (Grave and Tanem 1999). Therefore, long-acting NSAID preparations achieving an increased overall compliance might be more dependable in reducing or preventing central sensitisation. Mavacoxib is an orally administered preferential COX-2 inhibitor which has a long mean half-life of 44 days in a typical patient population (Cox and others 2011). This long half-life enables monthly dosing after a second loading dose which is administered 14 days after the first dose. All other oral NSAIDs approved for the treatment of pain and inflammation associated with canine OA are administered on a daily basis.
This report details the results of a randomised positive-controlled study to gain European regulatory approval with the European Medicines Agency (EMA) for mavacoxib. The study evaluated mavacoxib administered orally at 2 mg/kg, in comparison with carprofen, for its efficacy and safety in the treatment of pain and inflammation associated with OA in dogs under field conditions.
Materials and methods
A controlled multi-site study was conducted in compliance with VICH (International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products) guidelines for Good Clinical Practice (VICH 2000) at 12 veterinary practices located throughout France. Approval was obtained from the appropriate regulatory authority in France and satisfied national regulatory and animal welfare standards and requirements. The protocol was subjected to review and satisfied the sponsor's animal welfare and ethical committee. Informed consent was obtained from each one of the dog's owners, or their agents, prior to enrolment.
Animal selection
Dogs greater than one year of age presenting as veterinary patients with clinical and radiographic signs of OA were evaluated for possible inclusion in the study. At initial presentation, dogs underwent screening procedures to determine study eligibility. Dogs received a complete general physical examination, and blood and urine were collected for routine clinical pathology, including complete blood cell count, serum biochemical profile and urinalysis. The severity of clinical signs of OA was evaluated by both the ‘examining veterinarian’ and the dog's ‘owner’. The dog owner's evaluation focused on three categories; lameness, musculoskeletal pain and quality of life taking into account the condition of the pet over the previous two days. The examining veterinarian also focused on the three categories: general musculoskeletal condition, lameness/weight bearing and pain on palpation/manipulation of joints on the day of the examination. Each dog was evaluated whilst walking and trotting and its gait was assessed whilst turning in a tight circle or whilst going up and down stairs. After completion of the evaluations at exercise, each dog was observed while standing for signs of weakness, asymmetric limb trembling, spasms, and asymmetry of limb carriage or weight bearing, including elevation of limbs contra-lateral to those affected to assess the degree of resistance. Each category was scored with a severity grade from 0 (clinically normal) to 4 (nearly incapacitated) as detailed in Appendix 1.
Dogs selected for inclusion in the study were considered to be in good general health based on physical examination and clinical pathology results, and had clinical signs of OA as evidenced by positive scores in one or more of the above categories when assessed by both the owner and examining veterinarian at screening and again on day 0 prior to treatment. The presence of OA in at least one of the affected joints was confirmed by radiography.
Prior to enrolment, dogs had not received any treatment with systemic NSAIDs for at least 30 days, short-acting topical or systemic corticosteroids for at least 14 days, intermediate or long-acting corticosteroids for 30 days, repository anti-inflammatory drugs for at least 120 days, intra-articular injections of any type for approximately one year, and disease modifying agents (e.g. glycosaminoglycans, chondroitin sulfate or sodium hyaluronate) for at least three weeks. Dogs known or suspected to have gastric or duodenal ulceration, inflammatory bowel disease, liver disease, kidney disease, uncontrolled endocrine disease, congestive heart failure, internal soft tissue injury (e.g. contusions of the abdominal organs as a result of trauma) or bleeding disorders, most of which are also label contraindications or cautions for the use of daily NSAIDs, were excluded from participation. Dogs for which surgical intervention was anticipated, or for which the presenting lameness was known to be related to neoplasia, primary neurological disorder, immunological disorder (e.g. rheumatoid arthritis), infection or non-healed fracture, were also excluded from enrolment. Dogs undergoing physical therapy were only eligible for enrolment if, in the opinion of the examining veterinarian, the presenting orthopaedic condition was stable. Dogs on aggressive weight loss programmes, females that were pregnant or lactating, or animals intended for breeding were also excluded from enrolment. Concurrent treatment with topical or systemic anti-inflammatory drugs, or tetracycline class antimicrobials was not permitted during the study. Administration of other concurrent medications was permitted but had to be recorded.
Treatments
Dogs that met the inclusion criteria were allocated to treatment at the time of enrolment by use of a randomised block schedule that was produced in advance of the study. The block size was four, with two mavacoxib-treated and two carprofen-treated dogs in each block. Day 0 was defined as the day of first treatment administration. Each dog received treatment with either mavacoxib (Trocoxil, Zoetis) at 2 mg/kg monthly to day 104 with a loading dose administered 14 days after the first dose, or carprofen (Rimadyl, Zoetis) at 4 mg/kg once daily to day 134. In order to maintain masking of all individuals involved in the study, dogs allocated to treatment with mavacoxib were also administered placebo for carprofen daily for the same duration as mavacoxib treatment, and dogs allocated to treatment with carprofen were also administered placebo for mavacoxib on the same schedule as active mavacoxib. Placebos for mavacoxib and carprofen were the commercial formulations and presentations without the active ingredients.
Dose calculations for study treatments were performed using the body weight determined during the most recent physical examination. Mavacoxib/mavacoxib placebo tablets were administered either with food or immediately prior to feeding.
Assessments
Following treatment administration on day 0, dogs were re-examined at regular intervals during the study. Owner assessments were performed on day 0 prior to treatment, and thereafter on approximately days 2, 7, 14, 44, 74, 104 and 134. Examining veterinarian assessments were performed on day 0 prior to treatment and thereafter on approximately days 14, 44, 74, 104 and day 134. General physical examinations were also performed on the same days as the examining veterinarian assessments together with collection of blood or blood and urine for analysis. Any abnormal health observations irrespective of their nature and severity made by either owner or veterinarian were recorded according to VICH (2000,).
Efficacy outcome measures
Scores for each of the categories (owner assessment of lameness, musculoskeletal pain and quality of life, and examining veterinarian assessment of lameness/weight bearing, pain on palpation/manipulation of joints and general musculoskeletal condition) were summarised by treatment for each assessment day. For each assessment conducted after day 0, the improvement in each score was determined for each dog.
The primary efficacy endpoint was based on the owner assessment scores for the categories lameness, musculoskeletal pain and quality of life on day 44 compared with day 0. A successful ‘overall improvement’ was defined as an improvement in at least one of the three categories of owner assessment with no worsening in either of the other two categories, or improvement in at least two of the three categories with no change or a worsening of the third category. It was predetermined that any dog that was withdrawn from the study due to apparent lack of efficacy or inadequate improvement would be considered a treatment failure. The overall objective of this study was to demonstrate non-inferiority for ‘overall improvement’ of mavacoxib compared with carprofen using a 15 per cent non-inferiority margin. To assess non-inferiority, a 90% CI was calculated on the difference in the per cent of dogs showing ‘overall improvement’ between mavacoxib and carprofen treatment groups (Newcombe 1998). If the lower bound of the CI was greater than the non-inferiority margin (i.e. −15 per cent), then mavacoxib was considered to be non-inferior to carprofen.
The study results were analysed in two ways. The primary analysis was ‘per-protocol’, with data being excluded from the analysis if significant deviations from the study protocol had occurred. The secondary analysis was ‘intent to treat/all randomised animals’, in which data from all cases which had been randomised and dosed with either mavacoxib or carprofen on day 0 were included.
Safety outcome measures
All enrolled dogs that were administered at least one dose of test article were included in the safety summary. Frequencies of dogs experiencing at least one abnormal health event were identified and listed by clinical sign. For each continuous haematology and serum chemistry measure, summary statistics (mean, median, sd, minimum and maximum) were calculated by treatment and time point.
Results
Animals
Dogs were enrolled from 12 veterinary practices in France. In all, 139 dogs were screened for suitability, of which 124 were suitable. Body weights ranged from 4.8 to 72.6 kg on the first day of dosing and ages ranged from 1 to 15 years. Overall, 62 dogs were treated with mavacoxib (mean age 10.1 years) and 62 were treated with carprofen (mean age 9.3 years). Dogs were predominantly purebred and the majority were comprised of large/giant breeds. Male, female, neutered male and neutered female dogs were enrolled in each treatment group.
More than 40 concomitant medications were administered concurrently with either mavacoxib or carprofen during the study. The types of medications were extensive and included many agents routinely used in general veterinary practice, such as antimicrobials, sedatives, anaesthetics, vaccines, antiparasitics and topical skin therapy preparations.
Comparative efficacy assessment
There was a rapid improvement observed in some dogs in both treatment groups, with a reduction of apparent clinical signs as early as two days after the first treatment, which was the first post-treatment assessment time. Fig 1 shows the patient response rate over time with respect to ‘overall improvement’ as assessed by their owners.
FIG 1:
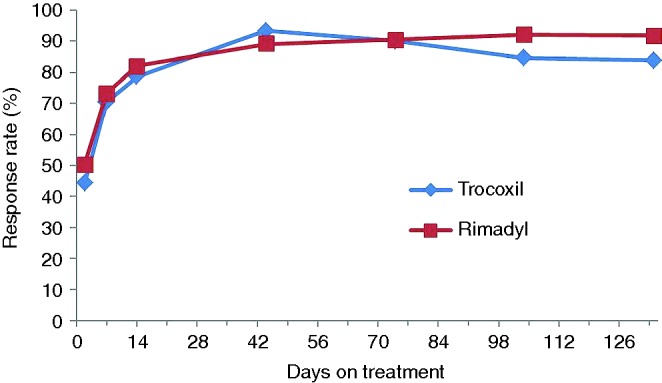
Overall improvement with time. Owner assessment – per-protocol animals (those patients that did not deviate significantly from the study protocol)
Of the 124 dogs enrolled, eight dogs (one treated with mavacoxib and seven treated with Rimadyl) were not included in the ‘per-protocol’ efficacy assessment for day 44 (for details see Table 1). No dogs were withdrawn because of an apparent lack of efficacy or inadequate improvement. At day 44, 57/61 dogs (93.4 per cent) treated with mavacoxib showed an ‘overall improvement’ using data from owner assessments, compared with 49/55 (89.1 per cent) of carprofen-treated animals (‘per-protocol’ analysis), and the percentages for the ‘intent to treat/all randomised animals’ analysis were almost identical (Table 1). Non-inferiority was demonstrated for both analyses. Veterinary assessments reflected those of the owners with all assessed parameters improving up to day 44 after which the level of improvement was sustained throughout the study up to day 134 and were very similar for both drugs. Fig 2 depicts the patient response in relation to the veterinary assessment of pain on palpation/manipulation of joints over time for per-protocol animals.
TABLE 1:
Primary efficacy analysis: owner assessed overall improvement
| Mavacoxib |
Carprofen |
90% CI |
||||
|---|---|---|---|---|---|---|
| Proportion of dogs improved | Percentage of dogs improved | Proportion of dogs improved | Percentage of dogs improved | Difference in percentage improved | Lower | Upper |
| Per-protocol (those patients that did not deviate significantly from the protocol) | ||||||
| 57/61* | 93.4 | 49/55† | 89.1 | 4.4 | −4.6 | 13.9 |
| Intent to treat/all randomised animals | ||||||
| 57/61 | 93.4 | 52/58 | 89.7 | 3.8 | −5.0 | 13.0 |
*F0508 was withdrawn from the study on day 37 in order to permit corticosteroid therapy for idiopathic vestibular syndrome/cerebrovascular accident. Hence, the total number of mavacoxib cases is 61 not 62
†Three carprofen-treated cases (F0219, F0509 and F0808) were excluded from the ‘per-protocol’ analysis as a result of deviating from the protocol.
F0209 was withdrawn from study and euthanased on day 43
F0403 was withdrawn from study on day 2 having ingested rodenticide
F1107 was withdrawn from study on day 41 with a liver lymphoma
F0805 was withdrawn from study for non-medical reasons prior to day 44
FIG 2:
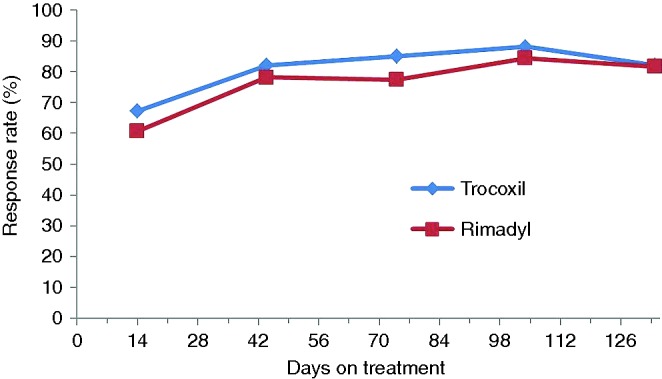
Pain on palpation/manipulation of joints. Veterinary assessment – per-protocol animals (those patients that did not deviate significantly from the study protocol)
Safety assessment
A total of 59 ‘adverse events’ (AEs) were reported during the study, an ‘AE’ being defined as ‘any observation in animals that is unfavourable and unintended and occurs after the use of a veterinary product or investigational veterinary product, whether or not considered to be product related’ (VICH 2000). Of the 59 AEs, a total of 29 AEs were reported in 26 mavacoxib-treated dogs and 30 were reported in 25 carprofen-treated dogs (see Table 2). All AEs were assessed using the ABON system of causality assessment (Committee for Veterinary Medicinal Products (CVMP) 2004), where A=probable, B=possible, O=unclassifiable/unassessable and N=unlikely to be drug related, by the sponsor at the end of the study. The assessment of causality recognised that NSAIDs are considered to have the potential to cause or exacerbate digestive tract, renal and hepatobiliary disorders as is reflected by each of their respective summary of product characteristics (Dictionnaire Medicaments Veterinaires et des produits de Sante Animale 2013, Veterinary Medicine Directorate 2013).
TABLE 2:
Categories of adverse events
| Category of adverse event | Mavacoxib Case number |
Clinical severity of event | ABON* classification | Carprofen Case number |
Clinical severity of event | ABON* classification |
|---|---|---|---|---|---|---|
| Digestive tract disorders | F0206 | Minor, transient | B | F0201 | Fatal | A |
| F0212 | Minor, transient | N | F0205 | Minor, transient | B | |
| F0216 | Minor, transient | B | F0209 | Fatal | B | |
| F0401 | Minor, transient | B | F0404 | Minor, transient | B | |
| F0804 | Minor, transient | N | F1013 | Minor, transient | B | |
| F1003 | Minor, transient | N | ||||
| F1108 | Minor, transient | B | ||||
| F1201 | Minor, transient, occurred twice | B | ||||
| F1205 | Minor, transient | B | ||||
| F1206 | Minor, transient | B | ||||
| Skin and appendages disorders | F0204 | Minor, transient | N | F0205 | Minor, long-term | N |
| F0216 | Minor, transient | N | F0405 | Minor, transient | N | |
| F0218 | Minor, transient | N | F0509 | Minor, transient | N | |
| F0305 | Minor, transient | N | F0606 | Minor, long-term | N | |
| F0310 | Minor, transient | N | F0702 | Minor, transient | N | |
| F0311 | Minor, transient | N | F0801 | Minor, transient | N | |
| F0504 | Minor, transient | N | F0814 | Minor, transient | N | |
| F0906 | Minor, transient | N | F0907 | Minor, transient | N | |
| F1009 | Minor, transient | N | F1011 | Minor, transient | N | |
| Neurological disorders | F0302 | Minor, transient | N | F0205 | Significant, long-term | N |
| F0304 | Significant, transient | N | F0910 | Serious, long-term | N | |
| F0508 | Significant, transient | N | ||||
| Respiratory tract disorders | F0108 | Serious/critical | N | F0107 | Significant, transient | N |
| F0408 | Minor, transient | N | F0219 | Serious, long-term | N | |
| Reproductive system disorders | F0402 | Significant, recurrent | N | F0207 | Minor, transient | N |
| F1001 | Significant, recurrent | N | ||||
| Musculoskeletal disorders | F0102 | Significant, possibly long-term | N | F0205 | Significant, possibly recurrent | N |
| F0512 | Significant, transient | N | ||||
| Renal and urinary disorders | F0308 | Serious, long-term | N | |||
| Systemic disorders | F0401 | Fatal | B | F0403 | Serious/critical | N |
| F0501 | Minor, transient | N | ||||
| F0903 | Significant, possibly recurrent | N | ||||
| Eye disorders | F0201 | Significant, possibly long-term | N | |||
| Hepatobiliary disorders | F1107 | Serious/critical | N | |||
| Behavioural disorders | F1111 | Minor/transient | N |
*ABON classification of causality: A=Probable; B=Possible; O=Unclassifiable/unassessable; N=Unlikely
‘Digestive tract disorders’ had a relatively high incidence (see Table 2), being only surpassed by minor skin disorders like pyoderma, traumatic wounds and dermatitis which made up the ‘Skin and appendages disorders’ category. This incidence of ‘Digestive tract disorders’ is in line with a recently published systematic review of NSAID-induced AEs where the most commonly observed clinical signs were related to the digestive tract (Monteiro-Steagall and others 2013). Table 3 indicates the severity and duration of reported ‘Digestive tract disorders’; all such mavacoxib cases were minor and transient: three of the five carprofen cases were minor and transient. Only one AE report related to ‘Renal and urinary disorders’; this mavacoxib-treated patient (F0308) presented with an undifferentiated anaplastic adenocarcinoma involving the right kidney. This patient had normal blood results at enrolment. The one AE report classified primarily as a ‘Hepatobiliary disorder’, case F1107, a carprofen-treated patient, had a hepatic lymphoma. This dog had minor increases in blood liver parameters (alanine aminotransferase (ALT), aspartate aminotransferase) and creatinine kinase (CK) at the time of prescreening but was deemed to be in good health and eligible for enrolment. Four weeks later, alkaline phosphatase, ALT, CK and γ-glutamyltransferase levels were elevated and total protein was reduced but the dog remained in the study beyond this time point.
TABLE 3:
Digestive tract disorders: type and duration
| Mavacoxib Case # |
Type and duration of adverse event | Carprofen Case # |
Type and duration of adverse event |
|---|---|---|---|
| F0206 | Vomited once, reported retrospectively to veterinarian, not treated | F0201 | Multiple vomiting events postanaesthesia and surgery, gastric and intestinal ulceration, patient died despite prolonged intensive therapy |
| F0212 | Infected oral wounds, treated with antimicrobial | F0205 | Diarrhoea, treated with antimicrobial |
| F0216 | Loose faeces on one occasion, reported retrospectively to veterinarian, not treated | F0209 | Gastric ulceration, patient was in extremis, euthanased |
| F0401 | Vomited once, reported retrospectively to veterinarian, not treated | F0404 | Vomited several times, gastritis, treated medically |
| F0804 | Gingivitis, treated with antimicrobial | F1013 | Gastritis, not treated |
| F1003 | Vomited foreign bodies, treated medically | ||
| F1108 | Vomited once, reported retrospectively to veterinarian, not treated | ||
| F1201 | Mild diarrhoea on two occasions, reported retrospectively to veterinarian, not treated | ||
| F1205 | Vomited once, reported retrospectively to veterinarian, not treated | ||
| F1206 | Haemorrhagic colitis on one day, reported retrospectively to veterinarian, not treated |
Twelve patients failed to complete the study (see Table 4), five mavacoxib-treated dogs and seven treated with Rimadyl; one of these seven carprofen cases (F0805) was withdrawn for a reason other than AEs (protocol non-compliance). Using the ABON system of causality assessment, four of the five mavacoxib-treated cases were assessed as unlikely (N) to be drug associated and one case (F0401) as possibly (B) associated. Of the six carprofen-treated cases, four were assessed as unlikely (N) to be drug associated, one (F0209) as possibly (B) drug associated and one (F0201) as probably (A) drug associated. Both F0209 and F0201 had serious ‘digestive tract disorders’ with a fatal outcome (see Tables 2 and 3).
TABLE 4:
Reason for withdrawal from or non-completion of the study
| Case # | Treatment | Adverse event | ABON* classification |
|---|---|---|---|
| F0102 | Mavacoxib | Acute spinal injury requiring steroid therapy | N |
| F0308 | Mavacoxib | Adenocarcinoma involving right kidney | N |
| F0108 | Mavacoxib | Acute laryngeal paralysis | N |
| F0508 | Mavacoxib | Idiopathic vestibular syndrome/cerebrovascular accident requiring steroid therapy | N |
| F0401 | Mavacoxib | Septicaemia, with gastrointestinal ulceration | B |
| F0201 | Carprofen | Gastrointestinal ulceration | A |
| F0209 | Carprofen | Gastrointestinal ulceration | B |
| F0403 | Carprofen | Rodenticide poisoning, owner requested withdrawal | N |
| F0910 | Carprofen | Idiopathic vestibular syndrome/cerebrovascular accident requiring steroid therapy | N |
| F1107 | Carprofen | Hepatic lymphoma | N |
| F0907 | Carprofen | Facial eczema requiring steroid therapy | N |
| F0805 | Carprofen | Not applicable withdrawn for non-compliance | Not applicable |
*ABON classification of causality: A=Probable; B=Possible; O=Unclassifiable/unassessable; N=Unlikely
The one mavacoxib-treated case (F0401) which was withdrawn from the study and attributed a ‘possible’ causality associated with mavacoxib had a septicaemia. As well as septic foci in multiple organs, this patient had some gastrointestinal lesions but these might well have been secondary to the septicaemia as the patient had not exhibited any gastrointestinal clinical signs.
Comparison of clinical biochemistry and haematology from samples taken at screening, the efficacy endpoint (day 44) and study completion demonstrated some differences. Most of these are unlikely to be of clinical importance as they were generally marginal.
The means and medians for erythrocyte count, haematocrit, urea, creatinine and albumin remained within the reference ranges for both treatments throughout the study (see Table 5).
TABLE 5:
Clinical pathology variables
| |
Screening |
Day 134 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Ref. range | Treatment | Mean | sd | Median | Min./max. | Mean | sd | Median | Min./max. |
| Erythrocyte count (mm³) | 5.5–8.5 | Mavacoxib | 6.5 | 0.91 | 6.53 | 3.8/9.66 | 6.8 | 1.09 | 6.63 | 4.49/10.67 |
| Carprofen | 6.5 | 0.78 | 6.5 | 4.16/8.27 | 7.0 | 0.92 | 6.96 | 4.90/10.36 | ||
| Haematocrit (%) | 37–55 | Mavacoxib | 46.4 | 6.2 | 45.3 | 28.1/62.6 | 49.1 | 7.5 | 49.8 | 34.4/79 |
| Carprofen | 46.6 | 5.7 | 46.7 | 31.6/57.6 | 51.4 | 7.5 | 51.0 | 36.9/79.9 | ||
| Albumin (g/L) | 20–40 | Mavacoxib | 30 | 3 | 30 | 21/37 | 31 | 3 | 31 | 25/39 |
| Carprofen | 30 | 3 | 31 | 24/38 | 31 | 3 | 32 | 24/44 | ||
| Urea (g/L) | 0.15–0.5 | Mavacoxib | 0.4 | 0.15 | 0.35 | 0.15/0.95 | 0.5 | 0.2 | 0.46 | 0.23/1.19 |
| Carprofen | 0.4 | 0.14 | 0.32 | 0.15/0.91 | 0.4 | 0.17 | 0.37 | 0.15/1.12 | ||
| Creatinine (mg/L) | 0–15 | Mavacoxib | 8.5 | 2.2 | 8.0 | 5.0/15.0 | 9.5 | 2.9 | 9.0 | 4.0/22.0 |
| Carprofen | 8.4 | 2.1 | 8.0 | 5.0/16.0 | 9.2 | 2.4 | 9.0 | 5.0/16.0 | ||
Discussion
The two preferential COX-2 inhibitors, mavacoxib and carprofen, had a similar good efficacy and safety profile. The increase in patient response up to 44 days and the sustained benefit seen throughout the study up to day 134 support the premise that continuous use of NSAIDs over the long-term provides additional benefit in the management of OA as compared with short-term treatment (Innes and others 2010). Mansa and others reported that the number of patients improving with carprofen treatment increased between day 14 and day 84 of treatment and that some patients that had improved by day 14 demonstrated further improvement by day 84 (Mansa and others 2007). Although the patients were unaware that they were receiving an active anti-inflammatory agent, both the owners and the examining veterinarians, who were making assessments, were aware that every dog on study was being positively treated. Consequently, there was the potential for an ‘indirect’ placebo effect. This potential artefact could have been avoided by having a third treatment group receiving placebo alone. However, as there were recognised active comparators available, providing placebo long-term treatment for a painful condition would be unethical and be contrary to good animal welfare. An alternative approach might have been to have a treatment group that received placebo for a short period initially but one of the aims of this study was to investigate long-term effects. The study reported here was conducted to gain European regulatory approval for mavacoxib; following the guidance of EMA, it was designed as a positive-controlled study to demonstrate that monthly administration of mavacoxib is at least as good as (i.e. non-inferior) daily administration of carprofen. Carprofen was selected as a comparator as it is generally recognised to be effective in practice and its efficacy has been demonstrated in placebo studies, for example, Vasseur and others (1995) and U.S. Food and Drug administration (1996). The demonstration of non-inferiority does not automatically imply efficacy (e.g. if the comparator molecule has no proven efficacy and/or the methodology used to assess efficacy is insensitive). Great care has to taken in designing and interpreting the results of ‘non-inferior studies’ (e.g. magnitude of the non-inferiority margin, Freise and others 2013).
It should be noted that there is good evidence from other studies for both mavacoxib's and carprofen's efficacy in negative-controlled studies and/or studies employing objective assessment tools (e.g. Vasseur and others 1995, Lees and others 2009, Walton and others 2014). Ideally, objective rather than subjective assessment tools should be used for efficacy assessments, that is, force plate analysis. This study was designed as a multi-centre field study and for practical reasons force plate analysis was not possible as the technical equipment is typically only available in clinical research facilities. It has to be acknowledged that the tool used in the study described here was subjective and had not been validated and published but expert opinion was sought and used in its design. There are now a number of validated subjective tools to assess chronic pain in dogs related to OA (Wiseman-Orr and others 2006, Brown and others 2008, Hercock and others 2009, Hielm-Björkmann and others 2009, Walton and others 2013), but at the time the study was performed, these subjective tools (LOAD, HCPI, CBPI) were either not yet available or validated. Further, it should be noted that the EMA had previous experience with similar assessment tools to that reported here. Consequently, the authors decided to use the categorical assessment tool described here. It has to be said that the owner assessments in particular may have given a better judgement of the ‘quality of life’ improvement than a purely objective assessment such as force plate analysis and the categorical assessments are applicable to a more comprehensive set of affected joints.
The potential ‘indirect placebo effect’, non-inferiority comparison and use of a subjective assessment tool might be considered weaknesses in the study design but they represent reasonable compromises that satisfy animal welfare and ethical considerations. The efforts made to ensure blinding of study participants as to which patients received which drug provide a degree of robustness to the study results.
In this study, all abnormal health observations were recorded in alignment with VICH Good Clinical Practice (VICH 2000). During the course of the study, AEs were reported in 51 dogs (26 mavacoxib-treated and 25 carprofen-treated). This number must be considered in the context of the study, that is, the average age of the dogs at enrolment was more than nine years, owners and investigators were asked to record all abnormal health observations whatever their causality and severity and that the treatment period was four-and-a-half months during which it would not be unusual for dogs of this age to present with some abnormal health observations. Adverse reactions affecting the digestive tract, the renal and the hepatic systems have been described for NSAIDs in general approved in veterinary medicine. However, in this study, a ‘probable’ causality assessment was only attributed to one case, a carprofen-treated patient.
Review of all AEs recorded in the study indicates that the profile of AEs in mavacoxib-treated dogs is generally similar with regard to type, severity and duration to that seen in dogs dosed daily with carprofen. As the study was designed to provide adequate power for the ‘overall improvement’ calculation but not sufficiently powered to detect statistical differences in AEs, a statistical analysis was not conducted. It is worth noting that the aforementioned AEs were similar whether the treatments could be discontinued, thereby rapidly reducing drug exposure (daily, carprofen) or not (monthly, mavacoxib). Indeed, all ‘digestive tract disorders’ reported in mavacoxib-treated patients were very transient despite continued mavacoxib exposure (see also Table 3 for more details).
In this study, the monthly administration of mavacoxib achieved at least the same anti-inflammatory effects as did the daily administration of carprofen without an increase in the incidence and severity of suspect adverse reactions.
Acknowledgments
The authors would like to thank all the investigators and their assistants along with the statisticians who worked on this project. The study was funded by Zoetis, formerly known as Pfizer Animal Health; all authors were employees of Zoetis at the time of study conduct.
References
- BROWN D. C., BOSTON R. C., COYNE J. C. & FARRAR J. T. (2008) Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. Journal of the American Veterinary Medical Association 233, 1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS (CVMP) (2004) Harmonising the Approach to Causality Assessment for Adverse Reactions to Veterinary Medicinal Products. CVMP [Google Scholar]
- COX S. R., LIAO S., PAYNE-JOHNSON M., ZIELINSKI R. J. & STEGEMANN M. R. (2011) Population pharmacokinetics of mavacoxib in osteoarthritic dogs. Journal of Veterinary Pharmacology and Therapeutics 34, 1–11 [DOI] [PubMed] [Google Scholar]
- DICTIONNAIRE MEDICAMENTS VETERINAIRES ET DES PRODUITS DE SANTE ANIMALE (2013) Les editions du Point Veterinaire, 18e edition
- FREISE K. J., LIN T.-L., FAN T. M., RECTA V. & CLARK T. P. (2013) Evidence-based medicine: the diesign and interpretation of noninferioroty clinical trials in veterinary medicine. Journal of Veterinary Internal Medicine 27, 1305–1317 [DOI] [PubMed] [Google Scholar]
- GRAVE K. & TANEM H. (1999) Compliance with short-term oral antibacterial drug treatment in dogs. The Journal of Small Animal Practice 40, 158–162 [DOI] [PubMed] [Google Scholar]
- HERCOCK C. A., PINCHBECK G., GIEJDA A., CLEGG P. D. & INNES J. F. (2009) Validation of a client-based clinical metrology instrument for the evaluation of canine elbow osteoarthritis. Journal of Small Animal Practice 50, 266–271 [DOI] [PubMed] [Google Scholar]
- HIELM-BJÖRKMAN A. K., RITA H. & TULAMO R. M. (2009) Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. American Journal of Veterinary Research 70, 727–734 [DOI] [PubMed] [Google Scholar]
- INNES J. F., CLAYTON J. & LASCELLES B. D. (2010) Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. The Veterinary Record 166, 226–230 [DOI] [PubMed] [Google Scholar]
- JOHNSTON S. A. (1997) Osteoarthritis. Joint anatomy, physiology, and pathobiology. The Veterinary Clinics of North America. Small Animal Practice 27, 699–723 [DOI] [PubMed] [Google Scholar]
- LEES P., SIX R., KRAUTMANN M. & COX S. R. (2009) 11th International Congress of the European Association for Veterinary Pharmacology and Toxicology 12–16 July 2009
- MANSA S., PALMER E., GRONDAHL C., LONAAS L. & NYMAN G. (2007) Long-term treatment with carprofen of 805 dogs with osteoarthritis. The Veterinary Record 160, 427–430 [DOI] [PubMed] [Google Scholar]
- MONTEIRO-STEAGALL B. P., STEAGALL P. V. & LASCELLES B. D. (2013) Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs. Journal of Veterinary Internal Medicine 27, 1011–1019 [DOI] [PubMed] [Google Scholar]
- MOREAU D., CATHELAIN P. & LACHERETZ A. (2003) Comparative study of causes of death and life expectancy in carnivorous pets (II). Revue de Medecine Veterinaire 154, 127–132 [Google Scholar]
- NEUGEBAUER V. & SCHAIBLE H. G. (1990) Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat's knee. Journal of Neurophysiology 64, 299–311 [DOI] [PubMed] [Google Scholar]
- NEWCOMBE R. G. (1998) Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 17, 873–890 [DOI] [PubMed] [Google Scholar]
- SAMAD T. A., MOORE K. A., SAPIRSTEIN A., BILLET S., ALLCHORNE A., POOLE S., BONVENTRE J. V. & WOOLF C. J. (2001) Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410, 471–475 [DOI] [PubMed] [Google Scholar]
- SANDERSON R. O., BEATA C., FLIPO R. M., GENEVOIS J. P., MACIAS C., TACKE S., VEZZONI A. & INNES J. F. (2009) Systematic review of the management of canine osteoarthritis. The Veterinary Record 164, 418–424 [DOI] [PubMed] [Google Scholar]
- SINGH G. (2003) Treatment options for osteoarthritis. Surgical Technology International 11, 287–292 [PubMed] [Google Scholar]
- U.S. FOOD AND DRUG ADMINISTRATION (1996) NADA 141-053 Rimadyl®- Original Approval. Report # GCRN-127594
- VASSEUR P. B., JOHNSON A. L., BUDSBERG S. C., LINCOLN J. D., TOOMBS J. P., WHITEHAIR J. G. & LENTZ E. L. (1995) Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. Journal of the American Veterinary Medical Association 206, 807–811 [PubMed] [Google Scholar]
- VEIGA A. P., DUARTE I. D., AVILA M. N., DA MOTTA P. G., TATSUO M. A. & FRANCISCHI J. N. (2004) Prevention by celecoxib of secondary hyperalgesia induced by formalin in rats. Life Sciences 75, 2807–2817 [DOI] [PubMed] [Google Scholar]
- VETERINARY MEDICINE DIRECTORATE (2013) www.vmd.defra.gov.uk/Product informationDatabase/ [Google Scholar]
- VICH (2000) International Co-operation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products, Good Clinical Practice, June 2000 effective 01 July 2001
- WALTON M. B., COWDEROY E., LASCELLES D. & INNES J. F. (2013) Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. PLoS ONE 8, e58125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTON M. B., COWDEROY E., WUSTEFELD-JANSSENS B., LASCELLES D. & INNES J. (2014) Mavacoxib and meloxicam for canine osteoarthritis: a randomised clinical comparator trial. The Veterinary Record 175, 280. [DOI] [PubMed] [Google Scholar]
- WISEMAN-ORR M. L., SCOTT E. M., REID J. & NOLAN A. (2006) Validation of a structured questionnaire as an instrument to measure chronic pain in dogs on the basis of effects on health-related quality of life. American Journal of Veterinary Research67, 1826–1836 [DOI] [PubMed] [Google Scholar]


