Highlights
-
•
Abdominal inflammatory myofibroblastic tumor (IMFT) macroscopically appear as mass lesions that grow slowly and sometimes cause destruction of adjacent structures. The exact diagnosis can only be made with histopathological examination of permanent tissue. Resection with negative surgical borders is the most suitable surgical technique. The same principles also apply to IMFT developing on the basis of actinomycosis because it is quite difficult to predict at the preoperative period that the lesion developed secondary to actinomycosis. The diagnosis is almost always made with histopathological examinations. Affected patients should receive prolonged antibiotherapy once the diagnosis is confirmed.
Keywords: Inflammatory myofibroblastic tumor, Actinomycosis, Colon, Immunhistochemical stain
Abstract
Introduction
Inflammatory myofibroblastic tumors (IMFTs) are neoplastic lesions that are either benign or have low-grade malignancy potential. Although the etiopathogenesis is not entirely clear, many factors play a role in their development, including trauma, autoimmune disorders, and infectious and inflammatory processes. However, IMFTs caused by Actinomyces spp. infection are rare, with a limited number of cases reported in the literature.
Presentation of case
A 30-year-old woman was admitted to our clinic with abdominal pain and a palpable abdominal mass. Contrast-enhanced computed tomography revealed a tumoral lesion (11 × 10 × 7 cm) in the right colon. A right hemicolectomy and ileocolic anastomosis were performed, during which almost complete obstruction of the lumen by the 7.5 × 7.0 × 5.0 cm tumor was observed. Histopathology and immunohistochemical findings revealed that the tumor was consistent with an IMFT that developed from an Actinomyces infection. The patient was then placed on amoxicillin and doxycycline therapy.
Conclusion
This case demonstrates that the development of IMFT secondary to actinomycosis is difficult to predict in the preoperative period. Once an exact diagnosis is confirmed by histopathologic examination, affected patients should receive prolonged antibiotherapy.
1. Introduction
Inflammatory myofibroblastic tumors (IMFTs) are pseudosarcomatous inflammatory lesions that typically show a benign behavioral pattern [1]. However, these tumors have the potential to locally recur and invade neighboring tissues, and though rare, they can also metastasize to distant sites. These lesions can be found in almost all organs and tissues of human body, though they most commonly occur in the lungs or within the abdominal cavity [1]. The most common extrapulmonary sites are the omentum and mesentery, but lesions can also be found in the stomach, liver, gallbladder, spleen, pancreas, and colon [1–3]. Despite being seen in almost all age groups, IMFTs most commonly occur in children and young adults, and are more frequent in women than in men. Whether IMFTs are an inflammatory lesion or a true neoplasm remains controversial. Many factors have been implicated in their etiopathogenesis, including the presence of autoimmune disorders, bacterial or viral infections, trauma, and undergoing a surgical operation. This report describes a rare case of a colonic IMFT that developed secondary to an Actinomyces infection.
2. Presentation of case
A 30-year-old woman was referred to our outpatient clinic with abdominal pain and a palpable abdominal mass. She had fatigue and malaise concurrent with a mass in the lower-right quadrant of her abdomen that had been progressively growing for six months. She stated that she had given birth to three children and undergone a Caesarean section one year ago, and had not used an intrauterine device (IUD) for birth control. Physical examination revealed a 10 × 10 cm mobile mass in the lower-right quadrant of her abdomen. Blood tests were as follows: hemoglobin, 10.5 g/dL (normal: 12.5–16.0 g/dL); hematocrit, 33.8% (normal: 37–47%); mean corpuscular (MC) volume, 62 L (normal: 78–100 L); MC hemoglobin, 19 pg (normal: 27–33 pg); MC hemoglobin concentration, 31.1 g/dL (normal: 32.5–35.2 g/dL); ferritin, 52 ng/mL (normal: 30–400 ng/mL); total iron-binding capacity, 256 μg/dL (normal: 155–300 μg/dL); vitamin B12, 370 pg/mL (normal: 191–663 pg/mL); folic acid, 6.8 ng/mL (normal: 3.1–17.5 ng/mL); white blood cell count, 11,900 μL (normal: 4100–11,200/μL); platelet count, 468,000 μL (normal: 150–400,000 μL); C-reactive protein, 71 mg/dL (normal: 0–5 mg/dL). Other biochemical blood tests and urinalysis were normal. An abdominal plain film was also normal, but abdominal ultrasonography showed conglomerated bowel loops in the pericecal region in the lower-right quadrant. Contrast-enhanced abdominal computed tomography revealed marked mural thickening in the terminal ileum and colon segment, a heterogeneous appearance of the neighboring mesentery and pericolic fatty tissue, and an isodense mass (11 × 10 × 7 cm) in the ascending colon (Fig. 1). These findings suggested plastroned appendicitis, colonic tumor, or granulomatous disease, and the decision was made to proceed with surgery. A laparotomy was performed via a midline incision, and exploration revealed a 10 × 10 cm mass lesion that was 10 cm distal to ileocecal valve. Severe edema and lymphadenopathies in the mesenteric tissues surrounding the mass were observed. The mass was considered malignant, and a standard right hemicolectomy and ileocolic anastomosis were performed. Macroscopic examination of the tumor specimen showed a 7.5 × 7.0 × 5.0 cm tumoral lesion that nearly completely obstructed the bowel lumen. Histopathologic findings were consistent with an IMFT, which included foci of Actinomyces (Fig. 2). The tumor cells were immunopositive for vimentin (Fig. 3) and desmin, and negative for CD34 and S-100. No tumor tissue was found in any of the 30 lymph nodes that were also biopsied. These findings indicate that the tumor was an inflammatory pseudotumor that developed secondary to an Actinomyces infection. The patient was discharged on the sixth postoperative and placed on amoxicillin and doxycycline therapy for an additional six months.
Fig. 1.
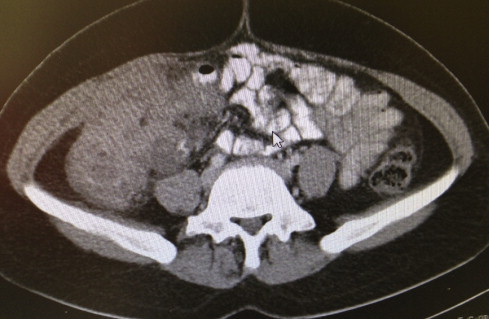
Contrast-enhanced abdominal tomography. Marked mural thickening was observed in the terminal ileum and colon segment, and neighboring mesentery and pericolic fatty tissue had a heterogeneous appearance. An 11 × 10 × 7 cm isodense mass was seen in the ascending colon.
Fig. 2.

Histopathology of the tumor. Hematoxylin and eosin staining revealed (A) bland fusiform elements arranged in fascicules, with (B) lymphocytes, plasma cells, and eosinophils, indicative of an inflammatory myofibroblastic tumor. (C) The bland fusiform elements of the tumor contained Actinomyces (basophilic clusters of bacteria with a peripheral, more eosinophilic zone of radiating filaments surrounded by acute inflammation) (A: 100 × ; B: 200 × ; C: 100 × ).
Fig. 3.

Immunohistochemical analysis. The tumor cells were immunopositive for vimentin (100 × ).
3. Discussion
IMFTs are typically benign inflammatory lesions of soft tissue, though they can potentially invade neighboring tissues, appearing both clinically and radiologically as malign tumors, and are thus referred to as pseudotumors. Because of their potential for invasion, local recurrence, and distant organ metastasis, IMFTs are defined by the World Health Organization as neoplasms with intermediate biological potential [1]. The histopathologic properties of these lesions have led to a variety of designations, including inflammatory pseudotumors, plasma cell granulomas, inflammatory myofibrohistiocytic proliferation, histiocytoma, xanthoma, fibroxanthoma, and xanthomatous pseudotumors [1].
IMFTs occur in patients with rheumatoid arthritis, systemic lupus erythematosus, and Sjögren’s syndrome, suggesting that autoimmunity plays a role in their development [1]. Recent cytogenetic studies have shown a relationship between IMFTs and mutations in the gene encoding anaplastic lymphoma kinase [1]. However, a significant majority of patients with IMFT have malaise, loss of appetite, anemia, fever, and weight loss, suggesting a possible role of infectious diseases in the etiology [1]. Infectious agents implicated in the emergence of IMFTs include the Epstein–Barr virus, Campylobacter jejuni, Mycobacterium avium-intracellulare, Escherichia coli, Pseudomonas veronii, Pasteurella haemolytica, Bacteroides corrodens, Coxiella burnetii, and Actinomyces israelii [1].
A. israelii is a gram-positive anaerobic or microaerophilic filamentous bacterium that is a component of the normal flora within the oropharynx, bronchial tree, upper gastrointestinal system, and female genitalia [2–7]. This bacterium only invades the mucosal barrier and adjacent tissues when injury occurs [2,7], which induces an inflammatory response and facilitates pseudotumor development [6]. Actinomyces infiltration is the cause of actinomycosis, defined as a chronic, progressive, suppurative disease characterized by multiple abscesses, drained sinuses, abundant granulation tissue, and dense fibrous tissue [2,5,7] Abdominal actinomycosis constitutes 20% of all actinomycosis cases [2]. It mostly involves the ileocecal region and right colon, but can also affect the retroperitoneum, perianal region, urinary bladder, liver, spleen, and sometime the stomach [5,6]. The most common predisposing factor for abdominal actinomycosis is prolonged IUD use [2,3]. Other predisposing factors include appendicitis, diverticulitis, intestinal perforation, peptic ulcer perforation, cholecystectomy, thyphoid fever, amebic dysentery, trauma, and hemorrhagic pancreatitis [2,7]. Although the patient in the present case had no history of IUD use, she had prolonged discharge that terminated spontaneously from the right corner of the surgical incision from the caesarean section.
Abdominal actinomycosis can manifest as a variety of symptoms, including nonspecific abdominal pain, malaise, appetite loss, fever, weight loss, abdominal mass, postoperative foul smelly discharge, and signs of intestinal obstruction [1]. Laboratory tests generally indicate an elevated erythrocyte sedimentation rate, and leukocytosis, neutrophilia, and elevated C-reactive protein levels, as demonstrated in the present case. However, it is not possible to diagnose abdominopelvic actinomycosis solely by clinical and biochemical parameters, as these mimic chronic infectious disorders or malignant abdominal tumors. Abdominal actinomycosis has no specific radiological feature [2], and can appear to invade neighboring tissues as malignant tumors do, with mass-like dense granulation tissue and fibrosis.
Similarly, it is difficult to differentiate abdominal actinomycosis-induced IMFTs from IMFTs secondary to other causes, which perhaps can only be distinguished by the frequent appearance of abscesses, sinuses, or fistulas. Nevertheless, the presence of yellowish-brown sulfur granules and/or branching gram-positive non-acid resistant bacilli in tumoral lesions can be a diagnostic indicator. In addition, bacteria may proliferate in anaerobic culture [8]. Newer molecular methods, such as polymerase chain reaction, are increasingly being used for diagnosis of Actinomyces sp. However, histopathologic evaluation should ultimately be performed for diagnosis/differential diagnosis of a tumoral component, as pseudotumors can contain varying ratios of spindle cells, myofibroblasts, and inflammatory cell infiltrates such as plasma cells, lymphocytes, and histiocytes. Immunohistochemical staining, such as for smooth-muscle actin, desmin, activin receptor-like kinase-1, creatine, cyclooxygenase-2, and vascular endothelial growth factor, are most commonly used for differential diagnosis of the disease.
The management of abdominopelvic actinomycosis is dependent on both the extent of the disease and the condition of the patient [9]. Surgical removal of the tumoral lesion with resection or drainage, supplemented with long-term antibiotic therapy is recommended, [9,10] such as with parenteral penicillin G [2,9] followed by peroral penicillin V or amoxicillin for up to 12 months [2,9,10]. Tetracycline, erythromycin, clindamycin can be given in those with penicillin allergy [2]. Ultrasonography or computed tomography are used to check treatment efficiency and recurrences. Our experiences have indicated that medical therapy should be pursued at least until symptoms and inflammatory signs subside, as actinomycosis is a resistant infection and can lead to severe fibrosis and granulation tissue.
In conclusion, this is the first report of actinomycosis-induced IMFT developing in the right colon. The development of IMFTs secondary to actinomycosis is difficult to preoperatively predict, and should be confirmed by histopathologic examination. Affected patients should receive prolonged antibiotherapy once the diagnosis is confirmed.
Conflict of interest
We declare that there is no conflict of interest.
Funding
None.
Ethical approval
Ethics committee approval was not required.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Gumus S and Yagmur Y performed surgical procedure; Akbulut S and Yagmur Y designed the report; Akbulut S and Demircan F conducted the literature review and wrote the report; Sogutcu N provided histopathologic information.
Guarantor
Sami Akbulut, Yusuf Yagmur (Prof Dr) (Head of Department of Surgery).
References
- 1.Yagmur Y., Akbulut S., Gumus S. Mesenteric inflammatory pseudotumor: a case report and comprehensive literature review. J. Gastrointest. Cancer. 2014;45(4):414–420. doi: 10.1007/s12029-014-9642-7. [DOI] [PubMed] [Google Scholar]
- 2.Das N., Lee J., Madden M., Elliot C.S., Bateson P., Gilliland R. A rare case of abdominal actinomycosis presenting as an inflammatory pseudotumour. Int. J. Colorectal. Dis. 2006;21(5):483–484. doi: 10.1007/s00384-004-0668-3. [DOI] [PubMed] [Google Scholar]
- 3.Evans J., Chan C., Gluch L., Fielding I., Eckstein R. Inflammatory pseudotumour secondary to actinomyces infection. Aust. N. Z. J. Surg. 1999;6:467–469. doi: 10.1046/j.1440-1622.1999.01602.x. [DOI] [PubMed] [Google Scholar]
- 4.Radhi J., Hadjis N., Anderson L., Burbridge B., Ali K. Retroperitoneal actinomycosis masquerading as inflammatory pseudotumor. J. Pediatr. Surg. 1997;32(4):618–620. doi: 10.1016/s0022-3468(97)90721-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang X.X., Lin J.M., Xu K.J., Wang S.Q., Luo T.T., Geng X.X., Huang R.G., Jiang N. Hepatic actinomycosis: report of one case and analysis of 32 previously reported cases. World J. Gastroenterol. 2014;20(43):16372––16376. doi: 10.3748/wjg.v20.i43.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naf F., Enzler-Tschudy A., Kuster S.P., Uhlig I., Steffen T. Abdominal actinomycosis mimicking a malignant neoplasm. Surg. Infect. (Larchmt) 2014;15(4):462––463. doi: 10.1089/sur.2012.057. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.Y., Kwon H.J., Cho J.H., Oh J.Y., Nam K.J., Lee J.H., Yoon S.K., Kang M.J., Jeong J.S. Actinomycosis of the appendix mimicking appendiceal tumor: a case report. World J. Gastroenterol. 2010;16(3):395–397. doi: 10.3748/wjg.v16.i3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H.Y., Lee I.S., Kim S., I, Jung S.E., Kim S.W., Kim S.Y., Chung M.K., Kim W.C., Oh S.T., Kang W.K. Clinical features of abdominal actinomycosis: a 15-year experience of a single institute. J. Kor. Med. Sci. 2011;26(7):932–937. doi: 10.3346/jkms.2011.26.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz M., Akbulut S., Samdanci E.T., Yilmaz S. Abdominopelvic actinomycosis associated with an intrauterine device and presenting with a rectal mass and hydronephrosis: a troublesome condition for the clinician. Int. Surg. 2012;97(3):254–259. doi: 10.9738/CC121.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagmurdur M.C., Akbulut S., Colak A., Aygun C., Haberal M. Retroperitoneal fibrosis and obstructive uropathy due to actinomycosis: case report of a treatment approach. Int. Surg. 2009;94(4):283–288. [PubMed] [Google Scholar]


