SUMMARY
In addition to direct effects on virus infectivity, antibodies mediate antibody-dependent cellular cytotoxicity (ADCC), the killing of an antibody-coated virus-infected cell by cytotoxic effector cells. Although ADCC has been suggested to protect against HIV, the relationship between HIV-specific ADCC antibodies at the time of HIV exposure and infection outcome in humans remains to be assessed. We evaluated the ADCC activity of passively acquired antibodies in infants born to HIV-infected mothers. ADCC levels were higher in uninfected than infected infants, although not significantly. Increase in ADCC antibody activity in infected infants was associated with reduced mortality risk. Infant ADCC positively correlated with the magnitude of IgG1 binding and IgG1 levels were associated with survival in infected infants. Infant IgG3-binding antibodies were not associated with infected infant survival. These data suggest a therapeutic benefit of pre-existing HIV-specific ADCC antibodies and support a role for eliciting ADCC-mediating IgG1 in HIV vaccines.
INTRODUCTION
Rational design of an effective vaccine against HIV requires understanding the functional characteristics of antibodies capable of preventing virus transmission or providing a therapeutic benefit. One function of antibodies is antibody-dependent cellular cytotoxicity (ADCC) and HIV-specific ADCC activity has been suggested to provide a protective and/or therapeutic effect in multiple settings. Evidence for a therapeutic effect in humans comes from studies showing that de novo ADCC antibody responses are inversely associated with viral load and higher in viral controllers than progressors (Reviewed in Lewis, 2014). However, human data on whether or not ADCC antibodies are protective if present at the time of exposure (pre-existing antibodies) are more limited. In the RV144 vaccine trial, vaccine-induced ADCC antibodies correlated with reduced infection risk in an exploratory analysis of individuals with low plasma IgA (Haynes et al., 2012). Furthermore, ADCC activity in the index case has been associated with protection in the setting of mother-infant transmission. There, high maternal breast milk HIV-specific ADCC activity correlated with reduced risk of infant infection via breastfeeding (Mabuka et al., 2012). Additional support for the protective role of ADCC antibodies comes from studies in macaques that have shown vaccine-induced ADCC responses correlate with lower viral loads and/or delayed disease progression following simian immunodeficiency virus (SIV) challenge (Reviewed in Lewis, 2014). Collectively, these findings support the hypothesis that ADCC antibodies present at the time of HIV exposure may have a role in protecting against HIV acquisition or modulating viral load in those who become infected. However, translating results from macaque studies and hypothesis-generating studies in humans to more definitive human studies is critical for determining the importance of pre-existing antibodies in protection.
HIV mother-to-child transmission (MTCT) is a unique setting in which to examine the protective role of ADCC antibodies present at exposure because maternal IgG crosses the placenta during pregnancy. Thus, infants born to HIV-infected mothers have HIV-specific antibodies present in circulation at birth that may provide protection during virus exposure, particularly during breastfeeding. Several early studies of ADCC in MTCT showed no correlation of infant or maternal ADCC and infection risk (Broliden et al., 1993; Jenkins et al., 1994; Ljunggren et al., 1990; Mabondzo et al., 1995; Pugatch et al., 1997). However, these studies may have been limited in their ability to detect a protective effect of ADCC antibodies based on techniques available, including: 1) use of lab-adapted viruses that do not represent transmitted strains; 2) infant infection status was often determined by ELISA at 15 months, and thus, timing of infection (including in utero infections) could not be verified; and 3) infant ADCC activity was measured at various ages up until 2 years, at which point passively transferred antibodies may not be relevant and de novo responses may have been measured. With advances in infant diagnosis/follow-up and improvements in ADCC methods, we are now more aptly positioned to determine if pre-existing ADCC antibodies in HIV-exposed infants influence virus acquisition or disease progression.
In this study, we evaluated passively acquired ADCC antibody activity in plasma near the time of birth from infants born to HIV-infected mothers. We hypothesized that this pre-existing HIV-specific ADCC antibody activity in infants would provide a protective and therapeutic benefit to infants exposed to HIV via breastfeeding. We found that both ADCC activity and the magnitude of IgG1 but not IgG3 antibody binding were significantly associated with a decreased risk of mortality in infants who became infected. These results suggest that pre-existing HIV-specific IgG1-mediated ADCC activity may provide a therapeutic benefit in individuals who become infected and is an important component to consider for a HIV vaccine.
RESULTS
Passively acquired ADCC antibody activity and infant infection risk
To investigate the impact of HIV-specific, ADCC-mediating passively acquired antibodies on infant infection and disease progression, we examined ADCC responses in 72 infants who were HIV RNA negative at birth and were continually exposed to HIV via breastfeeding. We focused on infants with a plasma sample from the first week of life because passively acquired antibodies are the highest at this time and because the first weeks of life are when infants are at the highest risk of breastfeeding infection (Nduati et al., 2000). Of the 72 infants who met the study criteria, 21 became infected and were detected as HIV-positive at the following visits: week 2 (n=1), week 6 (n=10), week 14 (n=1), month 6 (n=1), months 7–24 (n=8).
To select a representative envelope antigen to measure infant ADCC activity against, we first ran 8 HIV gp120s from diverse clades against 6 plasmas. This experiment suggested that although absolute values of ADCC activity varied, a similar pattern of ADCC antibody activity was observed across envelope antigens (Figure S1A). We selected the BL035 gp120 because it was representative of the results with the different gp120s (Figure S1B) and because it was cloned from an early infant virus in the cohort.
We then measured the ADCC antibody activity of the 72 infant plasmas against BL035. Overall, uninfected infants had higher mean ADCC activity than infected infants (36.7% vs. 26.3%; Figure 1A), however this association was not statistically significant (p=0.12). In a logistic regression controlling for maternal viral load, a known risk factor for MTCT, there was not a significant association between infant ADCC activity and risk of infection (Odds Ratio (OR): 0.99, 95% CI 0.97 to 1.01, p=0.26). When analysis was restricted to infants infected within the first six weeks of life (when passive ADCC antibody levels are highest), similar results were obtained: uninfected infants had higher passive ADCC levels than infants who became infected, but the different was not statistically significant (Figure S2A–B).
Figure 1. Infant ADCC Antibody Responses.
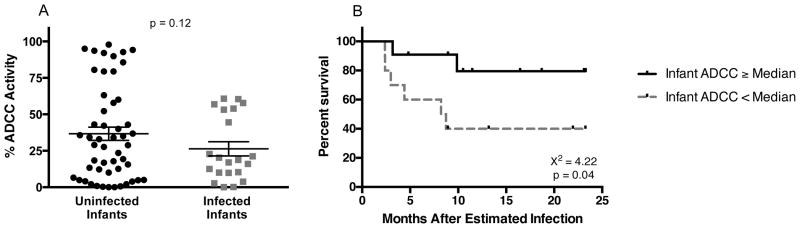
(A) Passively acquired infant ADCC responses are shown in relation to infection outcome. Results are normalized to a positive control (HivIg) and data are represented as mean ± SEM. (B) Kaplan-Meier estimates for infected infants with infant ADCC antibody activity ≥ infected infant cohort median ADCC activity (solid line) and infected infants with ADCC antibody activity < median (dashed line).
Passively acquired ADCC antibody activity and survival in HIV-infected infants
While sterilizing immunity is the gold standard measure of protection, pre-existing ADCC antibodies may also provide a therapeutic benefit in those who acquire HIV. Thus, as part of a pre-specified analysis plan, we examined time to mortality after infection to determine the impact of passively acquired ADCC antibodies on clinical outcome. In the 21 infected infants, there were 8 deaths during follow-up (38%). In a Cox-proportional hazards model, each 10% increase in ADCC antibody activity was associated with a 49.1% reduction in risk of mortality (p=0.033). Additionally, when comparing Kaplan-Meier survival functions for infected infants with ADCC ≥ the infected infant cohort median with those infants who had ADCC antibody activity < median, the survival curves were significantly different (X2 = 4.22, p=0.04; Figure 1B). Set point viral loads (Richardson et al., 2003) were only available for a subset of the infected infants. Among this smaller group (N=10), there was a trend for a negative association between pre-existing ADCC antibody activity and set point viral load (r = −0.59, p=0.074).
Maternal ADCC antibody activity and infant infection risk
In many published MTCT studies, maternal antibodies were measured in an attempt to define immune correlates of protection in infants. To address whether maternal antibody activity correlates with protection and whether maternal antibodies provide a similar measure of ADCC activity as passively acquired antibodies in the infants, ADCC activity in the 72 corresponding mothers was examined. Plasma from the third trimester or delivery was chosen based on the fact that the majority of passive antibody transfer occurs late in pregnancy and thus the maternal antibody repertoire during this time should most closely resemble the passively acquired antibodies present in infants at birth (Reviewed in Palmeira et al., 2012). Maternal ADCC antibody activity correlated with infant responses (r=0.75, p<0.0001; Figure 2A), and maternal ADCC levels were higher than those in the infants, with a median fold difference in ADCC activity of 1.7. However, unlike their infants, the mothers had nearly identical ADCC antibody levels of 44.2% for transmitters and 42.1% for non-transmitters (p=0.81; Figure 2B). This relationship remained non-significant when controlling for maternal viral load in a logistic regression analysis (OR: 1.00, 95% CI: 0.99 to 1.02, p=0.76). Additionally, in contrast to infant samples, there was not a significant association between maternal ADCC activity and infected infant survival in a Cox-proportional hazards model (Hazard ratio (HR): 0.99, p=0.30) or when comparing Kaplan-Meier estimates (Figure 2C), suggesting a unique role of passively acquired antibodies in the observed association with survival.
Figure 2. Maternal ADCC Antibody Responses.
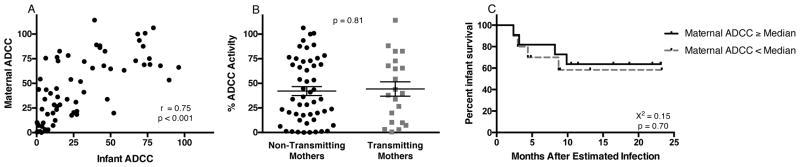
(A) Correlation between maternal and infant ADCC antibody responses. (B) maternal ADCC responses are shown in relation to infant infection outcome. Results are normalized to a positive control (HivIg) and data are represented as mean ± SEM. (C) Kaplan-Meier estimates for infected infants with maternal ADCC antibody activity ≥ the maternal transmitter cohort median ADCC activity (solid line) and infected infants with maternal ADCC antibody activity < median (dashed line).
Passively acquired HIV-specific neutralizing and binding antibodies in HIV-exposed infants
To determine whether the association between passively acquired ADCC activity and infected infant survival was unique to the ADCC antibody function, we also examined the impact of neutralizing antibodies (NAbs) on infant infection and survival using available IC50 data for 8 viruses representing 4 clades (Lynch et al., 2011). In the 72 infants included in this study, NAb breadth and potency were not associated with infant infection status (Figure S3A–B), as observed with a larger group of infants in the prior study (Lynch et al., 2011). There was a moderate, but significant, association between infant ADCC activity and NAb breadth (r=0.50, p<0.0001) and potency (r=0.57, p<0.0001) (Figure S3C–D). Nevertheless, in a Cox-proportional hazards model, there was not a significant association between passively acquired NAb breadth (HR: 0.86, p=0.35) or potency (HR: 0.94, p=0.34) and infected infant survival. Additionally, there was no correlation between neutralization IC50 and infected infant survival for each of the 8 individual viruses (data not shown).
We also examined HIV-specific IgG binding titers (end point titers (EPTs)), which encompass neutralizing and non-neutralizing antibodies, including those that mediate ADCC activity. These titers correlated with ADCC antibody activity (r=0.59, p<0.0001), but similarly did not differ between infected and uninfected infants (p=0.94) (Figure S3E–F). We observed a trend in association between these EPTs and infant survival (HR: 0.80, p=0.091).
Passively acquired IgG1 and IgG3 in infant ADCC
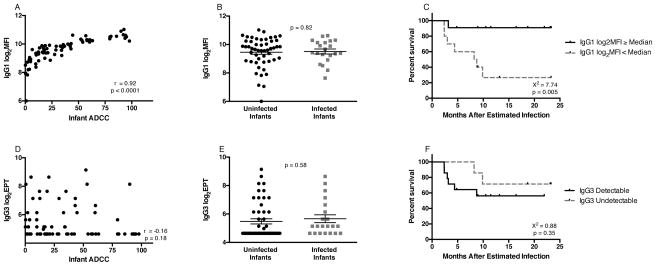
We next sought to understand whether a particular IgG subclass of passively transferred antibodies was associated with the survival benefit observed in infected infants. ADCC is reported to be predominately mediated by IgG1 and IgG3, and thus we focused on these subclasses. Infant ADCC antibody activity was strongly correlated with IgG1 antibody binding, as measured by binding to cell surface gp120 (r=0.92; p<0.0001; Figure 3A). There was no difference in surface IgG1 antibody binding between infected (mean log2MFI: 9.51) and uninfected infants (mean log2MFI: 9.46) (p=0.82; Figure 3B). In infected infants, however, there was a strong association between surface IgG1 binding and survival (HR: 0.24; p=0.005), with higher log2MFI associated with increased survival in a Cox-proportional hazards model. Furthermore, comparing Kaplan-Meier survival functions for infected infants with a log2MFI ≥ the cohort median with those infants who had log2MFI activity < the cohort median, the survival curves were significantly different (X2=7.74, p=0.005; Figure 3C).
Figure 3. IgG1 and IgG3 HIV-Specific Responses in Infants.
Correlation of infant ADCC antibody activity and IgG1 log2MFI responses (A) or IgG3 log2EPT (D). IgG1 (B) or IgG3 (E) responses in relation to infant infection status; mean ± SEM are shown. Kaplan-Meier estimates for infected infants with IgG1 log2MFI ≥ infected infant cohort median (solid line) and infected infants with IgG1 log2MFI < cohort median (dashed line) (C) or IgG3 levels detectable at a 1:50 dilution (solid line) vs. undetectable (dashed line) (F).
IgG3 binding was measured by ELISA because, similar to previous reports (Smalls-Mantey et al., 2012), we could not detect IgG3 surface binding by the flow cytometry-based method. IgG1 surface binding MFI and gp120-specific ELISA binding titers correlated (data not shown), demonstrating that the assays provide similar measures. Overall, HIV-specific IgG3 was detected in 34 (47.22%) of infant samples. IgG3 did not correlate with infant ADCC activity (r=−0.16, p=0.18; Figure 3D). Similarly, IgG3 binding was not associated with infant infection (p=0.58; Figure 3E) or survival in infected infants (Figure 3F).
Kinetics of ADCC antibody responses in infected infants
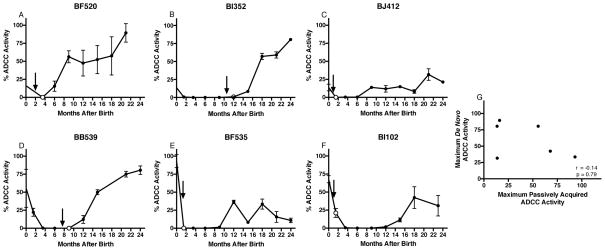
In macaques, higher passive NAb levels have been linked to more robust de novo responses (Haigwood et al., 2004; Ng et al., 2010), raising the possibility that the passive ADCC activity in infants was a surrogate measure for de novo ADCC responses contributing to infection control. To test this possibility, longitudinal plasma samples from six infected infants in the study were tested for ADCC activity. The highest passively acquired ADCC antibody activity was detected within the first week of life and declined to undetectable levels in all six infants prior to a subsequent increase in de novo ADCC activity after infection (Figure 4A–F). De novo ADCC antibody activity could be detected as early as two months post infection, however responses appeared delayed in infants infected within six weeks of birth. In four cases, the maximum de novo ADCC activity was higher than that of the passively acquired activity measured at birth. There was no correlation between passively acquired ADCC antibody activity measured at birth and maximum de novo activity measured after infection (r=−0.14, p=0.79; Figure 4G) suggesting that our finding showing an association with passive ADCC antibodies and infant outcome was not confounded by an association between passive and de novo responses.
Figure 4. Kinetics of ADCC Antibody Responses in HIV-Infected Infants.
ADCC antibody activity in plasma in longitudinal samples from 6 HIV-infected infants. Three had passively acquired ADCC activity < infected infant cohort median (A,B,C) and three had activity > median (D,E,F) Black arrow indicates estimated time of infection defined as the midpoint between the last negative and first positive HIV DNA test. Unfilled dot represents first HIV-DNA positive test. Results represent mean ± SD for 2 independent experiments in duplicate. (G) Correlation between the passively acquired ADCC antibody response (measured in the first week of life) and maximum de novo ADCC activity measured after infection, for the six infants.
DISCUSSION
Defining the role of HIV-specific antibodies in protection in natural infection settings is important to inform rational vaccine design. Insights from animal models and studies of viral control in chronic HIV infections provide indirect support for ADCC antibodies in protection, but these settings do not directly address the efficacy of antibodies present at the time of exposure in humans. In this study, we sought to understand whether pre-existing ADCC antibody activity provided protection from infection and/or disease progression in HIV-exposed infants. Here we report an association between pre-existing HIV-specific ADCC antibody activity and better clinical outcome in humans.
We found that uninfected infants had higher ADCC antibody activity than infected infants, however, this association was not statistically significant. Our power to detect a truly significant difference was limited by the small numbers of infected infants, particularly when considering only those infants infected in the first six weeks of life. Prior studies of similar or smaller size also did not detect a significant association between ADCC antibody activity and infant infection risk, although these studies were limited by the lack of plasma samples from the most relevant window for measuring protection, lack of relevant envelope antigens, and/or imprecise measures of infection timing (Broliden et al., 1993; Jenkins et al., 1994; Ljunggren et al., 1990; Pugatch et al., 1997). Thus, larger studies using well-timed samples tested against antigen representing circulating viruses are needed to clarify if higher pre-existing ADCC activity in infants is a significant correlate of protection from infection.
A correlate of protection was evident when examining clinical outcome. In infected infants, each 10% increase in ADCC activity was associated with a 49.1% reduction in the risk of death. Interestingly, two older studies also observed a positive association between ADCC antibody activity and clinical outcome in infected infants even though ADCC activity was measured at various ages (Broliden et al., 1993; Ljunggren et al., 1990). Due to this variation in sample timing, the contribution of de novo versus pre-existing ADCC antibodies in protection was unclear and de novo responses may have been primarily measured in these older studies. In our results, de novo ADCC levels did not correlate with passively acquired activity, further supporting the role of pre-existing antibodies in the survival benefit we observed. As binding titers measure a contribution of both non-neutralizing (including ADCC) and neutralizing antibodies, the role of ADCC antibodies in survival was also indirectly supported by the trend between IgG binding titers and survival. Additionally, the observation that passive NAbs were not associated with outcome suggests that the infant ADCC activity measured was not a surrogate for overall HIV-specific antibody activity, but rather was specific for the ability of antibodies to mediate ADCC.
In addition to the positive association between pre-existing ADCC antibody activity and infected infant survival, we also observed a trend towards a negative correlation between infant ADCC and set point viral load. Unfortunately, our ability to detect a true association between ADCC and set point was limited by the sparse set point viral load data for the infected infants. Nevertheless, the observed trend supports the hypothesis that pre-existing antibodies may have acted to clear infected cells, thereby lowering set point viral loads and protecting against disease progression. This hypothesis is supported by the fact that infant set point viral loads are predictive of HIV disease progression (Obimbo et al., 2009; Palumbo et al., 1998), and is consistent with macaque vaccine studies showing pre-existing ADCC antibody activity is associated with lower viremia in macaques infected by virus challenge (Reviewed in Lewis, 2014). The association between pre-existing ADCC activity and viral control should be analyzed in future human vaccine studies to understand if ADCC provides a therapeutic effect in individuals who become infected.
Interestingly, our results suggest that IgG1, and not IgG3, was important for the ADCC activity and survival effect observed in this study. While IgG1 and IgG3 are major mediators of ADCC activity, recent data from RV144 suggested that vaccine-induced IgG3 was important for the ADCC activity and protective effect observed in that trial (Chung et al., 2014; Yates et al., 2014). In our study, IgG3 levels did not correlate with risk of infant infection or survival in infected infants. IgG1 levels, however, did directly correlate with survival in infected infants. This difference between our study and the RV144 results highlights differences in antibodies elicited by natural infection versus the ALVAC/AIDSVAX vaccination method and suggests that IgG1 ADCC-mediating antibodies could be an important component of an effective vaccine response.
De novo ADCC activity was detected as early as 2 months post-infection and 6 months of age in our study, which is earlier than has previously been reported for infant HIV-specific ADCC antibodies (Pugatch et al., 1997). Although numbers are small, there was evidence that responses were delayed in infants infected within six weeks of birth. A delay in de novo responses may be due to the detrimental impact of early HIV-infection on the developing immune system (Reviewed in Tobin and Aldrovandi, 2013). While adult ADCC antibody responses have been suggested to peak at approximately 6 months post-infection (Dugast et al., 2014), infant de novo responses continued to increase for over a year post infection in three infants. These ADCC levels exceeded those acquired by passive transfer and this difference from adults may be due to the higher peak viral loads and thus increased antigenic stimulation observed in infants (Richardson et al., 2003).
Unexpectedly, transmitting and non-transmitting mothers had nearly identical ADCC levels and in those mothers who transmitted, maternal ADCC antibody levels were not predictive of infant survival. This difference between maternal and infant ADCC activity may be partially explained by the timing of maternal sampling. We sampled maternal plasma primarily from the third trimester of pregnancy as the majority of passive antibody transfer occurs during this time. However, as reports suggest that the majority of passive transfer occurs within the last few weeks of pregnancy (Palmeira et al., 2012), samples from earlier in the third trimester may not be representative of the infant antibody repertoire at birth, particularly if there is variation in gestational age. This variation in the time when the infant was born in relation to when the maternal antibodies were sampled may have allowed us to discriminate the effect of maternal versus passive antibodies in protection. A more intriguing possibility is that the differences between maternal and infant ADCC are due to differences in passive transfer of antibodies to the infants. IgG subclass and the glycosylation profile of antibodies impact ADCC activity and have also been suggested to be differentially transferred across the placenta (Ackerman et al., 2013; Ferrante et al., 1990; Hashira et al., 2000; Simister, 2003; Williams et al., 1995; Wren et al., 2013), suggesting that maternal ADCC antibody levels may not always be indicative of infant repertoires. In any case, these results have important implications given that prior studies often focused on the relationship between maternal antibody responses, rather than infant responses, and protection. The observed differences in infant and maternal responses suggest that maternal samples may not be an accurate surrogate measure of passively acquired responses in infants for some antibody functions such as ADCC.
In summary, we show that pre-existing HIV-specific ADCC antibody activity is associated with survival in HIV-infected infants. This association was detected when measuring infant passive antibody levels, but not maternal antibodies. Infected infant survival was also associated with HIV-specific IgG1, and not IgG3, levels and was not linked to de novo ADCC activity. These data support a role for developing vaccines that are designed to elicit ADCC-mediating IgG1 antibodies.
EXPERIMENTAL PROCEDURES
Study Design
Plasma samples were from the Nairobi Breastfeeding Trial conducted in the mid-1990s, before antiretrovirals were used for prevention of MTCT (Nduati et al., 2000). Infants were tested for HIV DNA at birth, 6 weeks, 14 weeks, and every 3 months until 2 years of age. For those infants who tested positive, samples prior to the first HIV DNA positive test were tested for HIV RNA to more precisely define infection timing. Time of infection was estimated as the midpoint between the last negative HIV DNA or RNA test and the first positive test. Infant samples were selected for study based on the following criteria: 1) HIV DNA and RNA negative at birth; 2) breastfed for ≥3 months; 3) remained HIV-negative for at least 6 months and throughout all follow-up if in the uninfected arm; and 4) availability of a plasma sample from the first week of life. Based on these criteria, 72 infants were included, 21 of who became infected during follow-up. Corresponding maternal plasma samples from the third trimester of pregnancy (n=69) or delivery (n=3) were also selected for testing. Longitudinal plasma samples were analyzed for 6 infants. These infants were chosen based on the availability of multiple samples prior to and post infection.
Rapid Fluorometric Antibody-Dependent Cellular Cytotoxicity Assay
ADCC was measured using the rapid fluorometric ADCC (RFADCC) assay as previously described (Gomez-Roman et al., 2006; Mabuka et al., 2012). Target cells were coated with BL035.W6M.Env.C1 (BL035) gp120 (Immune Technology Corp.). Plasma samples were run at 1:5000; which detected a wide range of ADCC activity while avoiding a prozone effect. Samples were run in duplicate and in comparison studies, all samples were run with the same peripheral blood mononuclear cell (PBMC) donors to reduce donor variability. Infant samples were run 3 times in duplicate with 2 PBMC donors and maternal samples were run 2 times in duplicate with 1 donor. Pooled IgG from HIV-positive individuals, HivIg (NIH AIDS Reagent Program), was used as a positive control. In a pilot study, 4 infant plasmas and 2 plasma pools (HivIg and pooled plasma from 30 chronically infected Kenyans (VA Pool)) were tested in duplicate against target cells coated with different gp120 antigens (BL035.W6M.Env.C1, MG505.W0M.Env.H3, MK184.W0M.Env.G3, Bal, WITO4160, YU2, CAP210.2.00, and Du422.1; Immune Technology Corp). Final reports of ADCC activity were normalized to HivIg, which was set at 100%.
HIV-Specific Total IgG and IgG3 ELISAs
HIV-envelope specific ELISAs were performed as previously described (Mabuka et al., 2012), with the following modifications. ELISA plates were coated with BL035 gp120 at 25ng/well (total IgG ELISA) or 50ng/well (IgG3 ELISA). Plasma dilutions started at 1:25,000 (total IgG) or 1:50 (IgG3) and were titrated two-fold. If IgG EPT was less than 1:25,000, 2-fold dilutions were conducted starting at 1:1000. For total IgG, samples were detected with goat anti-human IgG-HRP diluted 1:3000. IgG3 samples were incubated with mouse anti-human IgG3-biotin (SouthernBiotech) at 2 μg/mL and then with streptavidin-HRP at 1 μg/mL. EPT was defined as the plasma reciprocal dilution at which the average OD value was ≥2 times the average OD value of background measured against HIV-uninfected plasma. Samples were run in duplicate 2 times. If the sample did not reach EPT at the lowest dilution tested, the EPT was set as the midpoint between 0 and the lowest dilution tested.
Detection of IgG1 Cell-Surface Binding
To measure the magnitude of HIV-specific IgG1 binding to gp120-coated cells, the following protocol was adapted from Smalls-Mantey, et al.2012. CEM-NKr cells were coated with BL035 gp120 at 15μg/1 million cells. Next, 25,000 CEM-NKr cells were incubated with 1:1000 patient plasma run in duplicate. Samples were washed and stained with mouse anti-human IgG1-Alexa-488 at a 1:1000 dilution (SouthernBiotech). Median fluorescence intensity (MFI) was quantified by flow cytometry.
Statistical Analysis
The statistical plan for infant and maternal ADCC antibody responses were established prior to data collection and based on literature suggesting ADCC activity may impact acquisition and clinical outcome, thus negating the need to adjust for multiple comparisons (Savitz and Olshan, 1995). Following the observed positive association between infant ADCC antibody activity and survival, experiments and analyses were conducted to further explore the mechanism and antibody subclass responsible for the effect. Independent groups were compared by two-sided Welch’s t-test. Logistic regression analysis was used to compare independent groups and control for maternal viral load. Survival analyses were conducted using Cox-proportional hazards models and Kaplan-Meier estimates with log-rank tests. Correlations were estimated by the Spearman rank method. ELISA and MFI data were log2-transformed and viral load data were log10-transformed for all analyses. Statistical analyses were performed using STATA version 12.1 (College Station, TX).
Supplementary Material
Acknowledgments
This project was supported by NIH grant R01 AI076105 and K24 HD054314. CM was supported by NIH training grant T32 AI083203 and fellowship F30 AI112385. We would like to thank Dr. John Lynch for neutralization data and participants of the Nairobi Breastfeeding Trial, without whom this study would not have been possible.
Footnotes
AUTHOR CONTRIBUTIONS
CM, JO, and GJS designed the study. CM performed all experiments and statistics with guidance from BR. RN oversaw the original Nairobi Breastfeeding Trial and sample collection. CM and JO wrote the paper with input from co-authors.
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broliden K, Sievers E, Tovo PA, Moschese V, Scarlatti G, Broliden PA, Fundaro C, Rossi P. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin Exp Immunol. 1993;93:56–64. doi: 10.1111/j.1365-2249.1993.tb06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Sci Transl Med. 2014;6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- Dugast AS, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, Ackerman ME, Streeck H, Klasse PJ, Moore JP, et al. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur J Immunol. 2014;44:2925–2937. doi: 10.1002/eji.201344305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Ped Infect Dis J. 1990;9:S16–S24. [PubMed] [Google Scholar]
- Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Haigwood NL, Montefiori DC, Sutton WF, McClure J, Watson AJ, Voss G, Hirsch VM, Richardson BA, Letvin NL, Hu SL, et al. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J Virol. 2004;78:5983–5995. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashira S, Okitsu-Negishi S, Yoshino K. Placental transfer of IgG subclasses in a Japanese population. Pediatr Int. 2000;42:337–342. doi: 10.1046/j.1442-200x.2000.01245.x. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M, Landers D, Williams-Herman D, Wara D, Viscarello RR, Hammill HA, Kline MW, Shearer WT, Charlebois ED, Kohl S. Association between anti-human immunodeficiency virus type 1 (HIV-1) antibody-dependent cellular cytotoxicity antibody titers at birth and vertical transmission of HIV-1. J Infect Dis. 1994;170:308–312. doi: 10.1093/infdis/170.2.308. [DOI] [PubMed] [Google Scholar]
- Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142:46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren K, Moschese V, Broliden PA, Giaquinto C, Quinti I, Fenyo EM, Wahren B, Rossi P, Jondal M. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis. 1990;161:198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- Lynch JB, Nduati R, Blish CA, Richardson BA, Mabuka JM, Jalalian-Lechak Z, John-Stewart G, Overbaugh J. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J Virol. 2011;85:5252–5261. doi: 10.1128/JVI.02216-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabondzo A, Rouvier P, Raoul H, Le Naour R, Courpotin C, Herve F, Parnet-Mathieu F, Lasfargues G, Dormont D. Relationships between humoral factors in HIV-1-infected mothers and the occurrence of HIV infection in their infants. Clin Exp Immunol. 1995;102:476–480. doi: 10.1111/j.1365-2249.1995.tb03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLos Pathogens. 2012;8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obimbo EM, Wamalwa D, Richardson B, Mbori-Ngacha D, Overbaugh J, Emery S, Otieno P, Farquhar C, Bosire R, Payne BL, et al. Pediatric HIV-1 in Kenya: pattern and correlates of viral load and association with mortality. JAIDS. 2009;51:209–215. doi: 10.1097/qai.0b013e31819c16d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo PE, Raskino C, Fiscus S, Pahwa S, Fowler MG, Spector SA, Englund JA, Baker CJ. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–761. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- Pugatch D, Sullivan JL, Pikora CA, Luzuriaga K. Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. WITS Study Group. Women and Infants Transmission Study. J Infect Dis. 1997;176:643–648. doi: 10.1086/514085. [DOI] [PubMed] [Google Scholar]
- Richardson BA, Mbori-Ngacha D, Lavreys L, John-Stewart GC, Nduati R, Panteleeff DD, Emery S, Kreiss JK, Overbaugh J. Comparison of human immunodeficiency virus type 1 viral loads in Kenyan women, men, and infants during primary and early infection. J Virol. 2003;77:7120–7123. doi: 10.1128/JVI.77.12.7120-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142:904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, Hallahan CW, Wong H, Liu B, You L, et al. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol. 2012;86:8672–8680. doi: 10.1128/JVI.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev. 2013;254:143–169. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Arkwright PD, Rudd P, Scragg IG, Edge CJ, Wormald MR, Rademacher TW. Short communication: selective placental transport of maternal IgG to the fetus. Placenta. 1995;16:749–756. doi: 10.1016/0143-4004(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Wren LH, Stratov I, Kent SJ, Parsons MS. Obstacles to ideal anti-HIV antibody-dependent cellular cytotoxicity responses. Vaccine. 2013;31:5506–5517. doi: 10.1016/j.vaccine.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Yates NL, Liao HX, Fong Y, DeCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, et al. Vaccine-Induced Env V1-V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon After Vaccination. Sci Transl Med. 2014;6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.