Abstract
To understand how function arises from the interactions between neurons, it is necessary to use methods that allow the monitoring of brain activity at the single-neuron, single-spike level and the targeted manipulation of the diverse neuron types selectively in a closed-loop manner. Large-scale recordings of neuronal spiking combined with optogenetic perturbation of identified individual neurons has emerged as a suitable method for such tasks in behaving animals. To fully exploit the potential power of these methods, multiple steps of technical innovation are needed. We highlight the current state-of-the-art in electrophysiological recording methods, combined with optogenetics, and discuss directions for progress. In addition, we point to areas where rapid development is in progress and discuss topics where near-term improvements are possible and needed.
INTRODUCTION
How does the brain orchestrate perceptions, thoughts, and actions from the activity of its neurons? Addressing these challenging questions requires methods capable of isolating, identifying and manipulating statistically representative fractions of the neurons of the investigated circuits at single-neuron and single-spike resolution during behavior (Alivisatos et al., 2013; Buzsáki, 2004; Carandini, 2012; Marblestone et al., 2013; Nicolelis et al., 1997). Electrical recording of extracellular action potentials (‘spikes’) is among the oldest neuronal recording techniques (Adrian and Moruzzi, 1939) and its physical principles are well understood (Buzsáki et al., 2013; Einevoll et al., 2013; Logothetis, 2003; Nádasdy Z. et al., 1998). Electrical recordings have the additional advantage of simultaneously detecting the superimposed synaptic activity of neurons in the form of local field potentials (LFPs).
While the number of simultaneously-recorded neurons has doubled every seven years over the past several decades (Stevenson and Kording, 2011), the widespread adoption of large-scale recording methods by the neuroscience community has generally lagged behind. However, significant recent technological innovations are now bringing large-scale recording methods into the mainstream, thereby enabling progressively more advanced experiments and analyses – and associated challenges. To meet the expectations of the BRAIN Initiative (http://www.nih.gov/science/brain/), this trend of innovation coupled with translation over multiple technologies becomes increasingly important.
Relating the activity patterns of circuit components to behavior is a powerful method for inferring their role in organizing behavior. Testing their causal role, however, requires additional steps. Optogenetics has recently emerged as the appropriate method for fast manipulation of genetically identified neuron types. While optogenetic methods have the sufficient temporal speed for interacting with neuronal circuits, their spatial resolution in currently used instantiations is typically very poor. Another limitation is the lack of multisite light delivery methods for flexible circuit control in freely behaving small rodents. Thus, new techniques are needed to validate hypotheses derived from the correlation measures between the spatiotemporal coordination of neurons and behavior.
To harness the maximum potential of combining large-scale recording methods, progressive innovation is needed at various levels of device integration. Our Primer focuses on technologies developed for small-size animals. However, the discussed methods can be adapted to non-human primates as well, and several recent innovations with specific solutions for primates have been reviewed recently (Cavanaugh et al., 2012; Diester et al., 2011; Gerits et al., 2012; Gray et al., 2007). Three issues are addressed: (I) state-of-art current methods and barriers, (II) approaches to make the current upper limit more accessible to mainstream neuroscience, and (III) a look ahead to technology innovations required to increase the upper limit of recording sites by one or two orders of magnitude. A flow chart of large-scale recording and the closed-loop targeted feedback perturbation of neurons in their native networks is illustrated in Figure 1.
Figure 1. Flow chart of large-scale silicon probe recordings of unit and LFP activity combined with optogenetic manipulation of circuits.

The components of the chart are discussed in this Primer. While large-scale recordings and optogenetic perturbations can be implemented separately, their combination provides a powerful tool for circuit analysis. FPGA, field-programmable gate array.
Sensing neuronal activity from the extracellular space
Neuronal activity gives rise to transmembrane current and the superposition of multiple potentials spreading through the extracellular medium can be measured as voltage (Ve) , referred to as local field potential (LFP). The somato-dendritic tree of the neurons forms an electrically conducting interior surrounded by a highly insulating membrane with hundreds to thousands of synapses located along it. They can conduct either inward (excitatory) or outward (hyperpolarizing, most often inhibitory) currents. The amplitude and time-varying patterns of the LFP depend on the proportional contributions of the multiple current sources and are modified by the various properties of the brain tissue (Buzsáki et al., 2012; Einevoll et al., 2013). Because the amplitude of Ve inversely scales with the distance between the source and the recording site, the larger the distance, the less informative is the measured LFP regarding its origin.
Action potentials generate the largest-amplitude currents across the somatic membrane and can be detected as extracellular ‘unit’ or spike activity. In addition to the distance between the neuron and the recording electrode, the magnitude of the extracellular spike depends on the size and shape of the neuron (Buzsáki et al., 2012; Einevoll et al., 2013). Extracellular spikes are negative near the soma (sink), corresponding to the fastest rate of Na+ influx into the cell body (Figure 2).
Figure 2. Electric signals in the extracellular space.

(A) Simultaneous intracellular and extracellular recordings of action potential in a hippocampal CA1 neuron in vivo. Note that the trough and peak of the extracellular unit spike (0.1 Hz-5 kHz) correspond to the maximum rate of rise and maximum rate of fall of the intracellular action potential. (B) Extracellular contribution of an action potential (‘spike’) to the LFP in the vicinity of the spiking pyramidal cell. The magnitude of the spike is normalized. The peak-to-peak voltage range is indicated by the color of the traces. Note that the spike amplitude decreases rapidly with distance from the soma. The distance-dependence of the spike amplitude within the pyramidal layer is shown (bottom left panel) with voltages drawn to scale, using the same color identity as the traces in the boxed area. The same traces are shown normalized to the negative peak (bottom right panel). (C) Multisite electrodes can estimate the position of the recorded neurons by triangulation of extracellular voltage measurements. Distance of the electrode tips from a single pyramidal cell is indicated by arrows. The spike amplitude of neurons (>60 µV) within the gray cylinder (50 µ m radius), containing ∼100 neurons, can be used for effective separation by current clustering methods. (D) Coherence maps of gamma activity (30–90 Hz) in the hippocampus of a freely behaving rat. The 10 example sites (black dots) served as reference sites, and coherence was calculated between the reference site and the remaining 255 locations of an 8-shank (32-site vertical linear spacing at 50 µm) probe. (A) Reproduced from (Buzsáki et al., 1996). (B) Reproduced from (Buzsáki et al., 2012). (C) Reproduced from (Buzsáki, 2004). (D) Reproduced from (Berenyi et al., 2014).
In cortical structures, the bodies and apical dendrites of densely-packed pyramidal neurons lie parallel to each other, making the separation of neurons on the basis of their extracellular spikes difficult. On the other hand, such architecturally regular geometry is favorable for estimating afferent-induced synaptic inputs to the circuit since afferents in cortical layers run perpendicular to the dendritic axis, and this arrangement facilitates the combination of synchronously active synaptic/transmembrane currents by superposition (Figure 2B). In summary, both identification of spikes of different neurons and the inference of population synaptic activity from the extracellular voltage require high-resolution spatiotemporal sampling of the extracellular space, ideally equal to or smaller than the diameter of neuronal cell bodies.
Hardware components for large-scale monitoring of neuronal activity
Recording electrodes
Action potentials can be detected as voltage by placing a conductor, such as a wire, in close proximity to the neuron (Adrian and Moruzzi, 1939). Because neurons of the same class generate largely identical action potentials, identifying an individual neuron from its extracellular spikes is possible by moving the electrode tip substantially closer to the cell body of one neuron than to the neighboring neurons. To record another neuron or dissociate the spike signals of two different nearby neurons, more than one electrode is needed.
The use of two or more recording sites in close proximity allows for the triangulation of distances between the spike-emitting cell bodies and the electrodes because the spike amplitude waveform morphology changes as a function of distance and direction from the neuron (Drake et al., 1988). Often, this task is accomplished with two or four twisted wires (dubbed “stereotrodes’ or “tetrodes”; (McNaughton et al., 1983; Wilson and McNaughton, 1993). Ideally, the tips are separated in three-dimensional space so that the point-source localization of several neurons is possible (Drake et al., 1988; Perlin and Wise, 2004; Wilson and McNaughton, 1993). Typical types of wire tetrodes can “hear” hippocampal CA1 pyramidal cells in rats as far as 140 µm laterally. A cylinder with this radius contains ∼1000 neurons, which is the number of cells theoretically recordable by a single tetrode (Henze et al., 2000); these estimates vary by neuron size, type and brain region). Yet, in practice, only 5–15 neurons can be reliably separated in limited-duration recordings. The low firing-rates of many neurons (Buzsáki and Mizuseki, 2014; Schwindel et al., 2014; Shoham et al., 2006), as well as low amplitudes of the majority of detected spikes (Fig. 2; <40 µV; (Schomburg et al., 2012) preclude a reliable neuron isolation with currently available clustering algorithms (Harris et al., 2000). Furthermore, mechanical damages associated with probes movements may damage neuronal processes, or tear small vessels (Claverol-Tinture and Nadasdy, 2004; Kozai et al., 2010; Seymour and Kipke, 2007; Tsai et al., 2009).
Electrical recording from neurons is invasive. Therefore, the desire for large-scale monitoring of neurons with many recording sites in a small volume and the desire to minimize the tissue damage inflicted by the electrodes compete with each other (Scott et al., 2012). Micro-Electro-Mechanical System (MEMS)-based recording devices can reduce the technical limitations inherent in wire electrodes because with the same amount of tissue displacement, the number of monitoring sites can be substantially increased (Kipke et al., 2008; Najafi and Wise, 1986), and a tapered tip is readily achieved. Most micromachined electrode arrays (‘silicon probes’; Fig. 3) are formed by photolithographic patterning of thin films of conductors and insulators on silicon substrate. Such a fabrication process allows tailoring the size, shape and the arrangement of the electrodes according to the neural density and local circuit architecture of a specific brain region. Advances in lithography, metal deposition procedures and quality control, an ever-growing number of probe architectures have been developed over the years to meet experiment-guided specific requirements (Bartels et al., 2008; Hofmann et al., 2006; Jamille and David, 2002; Ludwig et al., 2006; McCreery et al., 2006; Motta and Judy, 2005; Musallam et al., 2007; Neves and Ruther, 2007; Nordhausen et al., 1996; Rennaker et al., 2005; Seidl et al., 2011; Wise et al., 2004). The resulting silicon devices enable high-density LFP recordings and large-scale single unit recordings in a variety of brain structures and species (Fig. 4; (Agarwal et al., 2014; Bartho et al., 2004; Blanche et al., 2005; Broome et al., 2006; Csicsvari et al., 2003; Du et al., 2011; Fujisawa et al., 2008; Lin et al., 2014; Montgomery et al., 2008; Pouzat et al., 2002)).
Figure 3. Silicon probes designed for unit sampling and high spatial density monitoring of LFP.

A. Six-shank ‘decatrodes’ (Buzsáki64sp probe from NeuroNexus) for recording and effective separation of single neurons spikes. Vertical separation of the recording sites (160 µm2): 20 µm. Shanks are 15 µm thick and 52 µm wide. B. Eight-shank probe for large spatial coverage (2.1 mm × 1.6 mm; Buzsáki256 probe from NeuroNexus). Recording sites (160 µm2) are separated by 50 µm. Shanks taper from 96 µm to 13 µm at the tip.
Figure 4.
High-quality unit recordings from the superficial layers of the prefrontal cortex (traces 1–64) and the hippocampal CA1 pyramidal layer (traces 65–96). 400 msec epoch during sleep. * indicates sharp wave ripple. Data reproduced from (Fujisawa et al., 2008).
Further advances in the electrode design and arrangement require to push beyond existing technology capabilities to achieve higher recording densities. One of these is the need to reduce probe dimensions to enable probe designs of about the same size, but having significantly more electrode sites. Such a goal is limited mainly by the width of the embedded conductive leads (also called traces or interconnects) that are needed to connect each electrode site to an extracranial bond pad for connection to the external electronics interface. Experience in rodents suggests that when the width of the probe shanks is wider than >60 µm (assuming a shank thickness less than 20 µm), the unit recording quality of the probe decreases rapidly, perhaps due to compression or damage to the dendrites of the surrounding neurons (Claverol-Tinture and Nadasdy, 2004). There is a viable technology roadmap for developing next-generation fine-featured probes for high-resolution recording. One approach involves incremental advances in current MEMS microfabrication processes that result in smaller minimum feature sizes. A related approach involves using alternative MEMS technologies that intrinsically support finer features, e.g., electron-beam lithography (Du et al., 2011). A third MEMS-based approach is to integrate active electronic components in the probe shanks to provide electronic switching that reduces the lead count, and thereby reduces the required shank dimensions (Seidl et al., 2011). Efforts are under way to manufacture a single-shank (50 µm wide) probe with up to 960 closely-spaced (20 µm) contacts, of which 384 can be selected for simultaneous recording at a given time (HHMI-Allen Institute-Gatsby Foundation-Wellcome Trust Consortium), thus enabling more powerful experimental approaches to understanding the interaction of neurons and neuronal pools. A non-MEMS approach is to use small diameter (less than 7 microns), parylene coated carbon fibers to create fine-featured microelectrode arrays having large numbers of ultra-small microlectrodes (Kozai et al., 2012).
A second advance in probe design space is to optimize shank geometry and recording site positions for maximal dense local sampling of neurons, which is important for capturing the dynamics of neuronal ensembles with complex connectivity patterns. In particular, one goal is to minimize the “backside shielding” effect of typical planar silicon probes that have electrode sites all on one side (the “topside”; (Moffitt and McIntyre, 2005)). One design approach for minimizing backside shielding is to locate electrode sites at or near the edge of the shank, perhaps in concert with additional sites located off the edge (Seymour et al., 2011). An alternative approach is to extend planar architectures to manufacture double-sided electrodes (Du et al., 2009; Perlin and Wise, 2004). A third design space opportunity is three-dimensional microelectrode arrays, which, in principle, offer high-resolution functional mapping of local circuits (Wise et al., 2008). Experiments indicate that with three-dimensional triangulation the source localization of neurons can precise as 17 µm (Du et al., 2009). Three-dimensional electrode arrays present a difficult technical challenges involving advanced hermetic packaging having micron-level precision, complex lead transfers for hundreds of leads, and sophisticated surgical techniques, combined with important practical needs for reliability and manufacturability (Bai et al., 2000; Hoogerwerf and Wise, 1994; Neves and Ruther, 2007; Pang et al., 2005; Perlin and Wise, 2010; Yao et al., 2007). Furthermore, inserting three-dimensional probes into the vessel-rich neocortex (Tsai et al., 2009) without compromising the neuropil will remain a serious challenge.
The above discussion of probe geometry can help estimate the optimal configuration of future probes and the maximum yield of simultaneously recordable neurons. For the purpose of recording and separating the maximum number of neurons, the recording sites should tile the entire shank surface of the probe, which should, in turn, have minimal dimensions. Putting aside the problem of interconnects for a moment, and assuming the need for at least 8×8 µm2 recoding sites with 10 µm center-to-center separation, 200 sites per shank on a 20-µm wide probe can cover the entire cortical gray matter (<2 mm). Adding recording sites on both sides would double the number of monitoring sites and increase the unit yield (Du et al., 2009). Placing three shanks in ≤100 µm triangle would allow effective three-dimensional triangulation of cortical neurons in all cortical layers. Such a probe could, in principle, record from between 1000 to 5000 neurons and determine the three-dimensional position of both cell bodies and the main dendrites of each neuron. These technical improvements are within reach but will require high-density off-chip lead transfers, probably in conjunction with on-probe circuitry (Al-Ashmouny et al., 2012; Bai and Wise, 2001; Perlin and Wise, 2010; Wise et al., 2004). We should emphasize that even slim-shank probes come at the expense of tissue damage, fractional displacement volume and associated disruption of physiological activity that needs to be carefully investigated (Claverol-Tinture and Nadasdy, 2004; Polikov et al., 2005; Tsai et al., 2009)
Unfortunately, there is not a ‘one fit for all’ solution for probe design. Various neuron types have different sizes, dendritic configurations and densities and require ‘custom-fit’ probes for effective neuron isolation. This area of research could benefit tremendously from appropriate modeling of the extracellular space populated with realistic neuron geometries (Reimann et al., 2013), glia and vessels and physiological spiking patterns. In turn, virtual electrodes could be embedded into such a ‘neuron cube’ to identify the most appropriate probe geometry, recording site configurations and neuron shielding effects. Such a neuron cube model could also offer ‘ground truth’ for the neuron-spike identity problem and be exploited for perfecting unit clustering algorithms.
Major questions in neuroscience address circuit changes and stability over extended time periods, and dealing with such problems presents important challenges for long-term, high-quality unit recordings. Although several laboratories have reported extended recordings for weeks and even months with diverse types of microelectrodes in various species (Chestek et al., 2011; Jackson and Fetz, 2007; Kipke et al., 2003; Nicolelis et al., 1997; Rousche and Normann, 1998; Ruther et al., 2011; Suner et al., 2005), chronic performance of electrodes, to date, is unpredictable and highly variable. A major cause of probe failure over time is mechanical instability and neuron damage. Ultra-thin, and flexible probes can alleviate the mechanical damage associated with probe penetration by reducing the mechanical mismatch between probe and brain tissue, and allow the probe to move with the adjacent tissue and neurons (Bjornsson et al., 2006). Biodegradable materials, such as silk fibroin, patterned on Parylene substrate, can provide sufficient mechanical support for the probes to be inserted in the brain, while allowing free-flotation of the flexible probe after the silk layer is absorbed (Kim et al., 2010)(Tien et al., 2013). There is a tremendous need to improve the electrode/tissue interface to enhance the signal-to-noise ratio (SNR) and increase the yield of neurons per recording session. Conducting polymers such as PEDOT:PSS are used to decrease the electrochemical impedance mismatch between electrode and brain tissue interface and further reduce the chronic reactive tissue response (Abidian and Martin, 2008; Ludwig et al., 2006; Shain et al., 2003; Spataro et al., 2005). Combining electrical recordings with local delivery of electrical stimulation, chemical substances, such as neurotransmitters, and light for optogenetics (see below) will play an growing role in the exploration of neural circuit mechanisms (Chen et al., 1997; Cheung et al., 2003; Li et al., 2007; Seidl et al., 2011; Seidl et al., 2010).
Penetrating probes can isolate multiple single-neurons from any structure. However, increasing the number of shanks per probe can cause irreversible damage to brain tissue limiting the monitoring of large-scale neural dynamics occurring over contiguous areas of cortex. An emerging approach is to use surface electrodes. The NeuroGrid is an organic material–based, ultraconformable, biocompatible and scalable neural interface device that can acquire both LFPs and spikes from superficial cortical neurons without penetrating the brain surface (Khodagholy et al., 2014). The device is fabricated from “soft organic” material to allow conformation of the probe on the curvilinear surface of the brain, as well as improving the efficiency of ion to electron conversion (Khodagholy et al., 2011). In concert with advances in microelectronics and data processing such as CMOS technologies used in Multi-Electrode Arrays (MEAs) that can yield several thousands recordings sites, LFP and spike samples recorded by the NeuroGrid can be substantially increased (Hutzler and Fromherz 2004)(Lei et al., 2011; Muller et al., 2012) (Chichilnisky and Baylor, 1999; Meister et al., 1994).
Microdrives and probe-preamplifier interconnect
Implanted and ‘fixed’-depth electrodes have good mechanical stability but their unit yield is low. During surgical anesthesia the brain expands and shrinks, and therefore even precisely-placed electrodes move their position relative to neuronal bodies. An effective way of increasing unit yield is to place the electrodes several hundred µm above the target area and move them slowly (<100 µm steps a day) by a microdrive. The unproven assumption of this practice is that moving the shank tips so slowly, the neurons, glia and vasculature have time to move away and rebuild (Fujisawa et al., 2008). Advancement of the electrode can be achieved by microdrives (Fee and Leonardo, 2001; Vandecasteele et al., 2012; Wilson and McNaughton, 1993; Yamamoto and Wilson, 2008). The key requirements of a microdrive are precision, mechanical stability and minimal size and weight. Drives add addition weight and volume to the head-gear and are seriously limiting factors in experiments that require free movement of small rodents. In our experience, a head implant totaling 10% of the preoperative body weight does not impede free behavior. Assuming an adult weight of a 30-g mouse, the package weight budget should be <3 g, although for many experiments the weight should be even less.
Any small drift (<10 µm) between the electrode tip and the neuron(s) can affect recording stability. Such drifts are typically brought about by headshakes, intense grooming, banging the head stage against cage walls and, importantly, during plugging and unplugging the head connectors. The latter problem can be reduced significantly if the microdrive, containing the electrodes, is physically separated from the head connector by a flexible interconnect cable. Polyimide cables have been used successfully for such purpose because of their flexibility and integrated design with silicon probes (Berenyi et al., 2014; Stieglitz et al., 2005) (NeuroNexus Inc.). Cables can also be fabricated as an integral part of the probe so that bonds are required only at the connector end (Yao et al., 2007). With high site-count probes, however, there are still remaining challenges. Higher-density bonding technologies are needed to permit lead transfers on <20µm centers, and traditional connectors with >100 sites and thick cables are not practical for freely behaving small rodents. On-site multiplexing is one solution but size, weight and cost factors must be considered.
On-head signal amplification and multiplexing
A critical aspect of miniaturization is the deployment of signal multiplexers. Earlier versions of multiplexers had either low channel counts or limited high- or low-pass frequency characteristics for simultaneous recordings of both unit and LFP signals in the physiological range and required de-multiplexing and digitization of the transmitted multiplexed analog signals (Berenyi et al., 2014; Du et al., 2011; Harrison, 2008; Olsson et al., 2005; Szuts et al., 2011; Viventi et al., 2011). The recent availability of small size 32- and 64-channel digital multiplexers (RHD-2132 or RHD-2164 chip or die; Intan, Inc.) has accelerated the spread of high-channel-count recordings in physiological laboratories. The digital output of the signal multiplexers (Harrison, 2008) allows direct streaming of the neurophysiological data to the computer. Despite important advances in miniaturization, high-channel-count recordings (>100) in behaving mice and other small animals still remain a challenge because routing the probe sites to the inputs of the signal multiplexers still requires large connectors. Placing headstages permanently on the head of the animal (Berenyi et al., 2014) is an alternative but expensive solution and they add unwanted volume and weight. Given that only 15% of the weight of the functional 64-site electrode system comes from the digitizing headstage integrated circuit (the bulk coming from the printed-circuit board, connector, and sealing epoxy), the most significant development step in packaging and electronics towards a 1,000 site recording system usable in a behaving mouse would be a next-generation integrated circuits that would support larger numbers of channels and that could be intimately packaged with a high-density neural probe (Lei et al., 2011).
An ideal solution to size reduction is integrating the signal amplifiers and multiplexers to the probe end (Csicsvari et al., 2003; Olsson et al., 2005) or into the flexible interconnect cable. The current limitation of such improvement is the high cost of design and fabrication, which can exceed several million dollars. In principle, the active circuits can be placed on the probe shanks, effectively reducing the number of interconnects between the recording sites and electronics outside the brain. However, active circuits also occupy volume and limit the number of recording sites. Experience dictates that any structure >60 µm wide deteriorates the recording ability of probes. Furthermore, such brain-embedded circuits must operate at low enough power levels so that significant heating of the probe is avoided since heating can impact local neurons. Active circuits must also be electrically shielded or located such that the electric fields generated by the circuit components do not influence the spiking activity of nearby neurons (Jefferys, 1995).
Data transmission (cable, telemetry, data loggers)
Cables interconnecting the head stage and the recording system can be completely eliminated by telemetry or data loggers. While up to 128-channel telemetry systems have been successfully used in small animals (Greenwald et al., 2011; Harrison et al., 2011; Sodagar et al., 2007; Szuts et al., 2011), cable connections allows much higher bandwidth, higher channel counts and lower noise. On-probe multiplexing followed by analog-to-digital conversion, thresholding, and data compression can make use of the relatively sparse nature of neural recordings to boost the channel capacity by well over an order of magnitude in some situations (Olsson et al., 2005). Data loggers present another solution for specific applications, such as studying underground, underwater behaviors, social interactions or flying (Yartsev and Ulanovsky, 2013). Unfortunately, the power source required for telemetry adds additional weight and limits the duration of the experiments, while less power-intensive data loggers do not allow the experimenter to monitor the recorded data during the experiments. A combination of these two approaches may optimize their cost-benefit by storing all data on a data logger, and transmitting a few user-selected channels, when needed, via a telemeter and allowing bidirectional communication perturbation of circuits and recording (see below). Externally powered devices (Wentz et al., 2011) may provide solutions for long-term wireless interfacing.
Data acquisition and on-line processing
Recent rapid progress with on-head digital multiplexing (Harrison, 2008) and direct streaming of the neurophysiological data to the computer has made high-density recordings affordable even for small laboratories. Community-driven improvement of shared software (e.g., klustaviewas@googlegroups.com; http://neuroscope.sourceforge.net/; http://klusta-team.github.io/klustakwik/; http://open-ephys.org/) is expected to provide new solutions for flexible data display and fast processing of the recorded physiological and behavioral data for closed-loop interactions with brain circuits. These include on-line detection of particular LFP and unit firing patterns of isolated single spikes, bursts or arbitrary combinations of multiple site spike timing relationships associated with any chosen aspect of behavior or spatial localization of the animal to drive instantaneous feedback perturbation of multiple single neurons or sites (Stark et al., 2012). On-line or real-time processing of even complex neural data is becoming feasible by microprocessors and field-programmable gate arrays (FPGAs; (Muller et al., 2012) ).
Combination of large-scale unit recordings with optogenetics
The recent advent of optogenetics (Boyden et al., 2005; Deisseroth, 2011; Zemelman et al., 2002) provides a solution for identifying specific, genetically-defined neuronal subtypes in blind extracellular recordings by expressing light-sensitive opsins in a given neuronal population. Both activation and silencing strategies can be used for this purpose. Several laboratories have offered solutions for optical stimulation of local circuits combined with simultaneous neuronal recordings (Cardin et al., 2010; Halassa et al., 2011; Han et al., 2009; Kim et al., 2013; Kravitz et al., 2010; Lu et al., 2012; Rubehn et al., 2013; Voigts et al., 2013; Zhang et al., 2009). However, a number of technical issues must be addressed to exploit the full potential of combined large-scale recording and optogenetics, including local delivery of low-intensity light, application of appropriate stimulus waveforms, and replacement of large benchtop lasers with small head-mounted LEDs or laser diodes (see below).
Integrated recording-optogenetic methods can be used to accomplish at least three goals: (a) identification of genetically labeled neurons (optical ‘tagging’), (b) physiological characterization and classification of neuron types and (c) testing the causal roles of the identified neurons in the performance of local circuits.
Strategies for optogenetic tagging of single neurons
The optogenetic method can, in principle, be used to distinguish cortical principal cells from interneurons, identify neurons with specific neuromodulators (Madisen et al., 2012) and differentiate physiologically similar neurons projecting to different targets (Packer et al., 2013). The rapidly growing Cre mouse (Madisen et al., 2012; Taniguchi et al., 2011) and rat (Witten et al., 2011) lines, intersection virus methods (Gradinaru et al., 2010) and other novel ways of marking neurons pave the way to ever more precise definitions of neuronal identity (Packer et al., 2013; Scanziani and Hausser, 2009). However, because neurons are both embedded in circuits and contribute to circuit function, their perturbation can bring about secondary changes, which need to be separated from the primary action of optical stimulation (Fig. 5). On the one hand, variable expression levels of opsins can yield false negative effects. On the other hand, indirect in/activation of neurons can arise from an undesirable recruitment of non-tagged neurons by trans-synaptic effects. Optogenetic activation of principal neurons by light pulses can discharge their partner interneurons (Csicsvari et al., 1998; Miles, 1990) and, potentially other principal cells at short latency and high fidelity. Separation of directly and indirectly driven neurons by latency criteria is highly unreliable (Kvitsiani et al., 2013). Silencing principal cells suffer from the same technical problems since the connected partners can also decrease their rates. Optogenetic activation of inhibitory interneurons can induce a robust rate increase in the directly activated interneurons and suppressed spiking or no-change in the remaining neurons. Yet strong inhibition, brought about by strong light and synchronously discharging interneurons, may bring about rebound spiking in principal cells and possibly other interneurons (Fig. 5C; (Stark et al., 2013)). Tagging of inhibitory interneurons by optogenetic silencing is more reliable than by their activation, although disynaptic disinhibition can mediate rate decrease in opsin-free cells. Several complementary strategies can be used to increase the precision of optogenetic tagging of neurons, including the application of different waveform patterns, but perhaps more important is using minimum light intensities of focally applied light and monitoring the induced effects both locally and more distally from the light source (Fig. 5).
Figure 5. Ambiguity and disambiguation of neuron identity by optical tagging.
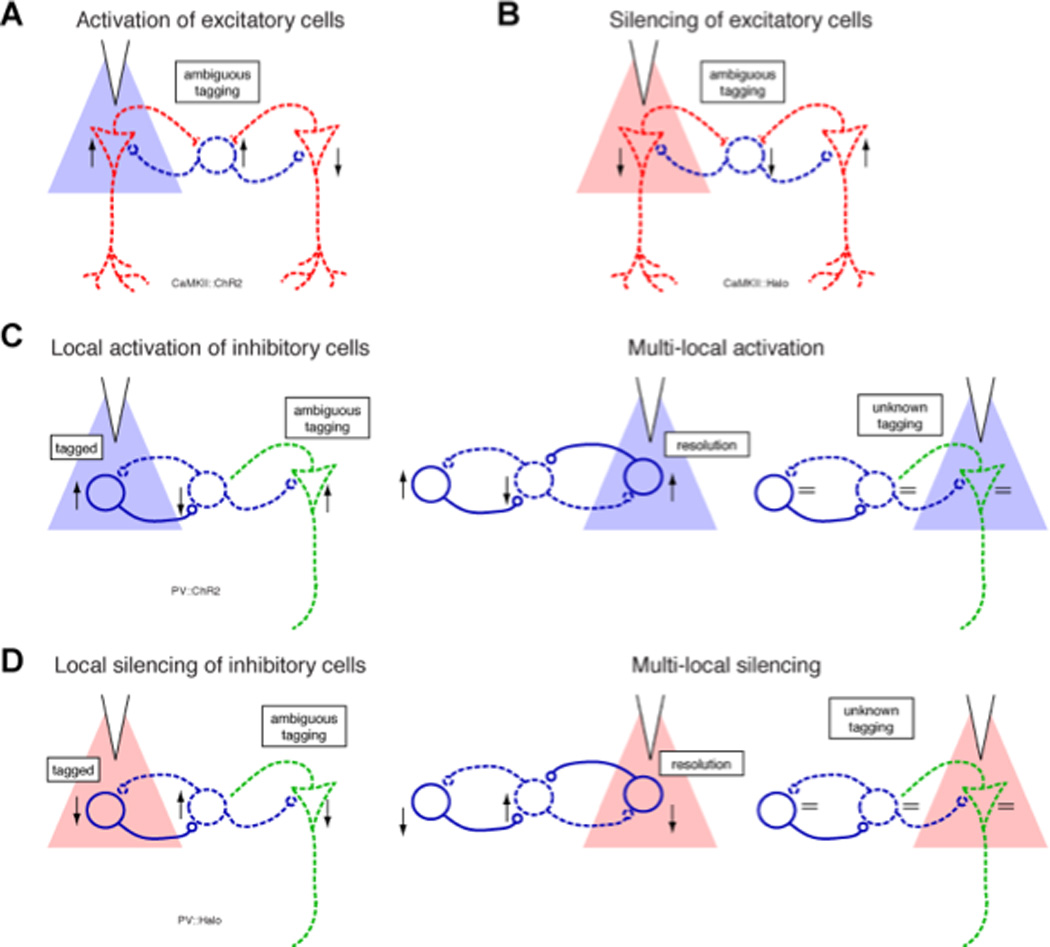
(A) Identification of ChR2-expressing excitatory cells (red) by light pulses is often ambiguous because they can drive nearby interneurons (blue) at a short-latency Since opsin expression level and spiking threshold may vary among principal cells, and since synaptic transmission from principal cells to interneurons is be rapid and strong, some principal cells may show decreased firing rates. (B) Similarly, silencing of several excitatory cells can bring about a short-latency rate decrease of interneurons and circuit-mediated rate increase of excitatory cells. (C) Strong optogenetic activation of inhibitory cells may also be ambiguous, since disynaptic disinhibition of opsin-free cells (dashed green; recorded on another shank) may also occur at a short latency. The ambiguity may be resolved or reduced if the neuron responds at a shorter latency upon direct illumination of the neighboring shank (central panel) but not if no change in firing rate is detected (right panel). (D) Silencing of inhibitory cells results in decreased spiking of illuminated opsin-expressing cells (direct effect), increased spiking of opsin-free cells (synaptic effect), and perhaps interneuron-mediated spiking decrease of other cells (disynaptic effect; green). The direct and disynaptic effects are particularly hard to differentiate upon prolonged global illumination, but may be disambiguated using sequential multi-site illumination.
Many of current technical problems of neuron identification and local circuit analysis arises from the use of large-diameter optical fibers (Cardin et al., 2010; Halassa et al., 2011; Han et al., 2009; Kravitz et al., 2010) or brain surface illumination (Huber et al., 2008; Lee et al., 2012; Moore and Wehr, 2013) at high light power. To activate neurons at a distance from a light source, high intensities (several mW or higher) are often used (Cardin et al., 2010; Halassa et al., 2011; Han et al., 2009; Huber et al., 2008; Kravitz et al., 2010; Moore and Wehr, 2013) . High-power photostimulation can generate photoelectric artifacts, activate/silence numerous neurons both directly and indirectly (Figure 5) and induce synchronous discharges in multiple neurons, making ‘spike sorting’ difficult or impossible. Such technical problems can be significantly reduced or eliminated by etching small-core (≤50 µm) optical fibers to a point (≤10 µm) and mounting them close (<40–50 µm) to the recording sites (Figure 6; (Royer et al., 2010; Stark et al., 2012)). Experiments have shown that less than 1 µW of light (<0.1 mW/mm2) is sufficient to activate ChR2-expressing neurons in vivo (Stark et al., 2012; Stark et al., 2014), and with the development of more sensitive opsins (Chuong et al., 2014), light requirements are expected to decrease. Such hybrid devices can eliminate photoelectric artifacts and reduce artificially induced overlapping spikes.
Figure 6. Diode probes for focal in/activation of neurons.
(A) Schematic of a single LED-fiber assembly. The LED is coupled to a 50-µm multimode fiber, etched to a point at the distal (brain) end. (B) Schematic of a drive equipped with a 6-shank diode probe with LED-fibers mounted on each shank. Etched optical fibers are attached ∼40 µm above the recordings sites on the silicon probe shanks. (C) Recording silicon probe integrated with a waveguide. Light transmission through the optical splitter waveguides integrated on the fabricated neural probe: (C, E) bright field microscope images; (D, F) dark field images. Light can be delivered to multiple shanks from a single fiber source via an optical splitter or different wave-length lights can be delivered to the same shank through an optical mixer. A-B, reproduced after (Stark et al., 2012), C-F, after (Wu et al., 2013).
The low light intensity requirement can eliminate the need for bench top lasers and optical cables that restrain the freedom of the animal’s movement. Instead, miniature light emitting diodes (LED) and/or laser diodes can be coupled to short, small-diameter (50 µm) multimode fibers and attached directly to the shanks of a silicon probe or tetrode (Figure 6). The small size and weight of these integrated ‘diode probes’ allow fast, multisite and multicolor optogenetic manipulations in freely moving animals with concurrent monitoring of the manipulated neurons (Royer et al., 2010; Royer et al., 2012; Stark et al., 2012). To replace the labor-intensive manual process, at least two approaches are being tested. The first is the monolithic integration of all optical and electrical components on the shanks of the silicon probe (Wu et al., 2013; Zorzos et al., 2010). Various waveguide configurations can be designed with photolithography and the distances between optical stimulation sites and recording electrodes can be precisely determined (Zorzos et al., 2010; Zorzos et al., 2012). At a given stimulation site, different wavelengths can be selected by switching between the light sources and allowing fast and complex manipulation of neural activity by exciting and silencing of the same neurons (Stark et al., 2012). Another approach is placing an assembly of micro-light-emitting diodes (µLEDs) in the vicinity of the recording sites (Kim et al., 2013). Either configuration will offer unmatched spatial precision and capability of targeted perturbation and recording from specified neuron types. Dramatic reduction of light intensity combined with dense recording of the surrounding neurons allows single neuron stimulation (Fig. 7) and estimation of the absolute numbers of directly-driven neurons in a small illuminated volume. Being able to assess the fraction of neurons that can affect a particular physiological pattern, perception, memory or overt behavior is an important goal of neuronal circuit analysis.
Figure 7. Optogenetic activation of a single neuron in the behaving mouse.
Autocorrelograms and optogenetically evoked responses (light blue rectangles) of pyramidal cells (red) and putative interneurons (blue) in a freely-moving CaMKII::ChR2 mouse during weak 50 ms light pulses (0.01 mW/mm2 at the center of the CA1 pyramidal layer). All neurons were recorded from the middle shank of the diode-probe (inset). Note robust response of a single pyramidal cell (boxed). Ten repetitions. No neurons were activated by light on the adjacent shanks. At higher intensities (>0.05 mW/mm2) multiple other neurons also increased their firing.
Characterization of neuron subtypes
To be able to identify diverse components of circuits, an iterative refinement of a library of physiological parameters is needed so that subsequently the various neurons can be recognized reliably by using purely physiological criteria without the need for optogenetics (Kvitsiani et al., 2013; Madisen et al., 2012; Pi et al., 2013; Royer et al., 2012; Stark et al., 2013). We are only beginning to understand how different methods of light delivery impact different neuron types. A hitherto unexploited path for characterizing the physiological properties of optically-tagged neurons is their input-output analysis. By analogy to neuron characterization by intracellular current injections (Ascoli et al., 2008) localized optical stimulation can be used to activate neurons and observe their characteristic response properties to pulses, sinusoid, white noise stimuli or more complex patterns. Although the photobleaching and light adaptation properties of the various opsins (Lin et al., 2009) are currently a potential obstacle with bright light sources, the availability of faster reacting and highly sensitive opsins (Berndt et al., 2011; Chuong et al., 2014; Gunaydin et al., 2010; Klapoetke et al., 2014; Zhang et al., 2011) will reduce such concerns. Comparison of pyramidal cells and interneurons has already demonstrated that ChR2 expressing parvalbumin interneurons follow optical responses much more efficiently than neighboring pyramidal neurons (Stark et al., 2013). Further experiments may validate the utility of such waveform characterization methods of neuron identity. Overall, combining optogenetic manipulation with high-density electrophysiological monitoring can offer high fidelity identification of circuit components, a prerequisite for a rational perturbation of identified neuron types and quantities for understanding their impact in circuit function (Roux et al., 2014; Roux and Buzsáki, 2015).
Studying circuits with correlational and perturbation methods
Perhaps the most critical goal of combining optogenetics with large-scale extracellular recordings is to identify the causal role of specific neuron classes in local circuit operations. Perturbation strategies can probe the involvement of particular neuron types in any defined computation, such as gain control, plasticity, oscillations, circuit stability and spike transfer modes. Closed-loop optogenetic activation or silencing of targeted neurons, combination of predetermined spike patterns in multiple cells contingent upon behavioral parameters, and/or selected features of the LFP can alter the timing of action potentials (Stark et al., 2013; Stark et al., 2012; Stark et al., 2014) and induce or suppress correlated firing between cells during movement, perception, leaning, memory, sleep or any other aspects of brain activity. Simultaneous monitoring of neuronal responses to optogenetic perturbation is critical for uncovering the mechanisms how each neuron type contributes to circuit performance. Optogenetics combined with large-scale extracellular recordings has already proven to be effective in studying the functional roles of specific GABAergic interneuron classes in both hippocampus and neocortex, as well as other brain regions (Alonso et al., 2012; Brown et al., 2012; Pi et al., 2013; Royer et al., 2012). Strong optogenetic activation of parvalbumin-expressing interneurons at gamma frequency was shown to coordinate the timing of sensory inputs relative to a gamma cycle and enhance signal transmission (Cardin et al., 2009; Carlen et al., 2012; Sohal et al., 2009), whereas at weaker activation produced theta resonance and excess spiking in nearby pyramidal cells (Stark et al., 2013). Local stimulation of pyramidal cells induced high frequency oscillations, typical of hippocampal ripples (Stark et al., 2014). Channelrhodopsin-2-assisted circuit analysis in the amygdala identified neurons critical in gating conditioned fear (Haubensak et al., 2010). Optogenetic activation of orexin neurons in the hypothalamus could change sleep choreography (de Lecea and Huerta, 2014). Periodic light stimulation of neurons of the thalamic reticular nucleus induced state-dependent neocortical spindles (Kravitz et al., 2010) or evoked generalized spike and wave discharges (Berenyi et al., 2012). On the other hand, tonic photoactivation of reticular neurons reduced focal seizures in the neocortex (Paz et al., 2013). Similarly, spontaneous seizures could be suppressed by optogenetic activation of parvalbumin interneurons in the hippocampus (Krook-Magnuson et al., 2013). Optogenetic activation of dentate gyrus neurons could induce false memories, making mice acting accordingly in a fear conditioning paradigm (Ramirez et al., 2013). This non-exhaustive list of experiments demonstrate the power of combined closed-loop recording-optogenetic methods for understanding complex neuronal interactions in intact and altered networks (Roux et al., 2014), and foretell how such methods could one day be harnessed for clinical applications.
Looking into the future
Good research practice stands on the foundation of accurate measurement. Our review has focused on the large-scale recording of neuronal spike data and the optogenetic manipulation of neurons, the appropriate combination of which can provide single neuron, single spike monitoring and control in behaving animals, not possible by other methods. Although several aspects of the combined recording/optogenetics technique are highly scalable and one can imagine exponential growth of the technology (Alivisatos et al., 2013; Marblestone et al., 2013), our aim was to discuss techniques that can be deployed currently or in the near future for the ultra-dense sampling of neuronal circuits. Combination of electrical recording and optogenetics with imaging (Grienberger and Konnerth, 2012; Yuste and Katz, 1991) can further enhance the power of these methods.
Spectacular progress has been made over the past few years in signal multiplexing, moving from expensive and bulky modular amplifier-digitizer systems to affordable, high-channel count signal multiplexed systems ((Berenyi et al., 2014); http://www.intantech.com/; http://open-ephys.org/). There are no longer financial constraints even for small labs to record from a few hundred channels. It is expected that channel count will greatly increase in the coming years, paralleled with a substantial decrease in the size of signal multiplexing devices. Silicon probes with several dozens and even hundreds of sites are currently commercially available and probes with even higher channel counts in both two- and three-dimensional configurations are being tested experimentally (Du et al., 2009; Riera et al., 2012). To make these techniques fruitful for experiments on mice and other small size animals will require orders of magnitude improvement in several key parameters, particularly on-probe signal multiplexing (Olsson et al., 2005). Substantially improved power efficiency of telemetry and data loggers or their external powering may eventually eliminate the need for cable connections between the animal and recording equipment. A foreseeable challenge with brain embedded electronics is that the electromagnetic fields needed for external powering may ephaptically stimulate neurons. Miniaturization and improved stability of micromanipulators to allow long-term recordings from multiple brain regions simultaneously are equally important steps.
Obtaining high-quality, high-density data from multiple interconnected structures is only the first necessary step. The second big challenge is to develop fast automatic or semi-automatic and validated algorithms for spike clustering with common standards to facilitate standardization and within- and across-laboratory replication of neuronal identification (Buzsáki, 2004; Einevoll et al., 2012). Finally, making sense of the large-scale and properly preprocessed data requires a further concerted effort (Packer et al., 2013). Effective dissemination of the newly developed methods and algorithms and sharing as well as maintaining well-curated data with common standards and seamless user accessibility ((Mizuseki et al., 2014); CRCNS.org) are among the key tasks ahead of us for advancing rapid progress.
Successful analysis of neuronal circuits requires not only large-scale recording of neurons at high speed but also their interactive control at the single-neuron, single-spike level. While optogenetics has emerged as an appropriate method for manipulating identified neurons at sufficient speed, substantial improvement is needed to achieve the desired spatial resolution at the single neuron level. Such development will require the integration of silicon recording technology with the local and programmable delivery of light, using either waveguides or neuron-size LEDs mixed with the recording sites (Kim et al., 2013; Wu et al., 2013). Neuron identity can be obtained online by optogenetics. Multi-color techniques can be deployed for activating and silencing the same neurons in the behaving animal (Stark et al., 2012) and for selective perturbation of excitatory and inhibitory neurons.
Optogenetic techniques are often cited as a solution for the long-waited mechanistic understanding of causal effects of neurons in their native environment (Boyden et al., 2005; Packer et al., 2013; Scanziani and Hausser, 2009). However, these aspects of optogenetics are often overstated since identifying the ‘cause’ by local perturbations in complex systems can be a daunting task. While tracking cause-effect relationships in linear systems (such as A causes B) is relatively straightforward, in complex systems with multiple re-entrant loops and emergent properties such as the brain, circuit perturbations can bring about multiple and hard-to-interpret consequences since A and B can have bidirectional or circular causal relationships. Because brain circuits are perpetually active and their components display qualitatively different interactions in different brain states, imposition of synthetic patterns has to compete with an ongoing brain-based program and can induce outcomes different from the native functions. These and other unexpected surprises of the perturbation technique (Miesenbock, 2009) emphasize the need for the focal delivery of light limited to the volume of recorded neurons and the application of control theory (Zhou et al., 1996). Despite these expected difficulties, multisite-multineuron close-loop control experiments offer unprecedented opportunities for the exploration of neuronal circuits. They can be used to induce, modify or annihilate network oscillations, test hypothesized rules of in vivo plasticity, examine the critical role of timing (e.g., by introducing controlled jitter of spike timing) and, perhaps most importantly, determine how synthetic spatiotemporal patterns of activity of identified neurons induce specific behaviors. This exercise amounts to understanding the neuronal syntax or brain coding.
Acknowledgments
Supported by National Institute of Health Grants NS34994, MH54671 and NS074015, the Human Frontier Science Program and the National Science Foundation (Temporal Dynamics of Learning Center Grant SBE 0542013). AB was supported by a Marie Curie FP7-PEOPLE-2009-IOF grant (No. 254780), EU-FP7-ERC-2013-Starting grant (No. 337075), the ‘Lendület’ program of the Hungarian Academy of Sciences. ES was supported by the Rothschild Foundation, the Human Frontiers in Science Project (LT-000346/2009-L), and the Machiah Foundation (20090098).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
Antal Berenyi is the founder and owner of Amplipex Ltd., Szeged, Hungary, which manufactures signal-multiplexed head stages and demultiplexing systems. Daryl Kipke is the founder and current Executive Director of NeuroNexus Technologies, Inc., a subsidiary of Greatbatch, Inc. The other authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29:1273–1283. doi: 10.1016/j.biomaterials.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Moruzzi G. Impulses in the pyramidal tract. The Journal of physiology. 1939;97:153–199. doi: 10.1113/jphysiol.1939.sp003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal G, Stevenson IH, Berenyi A, Mizuseki K, Buzsáki G, Sommer FT. Spatially distributed local fields in the hippocampus encode rat position. Science. 2014;344:626–630. doi: 10.1126/science.1250444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ashmouny KM, Chang SI, Yoon E. A 4 muW/Ch analog front-end module with moderate inversion and power-scalable sampling operation for 3-D neural microsystems. IEEE transactions on biomedical circuits and systems. 2012;6:403–413. doi: 10.1109/TBCAS.2012.2218105. [DOI] [PubMed] [Google Scholar]
- Alivisatos AP, Chun M, Church GM, Deisseroth K, Donoghue JP, Greenspan RJ, McEuen PL, Roukes ML, Sejnowski TJ, Weiss PS, Yuste R. Neuroscience. The brain activity map. Science. 2013;339:1284–1285. doi: 10.1126/science.1236939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nature neuroscience. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature reviews Neuroscience. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Q, Wise KD. Single-unit neural recording with active microelectrode arrays. IEEE transactions on bio-medical engineering. 2001;48:911–920. doi: 10.1109/10.936367. [DOI] [PubMed] [Google Scholar]
- Bai Q, Wise KD, Anderson DJ. A high-yield microassembly structure for three-dimensional microelectrode arrays. IEEE transactions on bio-medical engineering. 2000;47:281–289. doi: 10.1109/10.827288. [DOI] [PubMed] [Google Scholar]
- Bartels J, Andreasen D, Ehirim P, Mao H, Seibert S, Wright EJ, Kennedy P. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. Journal of neuroscience methods. 2008;174:168–176. doi: 10.1016/j.jneumeth.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. Journal of neurophysiology. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Berenyi A, Belluscio M, Mao D, Buzsáki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337:735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenyi A, Somogyvari Z, Nagy AJ, Roux L, Long JD, Fujisawa S, Stark E, Leonardo A, Harris TD, Buzsáki G. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. Journal of neurophysiology. 2014;111:1132–1149. doi: 10.1152/jn.00785.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. Journal of neural engineering. 2006;3:196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- Blanche TJ, Spacek MA, Hetke JF, Swindale NV. Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. Journal of neurophysiology. 2005;93:2987–3000. doi: 10.1152/jn.01023.2004. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. doi: 10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nature neuroscience. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nature reviews Neuroscience. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Mizuseki K. The log-dynamic brain: how skewed distributions affect network operations. Nature reviews Neuroscience. 2014;15:264–278. doi: 10.1038/nrn3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Penttonen M, Nadasdy Z, Bragin A. Pattern and inhibition-dependent invasion of pyramidal cell dendrites by fast spikes in the hippocampus in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9921–9925. doi: 10.1073/pnas.93.18.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M. From circuits to behavior: a bridge too far? Nature neuroscience. 2012;15:507–509. doi: 10.1038/nn.3043. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nature protocols. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, Wang KH, Boyden ES, Wurtz RH. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron. 2012;76:901–907. doi: 10.1016/j.neuron.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wise KD, Hetke JF, Bledsoe SC. A multichannel neural probe for selective chemical delivery at the cellular level. Biomedical Engineering, IEEE Transactions on. 1997;44:760–769. doi: 10.1109/10.605435. [DOI] [PubMed] [Google Scholar]
- Chestek CA, Gilja V, Nuyujukian P, Foster JD, Fan JM, Kaufman MT, Churchland MM, Rivera-Alvidrez Z, Cunningham JP, Ryu SI, Shenoy KV. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. Journal of neural engineering. 2011;8:045005. doi: 10.1088/1741-2560/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KC, Djupsund K, Yang D, Lee LP. Implantable multichannel electrode array based on SOI technology. Microelectromechanical Systems, Journal of. 2003;12:179–184. [Google Scholar]
- Chichilnisky EJ, Baylor DA. Receptive-field microstructure of blue-yellow ganglion cells in primate retina. Nature neuroscience. 1999;2:889–893. doi: 10.1038/13189. [DOI] [PubMed] [Google Scholar]
- Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sorensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nature neuroscience. 2014 doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverol-Tinture E, Nadasdy Z. Intersection of microwire electrodes with proximal CA1 stratum-pyramidale neurons at insertion for multiunit recordings predicted by a 3-D computer model. IEEE transactions on bio-medical engineering. 2004;51:2211–2216. doi: 10.1109/TBME.2004.834274. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Bartho P, Wise KD, Buzsáki G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. Journal of neurophysiology. 2003;90:1314–1323. doi: 10.1152/jn.00116.2003. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsáki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Frontiers in pharmacology. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nature methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nature neuroscience. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake KL, Wise KD, Farraye J, Anderson DJ, BeMent SL. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity. IEEE transactions on bio-medical engineering. 1988;35:719–732. doi: 10.1109/10.7273. [DOI] [PubMed] [Google Scholar]
- Du J, Blanche TJ, Harrison RR, Lester HA, Masmanidis SC. Multiplexed, high density electrophysiology with nanofabricated neural probes. PloS one. 2011;6:e26204. doi: 10.1371/journal.pone.0026204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Riedel-Kruse IH, Nawroth JC, Roukes ML, Laurent G, Masmanidis SC. High-resolution three-dimensional extracellular recording of neuronal activity with microfabricated electrode arrays. Journal of neurophysiology. 2009;101:1671–1678. doi: 10.1152/jn.90992.2008. [DOI] [PubMed] [Google Scholar]
- Einevoll GT, Franke F, Hagen E, Pouzat C, Harris KD. Towards reliable spike-train recordings from thousands of neurons with multielectrodes. Current opinion in neurobiology. 2012;22:11–17. doi: 10.1016/j.conb.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nature reviews Neuroscience. 2013;14:770–785. doi: 10.1038/nrn3599. [DOI] [PubMed] [Google Scholar]
- Fee MS, Leonardo A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. Journal of neuroscience methods. 2001;112:83–94. doi: 10.1016/s0165-0270(01)00426-5. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nature neuroscience. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Current biology : CB. 2012;22:1722–1726. doi: 10.1016/j.cub.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Goodell B, Lear A. Multichannel micromanipulator and chamber system for recording multineuronal activity in alert, non-human primates. Journal of neurophysiology. 2007;98:527–536. doi: 10.1152/jn.00259.2007. [DOI] [PubMed] [Google Scholar]
- Greenwald E, Mollazadeh M, Hu C, Wei T, Culurciello E, Thakor V. A VLSI Neural Monitoring System With Ultra-Wideband Telemetry for Awake Behaving Subjects. IEEE transactions on biomedical circuits and systems. 2011;5:112–119. doi: 10.1109/TBCAS.2011.2141670. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nature neuroscience. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nature neuroscience. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. Journal of neurophysiology. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Harrison RR. The design of integrated circuits to observe brain activity. Proceedings of the IEEE. 2008;96:1203–1216. [Google Scholar]
- Harrison RR, Fotowat H, Chan R, Kier RJ, Olberg R, Leonardo A, Gabbiani F. Wireless Neural/EMG Telemetry Systems for Small Freely Moving Animals. IEEE transactions on biomedical circuits and systems. 2011;5:103–111. doi: 10.1109/TBCAS.2011.2131140. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. Journal of neurophysiology. 2000;84:390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- Hofmann UG, Folkers A, Mosch F, Malina T, Menne KM, Biella G, Fagerstedt P, De Schutter E, Jensen W, Yoshida K, et al. A novel high channel-count system for acute multisite neuronal recordings. IEEE transactions on bio-medical engineering. 2006;53:1672–1677. doi: 10.1109/TBME.2006.877807. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf AC, Wise KD. A three-dimensional microelectrode array for chronic neural recording. IEEE transactions on bio-medical engineering. 1994;41:1136–1146. doi: 10.1109/10.335862. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler M, Fromherz P. Silicon chip with capacitors and transistors for interfacing organotypic brain slice of rat hippocampus. Eur J Neurosci. 2008;19:2231–2238. doi: 10.1111/j.0953-816X.2004.03311.x. [DOI] [PubMed] [Google Scholar]
- Jackson A, Fetz EE. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. Journal of neurophysiology. 2007;98:3109–3118. doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- Jamille FH, David JA. Handbook of Neuroprosthetic Methods. CRC Press; 2002. Silicon microelectrodes for extracellular recording. [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiological reviews. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Doublet T, Gurfinkel M, Quilichini P, Ismailova E, Leleux P, Herve T, Sanaur S, Bernard C, Malliaras GG. Highly conformable conducting polymer electrodes for in vivo recordings. Adv Mater. 2011;23:H268–H272. doi: 10.1002/adma.201102378. [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsáki G. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2014 Dec 22; doi: 10.1038/nn.3905. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim YS, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang KC, Zakin MR, Litt B, Rogers JA. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim RH, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipke DR, Shain W, Buzsáki G, Fetz E, Henderson JM, Hetke JF, Schalk G. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11:151–155. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. Independent optical excitation of distinct neural populations. Nature methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TD, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nature materials. 2012;11:1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TD, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, Kipke DR. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. Journal of neural engineering. 2010;7:046011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Ramakrishnan S, Shi P, Orcutt JS, Yuste R, Kam LC, Shepard KL. High-resolution extracellular stimulation of dispersed hippocampal culture with high-density CMOS multielectrode array based on non-Faradaic electrodes. Journal of Neural Engineering. 2011;8:044003. doi: 10.1088/1741-2560/8/4/044003. [DOI] [PubMed] [Google Scholar]
- Li Y, Baek K, Gulari M, Wise KD. A Drug-Delivery Probe with an In-Line Flowmeter Based on Trench Refill and Chemical Mechanical Polishing Techniques. In Sensors, 2007 IEEE. 2007:1144–1147. [Google Scholar]
- Lin CP, Chen YP, Hung CP. Tuning and spontaneous spike time synchrony share a common structure in macaque inferior temporal cortex. Journal of neurophysiology. 2014;112:856–869. doi: 10.1152/jn.00485.2013. [DOI] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Li Y, Pan J, Wei P, Liu N, Wu B, Cheng J, Lu C, Wang L. Poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate)-poly(vinyl alcohol)/poly(acrylic acid) interpenetrating polymer networks for improving optrode-neural tissue interface in optogenetics. Biomaterials. 2012;33:378–394. doi: 10.1016/j.biomaterials.2011.09.083. [DOI] [PubMed] [Google Scholar]
- Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. Journal of neural engineering. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marblestone AH, Zamft BM, Maguire YG, Shapiro MG, Cybulski TR, Glaser JI, Amodei D, Stranges PB, Kalhor R, Dalrymple DA, et al. Physical principles for scalable neural recording. Frontiers in computational neuroscience. 2013;7:137. doi: 10.3389/fncom.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery D, Lossinsky A, Pikov V, Liu X. Microelectrode array for chronic deep-brain microstimulation and recording. IEEE transactions on bio-medical engineering. 2006;53:726–737. doi: 10.1109/TBME.2006.870215. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. Journal of neuroscience methods. 1983;8:391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. Journal of neuroscience methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. The Journal of physiology. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Teeters J, Sirota A, Buzsáki G. Neurosharing: large-scale data sets (spike, LFP) recorded from the hippocampal-entorhinal system in behaving rats. F1000Research. 2014;3:98. doi: 10.12688/f1000research.3895.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt MA, McIntyre CC. Model-based analysis of cortical recording with silicon microelectrodes. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116:2240–2250. doi: 10.1016/j.clinph.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Sirota A, Buzsáki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PS, Judy JW. Multielectrode microprobes for deep-brain stimulation fabricated with a customizable 3-D electroplating process. IEEE transactions on bio-medical engineering. 2005;52:923–933. doi: 10.1109/TBME.2005.845225. [DOI] [PubMed] [Google Scholar]
- Muller J, Bakkum DJ, Hierlemann A. Sub-millisecond closed-loop feedback stimulation between arbitrary sets of individual neurons. Frontiers in neural circuits. 2012;6:121. doi: 10.3389/fncir.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam S, Bak MJ, Troyk PR, Andersen RA. A floating metal microelectrode array for chronic implantation. Journal of neuroscience methods. 2007;160:122–127. doi: 10.1016/j.jneumeth.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Nádasdy Z, Csicsvári J, Penttonen M, Hetke JKWGB. Extracellular recording and analysis of neuronal activity: from single cells to ensembles. In: Eichenbaum HB, Davis JL, editors. Neuronal Ensembles: Strategies for Recording and Decoding. New York: Wiley; 1998. [Google Scholar]
- Najafi K, Wise KD. An Implantable Multielectrode Array with on-Chip Signal-Processing. Ieee J Solid-St Circ. 1986;21:1035–1044. [Google Scholar]
- Neves HP, Ruther P. The NeuroProbes Project. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2007;2007:6443–6445. doi: 10.1109/IEMBS.2007.4353831. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LM. Reconstructing the engram: simultaneous, multisite, many single neuron recordings. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Nordhausen CT, Maynard EM, Normann RA. Single unit recording capabilities of a 100 microelectrode array. Brain research. 1996;726:129–140. [PubMed] [Google Scholar]
- Olsson RH, 3rd, Buhl DL, Sirota AM, Buzsáki G, Wise KD. Band-tunable and multiplexed integrated circuits for simultaneous recording and stimulation with microelectrode arrays. IEEE transactions on bio-medical engineering. 2005;52:1303–1311. doi: 10.1109/TBME.2005.847540. [DOI] [PubMed] [Google Scholar]
- Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nature neuroscience. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang C, Cham J, Nenadic Z, Musallam S, Tai YC, Burdick J, Andersen R. A new multi-site probe array with monolithically integrated parylene flexible cable for neural prostheses. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2005;7:7114–7117. doi: 10.1109/IEMBS.2005.1616146. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nature neuroscience. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin G, Wise K. The effect of the substrate on the extracellular neural activity recorded micromachined silicon microprobes. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2004;3:2002–2005. doi: 10.1109/IEMBS.2004.1403590. [DOI] [PubMed] [Google Scholar]
- Perlin GE, Wise KD. An Ultra Compact Integrated Front End for Wireless Neural Recording Microsystems. J Microelectromech S. 2010;19:1409–1421. [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of neuroscience methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Mazor O, Laurent G. Using noise signature to optimize spike-sorting and to assess neuronal classification quality. Journal of neuroscience methods. 2002;122:43–57. doi: 10.1016/s0165-0270(02)00276-5. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Reimann MW, Anastassiou CA, Perin R, Hill SL, Markram H, Koch C. A Biophysically Detailed Model of Neocortical Local Field Potentials Predicts the Critical Role of Active Membrane Currents. Neuron. 2013;79:375–390. doi: 10.1016/j.neuron.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Ruyle AM, Street SE, Sloan AM. An economical multi-channel cortical electrode array for extended periods of recording during behavior. Journal of neuroscience methods. 2005;142:97–105. doi: 10.1016/j.jneumeth.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Riera JJ, Ogawa T, Goto T, Sumiyoshi A, Nonaka H, Evans A, Miyakawa H, Kawashima R. Pitfalls in the dipolar model for the neocortical EEG sources. Journal of neurophysiology. 2012;108:956–975. doi: 10.1152/jn.00098.2011. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. Journal of neuroscience methods. 1998;82:1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- Roux L, Stark E, Sjulson L, Buzsáki G. In vivo optogenetic identification and manipulation of GABAergic interneuron subtypes. Curr Opin Neurobiol. 2014;26:88–95. doi: 10.1016/j.conb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Buzsáki G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology. 2015;88C:10–23. doi: 10.1016/j.neuropharm.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsáki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. The European journal of neuroscience. 2010;31:2279–2291. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nature neuroscience. 2012 doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubehn B, Wolff SB, Tovote P, Luthi A, Stieglitz T. A polymer-based neural microimplant for optogenetic applications: design and first in vivo study. Lab on a chip. 2013;13:579–588. doi: 10.1039/c2lc40874k. [DOI] [PubMed] [Google Scholar]
- Ruther P, Holzhammer T, Herwik S, Rich PD, Dalley JW, Paul O, Holtzman T. Compact wireless neural recording system for small animals using silicon-based probe arrays. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2011;2011:2284–2287. doi: 10.1109/IEMBS.2011.6090575. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Hausser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- Schomburg EW, Anastassiou CA, Buzsáki G, Koch C. The spiking component of oscillatory extracellular potentials in the rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11798–11811. doi: 10.1523/JNEUROSCI.0656-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindel CD, Ali K, McNaughton BL, Tatsuno M. Long-term recordings improve the detection of weak excitatory-excitatory connections in rat prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5454–5467. doi: 10.1523/JNEUROSCI.4350-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Du J, Lester HA, Masmanidis SC. Variability of acute extracellular action potential measurements with multisite silicon probes. Journal of neuroscience methods. 2012;211:22–30. doi: 10.1016/j.jneumeth.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl K, Herwik S, Torfs T, Neves HP, Paul O, Ruther P. CMOS-Based High-Density Silicon Microprobe Arrays for Electronic Depth Control in Intracortical Neural Recording. J Microelectromech S. 2011;20:1439–1448. [Google Scholar]
- Seidl K, Spieth S, Herwik S, Steigert J, Zengerle R, Paul O, Ruther P. In-plane silicon probes for simultaneous neural recording and drug delivery. J Micromech Microeng. 2010:20. [Google Scholar]
- Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594–3607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Seymour JP, Langhals NB, Anderson DJ, Kipke DR. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomedical microdevices. 2011;13:441–451. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2003;11:186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- Shoham S, O'Connor DH, Segev R. How silent is the brain: is there a "dark matter" problem in neuroscience? Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2006;192:777–784. doi: 10.1007/s00359-006-0117-6. [DOI] [PubMed] [Google Scholar]
- Sodagar AM, Wise KD, Najafi K. A fully integrated mixed-signal neural processor for implantable multichannel cortical recording. IEEE transactions on bio-medical engineering. 2007;54:1075–1088. doi: 10.1109/TBME.2007.894986. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Experimental neurology. 2005;194:289–300. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsáki G. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–1276. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Koos T, Buzsáki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. Journal of neurophysiology. 2012;108:349–363. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83:467–480. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson IH, Kording KP. How advances in neural recording affect data analysis. Nature neuroscience. 2011;14:139–142. doi: 10.1038/nn.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz T, Schuettler M, Koch KP. Implantable biomedical microsystems for neural prostheses. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2005;24:58–65. doi: 10.1109/memb.2005.1511501. [DOI] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- Szuts TA, Fadeyev V, Kachiguine S, Sher A, Grivich MV, Agrochao M, Hottowy P, Dabrowski W, Lubenov EV, Siapas AG, et al. A wireless multi-channel neural amplifier for freely moving animals. Nature neuroscience. 2011;14:263–269. doi: 10.1038/nn.2730. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien LW, Wu F, Tang-Schomer MD, Yoon E, Omenetto FG, Kaplan DL. Silk as a Multifunctional Biomaterial Substrate for Reduced Glial Scarring around Brain-Penetrating Electrodes. Advanced Functional Materials. 2013;23:3185–3193. [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, Lyden PD, Kleinfeld D. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecasteele M, M S, Royer S, Belluscio M, Berenyi A, Diba K, Fujisawa S, Grosmark A, Mao D, Mizuseki K, et al. Large-scale recording of neurons by movable silicon probes in behaving rodents. Journal of visualized experiments : JoVE. 2012:e3568. doi: 10.3791/3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nature neuroscience. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts J, Siegle JH, Pritchett DL, Moore CI. The flexDrive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Frontiers in systems neuroscience. 2013;7:8. doi: 10.3389/fnsys.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz CT, Bernstein JG, Monahan P, Guerra A, Rodriguez A, Boyden ES. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. Journal of neural engineering. 2011;8:046021. doi: 10.1088/1741-2560/8/4/046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wise KD, Anderson DJ, Hetke JF, Kipke DR, Najafi K. Wireless implantable microsystems: high-density electronic interfaces to the nervous system. Proceedings of the IEEE. 2004;92:76–97. [Google Scholar]
- Wise KD, Sodagar AM, Ying Y, Gulari MN, Perlin GE, Najafi K. Microelectrodes, Microelectronics, and Implantable Neural Microsystems. Proceedings of the IEEE. 2008;96:1184–1202. [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Stark E, Im M, Cho IJ, Yoon ES, Buzsáki G, Wise KD, Yoon E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. Journal of neural engineering. 2013;10:056012. doi: 10.1088/1741-2560/10/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Wilson MA. Large-scale chronically implantable precision motorized microdrive array for freely behaving animals. Journal of neurophysiology. 2008;100:2430–2440. doi: 10.1152/jn.90687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Gulari MN, Casey B, Wiler JA, Wise KD. Silicon Microelectrodes with Flexible Integrated Cables for Neural Implant Applications; In Neural Engineering, 2007 CNE '07 3rd International IEEE/EMBS Conference on; 2007. pp. 398–401. [Google Scholar]
- Yartsev MM, Ulanovsky N. Representation of three-dimensional space in the hippocampus of flying bats. Science. 2013;340:367–372. doi: 10.1126/science.1235338. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Laiwalla F, Kim JA, Urabe H, Van Wagenen R, Song YK, Connors BW, Zhang F, Deisseroth K, Nurmikko AV. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. Journal of neural engineering. 2009;6:055007. doi: 10.1088/1741-2560/6/5/055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Doyle JC, Glover K. Robust and optimal control. Upper Saddle River, N.J: Prentice Hall; 1996. [Google Scholar]
- Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Optics letters. 2010;35:4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Optics letters. 2012;37:4841–4843. doi: 10.1364/OL.37.004841. [DOI] [PMC free article] [PubMed] [Google Scholar]





