Background: Based on qualitative pH probes, loss of Fab1/PIKfyve was thought to impair vacuolar/lysosomal acidification.
Results: Using several quantitative assays, vacuoles/lysosomes remained acidic in Fab1/PIKfyve-inhibited cells in a V-ATPase-dependent manner.
Conclusion: Fab1/PIKfyve is not necessary to maintain the steady-state vacuolar/lysosomal pH.
Significance: Contrary to current thought, we propose a model in which Fab1/PIKfyve is dispensable to maintain vacuolar/lysosomal acidification.
Keywords: Lysosomal Acidification, Lysosome, Organellar pH Homeostasis, Organelle, Phosphoinositide, Yeast
Abstract
Lysosomes and the yeast vacuole are degradative and acidic organelles. Phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2), a master architect of endolysosome and vacuole identity, is thought to be necessary for vacuolar acidification in yeast. There is also evidence that PtdIns(3,5)P2 may play a role in lysosomal acidification in higher eukaryotes. Nevertheless, these conclusions rely on qualitative assays of lysosome/vacuole pH. For example, quinacrine, an acidotropic fluorescent base, does not accumulate in the vacuoles of fab1Δ yeast. Fab1, along with its mammalian ortholog PIKfyve, is the lipid kinase responsible for synthesizing PtdIns(3,5)P2. In this study, we employed several assays that quantitatively assessed the lysosomal and vacuolar pH in PtdIns(3,5)P2-depleted cells. Using ratiometric imaging, we conclude that lysosomes retain a pH < 5 in PIKfyve-inhibited mammalian cells. In addition, quantitative fluorescence microscopy of vacuole-targeted pHluorin, a pH-sensitive GFP variant, indicates that fab1Δ vacuoles are as acidic as wild-type yeast. Importantly, we also employed fluorimetry of vacuoles loaded with cDCFDA, a pH-sensitive dye, to show that both wild-type and fab1Δ vacuoles have a pH < 5.0. In comparison, the vacuolar pH of the V-ATPase mutant vph1Δ or vph1Δ fab1Δ double mutant was 6.1. Although the steady-state vacuolar pH is not affected by PtdIns(3,5)P2 depletion, it may have a role in stabilizing the vacuolar pH during salt shock. Overall, we propose a model in which PtdIns(3,5)P2 does not govern the steady-state pH of vacuoles or lysosomes.
Introduction
Lysosomes are a degradative powerhouse in cells; they are enriched in the hydrolytic enzymes that digest nutrients, cellular debris, and even engulfed pathogens (1, 2). In addition, numerous channel and transporter proteins are embedded in the lysosomal membrane that control the transport of small molecules like amino acids, sugars, and ions into and out of the lysosome (1, 3–5). Hence, lysosome dysfunction can build up debris and impair cell metabolism, leading to a variety of lysosomal storage diseases (3). The yeast vacuole is the analogue of the lysosome, serving as the main degradative storage and detoxification compartment in yeast (6). Because of its functional similarities to the lysosome and its easy manipulation, the yeast vacuole is an excellent model system for studying lysosomal regulation and function.
Both lysosomes and vacuoles are highly acidic, which is crucial for proper lysosomal/vacuolar functions. The acidic pH is required for the delivery and activation of hydrolytic enzymes in the lysosome (6, 7). In addition, the H+ electrochemical gradient across the lysosomal membrane drives the transport of amino acids, ions, and metals into and out the lysosome/vacuole lumen (5, 8, 9). This H+ gradient is achieved by the vacuolar-type H+-ATPase (V-ATPase), a highly conserved multisubunit enzyme that pumps H+ from the cytosol into the vacuolar lumen by coupling to ATP hydrolysis. The V-ATPase consists of a peripheral (V1) complex, the site of ATP hydrolysis, and an integral (V0) complex that forms the proton pore (10–12). The mammalian subunit a (Vph1p in yeast), a subunit of the V0 complex, is important for connecting the V1 and V0 on the membrane (12, 13). ATP hydrolysis by the V1 complex drives the rotation of the V0 complex and allows the translocation of protons across the vacuolar membrane (13–17).
The V-ATPase is regulated by a number of different mechanisms, one of which is believed to be phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2)3 (18, 19). PtdIns(3,5)P2 is a low abundance phosphoinositide found mainly on the vacuolar membrane in yeast and on endolysosomes in higher eukaryotes (18, 20, 21). PtdIns(3,5)P2 is involved in a variety of cellular functions including controlling lysosome/vacuole size, membrane recycling, and ion transport (22–25).
Deletion of genes involved in the synthesis of PtdIns(3,5)P2, including FAB1, which encodes the phosphatidylinositol 3-phosphate 5-kinase, impairs the vacuolar accumulation of quinacrine, a fluorescence base that generally accumulates in acidic compartments (25–28). This led to the belief that PtdIns(3,5)P2 is required to maintain an acidic vacuolar lumen. In fact, recently, PtdIns(3,5)P2 was suggested to bind to the V0 complex to stabilize the assembly of the V-ATPase and increase its activity during salt stress (29). In contrast, the role of PtdIns(3,5)P2 in establishing acidic lysosomes in higher eukaryotes is unclear. Some studies have suggested that acidotropic probes like acridine orange accumulate in enlarged lysosomes induced by antagonists of PIKfyve, the mammalian ortholog of Fab1 (30). Others have suggested that LysoTracker, another acidotropic agent, does not accumulate in enlarged lysosomes in PIKfyve-inhibited cells (31, 32). However, these acidotrophic dyes are not quantitative and do not provide information about the actual pH in PtdIns(3,5)P2-depleted cells.
In this study, we measured the lysosomal and vacuolar pH to better understand the role of PtdIns(3,5)P2 in lysosome/vacuolar acidification. To determine the vacuolar pH, we employed the pH-sensitive fluorescent dye 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (cDCFDA), and super-ecliptic pHluorin. cDCFDA was used previously to measure the vacuolar pH in Cryptococcus neoformans by fluorimetry (33). In addition, the super-ecliptic pHluorin, a pH-sensitive GFP variant, can be targeted to the vacuolar lumen to query the pH status of yeast vacuoles (34). We also utilized ratiometric imaging of lysosomes labeled with FITC-dextran to quantitate the lysosomal pH (35). Using these methods, we revealed that yeast vacuoles lacking PtdIns(3,5)P2 were as acidic as wild-type cells. Similarly, both control and PIKfyve-abated mammalian cells exhibited similarly acidic lysosomes.
EXPERIMENTAL PROCEDURES
Media and Reagents
7-Amino-4-chloromethylcoumarin (CMAC), cDCFDA, FITC-dextran, LysoTracker DND-99, fetal bovine serum, Hanks' balanced salt solution, and DMEM were purchased from Life Technologies. Quinacrine, nigericin, and monensin were purchased from Sigma-Aldrich. Yeast media and nutrients were from Biobasic (Toronto, Ontario, Canada). Concanamycin A, concanavalin A, and carbonyl cyanide m-chlorophenylhydrazone (CCCP) were purchased from BioShop (Burlington, Ontario, Canada). Apillimod was purchased from Toronto Research Chemicals. MF4 was a kind gift from Dr. Kevan Shokat (UCSF).
Yeast Strains
The strains used in this study are listed in Table 1. We employed the PCR-based gene deletion method described in Ref. 36, using F1 and R1 primer plasmid-specific sequences, to generate vph1Δ in the SEY6210 background. DNA for gene deletions was generated using the Phusion high-fidelity DNA polymerase (New England Biolabs). Yeast transformation was performed according to standard procedures using the lithium acetate method, and homologous recombination was confirmed by PCR. Mating was used to generate fab1Δ vph1Δ in the SEY6210 background, and fab1Δ Mup1-pHluorin and vph1Δ Mup1-pHluorin.
TABLE 1.
S. cerevisiae strains employed in this study
| Strain name | Genotype | Source |
|---|---|---|
| SEY6210 | MATα his3-Δ200 trp1-Δ901 leu2–3 ura3–52 lys2–801 suc2-Δ9 | S. Emr |
| SEY6211 | MATa leu2–3,112 ura3–52 his3-Δ200 trp1-Δ901 ade2–101 suc2-Δ9 | S. Emr |
| fab1Δ2 | SEY6210; fab1Δ::HIS3 | S. Emr |
| SRY13 | SEY6210; atg18::HIS3 | S. Emr |
| JGY145 | SEY6210; vac14Δ::TRP1 | S. Emr |
| SHY4 | SEY6210; vph1Δ::TRP1 | This study |
| SHY1 | SEY6210; fab1Δ::HIS3 vph1Δ::TRP1 | This study |
| BWY3818 | SEY6210; Mup1-pHluorin::KAN | B. Wendland |
| SHY2 | SEY6210; fab1Δ::HIS3 Mup1-pHluorin::KAN | This study |
| SHY3 | SEY6210; vph1Δ::TRP1 Mup1-pHluorin::KAN | This study |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | B. Andrews |
| BY4741 fab1Δ | Mat a fab1Δ::Kan his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | B. Andrews |
Cell Culture
The RAW264.7 (RAW) macrophage-like cell line was maintained in DMEM supplemented with 5% fetal bovine serum (Wisent Inc., Quebec, Canada) at 5% CO2 and 37 °C.
Vacuole Staining and Hyperosmotic Shock
Yeast cultures were grown overnight in synthetic complete (SC) medium (yeast nitrogen base without nitrogen and ammonium sulfate, sodium glutamate, and 2% glucose supplemented with appropriate amino acids) to an A600 of ∼0.6. Cells were washed and resuspended in SC medium buffered to pH 7.5. Vacuoles were labeled with 200 μm quinacrine for 10 min at 26 °C in 100 mm Hepes-KOH, pH 7.6. Cells were washed and resuspended in 25 μl of ice-cold 100 mm Hepes-KOH, pH 7.6, with 2% glucose. For CMAC staining, vacuoles were labeled with 100 μm CMAC in the dark for 15 min at room temperature in SC medium. Cells were washed and resuspended in 25 μl of SC medium. For cDCFDA staining, vacuoles were labeled with 50 μm cDCFDA for 1 h at 26 °C, washed, and resuspended in SC medium, pH 7.5. For osmotic shock, after cDCFDA staining, cells were exposed to 0.9 m NaCl for 10 min followed by fluorimetric measurement immediately after and for every 5 min over a period of 25 min.
Fluorimetry
About 10 ml of yeast cells was continuously grown in SC medium to A600 = 0.6. About 2 million cells were then incubated in 100 μl of yeast calibration medium (50 mm MES, 50 mm Hepes, 50 mm KCl, 50 mm NaCl, 0.2 m ammonium acetate, 10 mm NaN3, and 10 mm 2-deoxyglucose, pH 7.5) for 4 min without CCCP to obtain the basal fluorescence intensity. Subsequently, individual aliquots of 2 million cells were incubated for 4 min in yeast calibration medium containing 50 μm CCCP and set to pH 4.5–7.5. Fluorescence intensity was measured immediately. When necessary, cells were treated with 1 μm concanamycin A (ConA) for 1 h before calibration. Fluorescence intensity was measured with a fluorimeter (Hitachi F-2500) using an excitation scan between 400 and 520 nm and emission at 535 nm. The samples were measured in triplicate. Importantly, this assay is independent of absolute fluorescence intensity because each sample is internally calibrated; in other words, the basal fluorescence and calibration curves for each condition are all derived from a single population of cells labeled with cDCFDA. GraphPad Prism 6 software was used to calculate a calibration curve as a function of the measured values relative to the pH values of the calibration buffer. The data were then fitted to a sigmoidal dose-response curve for each sample. Statistical analysis is based on a minimum of three independent experiments.
Microscopy
Fluorescence, bright field, or differential interference contrast images of quinacrine, CMAC, and cDCFDA-labeled cells were obtained with a DM5000X Leica epifluorescence microscope. Time lapse imaging was captured using an Olympus IX81 quorum spinning disk microscope with PerkinElmer Volocity software. For ratiometric imaging, images were captured using a DM-IRB Leica epifluorescence microscope with MetaFluor software (MDS Analytical Technologies). The fluorescence intensity of FITC-labeled lysosomes and Mup1-pHluorin-labeled vacuoles was analyzed with ImageJ 1.47v.
Mup1-pHluorin and Imaging Analysis
Yeast culture were grown overnight in synthetic medium deficient in methionine to an A600 of ∼0.5. Methionine was added to the yeast culture at a concentration of 20 μg/ml and incubated for 2 h at 26 °C. Cells were then washed and resuspended in SC medium buffered to pH 7.5. Cells were seeded onto concanavalin A-coated glass coverslips for microscopy or ratiometric imaging analysis. Fluorescence intensity was measured until the signal was stable, and then 50 μm CCCP was added to the samples. The cells were allowed 10 min to equilibrate to media at pH 7.5. The fluorescence intensity was again measured until the signal was stable. Statistical analysis is based on 300–500 cells over a minimum of three independent experiments.
Lysosomal Labeling
RAW cells were grown on glass coverslips in DMEM supplemented with 10% fetal bovine serum and 5% CO2 at 37 °C. Cells were pulsed with 2 mg/ml FITC-dextran for 1 h and chased for 1 h at 37 °C. Cells were then treated with 200 nm MF4, 20 nm apilimod, 1 μm concanamycin A, or an equivalent volume of DMSO for 1 h. For time lapse imaging, cells were labeled with 1 μm LysoTracker for 10 min, and then five images/s were captured for 10 s. Live cell images were captured in an environmental chamber at 37 °C with 5% CO2.
Ratiometric Analysis of Mammalian Lysosomal pH
Ratiometric analysis of lysosomal pH was done as reported previously (35, 37). Briefly, resting cells in calibration medium (145 mm KCl, 10 mm glucose, 1 mm MgCl2, and 20 mm Hepes, pH 7.2) were imaged in a Leiden chamber maintained at 37 °C. The cells were rapidly excited by light transmitted through a 485- and a 438-nm excitation filter from an X-Cite 120 lamp (from XFO). The fluorescence ratio of 485/438 nm was measured until the signal was stable. Subsequently, 10 μm nigericin and 5 μm monensin were then added to the calibration media set to different pH values (7.5, 6.5, 5.5, and 4.5). For each calibration point, cells were incubated and imaged for 5 min to obtain a stable 485/438 nm fluorescence ratio. When necessary, cells were treated with concanamycin A before calibration. To quantify lysosomal pH, the fluorescence intensity of 125–400 randomly selected swollen and wild-type lysosomes in 100–300 cells was measured over three independent experiments. Graphpad Prism 6 software was used to calculate a calibration curve as a function of the measured values relative to the pH values of the calibration buffer (the data were fitted to a Boltzmann sigmoidal dose-response curve).
Phosphoinositide Labeling and HPLC-coupled Flow Scintillation
RAW cells were incubated for 24 h in inositol-free DMEM (MP Biomedicals) with 10 μCi/ml myo-[2-3H(N)] inositol (PerkinElmer Life Sciences), 10% dialyzed fetal bovine serum (Gibco), 4 mm l-glutamine (Sigma-Aldrich), 1× insulin-transferrin-selenium-ethanolamine (Gibco), 20 mm Hepes (Gibco), and 1× penicillin/streptomycin (Sigma-Aldrich). At the end of the incubation, macrophages were treated with 20 nm apilimod for 1 h. Cells were then treated with 600 μl of 4.5% perchloric acid (v/v) on ice for 15 min, scraped, and pelleted at 12,000 × g for 10 min. Pellets were washed with 1 ml of ice cold 0.1 m EDTA and resuspended in 50 μl of water. Phospholipids were deacylated with 500 μl of methanol/40% methylamine/1-butanol (45.7% methanol:10.7% methylamine:11.4% 1-butanol (v/v)) for 50 min at 53 °C. Samples were vacuum-dried and washed twice by resuspending them in 300 μl of water and drying. The dried samples were then resuspended in 450 μl of water, extracted with 300 μl of 1-butanol/ethyl ether/ethyl formate (20:4:1), vortexed for 5 min, and centrifuged at 12,000 × g for 2 min. The bottom aqueous layer was collected and extracted twice more. The aqueous layer was vacuum-dried and resuspended in 50 μl of water. Equal counts of 3H were separated by HPLC (Agilent Technologies) through an anion exchange 4.6 × 250-mm column (Phenomenex) with a flow rate of 1 ml/min and subjected to a gradient of water (buffer A) and 1 m (NH4)2HPO4, pH 3.8 (adjusted with phosphoric acid) (buffer B) as follows: 0% B for 5 min, 0 to 2% B for 15 min, 2% B for 80 min, 2 to 10% B for 20 min, 10% B for 30 min, 10 to 80% B for 10 min, 80% B for 5 min, and 80 to 0% B for 5 min. The radiolabeled eluate was detected by β-RAM 4 (LabLogic) with a 1:2 ratio of eluate to scintillant (LabLogic) and analyzed using Laura 4 software. Each of the phosphoinositides was normalized against the parent phosphatidylinositol peak.
Statistical Analysis
Experimental values are given as the mean of a minimum of three independent experiments and include standard error of the mean (S.E.). The population size is indicated in the text or figure legends. Comparisons between groups were made by Student's t test or using an ANOVA test followed by Tukey's post hoc test as appropriate.
RESULTS
Lysosomes Remain Acidic in PIKfyve-inhibited Cells
Lysosomes depend on their highly acidic milieu for optimal degradative capacity and to drive molecular transport across its membrane. Therefore, it is important to understand the mechanisms that establish and maintain lysosomal acidification. The role of PtdIns(3,5)P2 in controlling lysosomal acidification in mammalian cells remains unclear.
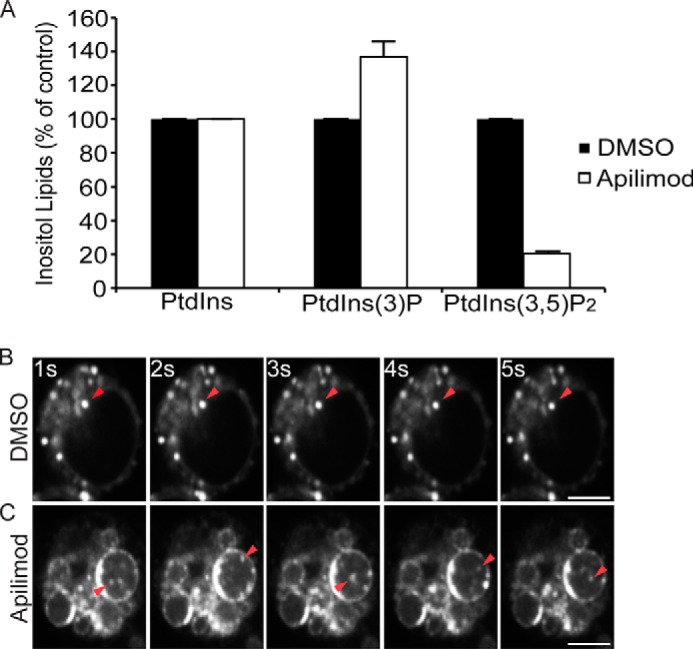
To better address this issue, we employed RAW macrophages as a model cell line given the importance of lysosomes in eliminating pathogens. RAW macrophages were treated for 1 h with 20 nm apilimod, a potent PIKfyve antagonist (38). Importantly, we limited PIKfyve inhibition to 1 h to avoid any nonspecific, indirect effects of prolonged PIKfyve abatement. First, we used myo-[2-3H]inositol labeling and HPLC-coupled flow scintillation to show that 20 nm apilimod treatment was sufficient to cause a ∼80% reduction in PtdIns(3,5)P2 levels relative to control cells and a concurrent increase in PtdIns(3)P, consistent with previous work (Fig. 1A and Ref. 38). The loss of PtdIns(3,5)P2 coincided with extensive vacuolation as observed previously (Fig. 1B and Ref. 39).
FIGURE 1.
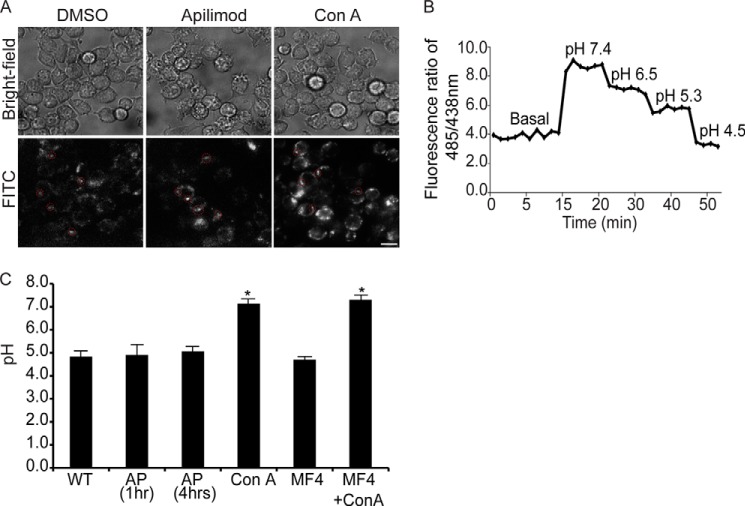
LysoTracker decorates the limiting membrane and intraluminar vesicles in swollen lysosomes induced by PIKfyve inhibition. A, levels of PtdIns, PtdIns(3)P, and PtdIns(3,5)P2 from [3H]myo-inositol-labeled cells after treatment with vector (DMSO) or 20 nm apilimod for 1 h. Levels are relative to PtdIns and normalized to vector-treated cells. B and C, RAW cells were treated with vector alone (B, DMSO) or with 20 nm apilimod (C) for 1 h followed by labeling with LysoTracker for 10 min. Live cells were then imaged at 5 frames/s for 10 s by spinning disc confocal microscopy. Each illustrated frame is 1 s apart, showing a 5-s sequence. Arrowheads point to a LysoTracker-positive lysosome (B) or to a moving ILV in apilimod-treated cells (C). See also supplemental Movie S1 and Movie S2. Scale bar = 5 μm.
Subsequently, cells were exposed to LysoTracker to label acidic compartments. In control cells, LysoTracker labeled punctate structures (Fig. 1B and supplemental Movie S1). In contrast, LysoTracker decorated the limiting membrane of vacuoles induced by PIKfyve inhibition (Fig. 1C). In addition, LysoTracker also associated with what appeared to be intraluminal vesicles (ILVs) within the swollen lysosomes (Fig. 1C). These ILVs moved freely within the vacuoles as depicted by live cell, time-resolved imaging (Fig. 1C, arrowheads, and supplemental Movie S2). Notably, swollen lysosomes were never homogenously filled with LysoTracker. We observed similar results when cells were treated with 200 nm MF4, a distinct PIKfyve antagonist (30) (data not shown). Overall, our data suggest that swollen lysosomes in PIKfyve-inhibited cells are still acidic.
Because LysoTracker provides only a qualitative indication of lysosomal pH, we used FITC, a ratiometric pH-sensitive fluorochrome, to quantitatively measure lysosomal pH by ratiometric imaging (35). Lysosomes were labeled by pinocytosis of FITC-labeled dextran. Because PtdIns(3,5)P2 may be involved in vesicular trafficking to lysosomes (40, 41), we first labeled lysosomes with FITC-dextran before treatment with MF4 or apilimod. Cells were then imaged and analyzed by ratiometric imaging as described under “Experimental Procedures” (Fig. 2, A and B). Control cells had a lysosomal pH of 4.8 ± 0.2, which is consistent with prior measurements (35, 37). Notably, the lysosomal pH in macrophages treated with apilimod or MF4 for 1 h was 4.9 ± 0.4 and 4.7 ± 0.1, respectively (Fig. 2C), numbers that were not significantly different from control cells. To test whether prolonged loss of PtdIns(3,5)P2 disrupted lysosomal pH, we treated cells for 4 h with 20 nm apilimod. Despite the protracted drug treatment, the lysosomal pH remained acidic (5.1 ± 0.2) and comparable with that of control cells (Fig. 2C). In contrast, lysosomal acidification was perturbed with ConA, a V-ATPase inhibitor, which increased the pH to 7.1 ± 0.2 (Fig. 2C). Importantly, ConA treatment also dissipated the lysosomal pH in RAW cells treated with PIKfyve inhibitors, suggesting that the V-ATPase was responsible for lysosome acidification in PIKfyve-hindered cells (Fig. 2C). Thus, lysosomal pH remains acidic after treatment with apilimod or MF4, indicating that PtdIns(3,5)P2 is not necessary for maintaining baseline lysosomal pH.
FIGURE 2.
Ratiometric imaging shows that lysosomes are acidic in PIKfyve-inhibited cells by ratiometric imaging. A, lysosomes in RAW cells were labeled with FITC-dextran for 1 h and chased for 1 h. Cells were then treated with vector alone (DMSO), with 20 nm apilimod to inhibit PIKfyve for 1 or 4 h, or with 1 μm ConA. Shown are corresponding bright field images for each treatment (top) and representative images of FITC-dextran labeled lysosomes (bottom). Note that images are composed of 2 × 2 binned pixels for increased signal intensity for ratiometric imaging. Randomized individual lysosomes were then selected using regions of interest (red dashed circles). B, for each condition, one field of cells was imaged live to acquire baseline FITC-dextran fluorescence followed by the addition of monensin and nigericin in high potassium media of known pH ranging from pH 7.4 to 4.5. This generated a calibration curve to convert fluorescence values to pH values. C, the lysosomal pH in RAW cells was calculated from cells treated with DMSO (based on n = 362 lysosomes), apilimod (AP) for 1 h (n = 412 lysosomes), apilimod for 4 h (n = 125 lysosomes), or ConA (n = 355 lysosomes). Values are means ± S.E. from a minimum of three different experiments. Using ANOVA and Tukey's post hoc test, we show that the lysosomal pH levels in DMSO-, apilimod-, and MF4-treated cells were not statistically different. *, statistical significant difference between ConA-treated cells versus samples not treated with ConA (p < 0.01).
Mup1-pHluorin Suggests That fab1Δ Vacuoles Are Acidic
We next examined the pH status of the enlarged vacuoles in fab1Δ yeast strains. We expected a defect in acidification given that past reports showed that quinacrine does not accumulate in the vacuoles of PtdIns(3,5)P2-deficient yeast (27, 28). We first employed the super-eclipitic pHluorin, a GFP variant in which fluorescence is potently quenched at low pH (34). pHluorin can be targeted to the lumen of yeast vacuoles by fusing it to Mup1 (34). Mup1 is a methionine transporter that localizes to the plasma membrane under low levels of methionine (34). However, excess extracellular methionine triggers the endocytosis of Mup1 followed by sorting into multivesicular bodies and trafficking to the vacuole for degradation (42). Consequently, by using the Mup1-pHluorin chimera, pHluorin accumulates in the vacuole in the presence of methionine (Ref. 34 and Fig. 3A).
FIGURE 3.
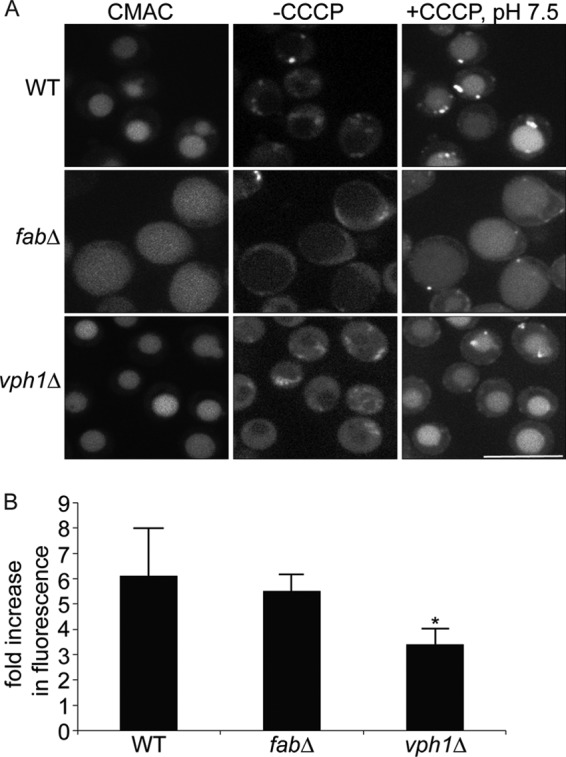
Vacuole-targeted pHluorin suggests that fab1Δ vacuoles are acidic. A, wild-type (WT), fab1Δ, and vph1Δ yeast cells carrying a genomic copy of Mup1-pHluorin were grown in the presence of methionine and stained with CMAC to label vacuoles. Cells were then mounted on concanavalin-coated slides and imaged live by spinning disc confocal microscopy before and after the addition of CCCP, a proton ionophore, in media set to pH 7.5. Scale bar = 10 μm. B, to measure changes in pHluorin intensity, images were obtained using epifluorescence ratiometric imaging. Regions of interest were then drawn over vacuoles to obtain the fluorescence intensity of pHluorin before and after CCCP addition. Fluorescence intensities were then background-corrected, and the fluorescence ratio after and before CCCP addition was calculated. Shown is the mean ± S.E. for wild-type (five experiments with a total of 514 vacuoles), fab1Δ (five experiments with a total of 372 vacuoles), and vph1Δ (three experiments with a total of 324 vacuoles) cells. Using ANOVA and Tukey's post hoc test, we show that there was no statistical difference between wild-type and fab1Δ cells, but there was a significant difference (*) between vph1Δ cells against control or fab1Δ cells (p < 0.01 for wild-type and p < 0.05 for fab1Δ).
To observe the pH status of vacuoles in PtdIns3,5P2-deficient yeast, fab1Δ Mup1-pHluorin and vph1Δ Mup1-pHluorin were generated. In the wild type, fab1Δ, and vph1Δ, there was a dim pHluorin-associated fluorescence signal in CMAC-labeled vacuoles, suggesting that pHluorin was being quenched by acidification (Fig. 3A). To better evaluate the vacuolar pH in these strains using pHluorin, we then measured its fluorescence intensity before and after alkalinization with the proton ionophore CCCP in media at pH 7.5. CCCP quickly dissipates the pH gradient and forces the vacuolar pH to equilibrate to the pH of the medium (43, 44). Wild-type, fab1Δ, and vph1Δ cells all exhibited an increase in the fluorescence intensity of vacuole-targeted pHluorin after alkalinization (Fig. 3A). But strikingly, when we quantified the ratio of fluorescence intensity after and before alkalinization, both wild type and fab1Δ showed similar -fold increases in fluorescence intensity, which were not significantly different from each other (6.1 ± 1.9 and 5.5 ± 0.67, respectively). By contrast, there was only a 3.4 ± 0.63-fold increase in pHluorin-based fluorescence in vph1Δ vacuoles (Fig. 3B). These data convey that fab1Δ and wild-type vacuoles are similarly acidic, whereas vph1Δ vacuoles are more alkaline.
Accumulation of Quinacrine and cDCFDA in Yeast Vacuoles
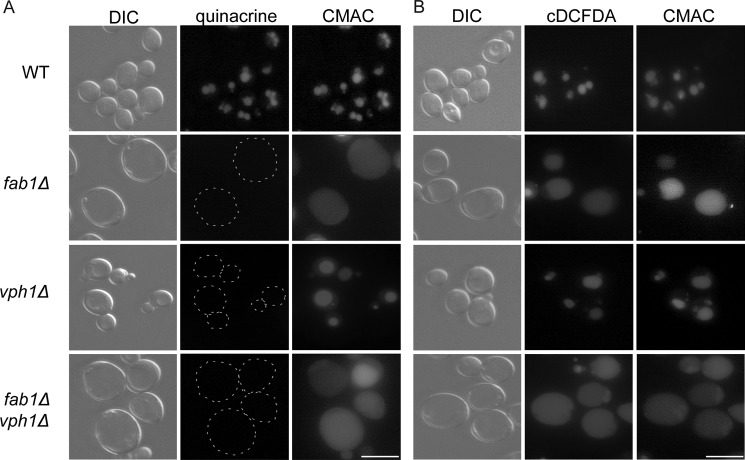
The Mup1-pHluorin assay produced an unexpected result, that is, fab1Δ and wild-type vacuoles appear to be similarly acidic. To ensure that our fab1Δ strain was not altered, we stained yeast vacuoles with quinacrine. As stated previously, quinacrine is a fluorescent dye that is thought to accumulate in the vacuole upon protonation. Therefore, quinacrine is often used to report on vacuolar acidification. Previous work had shown that quinacrine fails to accumulate in vacuoles in cells depleted for PtdIns(3,5)P2 (25–28). Consistent with the literature, we confirmed that quinacrine failed to accumulate in vacuoles, of fab1Δ, vph1Δ, and of the fab1Δ vph1Δ double mutant stained with CMAC, a vacuolar probe (Fig. 4A). In contrast, wild-type vacuoles, also identified by CMAC, were enriched in quinacrine (Fig. 4A).
FIGURE 4.
Vacuolar accumulation of quinacrine and cDCFDA. The vacuoles of wild-type, fab1Δ, vph1Δ, and fab1Δ vph1Δ double mutant yeast cells were labeled with CMAC and with either 200 μm quinacrine for 10 min (A) or 50 μm cDCFDA for 1 h (B). A, dashed lines outline cells with little to no quinacrine signal. Corresponding differential interference contrast (DIC) images are also shown. Live cell imaging was done with an epifluorescence microscope (scale bar = 10 μm).
To better understand the apparently contradictory data provided by the Mup1-pHluorin and the quinacrine assays, we employed an alternative method to quantitatively measure the vacuolar pH. The pH-sensitive fluorescent base cDCFDA had been shown previously to accumulate in and to measure the pH of vacuoles in C. neoformans (33). Therefore, we explored whether cDCFDA would be suitable to measure the pH of vacuoles in Saccharomyces cerevisiae. Foremost, we showed here that cDCFDA can accumulate in the vacuoles irrespective of apparent vacuolar acidification; cDCFDA accumulated in vacuoles identified by CMAC staining in wild-type, fab1Δ, vph1Δ, and fab1Δ vph1Δ yeast cells (Fig. 4B).
Quantitative pH Assays Indicate That fab1Δ Vacuoles Are Acidic
To quantify the vacuolar pH, cells were labeled with cDCFDA in SC medium. From a single pool of labeled cells, an aliquot was used to measure the baseline fluorescence intensity of cDCFDA by fluorimetry in SC medium at pH 7.5, recording the peak fluorescence intensity. After reading the baseline fluorescence, subsequent aliquots from the same culture were then treated with calibration buffer containing CCCP for 4 min to force the vacuolar pH to equilibrate to known media pH (pH 4–7), and the peak fluorescence intensity was recorded (Fig. 5A). These values were used to generate a standard curve of fluorescence intensity versus pH, which was then fit to a sigmoidal curve to extrapolate the baseline vacuolar pH for each strain. This method avoids the need for ratiometric fluorimetry, as each reading is an average of millions of cells and because calibration is internally controlled for each sample, i.e. the baseline and calibration readings use the same population of cells.
FIGURE 5.
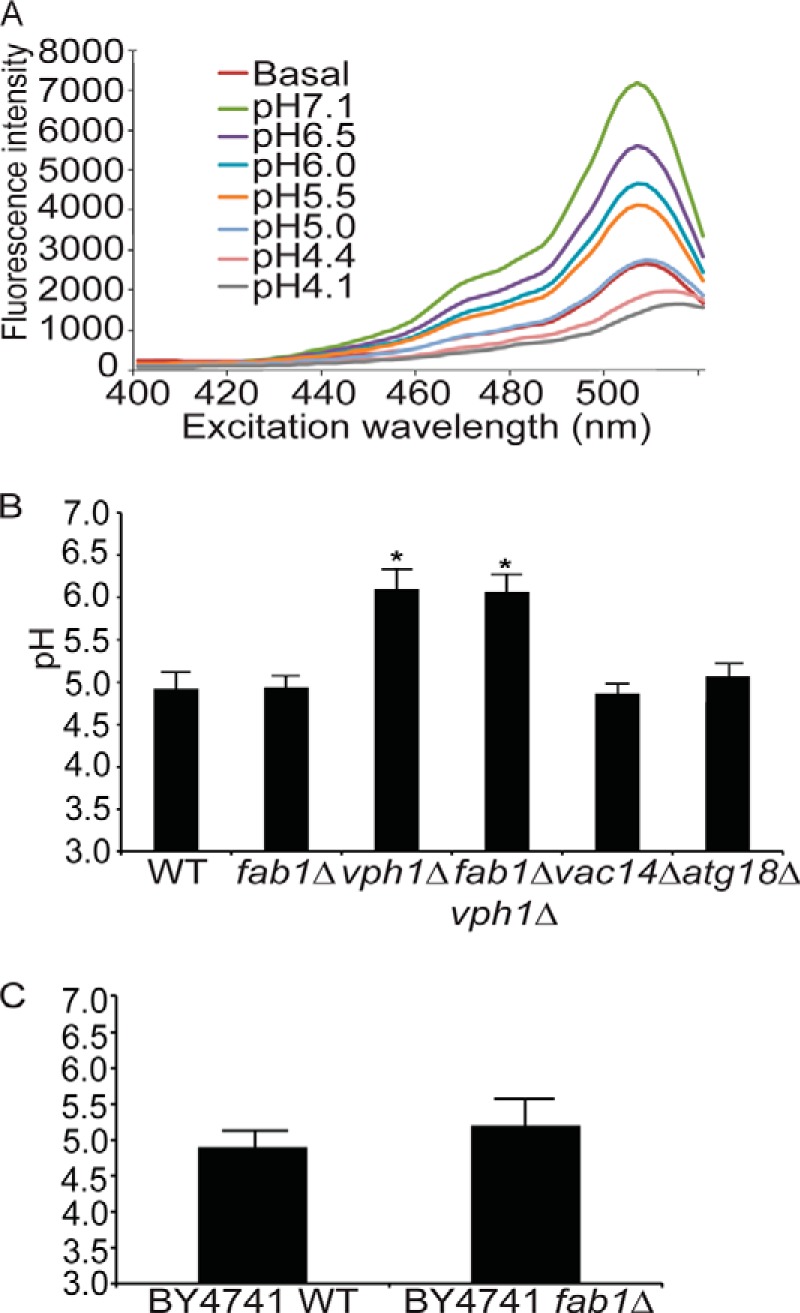
Quantification of vacuolar pH using cDCFDA-based fluorimetry. A, representative fluorimetry scan using wild-type yeast cells loaded with cDCFDA. A fluorescence intensity scan was obtained for resting yeast cells (Basal) followed by fluorescence intensity scans of cells from the same labeled population but in which vacuolar pH was equilibrated to the indicated pH by incubating them with CCCP for 4 min. B, peak intensities from calibration scans as shown in A were then used to generate calibration curves using a sigmoidal fit for the indicated strains to identify the basal pH. Using this method, we calculated the mean vacuolar pH and S.E. for wild type (n = 10, where each n is one independent experiment), fab1Δ (n = 10), vph1Δ (n = 3), fab1Δ vph1Δ double mutant (n = 3), vac14Δ (n = 3), and atg18Δ (n = 3). Using ANOVA and Tukey's post hoc test, there was a significant difference (*) for vph1Δ and vph1Δ fab1Δ against wild-type, fab1Δ, vac14Δ, and atg18Δ (p < 0.01) cells but not between wild-type, fab1Δ, vac14Δ, and atg18Δ cells. C, the vacuolar pH for wild type (n = 4) and fab1Δ (n = 3) in the BY4741 background strain. There was no significant difference between these strains and those from the SEY6210 background strain using Student's t test.
Importantly, we first assessed the effect of extracellular pH on the vacuole pH measured by cDCFDA fluorimetry by labeling cells in SC medium at pH 5.5 or 7.5 for 1 h. There was no observable difference in the apparent vacuolar pH in wild-type cells labeled in medium at pH 5.5 or 7.5 (data not shown). Therefore, all measurements were done with cells labeled in medium at pH 7.5. Strikingly, the steady-state vacuolar pH in fab1Δ cells was comparable to the wild type (pH 4.9 ± 0.1 and 4.9 ± 0.2, respectively, Fig. 5B), whereas a V-ATPase mutant, vph1Δ, had a more alkalinized vacuolar pH (pH 6.1 ± 0.2; Fig. 5B), which was significantly different from both the wild-type and fab1Δ cells. Importantly, the vacuolar pH of fab1Δ vph1Δ double mutant was 6.1 ± 0.2, comparable with vph1Δ (Fig. 5B). Because our assay produced similar vacuolar pH values for both the vph1Δ and fab1Δ vph1Δ strains, this suggests that cDCFDA fluorescence was not being quenched by other factors altered in fab1Δ cells that might lead to a falsely acidic reading of fab1Δ vacuoles. Lastly, the strains that we used in this study were based on the SEY6210 genetic background. Thus, we also measured the vacuolar pH in the BY4741 background and found that there was no significant difference between the wild type and fab1Δ (pH 4.9 ± 0.2 and 5.2 ± 0.4; Fig. 5C). This suggests that the genetic background did not account for the unexpected acidic vacuoles in fab1Δ identified by our assays.
We also tested whether the vacuolar pH remained intact upon deletion of VAC14 and ATG18, two key regulators of the PtdIns(3,5)P2 pathway. In vac14Δ cells, active Fab1 protein complexes fail to assemble, reducing PtdIns(3,5)P2 levels to 10% of the wild type (27, 28, 45, 46). In comparison, atg18Δ cells exhibit a 5–10-fold increase in the levels of PtdIns(3,5)P2 (47). Upon measuring the vacuolar pH, we observed no significant differences in the vacuolar pH between these strains and those of wild-type and fab1Δ cells (Fig. 5B). Thus, the steady-state vacuolar pH seems unaffected in cells deficient in or with elevated PtdIns(3,5)P2 levels.
The Acidification of fab1Δ Vacuoles Requires V-ATPase Activity
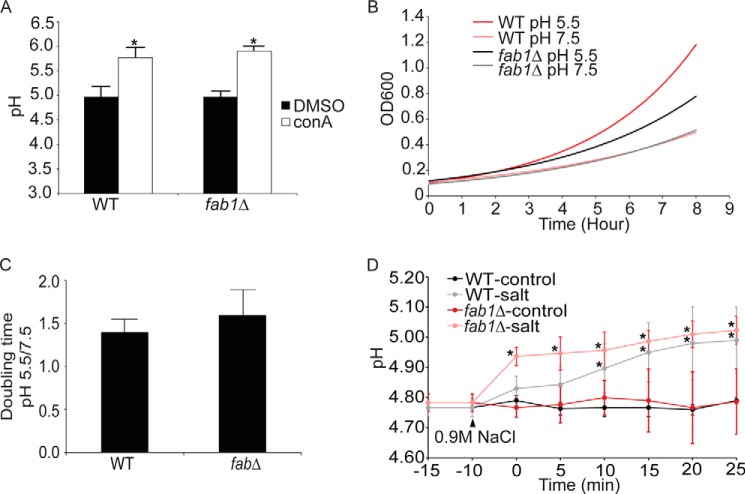
Our data strongly indicate that fab1Δ vacuoles are not defective in acidification. It remained possible that vacuole acidity in fab1Δ yeast was independent of V-ATPase activity. To test for this possibility we treated fab1Δ cells with ConA, a potent V-ATPase antagonist. Importantly, both wild-type and fab1Δ cells treated with ConA displayed an alkalinized vacuolar pH (pH 5.8 ± 0.2 and 5.9 ± 0.1, respectively; Fig. 6). This is consistent with our measurements obtained for vph1 and vph1Δ fab1Δ strains. Thus, we show here that fab1Δ vacuoles undergo a V- ATPase-dependent acidification. In addition, because V-ATPase mutants cannot grow in high pH media, we also tested whether fab1Δ cells could grow in media set to pH 5 or 7. We found that both wild-type and fab1Δ cells grew less well in media at pH 7 relative to media at pH 5 (Fig. 6B). However, because fab1Δ cells grow slower than wild-type cells even at pH 5, we normalized the growth rates for both strains at pH 7 media to pH 5 media. We found that there was little difference in the normalized growth rates between wild-type and fab1Δ cells (Fig. 6C). This is consistent with the reported observation that fab1Δ cells do not exhibit a vma growth defect typical of V-ATPase mutants, i.e. fab1Δ cells can grow in the presence of a high CaCl2 concentration and high pH (48).
FIGURE 6.
Basal vacuolar acidification in PtdIns(3,5)P2-deficient cells is dependent on V-ATPase activity. A, the vacuolar pH for cells labeled with cDCFDA and treated with 1 μm ConA or vector alone (DMSO) for 1 h. For each condition, three different independent experiments were performed. The mean ± S.E. is shown. Using Student's t test, there was a significant difference (*) between control (DMSO) and ConA-treated cells for either wild-type or fab1Δ cells (p < 0.01). B, overnight cultures grown in normal SC medium were cut back to A600 = 0.15 in SC medium set to pH 5.5 or 7.5. Cells were then incubated at 26 °C, and the A600 was measured each hour up to 8 h. Shown is a representative growth trace. C, the ratio between the doubling time of cells grown at pH 5.5 versus 7.5 from two independent experiments, each with three replicates. There was no significant difference in this ratio between wild-type and fab1Δ cells. D, the calibrated vacuolar pH for wild-type (n = 3) and fab1Δ (n = 3) cells before (−15 and −10 min) and after a 10-min exposure to 0.9 m NaCl (0 min) followed by pH measurements over 25 min at 5-min intervals. Using two-way ANOVA and Tukey's post hoc test, there was a significant difference (*) relative to the resting pH (−15 and −10 min) in the vacuolar pH in fab1Δ starting at 0 min and for the wild type 10 min after salt exposure (p < 0.05).
Recently, it was shown that PtdIns(3,5)P2 stimulates V-ATPase assembly during hyperosmotic shock, although the authors did not measure vacuolar pH (29). Salt shock is known to cause a 5–10-fold increase in PtdIns(3,5)P2 levels (20). Thus, we next tested whether salt shock might disturb the vacuolar pH in a Fab1-dependent manner. First, we observed a mild alkalinization of the vacuoles in both wild-type and fab1Δ cells exposed to 0.9 m NaCl over a period of 25 min (Fig. 6D). Interestingly, this alkalinization was not significant until 10 min post-salt shock in wild-type cells (Fig. 6D). By contrast, fab1Δ cells suffered a greater rate of alkalinization induced by salt shock, becoming significantly different immediately after the hyperosmotic stress (Fig. 6D). Thus, it appears that salt shock can disturb the vacuole pH and that PtdIns(3,5)P2-deficient cells are more prone to this effect than wild-type cells. This is supportive of the notion that PtdIns(3,5)P2 may play a role in stabilizing the V-ATPase during salt shock, as suggested previously (29).
Overall, using several quantitative assays, we report here that PIKfyve and Fab1 inactivation does not cause a defect in the steady-state pH of lysosomes and yeast vacuoles, respectively. This conclusion suggests that the role of PtdIns(3,5)P2 in vacuolar acidification is more nuanced than thought previously.
DISCUSSION
Depletion of PtdIns(3,5)P2 has been thought to alkalinize the yeast vacuole and, in some instances, to alkalinize lysosomes in higher eukaryotes (26, 29, 31, 32, 41). However, these studies employed qualitative assays that did not probe the actual pH of the yeast vacuole and of lysosomes. In contrast, we have provided quantitative evidence that suggests that the luminal pH of mammalian lysosomes and of the yeast vacuole remains acidic in PtdIns(3,5)P2-depleted cells.
The Lysosomal pH in PIKfyve-inhibited Mammalian Cells
Up to this point, the requirement for PIKfyve in maintaining the acidic lumen of lysosomes in higher eukaryotes has remained unclear. For instance, there is no apparent difference in acridine orange accumulation between control and MF4-treated mammalian cells (30). In contrast, others have suggested that cells treated with YM201636, another PIKfyve inhibitor, have reduced LysoTracker accumulation in swollen lysosomes (31).
Here, we show that LysoTracker does indeed decorate swollen lysosomes, indicating that they are still acidic. However, LysoTracker fluorescence was not present throughout the lumen of the swollen lysosomes, as might be expected. Instead, LysoTracker adhered to ILVs within and with the limiting membrane of swollen lysosomes. Others have reported this LysoTracker behavior in PIKfyve-hindered cells but interpreted it as a defect in acidification (31). Moreover, Hazeki et al. (50) depicted LysoTracker labeling of the limiting membrane of swollen lysosomes in PIKfyve-inhibited RAW cells, although they did not emphasize this effect. Although we cannot rule out that this LysoTracker behavior is specific to PIKfyve-inhibited cells, we speculate that LysoTracker also adheres to ILVs and to the limiting membrane of lysosomes in control cells; however, this is not resolvable because “normal” lysosomes are diffraction-limited when observed by light microscopy. We also speculate that because LysoTracker does not decorate the luminal space of swollen lysosomes, this may have led to a misinterpretation of the acidic state of lysosomes in PIKfyve-inhibited cells. Either way, acidotropic dyes like LysoTracker and acridine orange do not quantitatively assess pH. To resolve this caveat, we employed ratiometric imaging, which provides accurate and absolute pH values. Thus, using ratiometric imaging, we conclusively showed that lysosomes in control and PIKfyve-hindered cells retained a pH level of 4.8 to 4.9.
Overall, our results best support a model in which PIKfyve does not play a direct role in maintaining the acidic pH of mammalian lysosomes. Nevertheless, there are a few caveats to our study. First, it remains possible that chronic loss of PIKfyve (as in a gene knock-out of PIKfyve) may indirectly lead to less acidic lysosomes. However, given that lysosomes remained acidic even after 4 h of PIKfyve inhibition, this may be unlikely. Second, drug-treated mammalian cells retain small levels of PtdIns(3,5)P2 that may suffice to maintain acidic lysosomes. Yet, at these low levels of PtdIns(3,5)P2, many other defects are observed including lysosome swelling, phagosome maturation arrest, cytokine production impairment, and trafficking inhibition (38, 39, 51). Third, we do not exclude the possibility that extremely enlarged lysosomes may be more prone to alkalinization, as suggested elsewhere (31). Lastly, our observations may not apply to all mammalian cells or eukaryotic models.
The Vacuolar pH in fab1Δ Yeast Cells
Yeast vacuoles depleted of PtdIns(3,5)P2 fail to accumulate quinacrine (25–27), leading to the conclusion that these vacuoles are not acidic. However, our data suggest otherwise. First, we targeted the super-ecliptic pHluorin to the lumen of vacuoles. Both the wild-type and fab1Δ vacuoles displayed dim pHluorin-associated fluorescence after the addition of methionine. Strikingly, forced equilibration of the vacuole to pH 7.5 caused a similar large increase in fluorescence signal in control and fab1Δ vacuoles. This suggests that the fab1Δ vacuoles were as acidic as the wild-type cells. In contrast, vph1Δ cells exhibited a lesser increase in pHluorin fluorescence, consistent with a more alkaline vacuolar pH in these cells. Second, we employed cDCFDA-based fluorimetry to measure the vacuolar pH in S. cerevisiae by modifying a method used to quantify the vacuolar pH in C. neoformans (33). Consistent with the Mup1-pHluorin assay, the vacuolar pH of wild-type and fab1Δ cells was indistinguishable at pH 4.9. In addition, different levels of PtdIns(3,5)P2 present in fab1Δ, vac14Δ, and atg18Δ showed no effect on vacuolar pH.
The validity of these results is supported by two key observations: (i) the cDCFDA test reported a pH above 6 for both vph1Δ and fab1Δ vph1Δ mutants, making it unlikely that cDCFDA was reporting the behavior of another factor, like Ca+2, that might be altered in fab1Δ vacuoles and that might mask an alkaline vacuolar pH; and (ii) both wild-type and fab1Δ vacuoles became alkaline after V-ATPase inhibition. This result is also consistent with the fact that fab1Δ cells do not exhibit a vma phenotype, defined by the inability of V-ATPase mutants (vma) to grow in media with high CaCl2 and high pH (48). Overall, we present our data as evidence that fab1Δ vacuoles are as acidic as wild-type cells.
Recent elegant work indicates that PtdIns(3,5)P2 binds to the V-ATPase and helps to stabilize the V1-V0 assembly in vitro and in vivo (29). In addition, PtdIns(3,5)P2 appears to stimulate the ATPase activity of the V-ATPase using in vitro assays (29). However, it is important to note that the authors did not measure the actual pH of the vacuoles. Although seemingly contradictory, we believe these observations are not mutually exclusive with our data. First, we speculate that the vacuolar membrane is equipped with a pH-sensing protein that adjusts H+ efflux to offset reduced H+ influx, if indeed the V-ATPase is less active in the absence of PtdIns(3,5)P2. Although the identity of such a sensor in the lysosomal/vacuolar membrane is not known, there are examples of pH-sensing proteins in other systems; the activity of NHE3, an epithelial sodium/proton exchanger, is responsive to cytosolic pH (52, 53); Rim21 appears to sense external pH to modulate the Rim101 pathway, responsible for yeast adaptation to environmental pH (54, 55); even the V-ATPase may sense luminal endosomal pH to control Arf6 and ARNO membrane recruitment (56, 57). Second, a dearth of PtdIns(3,5)P2 may down-regulate specific channel and/or transporter activity that might reduce H+ efflux; as a precedent, PtdIns(3,5)P2 gates TRPML1, a lysosomal Ca+2 channel (24). Third, PtdIns(3,5)P2 may still control V-ATPase activity in response to a specific stimuli but be dispensable for steady-state conditions (29). Indeed, fab1Δ cells appeared to alkalinize more quickly than wild-type cells upon salt shock, consistent with this notion (Fig. 6D).
Lastly, despite an acidic vacuolar pH, quinacrine did not accumulate in fab1Δ vacuoles. There are at least two possibilities that might explain why quinacrine does not decorate fab1Δ vacuoles. First, quinacrine may require an unknown PtdIns(3,5)P2-dependent factor to enter or stay in the vacuole. For instance, quinacrine is known to bind ATP and other nucleotides and has been used to label ATP-rich vesicles (58, 59). Second, both quinacrine and chloroquine are weak bases that have been used to treat malaria, and in fact, chloroquine is often used to dissipate lysosomal pH (49, 60–62). Given that quinacrine is a weak base, we speculate that fab1Δ vacuoles may be more sensitive to quinacrine-mediated alkalinization than wild-type vacuoles, perhaps mimicking other stress conditions like hyperosmotic shock.
Summary
Overall, the work presented here makes use of potent quantitative tools including ratiometric imaging, fluorimetry, and quantitative fluorescence microscopy to show that mammalian lysosomes and yeast vacuoles retain a pH < 5.0 in cells depleted of PtdIns(3,5)P2, which is indistinguishable from control cells. We also showed that this is dependent on the V-ATPase. We argue that PtdIns(3,5)P2 has no role in maintaining a steady-state acidic pH in lysosomes/vacuoles, contrary to present thought.
Supplementary Material
Acknowledgments
We thank Dr. Kevan Shokat (UCSF) for kindly providing MF4. We thank Dr. Brenda Andrews (University of Toronto) for providing BY4741 wild-type and fab1Δ strains, Dr. Beverly Wendland (Johns Hopkins University) for kindly providing the Mup1-pHluorin::KanX yeast strain, and Dr. Scott Emr (Cornell University) for the fab1Δ, vac14Δ, and atg18Δ strains. We also thank Dr. Sergio Grinstein (Hospital for Sick Children, Toronto) for kindly allowing access to the ratiometric imaging system in his laboratory.
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (to R. J. B.).

This article contains supplemental Movie S1 and Movie S2.
- PtdIns(3,5)P2
- phosphatidylinositol 3,5-bisphosphate
- ANOVA
- analysis of variance
- cDCFDA
- 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- CMAC
- 7-amino-4-chloromethylcoumarin
- ConA
- concanamycin A
- DMSO
- dimethyl sulfoxide
- ILV
- intraluminal vesicle
- V-ATPase
- vacuolar-type H+-ATPase.
REFERENCES
- 1. Luzio J. P., Pryor P. R., Bright N. A. (2007) Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 [DOI] [PubMed] [Google Scholar]
- 2. Eskelinen E. L., Saftig P. (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta 1793, 664–673 [DOI] [PubMed] [Google Scholar]
- 3. Schneider L., Zhang J. (2010) Lysosomal function in macromolecular homeostasis and bioenergetics in Parkinson's disease. Mol. Neurodegener. 5, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruivo R., Anne C., Sagné C., Gasnier B. (2009) Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim. Biophys. Acta 1793, 636–649 [DOI] [PubMed] [Google Scholar]
- 5. Kiselyov K., Colletti G. A., Terwilliger A., Ketchum K., Lyons C. W., Quinn J., Muallem S. (2011) TRPML: transporters of metals in lysosomes essential for cell survival? Cell Calcium 50, 288–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S. C., Kane P. M. (2009) The yeast lysosome-like vacuole: endpoint and cross-roads. Biochim. Biophys. Acta 1793, 650–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klionsky D. J., Nelson H., Nelson N. (1992) Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J. Biol. Chem. 267, 3416–3422 [PubMed] [Google Scholar]
- 8. Miseta A., Kellermayer R., Aiello D. P., Fu L., Bedwell D. M. (1999) The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 451, 132–136 [DOI] [PubMed] [Google Scholar]
- 9. Russnak R., Konczal D., McIntire S. L. (2001) A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 276, 23849–23857 [DOI] [PubMed] [Google Scholar]
- 10. Ho M. N., Hirata R., Umemoto N., Ohya Y., Takatsuki A., Stevens T. H., Anraku Y. (1993) VMA13 encodes a 54-kDa vacuolar H(+)-ATPase subunit required for activity but not assembly of the enzyme complex in Saccharomyces cerevisiae. J. Biol. Chem. 268, 18286–18292 [PubMed] [Google Scholar]
- 11. Zhang J., Myers M., Forgac M. (1992) Characterization of the V0 domain of the coated vesicle (H+)-ATPase. J. Biol. Chem. 267, 9773–9778 [PubMed] [Google Scholar]
- 12. Manolson M. F., Proteau D., Preston R. A., Stenbit A., Roberts B. T., Hoyt M. A., Preuss D., Mulholland J., Botstein D., Jones E. W. (1992) The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H(+)-ATPase. J. Biol. Chem. 267, 14294–14303 [PubMed] [Google Scholar]
- 13. Leng X.H., Manolson M. F. (1998) Function of the COOH-terminal domain of Vph1p in activity and assembly of the yeast V-ATPase. J. Biol. Chem. 273, 6717–6723 [DOI] [PubMed] [Google Scholar]
- 14. Forgac M. (1999) Structure and properties of the vacuolar (H+)-ATPases. J. Biol. Chem. 274, 12951–12954 [DOI] [PubMed] [Google Scholar]
- 15. Graham L., Powell B., Stevens T. (2000) Composition and assembly of the yeast vacuolar H (+)-ATPase complex. J. Exp. Med. 203, 61–70 [DOI] [PubMed] [Google Scholar]
- 16. Tanabe M., Nishio K., Iko Y., Sambongi Y., Iwamoto-Kihara A., Wada Y., Futai M. (2001) Rotation of a complex of the γ subunit and c ring of Escherichia coli ATP synthase: the rotor and stator are interchangeable. J. Biol. Chem. 276, 15269–15274 [DOI] [PubMed] [Google Scholar]
- 17. Vik S. B., Antonio B. J. (1994) A mechanism of proton translocation by F1F0 ATP synthases suggested by double mutants of the a subunit. J. Biol. Chem. 269, 30364–30369 [PubMed] [Google Scholar]
- 18. Ho C. Y., Alghamdi T. A., Botelho R. J. (2012) Phosphatidylinositol 3,5-bisphosphate: no longer the poor PIP2. Traffic 13, 1–8 [DOI] [PubMed] [Google Scholar]
- 19. McCartney A. J., Zhang Y., Weisman L. S. (2014) Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays 36, 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dove S. K., Cooke F. T., Douglas M. R., Sayers L. G., Parker P. J., Michell R. H. (1997) Osmotic stress activates phosphatidylinositol 3,5-bisphosphate synthesis. Nature 390, 187–192 [DOI] [PubMed] [Google Scholar]
- 21. Ikonomov O. C., Sbrissa D., Shisheva A. (2001) Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J. Biol. Chem. 276, 26141–26147 [DOI] [PubMed] [Google Scholar]
- 22. Cooke F. T., Dove S. K., McEwen R. K., Painter G., Holmes A. B., Hall M. N., Michell R. H., Parker P. J. (1998) The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr. Biol. 8, 1219–1222 [DOI] [PubMed] [Google Scholar]
- 23. Ikonomov O. C., Sbrissa D., Dondapati R., Shisheva A. (2007) ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp. Cell Res. 313, 2404–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong X. P., Shen D., Wang X., Dawson T., Li X., Zhang Q., Cheng X., Zhang Y., Weisman L. S., Delling M., Xu H. (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gary J. D., Wurmser A. E., Bonangelino C. J., Weisman L. S., Emr S. D. (1998) Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 143, 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonangelino C. J., Catlett N. L., Weisman L. S. (1997) Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Cell. Biol. 17, 6847–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonangelino C. J., Nau J. J., Duex J. E., Brinkman M., Wurmser A. E., Gary J. D., Emr S. D., Weisman L. S. (2002) Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 156, 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dove S. K., McEwen R. K., Mayes A., Hughes D. C., Beggs J. D., Michell R. H. (2002) Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr. Biol. 12, 885–893 [DOI] [PubMed] [Google Scholar]
- 29. Li S. C., Diakov T. T., Xu T., Tarsio M., Zhu W., Couoh-Cardel S., Weisman L. S., Kane P. M. (2014) The signaling lipid PI(3,5)P2 stabilizes V1-Vo sector interactions and activates the V-ATPase. Mol. Biol. Cell 25, 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Lartigue J., Polson H., Feldman M., Shokat K., Tooze S. A., Urbé S., Clague M. J. (2009) PIKfyve regulation of endosome-linked pathways. Traffic 10, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jefferies H. B., Cooke F. T., Jat P., Boucheron C., Koizumi T., Hayakawa M., Kaizawa H., Ohishi T., Workman P., Waterfield M. D., Parker P. J. (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicot A. S., Fares H., Payrastre B., Chisholm A. D., Labouesse M., Laporte J. (2006) The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol. Biol. Cell 17, 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrison T. S., Chen J., Simons E., Levitz S. M. (2002) Determination of the pH of the Cryptococcus neoformans vacuole. Med. Mycol. 40, 329–332 [DOI] [PubMed] [Google Scholar]
- 34. Prosser D. C., Whitworth K., Wendland B. (2010) Quantitative analysis of endocytosis with cytolasmic pHluorin chimeras. Traffic 11, 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steinberg B. E., Huynh K. K., Brodovitch A., Jabs S., Stauber T., Jentsch T. J., Grinstein S. (2010) A cation counterflux supports lysosomal acidification. J. Cell Biol. 189, 1171–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 37. Botelho R. J., Hackam D. J., Schreiber A. D., Grinstein S. (2000) Role of COPI in phagosome maturation. J. Biol. Chem. 275, 15717–15727 [DOI] [PubMed] [Google Scholar]
- 38. Cai X., Xu Y., Cheung A. K., Tomlinson R. C., Alcázar-Román A., Murphy L., Billich A., Zhang B., Feng Y., Klumpp M., Rondeau J. M., Fazal A. N., Wilson C. J., Myer V., Joberty G., Bouwmeester T., Labow M. A., Finan P. M., Porter J. A., Ploegh H. L., Baird D., De Camilli P., Tallarico J. A., Huang Q. (2013) PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 20, 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim G. H., Dayam R. M., Prashar A., Terebiznik M., Botelho R. J. (2014) PIKfyve inhibition interferes with phagosome and endosome maturation in macrophages. Traffic 15, 1143–1163 [DOI] [PubMed] [Google Scholar]
- 40. Ikonomov O. C., Sbrissa D., Foti M., Carpentier J. L., Shisheva A. (2003) PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol. Biol. Cell 14, 4581–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rusten T. E., Rodahl L. M., Pattni K., Englund C., Samakovlis C., Dove S., Brech A., Stenmark H. (2006) Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol. Biol. Cell 17, 3989–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menant A., Barbey R., Thomas D. (2006) Substrate-mediated remodeling of methionine transport by multiple ubiquitin-dependent mechanisms in yeast cells. EMBO J. 25, 4436–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kasianowicz J., Benz R., McLaughlin S. (1984) The kinetic mechanism by which CCCP (carbonyl cyanide m-chlorophenylhydrazone) transports protons across membranes. J. Membr. Biol. 82, 179–190 [DOI] [PubMed] [Google Scholar]
- 44. Benz R., McLaughlin S. (1983) The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys. J. 41, 381–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Botelho R. J., Efe J. A., Teis D., Emr S. D. (2008) Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol. Biol. Cell 19, 4273–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alghamdi T. A., Ho C. Y., Mrakovic A., Taylor D., Mao D., Botelho R. J. (2013) Vac14 protein multimerization is a prerequisite step for Fab1 protein complex assembly and function. J. Biol. Chem. 288, 9363–9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dove S. K., Piper R. C., McEwen R. K., Yu J. W., King M. C., Hughes D. C., Thuring J., Holmes A. B., Cooke F. T., Michell R. H., Parker P. J., Lemmon M. A. (2004) Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 23, 1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sambade M., Alba M., Smardon A. M., West R. W., Kane P. M. (2005) A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 170, 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solomon V. R., Lee H. (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 625, 220–233 [DOI] [PubMed] [Google Scholar]
- 50. Hazeki K., Uehara M., Nigorikawa K., Hazeki O. (2013) PIKfyve regulates the endosomal localization of CpG oligodeoxynucleotides to elicit TLR9-dependent cellular responses. PLoS One 8, e73894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cai X., Xu Y., Kim Y. M., Loureiro J., Huang Q. (2014) PIKfyve, a class III lipid kinase, is required for TLR-induced type I IFN production via modulation of ATF3. J. Immunol. 192, 3383–3389 [DOI] [PubMed] [Google Scholar]
- 52. Babich V., Vadnagara K., Di Sole F. (2013) The biophysical and molecular basis of intracellular pH sensing by Na+/H+ exchanger-3. FASEB J. 27, 4646–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hayashi H., Szászi K., Coady-Osberg N., Orlowski J., Kinsella J. L., Grinstein S. (2002) A slow pH-dependent conformational transition underlies a novel mode of activation of the epithelial Na+/H+ exchanger-3 isoform. J. Biol. Chem. 277, 11090–11096 [DOI] [PubMed] [Google Scholar]
- 54. Obara K., Yamamoto H., Kihara A. (2012) Membrane protein Rim21 plays a central role in sensing ambient pH in Saccharomyces cerevisiae. J. Biol. Chem. 287, 38473–38481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lamb T. M., Xu W., Diamond A., Mitchell A. P. (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276, 1850–1856 [DOI] [PubMed] [Google Scholar]
- 56. Marshansky V. (2007) The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem. Soc. Trans. 35, 1092–1099 [DOI] [PubMed] [Google Scholar]
- 57. Hurtado-Lorenzo A., Skinner M., El Annan J., Futai M., Sun-Wada G. H., Bourgoin S., Casanova J., Wildeman A., Bechoua S., Ausiello D. A., Brown D., Marshansky V. (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 8, 124–136 [DOI] [PubMed] [Google Scholar]
- 58. Cao Q., Zhao K., Zhong X. Z., Zou Y., Yu H., Huang P., Xu T. L., Dong X. P. (2014) SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability. J. Biol. Chem. 289, 23189–23199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bodin P., Burnstock G. (2001) Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J. Cardiovasc. Pharmacol. 38, 900–908 [DOI] [PubMed] [Google Scholar]
- 60. Guha S., Coffey E. E., Lu W., Lim J. C., Beckel J. M., Laties A. M., Boesze-Battaglia K., Mitchell C. H. (2014) Approaches for detecting lysosomal alkalinization and impaired degradation in fresh and cultured RPE cells: Evidence for a role in retinal degenerations. Exp. Eye Res. 126, 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krogstad D. J., Schlesinger P. H., Gluzman I. Y. (1985) Antimalarials increase vesicle pH in Plasmodium falciparum. J. Cell Biol. 101, 2302–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lüllmann-Rauch R., Pods R., von Witzendorff B. (1996) The antimalarials quinacrine and chloroquine induce weak lysosomal storage of sulphated glycosaminoglycans in cell culture and in vivo. Toxicology 110, 27–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.