Summary
Neuromechanical principles define the properties and problems that shape neural solutions for movement. Although the theoretical and experimental evidence is debated, we present arguments for consistent structures in motor patterns, i.e. motor modules, that are neuromechanical solutions for movement particular to an individual and shaped by evolutionary, developmental, and learning processes. As a consequence, motor modules may be useful in assessing sensorimotor deficits specific to an individual, and define targets for the rational development of novel rehabilitation therapies that enhance neural plasticity and sculpt motor recovery. We propose that motor module organization is disrupted and may be improved by therapy in spinal cord injury, stroke, and Parkinson’s disease. Recent studies provide insights into the yet unknown underlying neural mechanisms of motor modules, motor impairment and motor learning, and may lead to better understanding of the causal nature of modularity and its underlying neural substrates.
Introduction
The principles of neuromechanics are a framework for understanding patterns of neural activity that generate movements in a healthy nervous system, in motor deficits, and how these patterns change through rehabilitation. Neuromechanics is the study of interactions between neural, biomechanical, and environmental dynamics that give rise to meaningful motor behaviors, and addresses the fundamental question: How does the activity of a neuron, a motor unit, or a muscle affect behavior? Neuromechanical studies reveal that the functional consequences of activity in any of these components cannot be interpreted independently, but must be interpreted in the context of all the forces acting on the body, including those from the external environment, from body structures, and from other muscles (Dickinson et al., 2000; Hooper and Weaver, 2000; Nishikawa et al., 2007). Depending on the neuromechanical context, a movement could be unaffected by, or critically dependent upon the timing and amplitude of a muscle’s activity. As a consequence, our ability to functionally interpret neural motor signals is intimately entwined with the properties of the neuromechanical system (Chiel and Beer, 1997; Chiel et al., 2009; Tytell et al., 2011). Here we will explore how the following neuromechanical principles provide insight into how the nervous system constructs and learns movements:
Motor abundance: For any given task, there are many functionally equivalent motor solutions.
Motor structure: The structure of motor patterns is shaped by biomechanical task relevance.
Motor variability: Motor variability is high where the effect on motor output is low.
Individuality: Individuals express different motor styles that depend on evolutionary, developmental, and learning processes.
Multifunctionality: Muscles can contribute to many actions; a few muscles can be combined in many ways to produce a wide range of different actions.
In the first section, we hypothesize that these neuromechanical principles and plasticity in the nervous system support the development of motor modules, which are defined as coordinated patterns of muscle activity that flexibly combine to produce functional motor behaviors (Bizzi and Cheung, 2013; Bizzi et al., 2008; d’Avella et al., 2003; Ting and McKay, 2007; Tresch and Jarc, 2009). While there is general consensus that structure exists in motor patterns, how they arise, whether they reflect neural structure, and whether they are functionally relevant are sources of lively debate. We argue that motor modules arise from neural plasticity in spinal and supraspinal structures, which is shaped by regularities in biomechanical interactions with the environment. Different expressions of motor modules across individuals may reflect how each individual explores a potentially difficult to search and nonlinear set of neuromechanical solutions for movement. As motor modules are refined over a lifetime, they may appear objectively optimal based on minimizing movement time, energy, or some other feature of the movement (Todorov and Jordan 2002; Scott 2004; Todorov 2004; Scott 2008; Shadmehr and Krakauer 2008). However, it is likely that motor modules are “slop-timal”, i.e. only locally optimal or just “good enough”, to balance competing costs of reliably generating motor actions versus exhaustive exploration or computation to produce new behaviors.
In the second section, we address how motor modules may provide a powerful framework to address current limitations that impede the development of more effective and individualized rehabilitation therapies (Giszter and Hart, 2013; Safavynia et al., 2011; Santello and Lang, 2015). Current clinical motor tests are focused on overall motor function such as walking speed, and are not intended to distinguish different task-specific deficits that underlie impairments (Cumberland Consensus Working Group et al., 2009; Mancini and Horak, 2010; Pardasaney et al., 2012). However, more directed task-specific training may be necessary to harness use-dependent neural plasticity, a common basis for rehabilitation across different neurological disorders (Figure 1). The level of motor disability may be most important for devising optimal strategies to fit individual patient needs (Dobkin, 2009). We will give three clinical examples of how motor modules can help to 1) identify individual-specific motor impairments, 2) assess the effects of rehabilitation, and 3) provide a framework for development of targeted therapies that enhance neural plasticity and sculpt motor recovery. We postulate that motor module organization is altered after central nervous system injury and disease (i.e., spinal cord injury, stroke, and Parkinson’s disease) and that quantifying this disruption may provide tremendous insight into individual-specific motor impairments as well as mechanisms of learning and refining motor behaviors during rehabilitation (Figure 1).
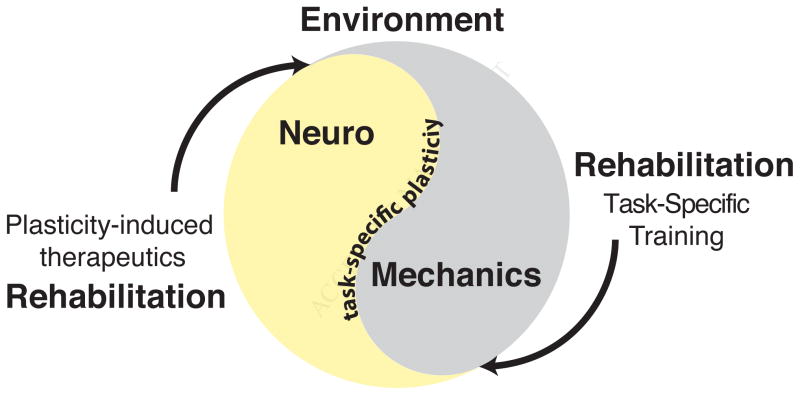
Figure 1. Neuromechanics and rehabilitation.
Movement is influenced by both the neural and biomechanical systems of the body and their interaction with the environment. Experience-dependent plasticity shapes the individual-specific patterns that determine how we move. Novel rehabilitation paradigms seek to restore motor function by enhancing endogenous neural plasticity through a number of mechanisms and to sculpt the plasticity via task-specific training.
Neuromechanical principles underlying motor module organization
In this first major section of the essay, we will elaborate on the characteristics of biomechanical systems that may lead to a modular organization for motor control. Modularity can be observed at many levels of motor performance, from muscle, kinetics, and kinematic measures, and in both the spatial and temporal organization of such measures. We will focus solely on what we consider to be the most basic level of modularity: time-synchronized activity of multiple muscles or motor units throughout the body. This level of modularity addresses a basic biomechanical constraint: muscle effects on motor output cannot be considered in isolation, but require the coordination of multiple muscles throughout the body (Chiel et al., 2009; Dickinson et al., 2000; Hooper and Weaver, 2000; Nishikawa et al., 2007; van Antwerp et al., 2007a). Upon this most basic level of modularity, structure and variability in timing, kinetics, and kinematics of movements can be constructed.
Typically, motor modules are characterized through linear decomposition techniques that are useful, but may not fully capture the true complexity of motor modules. Using signal processing methods, such as principal component analysis (PCA), independent component analysis (ICA) and nonnegative matrix factorization (NMF) (Lee and Seung, 1999; Ting and Chvatal, 2010; Tresch et al., 2006; Tresch et al., 1999), motor signals can be decomposed into underlying motor modules, also referred to as muscle synergies, that reflect consistent patterns of multi-muscle coordination that generate specific actions (Figure 2). More physiological and feature-based representations have been found in both sensory systems (Lee and Seung, 1999; Olshausen and Field, 2004) and motor control (Ting and Chvatal, 2010; Tresch et al., 2006) when using techniques that do not assume orthogonality (e.g PCA). In neural systems, non-negativity also appears to be important to reflect representations by spiking activity of neurons. While all of the current decomposition technique assume linear combinations of motor modules, it is unlikely that the motor modules are linearly additive in a global sense. However, using such methods can still be useful for revealing locally-linear mechanisms that the nervous system may use to represent complex nonlinearities in the environment (Olshausen and Field, 2004). Although current computational methods to analyze motor patterns have many limitations (Burkholder and van Antwerp, 2013; Steele et al., 2013; Ting and Chvatal, 2010; Tresch and Jarc, 2009; Zelik et al., 2014), and may not be directly interpretable in terms of neural mechanisms, they still provide useful tools for describing and understanding structure in motor coordination. Advances in computational methods are ongoing, but will not be discussed further here.
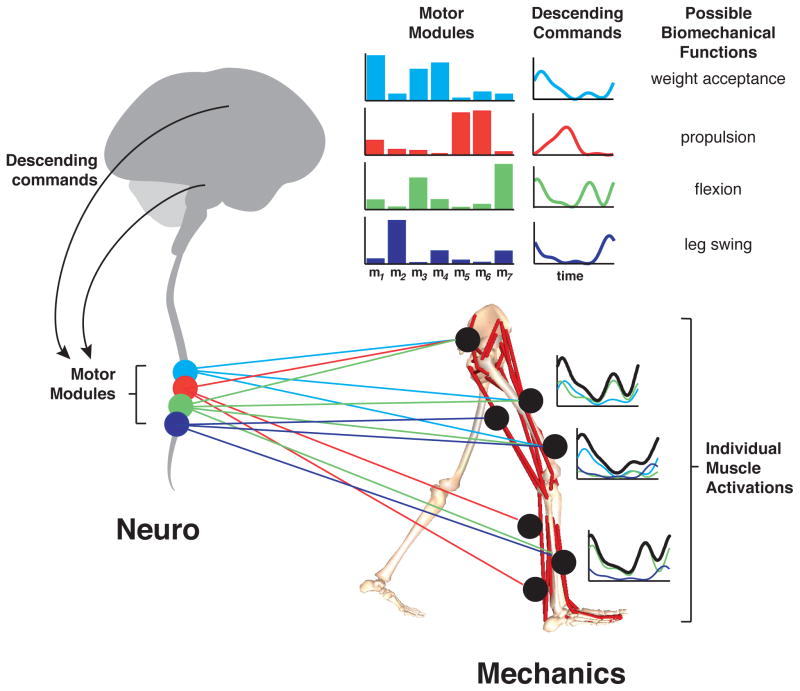
Figure 2. Motor modules define functional co-activation of muscles.
For walking, descending commands from the spinal cord, brainstem, and cortex can modulate spinal motor modules. Each motor module selectively co-activated multiple muscles with a characteristic level of activation (colored bars) to produce the mechanical output needed to achieve a given locomotor subtask (Clark et al., 2010; Neptune et al., 2009). The particular timing of recruitment (colored lines, top right) can vary across steps, across gait speeds, and environmental demands. The activity of individual muscles express unique temporal patterns of activity (black lines, bottom right) due to their different contributions to different motor modules (colored lines, bottom right).
Principle of motor abundance: motor modules reflect specific motor solutions selected from an abundance of possible solutions
For any given motor task or behavior there are generally a large number of “motor equivalent” solutions that can produce similar or functionally-equivalent behaviors. Because many motor solutions exist, there is no single correct or optimal motor pattern, e.g. different motor modules can equivalently perform the same motor task. This ability to choose from many solutions underlies the adaptability and robustness of biological systems. The concept of motor abundance (Latash, 2012) is critical for understanding that variations in movement solutions and variability in movements that are observed (Scholz and Schoner, 1999; Scholz et al., 2000; Valero-Cuevas et al., 2009). Within these “motor equivalent” solutions, there may be some that are less desirable than others for any number of reasons, including energetics, stability, and generalizability across tasks. However, finding optimal solutions may be challenging, as muscle activation patterns have complex and nonlinear relationships to biomechanical functions (Cullins et al., 2014).
Motor modules may reflect “good-enough” solutions for movement that provide stable and predictable motor outputs. Experimental evidence demonstrates that individuals exhibit consistent motor modules, in seemingly variable muscle activation patterns across multiple muscles, motor behaviors, and across species (Bizzi et al., 2008; Chvatal and Ting, 2013; Chvatal et al., 2011; d’Avella et al., 2003; Giszter et al., 2007; Ting, 2007; Ting and McKay, 2007; Torres-Oviedo and Ting, 2010). Different stable solutions can be identified throughout a lifetime. For example, default patterns for movement are established in the embryonic stage, during which spontaneous motor activity such as kicking and flailing are observed (Bekoff, 2001). These movement patterns are available at birth and can allow a fawn to run minutes after it is born. Human infants are born with the capacity for stepping and kicking (Yang et al., 2004), and through exploration (Smith and Thelen, 2003), movement patterns are refined and more are created throughout development (Dominici et al., 2011). Models of spinal circuitry and biomechanics suggest that “good enough” (e.g. suboptimal) solutions for movement that can be found in just a few iterations of random searching (Tsianos et al., 2014); once found, these solutions are likely to be reinforced by use-dependent neural plasticity.
Principle of motor structure: Motor modules reflect biomechanical task relevance
The biomechanical affordances and constraints of the body and environment shape the allowable structure and variability of motor patterns. Biomechanical affordances refer to the types of movements that are facilitated by body structure. Body structures define ways of moving that require little energy or neural control to produce. For example, simulations and robots that mimic the structure of the body can produce walking-like behaviors with little energy and without muscles or joint actuators (Collins et al., 2005; Kuo, 2007). Biomechanical constraints refer to movements that may be difficult or impossible to achieve with a given structure, or refer to the required neural input to achieve a movement, e.g. precise timing or activity of a particular muscle. In walking, biomechanical constraints limit knee extension and place constraints on step length in backward walking. The basic structure of motor patterns during a particular gait is defined by the sequence of subtasks: placing the foot on the ground, pushing against the ground for propulsion, and swinging the limb forward. Each subtask defines certain co-activation patterns of muscle activation across the limb (van Antwerp et al., 2007b; Zajac, 2002). Biomechanical affordances and constraints determine the how precise or variable these motor patterns must be. For example, in a simulation of single-legged locomotion, biomechanical “bottlenecks” and “don’t care” regions were identified that predicted the precision and variability of locomotor solutions found by a genetic algorithm. The highest fitness solutions all exhibited precise timing at the “bottleneck” of placing the leg and pushing it backward, which had a large effect on movement efficiency. In contrast, the solutions showed high variability in the “don’t care” region late in the stance phase, during which the model leg continued to move backwards but was no longer able to exert force (Beer et al., 1999). For example, distributions of motor neuron activation duration varies from one individual to another in Aplysia feeding behavior, but when motor neuron duration and timing play a critical role in a behavior such as closing its grasper to retract food, the distributions become similar across all individuals (Cullins et al., 2014). In contrast, there is high variability in the duration of motor neuron activity to close the grasper if the animal fails to grasp food, as the motor neuronal activity is no longer functionally relevant. Similar examples across many species and motor behaviors can be found where motor activity that does not directly contribute nor interfere with the task at hand is found to be highly variable both within and across individuals (Bernstein, 1967; Scholz and Schoner, 1999; Valero-Cuevas et al., 2009).
Motor modules may reflect biomechanical structures and the required coordination of neural signals to perform motor tasks. Motor modules identified experimentally have been associated with biomechanical functions necessary for walking and balance (Allen and Neptune, 2012; Chvatal et al., 2011; Clark et al., 2010; Neptune et al., 2009; Safavynia and Ting, 2013; Ting and Macpherson, 2005; Torres-Oviedo et al., 2006). In simulations, motor modules have been shown to emerge based on optimal control of multi-jointed and multi-legged systems and produce near-optimal motor performance (Berniker et al., 2009; Chhabra and Jacobs, 2006; Kurtzer et al., 2006; McKay and Ting, 2012; Todorov and Jordan, 2002). Modular control can reproduce essential features of movement in simulations of a frog leg (Berniker et al., 2009), and of human walking (Allen et al., 2013; Allen and Neptune, 2012; Neptune et al., 2009), and balance control (McKay and Ting, 2012). Motor modules may thus reflect an interaction between the neural and motor system, and may often align with coordination patterns that optimize energetic efficiency given biomechanical constraints (De Groote et al., 2014; McKay and Ting, 2012; Steele et al., 2013). Because motor structure reflects biomechanical task relevance, similarities in motor modules for the same task will exist. For example, there are substantial similarities in the most active muscles of motor modules used for walking at different speeds and for different balance strategies, although inter-individual differences also exist (Chvatal et al., 2011; Clark et al., 2010). As discussed below, biomechanical constraints cannot uniquely determine motor module structure in most cases.
Principle of motor variability: Motor module variations across individuals are high if the effect on motor output is low
Variations and variability in motor control also depend on biomechanical affordances and constraints, based on the reasonable assumption that the nervous system only regulates motor outputs that are directly relevant to task goals. Using biomechanical models in conjunction with optimality principles has predicted higher variability in ‘good enough’ regions of behavior, e.g. the uncontrolled manifold, theory of minimum intervention, and optimal feedback control (Bernstein, 1967; Scholz and Schoner, 1999; Todorov and Jordan, 2002; Valero-Cuevas et al., 2009). Biomechanical models can be used to determine the degree to which variability can occur without having a deleterious effect on performance. For isometric force production, the degree of variation in muscle activity in the finger is relatively constrained, allowing for little variability (Kutch and Valero-Cuevas, 2012; Valero-Cuevas et al., 1998), whereas the range of possible variations is much greater in the cat hindlimb (Sohn and Ting, 2013). These differences appear to match the variability in muscle activity measured experimentally. Variation in motor patterns may also endow a limb with other characteristics that may or may not matter to the movement. For example, increasing muscle activity to improve limb stability may reduce the need for precise neural control (Bunderson et al., 2008; Franklin et al., 2004; Sohn and Ting, 2013). Other equivalent solutions may be similar in energetics or stability, yet differ in motor pattern, causing subtle differences in movement (Sohn and Ting, 2013).
As a consequence of allowable variations to produce similar tasks, differences in the structure and number of motor modules that are specific to individuals have been identified across species and motor behaviors. The consistency of motor modules across biomechanical conditions within an individual suggest that they do not emerge from “online” optimization based on biomechanics, but represent preferred patterns of muscle coordination that are modulated across a class of movements. For example, the structure of motor modules for walking share similarities in the most active muscles, but the contributions of other muscles can vary substantially (Chvatal and Ting, 2012; Clark et al., 2010). Moreover, these same motor modules are recruited across walking speeds, and even in response to perturbations imposed during walking (Chvatal and Ting, 2012; Oliveira et al., 2012). Motor modules are used in kicking, swimming, and jumping in frogs and across different postural behaviors in cats and humans (Chvatal and Ting, 2013; Chvatal et al., 2011; Giszter et al., 2007; Hart and Giszter, 2004; Roh et al., 2011; Torres-Oviedo et al., 2006; Tresch et al., 1999), suggesting that they form a repertoire of whole limb actions. Motor modules in postural control can vary in structure and number across individuals and are preserved across different biomechanical configurations. Motor modules for weight support are characterized by extensor muscle activity, but the degree of activity in the hamstring muscles can vary substantially. Because motor variability is high where the effect on motor output is low, variations in motor modules may affect secondary characteristics of movement. Differences in motor modules can also reflect differences in kinematic strategies for postural control, and motor modules specific to one individual may not adequately reproduce muscle activity in another (Torres-Oviedo and Ting, 2010). Indeed, accuracy of human walking simulations is improved when individual-specific motor modules are included (Walter et al., 2014).
Principle of individuality: Motor modules are shaped by history and may generate individual movement styles
It has recently emerged as a general principle that individual — and not averaged — motor solutions solve neuromotor problems. Individuals may have their own “motor program styles,” i.e., they may show significant individual variations in outputs of the motor system that are both consistent within a given animal, and differ from one individual to another. Variations in “motor program styles” have been observed in a wide variety of animals (Calabrese et al., 2011; Golowasch et al., 2002; Marder and Goaillard, 2006; Prinz et al., 2004), and in humans (Nussbaum and Chaffin, 1997; Torres-Oviedo and Ting, 2010; Welch and Ting, 2008). Not all of these differences can be attributed to biomechanics, as the fidelity of human walking simulations using generic biomechanical models can be improved through consideration of individual movement patterns (Ting et al., 2012; Walter et al., 2014), and differences in the weightings of joint torque production can be used to synthesize different styles of walking (Liu et al., 2005). This illustrates that biomechanics in insufficient to determine motor patterns, allowing for many functionally-equivalent solutions.
Developmental processes, motor exploration, experience, and training all play a role in shaping individual movement styles. Motor exploration and variability are essential to the discovery of movement patterns that produce useful motor functions and do not necessarily follow rules of engineering approaches (Herzfeld and Shadmehr, 2014; Huang et al., 2008; Loeb, 2012; Smith and Thelen, 2003; Wu et al., 2014). The properties of the neuromechanical system may be such that only a few variations are required to identify “good enough” solutions (Tsianos et al., 2014). But, even after learning more “optimal” movement styles, subjects tend to revert to suboptimal, habitual patterns (de Rugy et al., 2012; Ganesh et al., 2010; Snaterse et al., 2011). Movement strategies for everyday tasks may appear optimal because they have been refined over both evolutionary time as well as a lifetime. Extensive, long-term training may be necessary to identify globally optimal movement strategies, which are sought by elite athletes, dancers, and musicians. Because motor history shapes individual movement styles, motor modules may differ and become different due to motor experience and training. Indeed, different movement patterns for grasping may be identified in musicians, shaped by their specific training (Gentner et al., 2010) and different musicians display different movement styles (Furuya and Altenmuller, 2013). This perspective on how we learn to move is consistent with activity-dependent plasticity after neural injury that is altered by the specificity, intensity, difficulty and complexity of motor training (Adkins et al., 2006; Fisher and Sullivan, 2001; Will et al., 2004). Similar challenges are posed by sports or classroom learning, where stable, slop-timal solutions may be difficult to change (Chi and Roscoe, 2002; Handford, 2006). For example, changing movement patterns is a risky endeavor for elite athletes, and Tiger Woods required two years without winning tournaments to reshape his golf swing (Eden, 2013). Understanding the costs of changing movement strategies is likely to play an important role in developing effective rehabilitation therapies.
As room for variability increases, the seemingly fixed and objective nature of motor modules dissolves. The more biomechanical constraints exist, the less opportunities for individual variation, and the more motor modules will tend to look energetically optimal. When characterizing the optimality of motor patterns in both neurologically normal and motor impaired individuals, it must always be asked: “with respect to what?” Although differences in walking style exist across gender, social status, and culture (Hall, 1976), these differences are much less than those observed in speech, a less biomechanically constrained motor task. Verbal communication can be equally good using different language-specific phonemes (Kuhl, 2004), which can be thought of as motor modules for speech. The degree of variability is directly related to the fact that variability in sound production does not cause the same devastating effects as in walking, where unfit variations may lead to a fall. Motor modules in speech production (Elemans, 2014; Gick and Stavness, 2013) may facilitate native-language speech, but cause distinctive accents and pronunciation errors when speaking a foreign language. Similarly, motor accents in bodily movements may also cause differences in a person’s ability to learn new motor tasks, and thus be an important consideration during rehabilitation.
Principle of multifunctionality: Motor modules may mediate multifuctionality of muscles for movement
While motor modules themselves are invariant, they do not produce stereotyped actions. Rather than constrain the nervous system, the ability to flexible combine modules actually facilitates adaptation and learning. Variability observed across different types of behaviors, and trial-by-trial variability can be accounted for by varying combinations of motor modules (Cheung et al., 2005; Hart and Giszter, 2004; Roh et al., 2011; Torres-Oviedo and Ting, 2007; Tresch et al., 1999). Rather than random noise in individual muscles or trajectories, variability across instances of movement may thus reflect differences in the descending drive to stored movement patterns (Churchland et al., 2006) that could facilitate motor exploration (Huang et al., 2008; Wu et al., 2014). Indeed, learning to perform a novel task is faster if it can be achieved by altering recruitment of a smaller number of motor modules rather than learning new control strategies for individual muscles (Berger et al., 2013). Consistent with findings in spinal CPGs where temporal rhythms can differentially recruit groups of muscles (McCrea and Rybak, 2008; Stein and Daniels-McQueen, 2002), this suggests that the temporal commands to motor modules can be more readily adapted than the modules themselves (McKay and Ting, 2012).
Although modularity is often taken to mean a reduction in dimension, this is true only within the context of specific behaviors. Because a few muscles mediate many motor behaviors muscles, many motor modules may exist to handle different motor behaviors or contexts. If one considers the very large number of different tasks that an animal or human may engage in over a lifetime, many different patterns are required (Zelik et al., 2014). Dimensional reduction may be an artifact of current algorithms for identifying modularity that work by reducing the dimensionality of data. The multifunctionality of limbs and bodies is critical for facilitating a large motor repertoire. If one considers just the simple on/off combinations of muscle activation patterns among n muscles, one obtains 2n possible joint torque patterns. Thus, the potential behavioral repertoire for coordination of multiple muscles is much greater than the total number of muscles or even motor units. (Chiel et al., 2009). When one further considers differences in level of muscle activation and relative timing of activations, the number of possibilities increases even further. If these possibilities are in turn combined with the effects of changes in posture, environment, or movement that can also modulate muscle function, the possibilities become very large. Consider the simple motor task of getting up from a chair, walking, turning, and then sitting down: the muscles of the body must be coordinated in a myriad of different patterns to accomplish all of the necessary subtasks. During challenging athletic activities or dancing, even more motor subcomponents must be mastered and properly deployed.
A large set of motor modules across the behavioral repertoire may facilitate multifunctionality, allowing the same muscles to perform different functions in different behavioral contexts. While having more motor modules than muscles may seem counterintuitive, a high-dimensional representation of actions defined by combinations of muscles may be more directly related to the resulting motor output. In contrast, the effects of individual muscles are highly nonlinear, variable and context dependent. Others have noted this previously:,Hughlings-Jackson (1889) noted that the muscles of the hand were represented in lower motor centers “in numerous different combinations, as simple and very general movements,” and in the highest centers “the same muscles are represented (re-re-represented) in innumerable different combinations, as most complex and most special movements.” The implication is that these areas are not simultaneously active, but represent the whole repertoire of hand movements. Physiologically, these many representations of movement could be mediated by neurons of the cortex, reticular formation, and spinal cord project to multiple muscles throughout the body. As an example, spinal motor neurons specialized for activating hip flexors in limb withdrawal reflex are not generally active in multiple types of limb movements (Berkowitz, 2007).
As a consequence, as representations of useful ensembles of muscles that produce actions, motor modules may improve the rapidity and robustness of searches for new movement patterns. Similar principles have been proposed to govern visual and sensory processing (Olshausen and Field, 2004) where different streams of visual input signals (e.g. retinal activity, or pixels) can represent the same object (cf. principle of motor abundance, above). It has been proposed that sensory processing is facilitated by representations of the inherent structure, or features, in complex natural scenes (cf. principle of motor structure, above). As the number of features far exceed the number of visual inputs, such representations form an overcomplete set of basis vectors that have more direct relationship to the objects in the environment than individual pixels (cf. principle of multifunctionality, above); such principles have also been identified in signal processing as a way to handle nonlinearities (Hastie et al., 2005; Olshausen and Field, 2004). In the motor system, the advantage is that motor modules can be recruited based on desired whole-limb or whole-body functions rather than requiring specific muscles activations to be computed (Safavynia and Ting, 2012, 2013; Ting and Macpherson, 2005; Ting and McKay, 2007). Moreover, amongst a vast dictionary of representations, only a few are used at a time to represent a given image or action, a phenomenon referred to as sparse coding (Olshausen and Field, 2004). Sparse coding is consistent with the existence of sensory and motor maps in which only neurons in small regions of the maps are active at any given time. Sparse representations have been proposed to enhance the efficiency of sensory processing and motor adaptation (Fiete et al., 2004; McKay and Ting, 2012; Ting and McKay, 2007), both of which are shaped by individual experience and developmental processes (cf. principles of variability and individuality, above). Similarly across biology, arguments for modularity have been made based on their ability to improve adaptability and robustness while decrease connectivity costs in neural networks and improve (Clune et al., 2013; Wagner et al., 2007).
Are motor modules encoded by the nervous system?
While the arguments presented are largely theoretical and indirect, some evidence for motor modules at a neurophysiological level does exist. Although it is possible for synchronous activity of motor neurons to arise without being directly linked to the same presynaptic neuron, the divergent structure of neurons in the cortex, brainstem, and spinal cord projecting to motorneurons and pre-motorneuronal pools can provide one type of neural substrate for the type of spatial modularity in muscle coordination discussed here. Studies stimulating the spinal cord and cortex reveal correlated outputs across motor pools (Overduin et al., 2012; Saltiel et al., 2001). During natural movement, shared common drive to motorneurons of the eye (Joshua and Lisberger, 2014), leg (Hart and Giszter, 2010) Krouchev, 2006 #346}, arm (Holdefer and Miller, 2002), and pelvic muscles (Asavasopon et al., 2014) have been demonstrated. Modularity in the temporal patterns of motor outputs (d’Avella et al., 2003; Flash and Hochner, 2005; Hart and Giszter, 2004; Ivanenko et al., 2003; McCrea and Rybak, 2008) likely have different, more dynamic representations in the nervous system such as in central pattern generators (McCrea and Rybak, 2008; Stein and Daniels-McQueen, 2002) Proprioceptive sensory feedback can also play a role in the expression of motor modules, providing inputs that are structured by the mechanics of the musculoskeletal system and environment in some cases (Cheung et al., 2005; Kutch and Valero-Cuevas, 2012). However, the existence of motor modules in the absence of sensory feedback (Gizster, (Cheung et al., 2005), during the production of voluntary movements using visual feedback (d’Avella et al., 2011), or that is at odds with sensory inflow (Chvatal et al., 2013; Safavynia and Ting, 2013; Torres-Oviedo et al., 2006) provide some evidence for the neural encoding of some motor modules. Evidence suggests that sensory feedback can modulate temporal patterning of recruitment to relatively fixed motor modules across different types of behaviors (Hart and Giszter, 2004; Kargo et al., 2010; McCrea and Rybak, 2008; Stein and Daniels-McQueen, 2002). Currently, the neural substrates for motor modularity remain largely elusive; however, studies of neuromotor impairments affecting the spinal cord, motor cortex, basal ganglia and other neuroanatomical structure may help to reveal the mechanisms of motor modularity.
Neuromechanics of motor impairment and rehabilitation
In the second major section of this essay, we turn to a consideration of the implications of motor modules for understanding motor impairment and their implications for rehabilitation. Although neurological pathologies affect neural mechanisms involved in movement, neuromechanical principles of motor abundance, motor structure, motor variability, individuality, and multifunctionality hold whether in skilled experts or in individuals with motor impairments. Deficits in motor module organization can, in turn, provide a clearer understanding and assessment of the nature of the motor impairments and how they can be improved, providing rational targets for novel therapies. Many of the current outcome measures, diagnostic techniques, and clinical tests focus on overall motor function, and lack the power to answer fundamental questions (i.e., why, what, and how) about a person’s deficits and ability to recover (Mancini and Horak, 2010; Pardasaney et al., 2012). Testing the effectiveness of novel therapies requires more specific methods to reveal heterogeneity of participants and characterize functional neurophysiologic adaptations due to treatments (Dobkin, 2007). For instance, why does a rehabilitation treatment help some but not others? What motor strategies were changed due to recovery or treatment? Further, motor modules provide a noninvasive assessment of the structure and variability of motor coordination that can be linked to scientific knowledge about the functions of the neural areas affected by injury or impairment. These can be combined with neurophysiological measure of neural connectivity (Belda-Lois et al., 2011; Krakauer et al., 2012; Whitall, 2004) that cannot typically be identified during movements, especially gait and balance tasks. Tracking changes in motor modules through recovery and rehabilitation can provide assessments of improvement as well as insights into the neural mechanisms of motor plasticity (Giszter and Hart, 2013; Safavynia et al., 2011; Santello and Lang, 2015).
As individual differences in motor modules are shaped through experience, appropriate rehabilitative training may be necessary to target individual-specific motor deficits in conjunction with novel plasticity-enhancing adjuvant therapies (Hayes et al., 2014b; Lovett-Barr et al., 2012). Use-dependent neural plasticity, the capacity of the nervous system to adapt in response to experience, is a critical yet relatively unexplored mechanism underlying rehabilitation (Kleim and Jones, 2008; Nudo et al., 1996a; Nudo et al., 2001; Nudo et al., 1996b; Wittenberg, 2009; Wittenberg and Schaechter, 2009). While plasticity plays a role in rehabilitation, endogenous and injury-induced neural plasticity only enables partial spontaneous recovery of motor function (Goshgarian, 2003; Kaegi et al., 2002), and the extent of recovery is slow, variable and frustratingly limited (Raineteau and Schwab, 2001). However, many exciting new developments in rehabilitation science are targeted at enhancing neural plasticity, including the use of stem cells (Isacson and Kordower, 2008; Lu et al., 2014; Takahashi and Yamanaka, 2006), neural stimulation (Benazzouz and Hallett, 2000; Hallett, 2000; Ruge et al., 2011; Stefan et al., 2000), therapeutic exercise (Courtine et al., 2009; Edgerton et al., 2006; Edgerton and Roy, 2009; Vaynman et al., 2003; Weishaupt et al., 2013), and acute intermittent hypoxia (Baker-Herman et al., 2004; Wilkerson and Mitchell, 2009). All of these therapies can provide a generalized enhancement of neural plasticity either locally or profusely, enhancing the potential for individuals to reorganize relevant neural circuitry necessary to improve movement (Kleim and Jones, 2008) but all of these therapies require this plasticity to be appropriately directed to improve motor function.
In the sections that follow, we discuss motor modules for walking and balance in three neurological disorders affecting different parts of the central nervous system: spinal cord injury, stroke, and Parkinson’s disease, each of which may provide insights to the neural bases of motor modules (Figure 3). Each of these neural impairments affects a specific part of the nervous system that has a different detrimental effect on the walking ability, reflecting the distributed control of posture and gait throughout the central nervous system (Takakusaki, 2013). Interneurons in the spinal cord may encode motor modules for locomotion and other lower limb tasks (Hart and Giszter, 2010; Roh et al., 2011; Saltiel et al., 2001), which can be flexibly recruited by spinal structures regulating the timing of locomotor patterns (McCrea and Rybak, 2008; Stein and Daniels-McQueen, 2002). Motor cortical activity may be more heavily involved during forms of walking requiring cortical inputs, such as visually-guided obstacle crossing (Drew et al., 2002). Cortical activity may encode motor modules as well (Capaday, 2002; Ethier et al., 2006), and/or recruit spinal motor modules (Rathelot and Strick, 2009). Spinal cord lesions affect both corticospinal connectivity, as well as the organization of the spinal cord below the site of the lesion, and can therefore affect the encoding and recruitment of modules in the spinal cord (Giszter and Hart, 2013; Roh et al., 2011; Tresch et al., 1999). It is also likely that there are separate and complementary motor modules encoded in cortical areas for both upper and lower limb movement (Hughlings-Jackson, 1889; Rathelot and Strick, 2009) as well as in reticular formation in the brainstem for simple arm movements (Riddle and Baker, 2010; Zaaimi et al., 2012), and for postural control (Deliagina et al., 2008; Schepens et al., 2008). Stroke may impair cortical motor modules as well as cortical recruitment of spinal motor modules. The basal ganglia, which are affected by Parkinson’s disease, may regulate the appropriate selection of motor modules in cortical and brainstem areas. In the following sections, we will discuss the different impairments in motor modules in spinal cord injury (SCI), stroke, and Parkinson’s disease (PD), and describe how targeted rehabilitative therapies may improve modular organization.
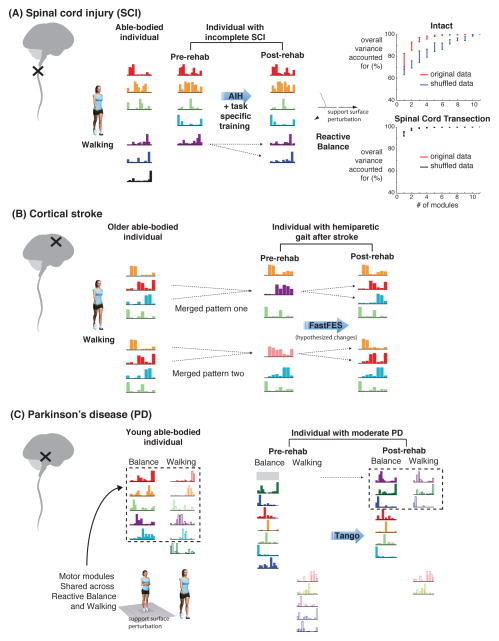
Figure 3. Different motor modules deficits and improvements in spinal cord injury, stroke, and Parkinson’s disease.
Colored bars represent motor modules, with the height of each bar representing the extent to which an individual muscle is part of that motor module. Color of motor modules across conditions and/or population (e.g., able-bodied to pre-SCI) represents similarity between motor modules. A) Spinal cord injury disrupts both descending connectivity and spinal organization. Accordingly, motor modules resembling those found in able-bodied individuals are reduced after incomplete spinal cord injury, and additional motor modules characterized by co-contraction can emerge (not shown) (Hayes et al., 2014a). After rehabilitation, motor modules may be reshaped and better resemble those in able-bodied individuals (Hayes et al., 2012). In animals with complete spinal cord transection, a few motor modules can account for a large degree of variance in muscle activity for reactive balance in response to support surface translations (Chvatal et al., 2013). In the intact condition, the total variance explained by an increasing number of motor modules is significantly different from the variance explained in randomly shuffled data, indicative of consistent structure in muscle activity (red vs. blue lines). However, after complete spinal transection, the variance explained by motor modules does not differ in from that obtained by randomly shuffling data, suggesting that no consistent structure exists (red vs. black lines). B) Stroke disrupts corticospinal drive, and impairs independent recruitment of joint actions. Motor modules for walking in the paretic leg are merged versions of those found in able-bodied individuals. Merging can occur between different modules that are associated with different motor deficits (Clark et al., 2010). After rehabilitation, splitting of motor modules is hypothesized to occur that would be associated with improved performance. C) Parkinson’s disease impairs basal ganglia function and is associated with inappropriate selection of motor patterns as well as cortical hyperexcitability. Accordingly, in individuals with PD, the number of motor modules in walking and reactive balance are similar to those found in healthy individuals (Rodriguez et al., 2013; Roemmich et al., 2014). However, in young, healthy adults, motor modules for reactive balance to support surface translation and overground walking are similar, suggesting a common subcortical origin for the recruited motor modules. In contrast, in individuals with PD that have balance impairments, motor modules from reactive balance and walking can appear to be completely distinct, consistent with increased attention and cortical control of gait. After rehabilitation, motor modules may become more similar across tasks, suggesting improved automatic, subcortical control of gait (Allen et al., 2014).
Loss of motor modules in spinal cord injury (SCI)
A few reports suggest that motor modules after spinal cord injury are lost or abnormally structured. The location of injuries to the spinal cord are highly variable, damaging and sparing different parts of the spinal cord and corticospinal connections that contribute to walking. Nearly 75% of persons with incomplete SCI regain some walking capacity (van Hedel et al., 2009) using assistive devices, but show little progression to unsupported overground walking (Field-Fote and Roach, 2011; van Hedel and Dietz, 2010). After incomplete SCI, the number of motor modules used in walking is reduced (Figure 3A) in both children and adults (Fox et al., 2013; Hayes et al., 2014a). While some of the motor modules resemble those found in able-bodied individuals, incomplete SCI subjects exhibited a wider range of module compositions, reflective of the heterogeneity inherent in incomplete SCI. Many of the pathological modules were characterized by co-contraction of agonist and antagonistic muscles (Fox et al., 2013; Hayes et al., 2014a), and many were statistically distinct from modules identified in healthy individuals (Figure 3A). Moreover, the expression of pathological modules may be specific to each individual’s gait deficits (Hayes et al., 2014a). In contrast, in animals with complete spinal transection, modules for reactive balance responses were absent (Chvatal et al., 2013) consistent with the need for brainstem connectivity. In contrast, muscles could be vigorously activated in an alternating rhythm by paw shake, which can be spinally-mediated. Although some modules were identified from successful balance trials, they were not statistically different from those extracted from randomly shuffled data (Figure 3A), suggesting that there was no meaningful motor structure in reactive balance after spinal transection (Chvatal et al., 2013).
Motor module analysis can provide insight into the motor deficits underlying impaired gait in SCI. For example, a module for eccentric braking was absent in all individuals with incomplete SCI, consistent with foot drop or slap that is often observed clinically. In general, motor modules exhibited abnormal co-activation of muscles and much broader temporal recruitment across the gait cycle (Hayes et al., 2014a). This suggests the use of a disorganized motor pattern, or patterns that stabilize the limb, rather than biomechanically efficient motor patterns that effectively achieve particular motor subcomponents during different phases of gait. Interestingly, the number of motor modules was reduced in able-bodied individuals when they used assistive devices that were matched to individuals with incomplete SCI. This suggests that the assistive devices provide biomechanical functions that may obviate the need to recruit particular motor modules. Analysis of such data could provide insight into the changes in motor module composition and recruitment necessary for improved motor function.
Neural plasticity induced by a novel breathing treatment may prepare the nervous system to be sculpted by task practice, which could then be assessed by motor module analysis. A promising strategy shown to improve respiratory and non-respiratory motor function in incomplete SCI is to induce spinal cord plasticity through exposures to modest bouts of low oxygen, e.g. acute intermittent hypoxia (AIH). In rodent SCI models, AIH induces motor plasticity (Dale and Mitchell, 2013; Dale-Nagle et al., 2010; Lovett-Barr et al., 2012; Mitchell, 2008; Vinit et al., 2009) through serotonin-dependent synthesis of brain-derived neurotrophic factor (BDNF)(Baker-Herman et al., 2004). Repetitive exposures to AIH elicits increased breathing capacity and locomotor performance in rodents with SCI (Lovett-Barr et al., 2012). In persons with incomplete SCI, a single bout of AIH increases plantar flexor muscle activity and torque output (Trumbower et al., 2012), and repetitive AIH over 5 consecutive days improves walking ability (Hayes et al., 2014b). Daily AIH just prior to walking training yields greater gains in walking function than either daily AIH or walking training alone (Hayes et al., 2014b). However, it is not known whether the effects of AIH simply boost the overall motor output or whether the combination of enhanced plasticity and training could improve the structure and recruitment of motor modules. Early evidence suggests that the number of motor modules is increased and the composition of motor modules changes after AIH (Hayes et al., 2012).
Even when motor patterns are highly variable across repetitions of the same movement, different movements, and across individuals, motor modules provide a way to identify consistent motor structure and track individual progress in rehabilitation. Motor module analyses can distinguish between completely random organization in motor outputs as opposed to highly variable recruitment of motor modules. The appearance of abnormal motor modules in SCI may be unstable, and could reflect either inappropriate neural activity, or a search process to re-learn useful motor coordination patterns. Tracking participants longitudinally across rehabilitation may inform how individuals search for and learn new motor modules.
Merging of motor modules after stroke
Motor modules provide a valuable way to analyze the consequences of stroke, which have effects that are quite different from spinal cord injury. The number of motor modules in individuals post-stroke is reduced on the paretic side due to a merging of modules, revealing impairments in whole-limb muscle coordination that correspond to observed motor deficits in the leg (Cheung et al., 2012; Clark et al., 2010) and arm. Merged modules reflect a decrease in the independence of muscular control, and are consistent with so-called “clinical muscle synergies” in stroke where abnormal coupling of muscles across the limb are observed in both the upper (Dewald et al., 1995) and lower extremities (De Quervain et al., 1996; Knutsson and Richards, 1979; Shiavi et al., 1987). In the lower limb, impaired motor modules appear to be due to a merging of two modules typically identified in the non-paretic and control legs (Figure 3B), consistent with a reduction in the independence of corticospinal drive to the spinal cord. The number of motor modules is correlated with reduced walking speed and clinical measures of balance and walking function, and biomechanical measures such as propulsion asymmetry and step length asymmetry (Bowden et al., 2010; Clark et al., 2010). Moreover, motor modules are better correlated with gait and balance function than are lower limb Fugl-Meyer assessments (Bowden et al., 2010) typically used to measure severity of motor impairment. Even when the number of modules is not reduced, impairments in the ability to flexibly recruit motor modules is also observed, such as a reduced ability to take longer, higher, or quicker steps (Routson et al., 2014), suggesting deficits in the descending control of motor modules.
The characteristics of merged motor modules also predict differences in gait impairments that may necessitate different rehabilitation approaches. This is important because self-selected walking speed is a common measure of rehabilitation effectiveness, yet speed (a functional output) can be achieved through different strategies (e.g., improved mechanical output from the paretic leg, or increased reliance on mechanical output from the nonparetic leg); within these strategies, a subject may use different muscular coordination patterns (improvement in neural control). One of the most common impairments post-stroke is the inability to adequately recruit the ankle plantarflexors of the paretic leg (Lamontagne et al., 2007; Turns et al., 2007), which is important for directing ground reaction forces (Bowden et al., 2006) and hip and leg extension (Peterson et al., 2010) in walking. Among individuals with a reduction in motor modules, two different types of plantarflexor impairment (Figure 3B) were found (Clark et al., 2010). Some were unable to independently activate the plantarflexors and the proximal extensors (hip abductors/extensors and knee extensors). In contrast, others could independently activate the plantarflexors but with inappropriate timing; these individuals also merged control of the proximal extensors with the hamstrings. Neuromechanical simulations reveal that both impairments lead to inadequate propulsion from the plantarflexors (Allen et al., 2013) suggesting that improving paretic plantarflexor recruitment is a critical component for rehabilitation. Further, both groups also had impaired swing of the paretic leg. In the first group (merged plantarflexor control) this occurred prior to swing, whereas in the second group (independent plantarflexor control but merged proximal extensors and hamstrings) this occurred during late swing. These results suggest that distinct rehabilitation approaches may be prescribed based on neuromechanical impairments identified through motor module analysis.
A novel gait retraining tool that combines fast treadmill walking and functional electrical stimulation (FastFES) is being developed that is targeted to sculpting plantarflexor motor modules for walking propulsion. Fast walking can help improve motor function in several ways. First, moderate exercise can be a promotor of motor plasticity (Lamontagne and Fung, 2004). Second, it may encourage motor exploration by requiring participants to walk at more challenging speeds, and provides opportunity for greater repetition (more steps) of practice, which enhances use-dependent plasticity. Third, it emphasizes biomechanical subcomponents of walking such as knee flexion and propulsion, promoting specific sculpting of motor modules. Further task-specific sculpting is provided through electrical stimulation of plantarflexors to improve paretic propulsion; stimulation also provides afferent feedback to enhance motor learning of new motor modules (Kesar et al., 2011; Reisman et al., 2013). FastFES has been shown to improve gait impairments, overground gait function, activity, and participation in individuals with chronic post-stroke hemiparesis (Awad et al., 2014a; Awad et al., 2014b; Knarr et al., 2013; Reisman et al., 2013) through improved plantarflexion (Knarr et al., 2013). However, changes in muscle activity, much less its structure have not been measured after FastFES.
Motor module analyses performed before and after rehabilitation may provide a more in-depth and mechanistic evaluation of the treatment effects, and this analysis can be used to further optimize the dosage and ingredients of the intervention. For example, in FastFES, motor module analysis could reveal whether FastFES improves abnormal muscle coupling, e.g. an increase in number of motor modules, and/or inappropriate timing, e.g. improved motor module timing in both the targeted (ankle plantarflexors) and non-targeted (proximal muscles). Similarly, after a different gait training program, merging of the plantarflexor motor module with other motor modules was improved in some individuals but was still inappropriately timed (Routson et al., 2013), suggesting the need for further rehabilitation.
In the upper extremity, altered structure and temporal recruitment of motor modules have also been observed after stroke that may be related to altered neural pathways. Corticomotorneuronal cells in motor cortex can directly project to spinal motor neuron pools, coordinating multiple muscles. However, they can also project to spinal interneurons, which in turn coordinate motor neurons (Rathelot and Strick, 2009). After stroke, reticulospinal neurons in the brainstem can also provide a limited degree of gross arm and hand function (Riddle and Baker, 2010; Riddle et al., 2009; Zaaimi et al., 2012). Given this anatomy, changes in the timing of largely intact motor modules in the upper extremity may reflect altered corticospinal drive to interneurons (Cheung et al., 2009). Merged motor modules in the paretic arm that correlate to the degree of impairment (Cheung et al., 2012) could reflect a greater impairment of corticospinal drive such that modules can no longer be independently activated; alternatively, the merged modules could be due to compensatory arm control from reticulospinal neurons. Interestingly, in the long-term chronic stroke survivors, even though some merged motor modules may still exist, other motor modules appear to fractionate, or split, perhaps as a compensation to improve performance. This may reflect greater capacity for experience-dependent plasticity in intact cortical areas in contrast to the brainstem or to the damaged cortical areas.
Altered selection of motor modules in Parkinson’s disease
Parkinson’s disease (PD) affects the functioning of the basal ganglia, which project to both cortical and brainstem motor areas. In two studies, only moderate decreases in motor modules (Figure 3C) were observed in PD patients compared to healthy individuals. Since spinal and cortical structures remain primarily intact in PD, it seems reasonable that the structure and recruitment of motor modules is not as severely and obviously impaired as in incomplete SCI and stroke (Rodriguez et al., 2013). However (see later in this section), the relationship of this deficit to basal ganglia dysfunction remains unclear, as the number of motor modules in PD was shown to be insensitive to the presence of dopaminergic medication that enhances basal ganglia function (Roemmich et al., 2014).
Deficits in walking and balance due to PD may reflect inappropriate selection of motor modules, which in turn may cause freezing of gait and postural instability. It has been proposed that the basal ganglia selectively inhibit competing motor programs, allowing the appropriate selection of motor pathways for movement (Mink, 1996; Mink, 2003). This hypothesis is consistent with evidence that individuals with PD have difficulty with set-shifting in both cognitive and motor tasks (Chong et al., 2000; Dirnberger and Jahanshahi, 2013). Moreover, impairments in cognitive set-shifting are associated with freezing of gait, which has been characterized as an inability to switch from gait initiation to walking (Factor et al., 2014). For postural control, this results in motor patterns that are inappropriate for a given biomechanical context. For example, after successful reactive balance responses during standing, individuals with PD continue to activate leg muscles when reacting to seated perturbations (Horak et al., 1992). This perseverance may also be related to other proposed functions of the basal ganglia, including reward prediction (Schultz et al., 1997), and habit formation (Yin and Knowlton, 2006). Therefore, it may be more important to examine the recruitment of motor modules in PD in different biomechanical contexts (Carpenter et al., 2004; Dimitrova et al., 2004a; Horak and Macpherson, 1996). For example, individuals with PD fail to adequately decrease muscle activity when changing from narrow to wide stance (Dimitrova et al., 2004b) or in response to different perturbations (Chong et al., 2000). Neuromechanical modeling studies demonstrate that frontal plane balance necessitates a decrease in muscle activity to maintain postural stability (Bingham et al., 2011). This inflexibility in the ability to appropriately recruit motor modules may contribute to postural instability and explain why individuals with PD preferentially select a narrower stance (Dimitrova et al., 2004a; Dimitrova et al., 2004b).
Adapted tango (AT) rehabilitation, specifically targeted at individuals with PD, may improve the appropriate recruitment of motor modules though exercise and practice of complex tasks. Increasing aerobic activity may enhance activity-dependent neural plasticity (Alberts et al., 2011; Hirsch and Farley, 2009). A link between activity, mental engagement and neural pathways may be primed by dancing, which involves complex, unfamiliar tasks such as walking backward, problem solving and movement improvisation. Furthermore, many individuals with PD have deficiencies in planning and executing complex, goal-directed behavior (Kliegel et al., 2005) and troubles with internally generating movement (Low et al., 2002). Therefore, alternating the leader and follower roles in AT may allow patients to focus on external cues, bypassing the dysfunctional basal ganglia and accessing circuitry involving the cerebellum, thalamus and cortex. During dance, the need for creativity, the exposure to novel steps, and the complex movement patterns could, through the mechanisms of neural plasticity, expand neural areas and improve neural pathways that facilitate movement. Improvements in clinical measures of balance and gait, as well as in symptom severity have been demonstrated after exercise for participants with PD (Corcos et al., 2013; Fisher et al., 2008; Hirsch et al., 2003; Li et al., 2012; Smania et al., 2010), including AT dance (Duncan and Earhart, 2012; Hackney and Earhart, 2010). These improvements in mobility, balance, spatial cognition, and disease severity may be retained for up to 3 months after AT in individuals with mild to moderate PD (Hackney and Earhart, 2009a, b, 2010; Hackney et al., 2007; McKee and Hackney, 2013).
Our early results suggest that changes in motor modules identified after AT are consistent with an increased automaticity of gait, shifting control of gait from cortical to subcortical structures. Increased cortical hyperexcitability is observed in animal models of PD (Petzinger et al., 2010). This may result in decreased automaticity of gait as control shifts from subcortical to cortical structures in order to compensate for the impaired ability of the basal ganglia to regulate ongoing movement (Petzinger et al., 2010). The increased reliance on attentional, i.e., cortical mechanisms for gait and balance could underlie difficulties in concurrently performing cognitive and motor tasks in individuals with PD (Hackney and Earhart, 2009b; Muslimovic et al., 2008; O’Shea et al., 2002). This inability to “walk and talk” is also impaired in some older adults (Woollacott and Shumway-Cook, 2002), and is associated with greater fall risk (Camicioli and Majumdar, 2010). Motor module analysis alone cannot directly reveal changes in the locus of motor control, but comparison of motor modules across behaviors mediated by different neural circuits can be instructive. For example, young, healthy adults used a common set of motor modules for both overground walking and brainstem-mediated reactive balance responses (Chvatal and Ting, 2013), as well as visually-guided anticipatory changes in gait that are likely to be mediated by cortical mechanisms (Chvatal and Ting, 2012). These motor modules may be organized in the spinal cord, and then recruited by spinal, brainstem, and cortical inputs. In a small sample of individuals with mild to moderate PD, there were no obvious deficits in the number and structure of motor modules used in either walking or reactive balance. In contrast to young, healthy adults, however, these modules were not shared across behaviors. Moreover, after intensive AT, the number of motor modules common to walking and reactive balance were improved in some individuals (Allen et al., 2014). These results suggest that the automaticity of gait control by subcortical structures is improved by AT (Figure 3C)
Motor module analysis coupled with neuromechanical modeling may allow us to interpret muscle activity during gait and postural tasks in PD patients and provide insight into dysfunction in nondopaminergic brainstem areas implicated in gait control that are now known to degenerate in PD.Postural and gait impairments may be unresponsive to dopaminergic pharmacotherapy, implying that they reflect nondopaminergic pathophysiology – and likely result from interactions between disease processes and compensatory mechanisms and strategies (Bloem et al., 2004). Recent work has also revealed deficits in other brain regions necessary for locomotor control (Mena-Segovia et al., 2004) (Bloem et al., 2001; Bohnen and Albin, 2011; Factor, 2008; Grabli et al., 2012; Melton et al., 2006). In particular, recent attention has focused on the neuroanatomy of brainstem areas including the pedunculopontine nucleus (PPN), which degenerates in PD as well as in related disorders such as progressive supranuclear palsy (Lee et al., 2000). PPN provides cholinergic input to the basal ganglia, brainstem, and spinal cord (Bohnen et al., 2009) and is closely localized to brainstem regions that have been identified in animal models as critical for regulating gait and postural tone (Takakusaki, 2013), and has been implicated as an upstream cause of abnormal spinal reflexes in PD patients (Meunier et al., 2000). Recent results suggest that this region may represent a promising new target for deep brain stimulation for postural and gait impairments and falls (Moro et al., 2010).
In summary, motor module analysis in PD may facilitate an understanding of the mechanisms of motor dysfunction and rehabilitation in PD. By examining motor modules across different movement types, a patient’s ability to appropriately select and modulate motor patterns may be evaluated. Changes in motor modules in response to various pharmacological and neural stimulation interventions may also reveal the neural mechanisms underlying motor deficits. Furthermore, they can be used in conjunction with neurophysiological measures to identify changes in the locus of neural control for movements due to neural degeneration and rehabilitation. As a consequence, the analysis of motor modules in PD and changes through AT may also provide insights into basic mechanisms of motor control and motor plasticity.
Clinical and scientific implications
We argue that examining motor modules in motor deficits and during recovery may provide more definite answers to the neural substrates of motor modularity. It remains an open question the degree to which motor modules are encoded by specific neuroanatomical structures or rapidly emerge from neuromechanical interactions. Studying rehabilitation in specific neural deficits can help to reveal how motor structure is altered and relearned through training, revealing processed underlying coordinated neural control of movement. Although motor modules as discussed in this essay may reflect only one aspect of neuromotor control processes, they provide a valuable first step towards analyzing motor patterns as a whole, allowing the previously impenetrable complexity and variability of motor signals to be managed. Computational methodologies that reveal the structure and variability of motor modules could lead to a powerful suite of diagnostic tools for movement that could be used for clinical, preclinical, and high-performance assessment of sensorimotor function. Ultimately these insights may drive hypothesis-driven neurophysiological and behavioral experiments to identify how and where motor modularity arise in the nervous system.
Acknowledgments
LHT is supported by NIH R01 HD46922, R21 HD075612, R01 HD081274, and NSF EFRI 1137229. HJC is supported by NSF IIS-1065489 and NIH NS087249. RDT is supported by NIH R01 HD081274, K12 HD055931, and Wings for Life Foundation. JLA is supported NIH R01 HD46922 and T32 NS007480-14. JLM is supported by NIH R21 HD075612 and the Atlanta Clinical and Translational Science Institute (ACTSI) KL2-Mentored Clinical and Translational Research Program (NIH KL2TR000455). MEH is supported by NIH R21 HD075612, Career Development Award Level-II (N0870-W) Department of Veterans Affairs R&D service, and NSF EFRI 1137229. TMK is supported by NIH K01 HD079584 and an American Heart Association (AHA) Scientist Development Grant AHA 13SDG1332000.
Footnotes
Author Contributions
All authors contributed to conceptualizing the organizational structure, content, and scope of the manuscript and approved the final document. LHT was primarily responsible for writing the manuscript. HJC and RDT contributed to the writing of the scientific portion of the manuscript and editing the entire document. RDT, MEH, JLM, JLA, TMK all contributed to the writing of the clinical portion of the manuscript. LHT and RDT developed figure 1. LHT, JLA, and JLM developed figure 2, LHT, HJC, JLA, and RDT developed figure 3.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol (1985) 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson’s disease. Exerc Sport Sci Rev. 2011;39:177–186. doi: 10.1097/JES.0b013e31822cc71a. [DOI] [PubMed] [Google Scholar]
- Allen JL, Kautz SA, Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech (Bristol, Avon) 2013 doi: 10.1016/j.clinbiomech.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, McKay JL, Ting LH. Rehabilitation improves generalization of muscle synergies across balance and walking in individuals with Parkinson’s disease. World Congress of Biomechanics; Boston, MA. 2014. [Google Scholar]
- Allen JL, Neptune RR. Three-dimensional modular control of human walking. J Biomech. 2012;45:2157–2163. doi: 10.1016/j.jbiomech.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asavasopon S, Rana M, Kirages DJ, Yani MS, Fisher BE, Hwang DH, Lohman EB, Berk LS, Kutch JJ. Cortical activation associated with muscle synergies of the human male pelvic floor. J Neurosci. 2014;34:13811–13818. doi: 10.1523/JNEUROSCI.2073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Reisman DS, Binder-Macleod SA. Do improvements in balance relate to improvements in long-distance walking function after stroke? Stroke Res Treat. 2014a;2014:646230. doi: 10.1155/2014/646230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Arch Phys Med Rehabil. 2014b;95:840–848. doi: 10.1016/j.apmr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Beer RD, Chiel HJ, Gallagher JC. Evolution and analysis of model CPGs for walking: II. General principles and individual variability. J Comput Neurosci. 1999;7:119–147. doi: 10.1023/a:1008920021246. [DOI] [PubMed] [Google Scholar]
- Bekoff A. Spontaneous embryonic motility: an enduring legacy. Int J Dev Neurosci. 2001;19:155–160. doi: 10.1016/s0736-5748(00)00089-7. [DOI] [PubMed] [Google Scholar]
- Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, Iosa M, Molinari M, Tamburella F, Ramos A, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil. 2011;8:66. doi: 10.1186/1743-0003-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–16. [PubMed] [Google Scholar]
- Berger DJ, Gentner R, Edmunds T, Pai DK, d’Avella A. Differences in adaptation rates after virtual surgeries provide direct evidence for modularity. J Neurosci. 2013;33:12384–12394. doi: 10.1523/JNEUROSCI.0122-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz A. Spinal interneurons that are selectively activated during fictive flexion reflex. J Neurosci. 2007;27:4634–4641. doi: 10.1523/JNEUROSCI.5602-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniker M, Jarc A, Bizzi E, Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci U S A. 2009;106:7601–7606. doi: 10.1073/pnas.0901512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. The Coordination and Regulation of Movements. New York: Pergamon Press; 1967. [Google Scholar]
- Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol. 2011;106:437–448. doi: 10.1152/jn.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Cheung VC. The neural origin of muscle synergies. Front Comput Neurosci. 2013;7:51. doi: 10.3389/fncom.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Cheung VC, d’Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Rev. 2008;57:125–133. doi: 10.1016/j.brainresrev.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248:950–958. 958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. White matter lesions in Parkinson disease. Nat Rev Neurol. 2011;7:229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24:328–337. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunderson NE, Burkholder TJ, Ting LH. Reduction of neuromuscular redundancy for postural force generation using an intrinsic stability criterion. J Biomech. 2008;41:1537–1544. doi: 10.1016/j.jbiomech.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ, van Antwerp KW. Practical limits on muscle synergy identification by non-negative matrix factorization in systems with mechanical constraints. Med Biol Eng Comput. 2013;51:187–196. doi: 10.1007/s11517-012-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A, Wright TM. Coping with variability in small neuronal networks. Integr Comp Biol. 2011;51:845–855. doi: 10.1093/icb/icr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli R, Majumdar SR. Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year Prospective Cohort Study. Gait Posture. 2010;32:87–91. doi: 10.1016/j.gaitpost.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002;25:370–376. doi: 10.1016/s0166-2236(02)02173-2. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, d’Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci. 2005;25:6419–6434. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci U S A. 2009;106:19563–19568. doi: 10.1073/pnas.0910114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VCK, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A. 2012;109:14652–14656. doi: 10.1073/pnas.1212056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra M, Jacobs RA. Properties of synergies arising from a theory of optimal motor behavior. Neural Computation. 2006;18:2320–2342. doi: 10.1162/neco.2006.18.10.2320. [DOI] [PubMed] [Google Scholar]
- Chi MTH, Roscoe RD. Reconsidering conceptual change: Issues in theory and practice. In: Limon M, Mason L, editors. The processes and challenges of conceptual change. The Netherlands: Kluwer; 2002. pp. 3–27. [Google Scholar]
- Chiel HJ, Beer RD. The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- Chiel HJ, Ting LH, Ekeberg O, Hartmann MJ. The brain in its body: motor control and sensing in a biomechanical context. J Neurosci. 2009;29:12807–12814. doi: 10.1523/JNEUROSCI.3338-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J Neurol Sci. 2000a;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A Central Source of Movement Variability. Neuron. 2006;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Macpherson JM, Torres-Oviedo G, Ting LH. Absence of postural muscle synergies for balance after spinal cord transection. J Neurophysiol. 2013;110:1301–1310. doi: 10.1152/jn.00038.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci. 2012;32:12237–12250. doi: 10.1523/JNEUROSCI.6344-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci. 2013;7:48. doi: 10.3389/fncom.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in non-stepping and stepping postural behaviors. J Neurophysiol. 2011;106:999–1015. doi: 10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clune J, Mouret JB, Lipson H. The evolutionary origins of modularity. Proc Biol Sci. 2013;280:20122863. doi: 10.1098/rspb.2012.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Ruina A, Tedrake R, Wisse M. Efficient bipedal robots based on passive-dynamic walkers. Science. 2005;307:1082–1085. doi: 10.1126/science.1107799. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28:1230–1240. doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Shaw KM, Gill JP, Chiel HJ. Motor neuronal activity varies least among individuals when it matters most for behavior. J Neurophysiol. 2014 doi: 10.1152/jn.00729.2014. jn 00729 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberland Consensus Working Group. Cheeran B, Cohen L, Dobkin B, Ford G, Greenwood R, Howard D, Husain M, Macleod M, Nudo R, et al. The future of restorative neurosciences in stroke: driving the translational research pipeline from basic science to rehabilitation of people after stroke. Neurorehabil Neural Repair. 2009;23:97–107. doi: 10.1177/1545968308326636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Portone A, Lacquaniti F. Superposition and modulation of muscle synergies for reaching in response to a change in target location. J Neurophysiol. 2011;106:2796–2812. doi: 10.1152/jn.00675.2010. [DOI] [PubMed] [Google Scholar]
- d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neuro. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- Dale EA, Mitchell GS. Spinal vascular endothelial growth factor (VEGF) and erythropoietin (EPO) induced phrenic motor facilitation after repetitive acute intermittent hypoxia. Respir Physiol Neurobiol. 2013;185:481–488. doi: 10.1016/j.resp.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci. 2010;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote F, Jonkers I, Duysens J. Task constraints and minimization of muscle effort result in a small number of muscle synergies during gait. Front Comput Neurosci. 2014;8:115. doi: 10.3389/fncom.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78:1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- de Rugy A, Loeb GE, Carroll TJ. Muscle coordination is habitual rather than optimal. J Neurosci. 2012;32:7384–7391. doi: 10.1523/JNEUROSCI.5792-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev. 2008;57:212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 ( Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. How Animals Move: An Integrative View. Science, New Series. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural Muscle Responses to Multidirectional Translations in Patients With Parkinson’s Disease. J Neurophysiol. 2004a;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Nutt J, Horak FB. Abnormal force patterns for multidirectional postural responses in patients with Parkinson’s disease. Exp Brain Res. 2004b;156:183–195. doi: 10.1007/s00221-003-1770-4. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Confounders in rehabilitation trials of task-oriented training: lessons from the designs of the EXCITE and SCILT multicenter trials. Neurorehabil Neural Repair. 2007;21:3–13. doi: 10.1177/1545968306297329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH. Motor rehabilitation after stroke, traumatic brain, and spinal cord injury: common denominators within recent clinical trials. Curr Opin Neurol. 2009;22:563–569. doi: 10.1097/WCO.0b013e3283314b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d’Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, et al. Locomotor primitives in newborn babies and their development. Science. 2011;334:997–999. doi: 10.1126/science.1210617. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26:132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- Eden S. Stroke of Madness: How has Tiger Woods managed to overhaul his swing three times? 2013 http://espn.go.com/golf/story/_/id/8865487/tiger-woods-reinvents-golf-swing-third-time-career-espn-magazine.
- Edgerton VR, Kim SJ, Ichiyama RM, Gerasimenko YP, Roy RR. Rehabilitative therapies after spinal cord injury. J Neurotrauma. 2006;23:560–570. doi: 10.1089/neu.2006.23.560. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain Res Bull. 2009;78:4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CP. The singer and the song: the neuromechanics of avian sound production. Curr Opin Neurobiol. 2014;28:172–178. doi: 10.1016/j.conb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Ethier C, Brizzi L, Darling WG, Capaday C. Linear summation of cat motor cortex outputs. J Neurosci. 2006;26:5574–5581. doi: 10.1523/JNEUROSCI.5332-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SA. Current status of symptomatic medical therapy in Parkinson’s disease. Neurotherapeutics. 2008;5:164–180. doi: 10.1016/j.nurt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SA, Scullin MK, Sollinger AB, Land JO, Wood-Siverio C, Zanders L, Freeman A, Bliwise DL, Goldstein FC. Freezing of gait subtypes have different cognitive correlates in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1359–1364. doi: 10.1016/j.parkreldis.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Physical therapy. 2011;91:48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Hahnloser RH, Fee MS, Seung HS. Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. J Neurophysiol. 2004;92:2274–2282. doi: 10.1152/jn.01133.2003. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Sullivan KJ. Activity-dependent factors affecting poststroke functional outcomes. Top Stroke Rehabil. 2001;8:31–44. doi: 10.1310/B3JD-NML4-V1FB-5YHG. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, Cen S, Gordon J, Jakowec M, Petzinger G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89:1221–1229. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flash T, Hochner B. Motor primitives in vertebrates and invertebrates. Curr Opin Neurobiol. 2005;15:660–666. doi: 10.1016/j.conb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Fox EJ, Tester NJ, Kautz SA, Howland DR, Clark DJ, Garvan C, Behrman AL. Modular control of varied locomotor tasks in children with incomplete spinal cord injuries. J Neurophysiol. 2013;110:1415–1425. doi: 10.1152/jn.00676.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, So U, Kawato M, Milner TE. Impedance control balances stability with metabolically costly muscle activation. J Neurophysiol. 2004;92:3097–3105. doi: 10.1152/jn.00364.2004. [DOI] [PubMed] [Google Scholar]
- Furuya S, Altenmuller E. Flexibility of movement organization in piano performance. Front Hum Neurosci. 2013;7:173. doi: 10.3389/fnhum.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh G, Haruno M, Kawato M, Burdet E. Motor memory and local minimization of error and effort, not global optimization, determine motor behavior. J Neurophysiol. 2010;104:382–390. doi: 10.1152/jn.01058.2009. [DOI] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010;20:1869–1874. doi: 10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Gick B, Stavness I. Modularizing speech. Front Psychol. 2013;4:977. doi: 10.3389/fpsyg.2013.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter S, Patil V, Hart C. Primitives, premotor drives, and pattern generation: a combined computational and neuroethological perspective. Prog Brain Res. 2007;165:323–346. doi: 10.1016/S0079-6123(06)65020-6. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Hart CB. Motor primitives and synergies in the spinal cord and after injury--the current state of play. Ann N Y Acad Sci. 2013;1279:114–126. doi: 10.1111/nyas.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Goldman MS, Abbott LF, Marder E. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol. 2002;87:1129–1131. doi: 10.1152/jn.00412.2001. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Plasticity in respiratory motor control - Invited review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. Journal of applied physiology. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Grabli D, Karachi C, Welter ML, Lau B, Hirsch EC, Vidailhet M, Francois C. Normal and pathological gait: what we learn from Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:979–985. doi: 10.1136/jnnp-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009a;41:475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord. 2009b;15:644–648. doi: 10.1016/j.parkreldis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Effects of dance on gait and balance in Parkinson’s disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil Neural Repair. 2010;24:384–392. doi: 10.1177/1545968309353329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31:173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- Hall ET. Beyond Culture. New York: Anchor Books; 1976. [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Handford C. Serving up variability and stability. In: Davids K, Bennet S, Newell K, editors. Movement System Variability. Champaign, IL: Human Kinetics; 2006. pp. 73–83. [Google Scholar]
- Hart CB, Giszter SF. Modular Premotor Drives and Unit Bursts as Primitives for Frog Motor Behaviors. J Neurosci. 2004;24:5269–5282. doi: 10.1523/JNEUROSCI.5626-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci. 2010;30:1322–1336. doi: 10.1523/JNEUROSCI.5894-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference and prediction. New York: Springer Science+Business Media; 2005. [Google Scholar]
- Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin Neurophysiol. 2014a Oct;125(10):2024–35. doi: 10.1016/j.clinph.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chvatal SA, Ting LH, Rymer WZ, Mitchell GS, Trumbower RD. Effect of single- day acute intermittent hypoxia on overground walking speed and muscle coordination in persons with incomplete spinal cord injury. Society for Neuroscience; New Orleans, LA: 2012. [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014b;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Shadmehr R. Motor variability is not noise, but grist for the learning mill. Nat Neurosci. 2014;17:149–150. doi: 10.1038/nn.3633. [DOI] [PubMed] [Google Scholar]
- Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur J Phys Rehabil Med. 2009;45:215–229. [PubMed] [Google Scholar]
- Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE. Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res. 2002;146:233–243. doi: 10.1007/s00221-002-1166-x. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Weaver AL. Motor neuron activity is often insufficient to predict motor response. Curr Opin Neurobiol. 2000;10:676–682. doi: 10.1016/s0959-4388(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Handbook of Physiology, Section 12. New York: American Physiological Society; 1996. Postural orientation and equilibrium; pp. 255–292. [Google Scholar]
- Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R, Diedrichsen J. Active learning: learning a motor skill without a coach. J Neurophysiol. 2008;100:879–887. doi: 10.1152/jn.01095.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughlings-Jackson J. Address in Medicine. The British Medical Journal. 1889;2:355–362. doi: 10.1136/bmj.2.1494.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacson O, Kordower JH. Future of cell and gene therapies for Parkinson’s disease. Ann Neurol. 2008;64(Suppl 2):S122–138. doi: 10.1002/ana.21473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari M, Scivoletto G, Castellano V, Macellari V, Lacquaniti F. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol. 2003;90:3555–3565. doi: 10.1152/jn.00223.2003. [DOI] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. A framework for using signal, noise, and variation to determine whether the brain controls movement synergies or single muscles. J Neurophysiol. 2014;111:733–745. doi: 10.1152/jn.00510.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaegi S, Schwab ME, Dietz V, Fouad K. Electromyographic activity associated with spontaneous functional recovery after spinal cord injury in rats. Eur J Neurosci. 2002;16:249–258. doi: 10.1046/j.1460-9568.2002.02076.x. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Ramakrishnan A, Hart CB, Rome LC, Giszter SF. A simple experimentally based model using proprioceptive regulation of motor primitives captures adjusted trajectory formation in spinal frogs. J Neurophysiol. 2010;103:573–590. doi: 10.1152/jn.01054.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, Binder-Macleod SA. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33:309–313. doi: 10.1016/j.gaitpost.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kliegel M, Phillips LH, Lemke U, Kopp UA. Planning and realisation of complex intentions in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1501–1505. doi: 10.1136/jnnp.2004.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knarr BA, Kesar TM, Reisman DS, Binder-Macleod SA, Higginson JS. Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors. J Neuroeng Rehabil. 2013;10:12. doi: 10.1186/1743-0003-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102:405–430. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nat Rev Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Hum Mov Sci. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH. Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol. 2006;95:493–504. doi: 10.1152/jn.00706.2005. [DOI] [PubMed] [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput Biol. 2012;8:e1002434. doi: 10.1371/journal.pcbi.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Fung J. Faster is better: implications for speed-intensive gait training after stroke. Stroke. 2004;35:2543–2548. doi: 10.1161/01.STR.0000144685.88760.d7. [DOI] [PubMed] [Google Scholar]
- Lamontagne A, Stephenson JL, Fung J. Physiological evaluation of gait disturbances post stroke. Clin Neurophysiol. 2007;118:717–729. doi: 10.1016/j.clinph.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Latash ML. The bliss (not the problem) of motor abundance (not redundancy) Exp Brain Res. 2012;217:1–5. doi: 10.1007/s00221-012-3000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–791. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J. 2000;41:167–184. doi: 10.3349/ymj.2000.41.2.167. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Maddalozzo G, Batya SS. Tai Chi and Postural Stability in Patients with Parkinson’s Disease. N Engl J Med. 2012:366. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CK, Hertzmann A, Popovic Z. Learning physics-based motion style with nonlinear inverse optimization. SIGGRAPH. 2005:1071–1081. [Google Scholar]
- Loeb GE. Optimal isn’t good enough. Biol Cybern. 2012;106:757–765. doi: 10.1007/s00422-012-0514-6. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32:3591–3600. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low KA, Miller J, Vierck E. Response slowing in Parkinson’s disease: a psychophysiological analysis of premotor and motor processes. Brain. 2002;125:1980–1994. doi: 10.1093/brain/awf206. [DOI] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239–248. [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JL, Ting LH. Optimization of Muscle Activity for Task-Level Goals Predicts Complex Changes in Limb Forces across Biomechanical Contexts. PLoS Comput Biol. 2012;8:e1002465. doi: 10.1371/journal.pcbi.1002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav. 2013;45:519–529. doi: 10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Leibson CL, Achenbach SJ, Bower JH, Maraganore DM, Oberg AL, Rocca WA. Fracture risk after the diagnosis of Parkinson’s disease: Influence of concomitant dementia. Mov Disord. 2006;21:1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pol S, Houeto JL, Vidailhet M. Abnormal reciprocal inhibition between antagonist muscles in Parkinson’s disease. Brain. 2000;123 ( Pt 5):1017–1026. doi: 10.1093/brain/123.5.1017. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia: Focused Selection and Inhibition of Competing Motor Programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Genetic basis for respiratory control disorders. Springer; 2008. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders? pp. 291–311. [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70:2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz Sa. Modular control of human walking: a simulation study. J Biomech. 2009;42:1282–1287. doi: 10.1016/j.jbiomech.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Biewener AA, Aerts P, Ahn AN, Chiel HJ, Daley MA, Daniel TL, Full RJ, Hale ME, Hedrick TL, et al. Neuromechanics: an integrative approach for understanding motor control. Integr Comp Biol. 2007;47:16–54. doi: 10.1093/icb/icm024. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996a;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24:1000–1019. doi: 10.1002/mus.1104. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996b;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Nussbaum MA, Chaffin DB. Pattern classification reveals intersubject group differences in lumbar muscle recruitment during static loading. Clin Biomech (Bristol, Avon) 1997;12:97–106. doi: 10.1016/s0268-0033(96)00056-3. [DOI] [PubMed] [Google Scholar]
- O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–897. [PubMed] [Google Scholar]
- Oliveira AS, Gizzi L, Kersting UG, Farina D. Modular organization of balance control following perturbations during walking. J Neurophysiol. 2012;108:1895–1906. doi: 10.1152/jn.00217.2012. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. 2012;76:1071–1077. doi: 10.1016/j.neuron.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardasaney PK, Latham NK, Jette AM, Wagenaar RC, Ni P, Slavin MD, Bean JF. Sensitivity to change and responsiveness of four balance measures for community-dwelling older adults. Phys Ther. 2012;92:388–397. doi: 10.2522/ptj.20100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Cheng J, Kautz SA, Neptune RR. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture. 2010;32:451–456. doi: 10.1016/j.gaitpost.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S141–145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D, Kesar T, Perumal R, Roos M, Rudolph K, Higginson J, Helm E, Binder-Macleod S. Time course of functional and biomechanical improvements during a gait training intervention in persons with chronic stroke. J Neurol Phys Ther. 2013;37:159–165. doi: 10.1097/NPT.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–2832. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KL, Roemmich RT, Cam B, Fregly BJ, Hass CJ. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin Neurophysiol. 2013;124:1390–1397. doi: 10.1016/j.clinph.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Fregly BJ, Hass CJ. Neuromuscular complexity during gait is not responsive to medication in persons with Parkinson’s disease. Ann Biomed Eng. 2014;42:1901–1912. doi: 10.1007/s10439-014-1036-2. [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VC, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol. 2011;106:1363–1378. doi: 10.1152/jn.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 2013 doi: 10.1016/j.gaitpost.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routson RL, Kautz SA, Neptune RR. Modular organization across changing task demands in healthy and poststroke gait. Physiol Rep. 2014:2. doi: 10.14814/phy2.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI, Coubes P, Rothwell JC. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain. 2011;134:2106–2115. doi: 10.1093/brain/awr122. [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Task-level feedback can explain temporal recruitment of spatially-fixed muscle synergies throughout postural perturbations. J Neurophysiol. 2012;107:159–177. doi: 10.1152/jn.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol. 2013;109:31–45. doi: 10.1152/jn.00684.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Torres-Oviedo G, Ting LH. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top Spinal Cord Inj Rehabil. 2011;17:16–24. doi: 10.1310/sci1701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel P, Wyler-Duda K, D’Avella A, Tresch MC, Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J Neurophysiol. 2001;85:605–619. doi: 10.1152/jn.2001.85.2.605. [DOI] [PubMed] [Google Scholar]
- Santello M, Lang KE. Are movement disorders and sensorimotor injuries pathologic synergies? When normal multi-joint movement synergies become pathologic. Front Hum Neurosci. 2015;8:1050. doi: 10.3389/fnhum.2014.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–2253. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G, Latash ML. Identifying the control structure of multijoint coordination during pistol shooting. Exp Brain Res. 2000;135:382–404. doi: 10.1007/s002210000540. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shiavi R, Bugle HJ, Limbird T. Electromyographic gait assessment, Part 2: Preliminary assessment of hemiparetic synergy patterns. Journal of rehabilitation research and development. 1987;24:24–30. [PubMed] [Google Scholar]
- Smania N, Corato E, Tinazzi M, Stanzani C, Fiaschi A, Girardi P, Gandolfi M. Effect of balance training on postural instability in patients with idiopathic Parkinson’s disease. Neurorehabil Neural Repair. 2010;24:826–834. doi: 10.1177/1545968310376057. [DOI] [PubMed] [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trends Cogn Sci. 2003;7:343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Snaterse M, Ton R, Kuo AD, Donelan JM. Distinct fast and slow processes contribute to the selection of preferred step frequency during human walking. J Appl Physiol (1985) 2011;110:1682–1690. doi: 10.1152/japplphysiol.00536.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ting LH. The cost of being stable: a quantitative approach to examine trade-off between effort and stability. American Society of Biomechanics; Omaha, Nebraska: 2013. [Google Scholar]
- Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci. 2013;7:105. doi: 10.3389/fncom.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stein PS, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci. 2002;22:6800–6809. doi: 10.1523/JNEUROSCI.22-15-06800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov Disord. 2013;28:1483–1491. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res. 2007;165:299–321. doi: 10.1016/S0079-6123(06)65019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Chvatal SA. Decomposing muscle activity in motor tasks: methods and interpretation. In: Danion F, Latash ML, editors. Motor control: theories, experiments, and applications. New York: Oxford University Press; 2010. pp. 102–138. [Google Scholar]
- Ting LH, Chvatal SA, Safavynia SA, Lucas McKay J. Review and perspective: neuromechanical considerations for predicting muscle activation patterns for movement. Int J Numer Method Biomed Eng. 2012;28:1003–1014. doi: 10.1002/cnm.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol. 2007;17:622–628. doi: 10.1016/j.conb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5:1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. 2006;96:1530–1546. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. 2007;98:2144–2156. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol. 2010;103:3084–3098. doi: 10.1152/jn.00960.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006;95:2199–2212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol. 2009;19:601–607. doi: 10.1016/j.conb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci. 1999;2:162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair. 2012;26:163–172. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Tsianos GA, Goodner J, Loeb GE. Useful properties of spinal circuits for learning and performing planar reaches. J Neural Eng. 2014;11:056006. doi: 10.1088/1741-2560/11/5/056006. [DOI] [PubMed] [Google Scholar]
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88:1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell ED, Holmes P, Cohen AH. Spikes alone do not behavior make: why neuroscience needs biomechanics. Curr Opin Neurobiol. 2011;21:816–822. doi: 10.1016/j.conb.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Venkadesan M, Todorov E. Structured variability of muscle activations supports the minimal intervention principle of motor control. J Neurophysiol. 2009;102:59–68. doi: 10.1152/jn.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Zajac FE, Burgar CG. Large index-fingertip forces are produced by subject-independent patterns of muscle excitation. J Biomech. 1998;31:693–703. doi: 10.1016/s0021-9290(98)00082-7. [DOI] [PubMed] [Google Scholar]
- van Antwerp K, Burkholder T, Ting L. Inter-joint coupling effects on muscle contributions to endpoint force and acceleration in a musculoskeletal model of the cat hindlimb. J Biomech. 2007a;40:3570–3579. doi: 10.1016/j.jbiomech.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Antwerp KW, Burkholder TJ, Ting LH. Inter-joint coupling effects on muscle contributions to endpoint force and acceleration in a musculoskeletal model of the cat hindlimb. J Biomech. 2007b;40:3570–3579. doi: 10.1016/j.jbiomech.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hedel HJA, Dietz V. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci. 2010;28:123–134. doi: 10.3233/RNN-2010-0508. [DOI] [PubMed] [Google Scholar]
- van Hedel HJA, Dietz V E.M.S.o.H.S.C.I.S Group. Walking During Daily Life Can Be Validly and Responsively Assessed in Subjects With a Spinal Cord Injury. Neurorehabil Neural Repair. 2009;23:117–124. doi: 10.1177/1545968308320640. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol. 2009;169:210–217. doi: 10.1016/j.resp.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP, Pavlicev M, Cheverud JM. The road to modularity. Nat Rev Genet. 2007;8:921–931. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- Walter JP, Kinney AL, Banks SA, D’Lima DD, Besier TF, Lloyd DG, Fregly BJ. Muscle synergies may improve optimization prediction of knee contact forces during walking. J Biomech Eng. 2014;136:021031. doi: 10.1115/1.4026428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt N, Li S, Di Pardo A, Sipione S, Fouad K. Synergistic effects of BDNF and rehabilitative training on recovery after cervical spinal cord injury. Behav Brain Res. 2013;239:31–42. doi: 10.1016/j.bbr.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Welch TD, Ting LH. A feedback model reproduces muscle activity during human postural responses to support-surface translations. J Neurophysiol. 2008;99:1032–1038. doi: 10.1152/jn.01110.2007. [DOI] [PubMed] [Google Scholar]
- Whitall J. Stroke rehabilitation research: time to answer more specific questions? Neurorehabil Neural Repair. 2004;18:3–8. doi: 10.1177/0888439003262876. author reply 9–11. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217:116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF. Neural plasticity and treatment across the lifespan for motor deficits in cerebral palsy. Dev Med Child Neurol. 2009;51(Suppl 4):130–133. doi: 10.1111/j.1469-8749.2009.03425.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Schaechter JD. The neural basis of constraint-induced movement therapy. Curr Opin Neurol. 2009;22:582–588. doi: 10.1097/WCO.0b013e3283320229. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- Wu HG, Miyamoto YR, Gonzalez Castro LN, Olveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci. 2014;17:312–321. doi: 10.1038/nn.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Lam T, Pang MY, Lamont E, Musselman K, Seinen E. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol. 2004;82:662–674. doi: 10.1139/y04-070. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac FE. Understanding muscle coordination of the human leg with dynamical simulations. J Biomech. 2002;35:1011–1018. doi: 10.1016/s0021-9290(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Zelik KE, La Scaleia V, Ivanenko YP, Lacquaniti F. Can modular strategies simplify neural control of multidirectional human locomotion? J Neurophysiol. 2014;111:1686–1702. doi: 10.1152/jn.00776.2013. [DOI] [PubMed] [Google Scholar]