Abstract
Objective
The aim of this RCT was to evaluate sucking organization in premature infants following a preterm infant multi-sensory intervention, the Auditory, Tactile, Visual, and Vestibular (ATVV).
Study Design
A convenience sample of 183 healthy premature infants born 29 - 34 weeks post-menstrual age (PMA) enrolled. Sucking organization was measured at baseline, then weekly assessments, during the infant's hospital stay.
Results
A quadratic trend was observed for number of sucks, sucks per burst, and maturity index, with the intervention group increasing significantly faster by day 7 (Model estimates for group*day: β = 13.69, p < 0.01; β = 1.16, p < 0.01; and β = 0.12, p < 0.05, respectively). Sucking pressure increased linearly over time, with significant between-group differences at day 14 (β = 45.66, p < 0.01).
Conclusion
ATVV infants exhibited improved sucking organization during hospitalization, suggestive that ATVV intervention improves oral feeding.
Keywords: Sucking skills, microstructure of infant sucking behaviors, preterm infants, multisensory intervention
INTRODUCTION
Review of the Literature
Premature infants are at risk for difficulties in feeding, social interaction and growth. Many premature infants exhibit a lower capacity for self-regulation, resulting in less behavioral alertness and hypersensitivity to stimulation.1 Feeding is critically important because it is a primary factor in infant growth and a major concern for both parents and clinicians. 2 Failure to coordinate breathing, sucking and swallowing predisposes the premature infant to apneic events, bradycardia, oxygen desaturation and fatigue during feeding.3 Inefficient oral feeding requires a large amount of energy expenditure by the premature infant and delays hospital progression.4 One of the challenges premature infants experience during the transiton to oral feeding is achieving and sustaining an alert behavioral state, such alertness being an indicator of appropriate behavioral organization.5 Slight arousal prior to feeding helps infants achieve an active awake state, conducive to oral feeding.6 Several oral and tactile sensory motor interventions have been suggested to support and enhance oral feeding, yet few if any have addressed how behavioral organization improves sucking behaviors.7
The Auditory, Tactile, Visual and Vestibular (ATVV) intervention is known to facilitate behavioral organization, specifically helping infants achieve alert states prior to feeding.2, 7 Recently we reported an increase in the frequency of alert behavioral states and orally directed behaviors (e.g., hand to mouth, sucking on hand) prior to feeding in response to a multi-sensory intervention. 8,9 Our previous work with the ATTV has demonstrated an increase in alert behavioral states as well as oral feeding efficacy, which we defined as the amount of oral intake per minute. What is unknown is how the ATVV influences sucking behavior during the first weeks of oral feeding.
Measurements and definitions of maturational progression of sucking behaviors in preterm infants offered in the current literature have been quite diverse. Measurements have often included pressure transducer obtained sucking records and clinical observation tools.10 Length-of-feeding assessments varied, with a 5-minute assessment being the most frequently used.11,12 Most investigators have used number of sucks (NOS) and number of sucks per burst (SPB) as a baseline estimate of feeding skills. Additional parameters may include quantity of milk ingested, average milk ingested per suction, proportion of time pausing and/or sucking pressures.9,12-14,15
Organized feeding behaviors during the early weeks of life have been linked to appropriate developmental outcome measures at 12 months of age.16,17 In most neonatal intensive care units (NICUs), general observations are made regarding feeding competence using indicators such as the rooting response or the infant's ability to initiate sucking.18 However, these observations do not address the quality of the infant's ability to feed. In this study, we used a comprehensive characterization of sucking skills including features of the microstructure of nutritive sucking behaviors: NOS, SPB, sucking pressure (MAMP, maximal adjusted mean pressure) and sucking maturity index (SMI, a combination of the three sucking parameters).19 These measures of nutritive sucking microstructure behaviors have been shown to improve with post-menstrual age (PMA).20
Thus, this research investigated whether the ATVV intervention enhanced maturation of nutritive sucking as defined as greater NOS, SPB, MAMP, and SMI in premature infants. In this study we hypothesized that: 1) acquisition of efficient sucking behaviors could serve as an indicator of improved behavioral organization and 2) the ATTV intervention would facilitate maturation of sucking behaviors.
MATERIALS/SUBJECTS AND METHODS
Study Setting
The research was conducted in two inner-city Midwestern community hospitals’ NICUs (level II [with extended capabilities] and level III NICU). The research was approved by the Institutional Review Boards from the University of Illinois, Chicago, and both clinical sites.
Study Design
The study employed a balanced two-group randomized trial design.21-23 After baseline data collection, 230 premature infants born at 29-34-weeks gestational age (GA) were randomly assigned to the ATVV intervention or a control group. Infant nutritive sucking indices were obtained at baseline and weekly during hospital stay.
Sample Selection
Infant eligibility criteria included birth between 29 and 34 weeks GA, no other major health problems, and clinically stable at enrollment. Eligible infants may have received prior ventilator support or other medical therapies. Infants were excluded if they were diagnosed with congenital anomalies, necrotizing enterocolitis, brain injury, chronic lung disease, HIV, or prenatal drug exposure. Infants were also excluded if their mothers were not their legal guardians.
Recruitment occurred at each site in the following manner. The in-hospital research nurse identified potential subjects and approached prospective mothers. After explaining the study and confirming the infants’ eligibility and the mothers’ willingness to participate, the nurses obtained informed consent. Then the infants were randomly assigned to the ATVV or control groups using computer generated lists of random numbers.
Randomization was centrally controlled. Two lists of random numbers were generated, one for each site, and balanced so that half the infants at each site were in the experimental group. The order of random assignment was then transferred from this list into sequential envelopes. To facilitate the mother's engagement with the study protocol, after the mother consented to her infant's participation, she selected the next envelope and the recruitment nurse entered the group assignment, date, and random generation number. This allowed us to confirm that assignment occured in the computer-generated random order.
Sample Description
Two hundred-thirty mother-infant dyads with at least 2 social-environmental risk factors were enrolled in the study. Twenty-six were deemed ineligible due to infant health conditions identified after enrollment. Seven infants who developed health conditions known to interfere with feeding (e.g., pulmonary hypertension, brohchopulmonry dysplasia) or transferred to other institution were excluded (Figure 1). Three infants were excluded due to an equipment malfunction or protocol deviation. Four infants were discharged prior to data collection. In addition, three mothers withdrew after consent but before data collection. Ethnic diversity was limited (Latina = 50.5%; African-American = 45.5%), thus, data for three mothers self-identified as being Asian or white were not included in the analysis.
Figure 1.
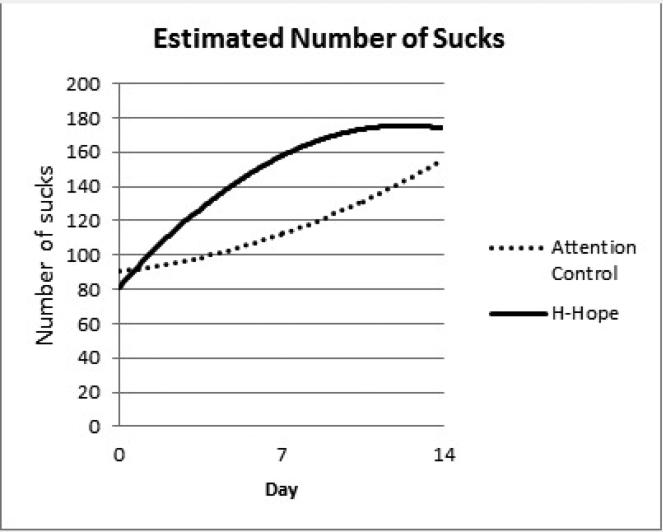
Panel 1 Estimated Number of Sucks
Therefore, 183 infants (n = 93 control, n = 90 ATVV) had baseline oral feeding assessments and were included in the analytic sample for this study. At day 7, 88 (ATVV = 40) infants remained hospitalized and only 47 (ATVV = 20) infants were hospitalized at day 14.
The ATVV Intervention
The ATVV provided 10 minutes of auditory (female voice), tactile (moderate touch stroking or massage) and visual (eye to eye) stimulation, followed by 5 minutes of vestibular stimulation (horizontal rocking).6,24
The stimuli were presented in gradual progression: auditory only; auditory and tactile; visual, added as the infant became alert. This increased the frequency of alert states in premature infants 33-36 weeks PMA7,6,25,26 and the frequency of orally directed behaviors.5,27 The ATVV was administered after the baseline feeding assessment at 32 weeks PMA and twice daily for five days per week for 20 minutes prior to a morning and early afternoon feeding by the mother or research nurse. Mothers and research team members were trained in ATVV intervention and reliability (>90% agreement with the ATVV checklist) was established and maintained. 26 To standardize the testing session, the ATVV was administered during each testing session by the research nurse.
The Control Condition
The control education was designed to provide a similar contact and staff attention but distinctly different content from the intervention. Infants received the current standard feeding and nursery care while their mothers received educational content incorporating premature infant care (e.g., bathing, sleep positions and habits, holding the baby, safety of infant equipment and car safety).
MEASURES
Microstructure of Nutritive Sucking Behaviors
Infant sucking was digitally recorded using the Medoff-Cooper Nutritive Sucking Apparatus (M-CNSA) supported by the AcqKnowledge 3.9.0 software (BIOPAC, Goleta, CA). The M-CNSA continuously measured negative pressure generated by the infant during nutritive sucking. The fluid flow rate was determined by the infant's sucking pressure using a Bionix nipple (Bionex Medical Technology, Toledo, Ohio). Thirty minutes prior to the scheduled feeding, the infant was brought into a dimly lit nursery with an open warming bed. Microstructure of sucking was measured at baseline and weekly thereafter until discharge for a ten minute time period. To standardize the feeding, a member of the research team conducted the feeding for the test sessions. The digital nutritive record was analyzed by Matlab 2007a (Natick, MA: The MathWorks) and a custom Matlab subroutine, Suck_Detect 1.1.12 software28 for the five minutes that were most representative of the ten minute feeding, according to procedures detailed elsewhere29. During this process, portions of the record during which the nipple was removed from the infant's mouth were deleted.29 Sucking parameters included NOS, NOS per burst and MAMP. The SMI was derived by calculating and averaging the Z-scores for total NOS, MSPB and MAMP across time points.
Covariates
Covariates considered in the analysis included the following infant characteristics abstracted from medical records: gender, plurality (singleton or multiple birth), delivery type, hospital site, birth GA, birth weight, small for GA, five minute Apgar score, infant morbidity (measured using a subset of the Problem-Oriented Perinatal Risk Assessment System [POPRAS] score30 to assess risk at baseline), chronological age, PMA at baseline assessment and number of days feeding experience prior to baseline assessment. Oral feeding was defined as consuming ≥ 10% of that day's nutrition orally. Maternal race/ethnicity was obtained by self-report.
Procedure
Sucking microstructure data were collected at baseline when the infant was to begin oral feeding prior to intervention and every seven days until hospital discharge. The baseline feeding assessment was conducted at the first oral feeding when possible, or within five days of initiation of oral feeding, then weekly until hospital discharge. The ten-minute feeding assessments were conducted during an early morning regularly scheduled feeding, using the MCNSA.31 For the intervention group, feeding assessments occurred immediately following the administration of the ATVV by a trained nurse.
Statistical Analysis
Descriptive statistics were calculated overall and by group (ATVV vs. Control) using chi-square tests and t-tests to examine group equivalence at baseline. Pearson's correlation coefficients and t-tests were used to examine the association between categorical and continuous infant characteristics, respectively, and the four oral feeding measures at baseline. Two-sided tests were used for hypothesis testing, controlling for a type I error probability of α = 0.05, with results having a p-value between 0.05 and 0.10 noted as a marginal result. Due to a significant drop in sample size (n = 15) after day 20, we focused our inferences of the sucking outcomes on days 0, 7 and 14 after intervention. Mixed-effects regression models were employed, with repeated outcome measures clustered within individuals to examine the effect of intervention on the outcomes over time. Random individual effects (e.g., random intercept and time trends and correlated error structures) were estimated to provide good fit and to account for correlations between the repeated measurements from the same infant. Best-fit random effects and covariance structure of the errors were conducted using the likelihood ratio tests for nested models, or fit statistics (e.g., Akaike information criterion [AIC] and Bayesian Information criterion [BIC]) for non-nested models. Residual analysis was performed for model diagnostic purposes and no severe departure from model assumptions, including normality, was detected.
The mean shape of the outcome trajectories over time was examined using polynomial time effects and adjusted for covariates. Interactions between group and time trend terms were examined to identify the shape of the intervention effect over time. Backward selection was used for selection of covariates that were significantly associated with the outcome variable in each model. Model parameters were used to estimate and test differences in the mean of each outcome by group at each timepoint (0 – baseline and 7 and 14 days post-baseline), using t-test generated from the model results.
RESULTS
The final analytic sample included 183 infants (female = 50.5 %). The average birth age was 32.58 weeks GA (SD = 1.48 weeks) with a mean birth weight of 1815.54g (SD = 350.15 g), a mean chronological age of 9.29 days (SD = 6.48 days) and a mean PMA 33.89 (SD = 1.00) at baseline. The mean number of days of oral feeding prior to baseline feeding assessment was 4.16 days (SD = 3.49 days). Mean baseline scores for sucking outcomes were 85.87 (SD = 74.48) for NOS, 28.05 mm Hg (SD = 17.49) for MAMP, 7.03 (SD = 8.57) for MSPB and -0.29 (SD = 0.82) for the SMI. Baseline characteristics and baseline sucking parameters were not significantly different between groups (Table 1a and b).
Table 1a.
Baseline Characteristics for Infants assigned to the ATVV Intervention and the Attention Control Group
| Infant Characteristics | ATVV (n = 90) % or mean (SD) | Attention Control (n = 93) % or mean (SD) | p-value* | |
|---|---|---|---|---|
| Sex | Female | 56.7 | 44.7 | 0.10 |
| Male | 43.3 | 55.3 | ||
| Plurality | Singleton | 85.6 | 84.0 | 0.74 |
| Twin/Triplet | 14.4 | 16.0 | ||
| Type of Delivery | NSVD | 54.4 | 51.6 | 0.53 |
| C-SEC | 45.6 | 48.4 | ||
| Site | A | 55.6 | 57.4 | 0.32 |
| B | 44.4 | 42.6 | ||
| Small for | Yes | 30.0 | 29.8 | 0.46 |
| Gestational Age | No | 70.0 | 70.2 | |
| Maternal | Latina | 50.0 | 51.1 | 0.88 |
| Race/Ethnicity | African-American | 50.0 | 48.9 | |
| Gestational Age, weeks | 32.5 (1.5) | 32.6 (1.4) | 0.63 | |
| Birth Weight, grams | 1791 (314) | 1839 (382) | 0.45 | |
| Apgar score 5 min | 8.4 (0.9) | 8.3 (1.1) | 0.73 | |
| Infant Morbidity Score at Delivery (POPRAS sub score)d | 43.3 (10.4) | 45.4 (10.8) | 0.18 | |
| Chronological age at baseline, days | 9.1 (6.2) | 9.5 (6.7) | 0.64 | |
| Postmenstrual age at baseline, weeks | 33.8 (1.0) | 34.0 (1.0) | 0.27 | |
| Number of days of oral feeding ≥10% before baseline | 4.0 (3.3) | 4.3 (3.7) | 0.50 | |
Chi-square test for categorical variables and t-test for continuous variables
Table 1b.
Baseline Sucking Parameters for Infants assigned to the ATVV Intervention and the Attention Control Group
| Sucking Parameters at Baseline | ATVV (n = 90) mean (SD) | Attention Control (n =9 3) mean (SD) | p-value* |
|---|---|---|---|
| Number of Sucks | 80.2 (68.5) | 91.4 (79.8) | 0.31 |
| Mean Adjusted Maximum Pressure | 28.1 (17.8) | 28.0 (17.3) | 0.98 |
| Mean Number of Sucks per Burst | 5.8 (6.0) | 8.2 (10.4) | 0.06 |
| Sucking Maturity Index | −0.36 (0.7) | −0.2 (0.9) | 0.25 |
T-test
NOS over time had an increasing quadratic mean pattern as indicated by the significant time group*day*day interaction (Table 2, top panel) and the gradually increasing, then leveling off trend (Figure 1, panel 1). Although the time trend terms for the control group (day and day*day terms) were not significant, the intervention group increased significantly faster (β = 13.69 for group*day, p < 0.01) and leveled over time (β = -0.84 for group*day*day, p < 0.05). When the means at each timepoint were estimated from the model, a between-group difference in the quadratic pattern resulted in a significant group effect at day 7 (estimated difference = 45.66, p < 0.01) but not at day 14 (Table 2, bottom panel). These patterns of change over time in NOS by group are depicted pictorially in Figure 2, panel 1.
Table 2.
Longitudinal model estimates of Sucking Outcomes, Beta Estimate (SE); n = 183
| Variable | NOS | MAMP | MSPB | SMI |
|---|---|---|---|---|
| Var-Cov Structure: Random Effects (Error Structure) | Random Intercept (Toeplitz 2) | Random Intercept (Unstructured) | Random Intercept and time (Unstructured) | Random Intercept and time (Unstructured) |
| Intercept | 66.60(9.62)*** | −108.95(41.00)** | 2.97(2.60) | 1.381(0.314)*** |
| Group | −9.11(10.75) | 1.05(2.55) | −2.11(1.23)* | −0.107(0.118) |
| day | 1.59(3.13) | 1.08(0.23)*** | −0.32(0.28) | 0.009(0.031) |
| day*day | 0.22(0.24) | 0.03(0.02) | 0.002(0.002) | |
| Group*day | 13.69(4.63)*** | 0.57(0.34)* | 1.16(0.41)*** | 0.123(0.046)** |
| Group*day*day | −0.84(0.35)** | −0.06(0.03)** | −0.007(0.003)* | |
| Small for Gestational Age | 0.245(0.116)** | |||
| Gestational Age, week | 3.70(1.18)*** | |||
| Birth Weight, kg | 9.95(3.71)** | 2.85(1.33)** | 0.500(0.155)*** | |
| Infant morbidity score at delivery (subset of POPRAS) | −0.24(0.11)** | |||
| Chronological age at baseline, day | 0.89(0.28)*** | |||
| Number of days of oral feeding ≥10% before baseline | 5.73(1.38)*** | 0.040(0.015)** | ||
|
Estimated mean difference between groups at different time point (ATVV- Attention Control group) | ||||
| Day 0 (n=183) | −9.11(10.75) | 1.05(2.55) | −2.11(1.23)* | −0.11(0.12) |
| Day 7 (n=89) | 45.66(15.23)*** | 5.04(2.62)* | 2.82(1.36)** | 0.43(0.15)*** |
| Day 14 (n=48) | 18.35(20.64) | 9.02(4.30)** | 1.38(1.39) | 0.33(0.19)* |
NOS: Number of Sucks; MAMP: Mean adjusted max pressure; MSPB: Mean sucks per burst; SMI: Sucking Maturity Index
p < 0.10(marginally significant)
p < 0.05
p < 0.01
Figure 2.
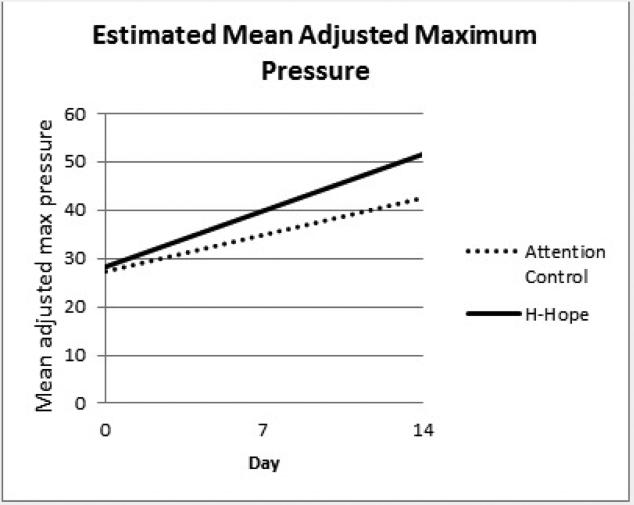
Panel 2 Estimated Mean Adjusted Maximum Pressure
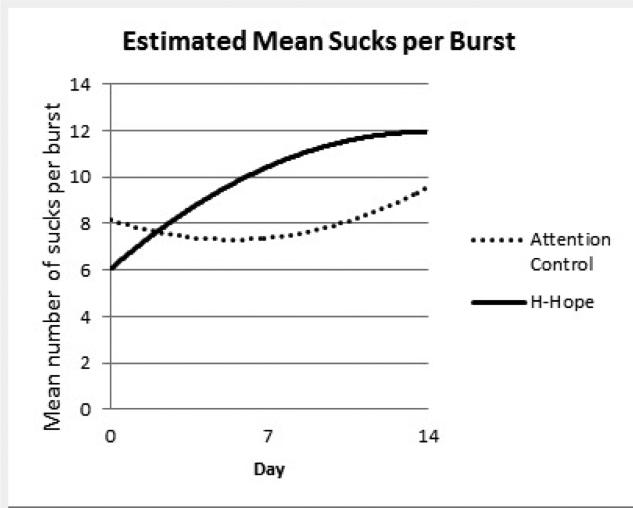
There was a significant increasing trend over time in MAMP for the control group (β = 1.08 for day, p < 0.01) and the ATVV group increased marginally faster than the control group over time (β = 0.57 for group*day, p = 0.096). When the means at each timepoint were estimated from the model, there was a marginally significant between-group difference in MAMP at day 7 and a significant difference at day 14 (about 9.0 mmHg, p < 0.05, Table 2, bottom panel; Figure 2, panel 2). Similar to the pattern of the NOS, the intervention group had a significantly higher increasing rate for mean MSPB (β = 1.16 for group*day, p < 0.01) which leveled off over time (β = -0.06 for group*day*day, p < 0.05) becoming non-significant by day 14 (Table 2, bottom panel; Figure 2, panel 3). The trend for the control group, as represented by the coefficients for day and day*day was not statistically significant. At day 7, the ATVV group had significantly higher mean MSPB, a profound increase considering a marginally lower mean at baseline (Table 2,Figure 2, panel 3).
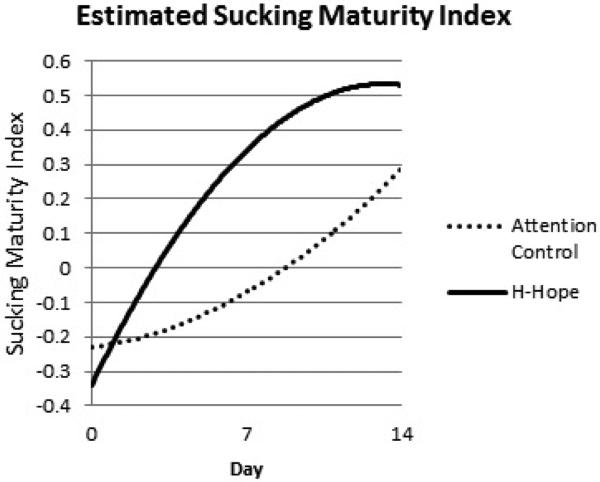
Over time, the mean SMI had a similar pattern as NOS and meanMSPB. The intervention group increased significantly faster by day 7 (a difference of 0.43, p < 0.01) and leveled off with a marginally significant SMI (a difference of 0.33, p = 0.099) at day 14 (Table 2, bottom panel; Figure 2, panel 4).
DISCUSSION
The current study tested a multi-sensory intervention which addressed biologic immaturity and social-environmental risk to enhance feeding behaviors in premature infants from initiation of oral feeding until discharge. Understanding the microstructure of sucking or feeding behaviors informed our understanding of the maturational progression of oral feeding in this sample of preterm infants.
It is well documented that sucking patterns in preterm infants mature over time. Amaizu 32 reported maturation of oral feeding skills such as lip seal, rate of milk intake, feeding efficiency (ml/min), percent of milk leakage, suck/expression ratio, suction musculature and sucking stage (presence and rhythmicity of suction and or expression) from the 1-2 to 6-8 days of oral feeding. In this study, sucking microstructure matured for both the control and ATVV groups. Lau14 reported that as feeding performance improved, sucking and swallowing frequency, bolus size and suction amplitude increased. Gewolb33 found that sucking and suck-swallow rhythms stabilize by 36 weeks PMA, and coordination of swallow-respiration and suck-swallow rhythms may be predictive of feeding, respirations, and neurodevelopmental progress/delays. A variety of studies by Medoff-Cooper and colleagues11,20,34 have shown significant maturational progress in preterm infants’ feeding behaviors from early oral feeding initiation until term. What we asked in this study was whether we could enhance or augment this maturational process by providing an intervention which has been shown to improve behavioral state and feeding organization in healthy premature infants.35
To assure there were no differences in baseline between the two groups of infants, we evaluated both infant characteristics and feeding measurements at baseline. At baseline, numbers of days of oral feeding prior to baseline was found to be the only predictor of the NOS an infant produced during the 5-minute assessment. The NOS, a reliable measurement of feeding organization, appears to be influenced by experience and helps to account for this finding.34 Most notable was the significant increase in the NOS at day 7 for the infants in the intervention group when compared to control. However, this difference was no longer seen at day 14, causing us to speculate that experience, maturation and other unidentified overarching factors influenced the NOS a premature infant was able to produce.
A similar trend was seen for the mean MSPB. As expected, NOS and mean MSPB were associated. However, the NOS do not always provide adequate information about sucking organization; rather, it is the length of the sucking burst as determined by the number of sucks within a sucking burst. We have shown in previous work that with increasing maturation, sucking bursts become longer, and sucking pauses or time between generation of sucking bursts become shorter.36 Unfortunately, we were not able to assess time between bursts due to the variability in the length of time an infant maintained sucking activity. However, the findings suggest that infants in the intervention group demonstrated a more mature sucking pattern at day 7 as compared to control.
The SMI follows the same trend of earlier achievement of skills at day 7 for infants in the intervention group. While we do not know if this earlier achievement can be used as a marker for long term developmental outcome, there is evidence from previous work that lower SMI at 40 weeks was predictive of lower 12-month developmental scores.11
Further work may qualify the interpretation of the current findings. It is also possible that the prognostic potential of a neonatal sucking assessment is underestimated. It should be emphasized that each feeding assessment was based on a standardized, single 5-minute sucking test. Nevertheless, the inherent variability of infant sucking performance, arousal level, day-to-day response and feeding-to-feeding response is well known. Such within-subject variability could have weakened the sensitivity of the method for detecting real differences between infant groups. A better estimate of an infant's maturational status may be derived from 2 or more tests, delivered the same day, and/or over consecutive days. Separately, it is important to determine whether test sensitivity varies as a function of time of day.
Limitations
We acknowledge the small sample size and the inclusion of relatively healthy preterm infants limits our ability to generalize the findings of this study. By day 14 the most healthy and older infants were discharged to home, with only the infants with either medical or feeding issues left in the nursery. We did not collect sucking data on a daily basis, which limited our ability to understand the variability of sucking behaviors day by day. An important strength of this study is that we were able to explore the relationship between an intervention which is noted to improve feeding efficiency and the microstructure of sucking behaviors.
In conclusion, it appears that the ATVV intervention contributed to earlier achievement of sucking organizational skills at day seven. Exploration of the strength of the relationship between earlier maturation of feeding skills and better developmental outcomes would greatly add to our understanding of preterm infant development. Further work is needed to verify and extend the present findings and to develop protocols with greater sensitivity for the detection of stable and reliable individual differences in neonatal sucking organization in a broader population of preterm infants.
SUMMARY TABLE.
What we know: The ATVV intervention enhances maturation of sucking patterns in preterm infants
What needs to be studied: The appropriate daily frequency of the intervention to ensure maximum sucking maturation
What can we do today to guide caregivers: The ATVV intervention is suited for caregivers to use in the clinical setting as part of the preterm infant feeding protocol during the initiation of oral feeding
What this study adds:
-Multi-sensory intervention improves early feeding behaviors in preterm infants
-Maturation of early feeding behaviors is seen during the first week of oral feeding
Figure 3.
Panel 3 Estimated Mean Sucks per Burst
Figure 4.
Panel 4 Estimated Sucking Maturity Index
Acknowledgements
This work was supported by grants from the National Institutes of Child Health and Human Development, the National Institute of Nursing Research (1 R01 HD050738-01A2), and the Harris Foundation to the University of Illinois at Chicago. We also wish to acknowledge the infants and mothers who participated in this research.
Footnotes
CONTRIBUTION OF FINDINGS
- - Prefeeding behavioral state to one appropriate for infant feeding
- - Sucking maturation during the first week of oral feedings
Conflicts of Interest: The material included in this manuscript is original research, has not been previously published and has not been submitted for publication elsewhere while under consideration. There is no competing financial interest in relation to the work described. The authors have nothing to disclose and declare no conflict of interest in any way related to this submission.
References
- 1.Feldman R, Eidelman AI. Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. Pediatrics. 2006;118:e869–e78. doi: 10.1542/peds.2005-2040. [DOI] [PubMed] [Google Scholar]
- 2.Barnard KE, Kelly JF. Assessment of parent-child interaction. In: Meisels SJ, Shonkoff JP, editors. Handbook of early childhood intervention. Cambridge University Press; New York: 1990. pp. 278–302. [Google Scholar]
- 3.Barlow S. Oral and respiratory control for preterm feeding. Curr Opin Otolaryngol Head Neck Surgery. 2009;17:179–86. doi: 10.1097/MOO.0b013e32832b36fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medoff-Cooper B, Bilker W, Kaplan JM. Sucking patterns and behavioral state in 1- and 2-day-old full-term infants. J Obstet Gynecol Neonatal Nurs. 2010;39:519–24. doi: 10.1111/j.1552-6909.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colonnese M. Rapid developmental emergence of stabel depolarization during wakefulness by inhibitory balancing of cortical network excitability. The JOurnal of Neuroscience. 2014;34:5477–85. doi: 10.1523/JNEUROSCI.3659-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White-Traut RC, Nelson MN, Silvestri JM, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Developmental Medicine and Child Neurology. 2002;44:91–7. doi: 10.1017/s0012162201001736. [DOI] [PubMed] [Google Scholar]
- 7.White-Traut RC, Nelson MN, Silvestri JM, Vasan U, Patel M, Cardenas L. Feeding readiness behaviors and feeding efficiency in response to ATVV intervention. Newborn and Infant Nursing Reviews. 2002;2:166–73. [Google Scholar]
- 8.White-Traut R, Rankin K, Pham T, Li Z, Liu L. Premature infants’ orally directed behavioral cues and behavioral state responses to a prefeeding multisensory Intervention. Infant Behavior & Development. 2014;37:583–96. doi: 10.1016/j.infbeh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White-Traut RC, Nelson MN, Silvestri JM, Patel MK, Kilgallon D. Patterns of physiologic and behavioral response of intermediate care preterm infants to intervention. Pediatric Nursing. 1993;19:625–9. [PubMed] [Google Scholar]
- 10.Palmer MM, Crawley K, Blanco IA. Neonatal Oral-Motor Assessment scale: a reliability study. Journal of Perinatology. 1993;13:28–35. [PubMed] [Google Scholar]
- 11.Medoff-Cooper B, Shults J, Kaplan J. Sucking behavior of preterm neonates as a predictor of developmental outcomes. Journal of developmental and behavioral pediatrics : JDBP. 2009;30:16–22. doi: 10.1097/DBP.0b013e318196b0a8. [DOI] [PubMed] [Google Scholar]
- 12.Cunha M, Barreiros J, Goncalves I, Figueiredo H. Nutritive sucking pattern--from very low birth weight preterm to term newborn. Early Human Development. 2009;85:125–30. doi: 10.1016/j.earlhumdev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno K, Ueda A. The maturation and coordination of sucking, swallowing, and respiration in preterm infants. The Journal of pediatrics. 2003;142:36–40. doi: 10.1067/mpd.2003.mpd0312. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta paediatrica. 2000;89:846–52. [PubMed] [Google Scholar]
- 15.White-Traut R, Norr K. An ecological model for premature infant feeding. Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN / NAACOG. 2009;38:478–89. doi: 10.1111/j.1552-6909.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medoff-Cooper BaS J. International Congress of Infant Studies. Lawrence Erlbaum Associates; Toronto Canada: 2002. Feeding Behaviors as an Index of Developmental Outcomes. 2002. [Google Scholar]
- 17.Medoff-Cooper B, Shults J, Kaplan J. Sucking behavior of preterm neonates as a predictor of developmental outcomes. J Dev Behav Pediatr. 2009;30:16–22. doi: 10.1097/DBP.0b013e318196b0a8. [DOI] [PubMed] [Google Scholar]
- 18.McGrath JM, Braescu AV. State of the science: feeding readiness in the preterm infant. The Journal of perinatal & neonatal nursing. 2004;18:353–68. doi: 10.1097/00005237-200410000-00006. quiz 69-70. [DOI] [PubMed] [Google Scholar]
- 19.Medoff-Cooper B. Nutritive sucking. In: Salkind NJ, editor. Encyclopedia of human development. Sage Publications; Thousand Oaks, CA: 2006. [Google Scholar]
- 20.Medoff-Cooper B, Bilker WB, Kaplan JM. Suckling behavior as a function of gestational age: A cross-sectional study. Infant Behavior & Development. 2001;24:83–94. [Google Scholar]
- 21.Hinkelmann KK. O. Design and analysis of experiments. J. Wiley; New York: 1994. [Google Scholar]
- 22.Keppel G. Design and analysis: A researcher's handbook. 2nd ed. Prentice-Hall; Englewood Cliffs, NJ: 1982. [Google Scholar]
- 23.Kirk RE. Experimental design: Procedures for the behavioral sciences. Brooks/Cole; Pacific Grove, CA: 1995. [Google Scholar]
- 24.Burns K, Cunningham N, White-Traut R, Silvestri J, Nelson MN. Infant stimulation: modification of an intervention based on physiologic and behavioral cues. J Obstet Gynecol Neonatal Nurs. 1994;23:581–9. doi: 10.1111/j.1552-6909.1994.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 25.White-Traut RC, Pate CM. Modulating infant state in premature infants. Journal of Pediatric Nursing. 1987;2:96–101. [PubMed] [Google Scholar]
- 26.White-Traut RC, Nelson MN. Maternally administered tactile, auditory, visual, and vestibular stimulation: Relationship to later interactions between mothers and premature infants. Research in Nursing and Health. 1988;11:31–9. doi: 10.1002/nur.4770110106. [DOI] [PubMed] [Google Scholar]
- 27.White-Traut R, Rankin K, Pham T, Li Z, Liu L. Premature infants’ orally directed behavioral cues and behavioral state responses to a prefeeding multisensory intervention. Infant Behavior & Development. 2014;37:583–96. doi: 10.1016/j.infbeh.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adnani F. Suck_Detect Handbook. University of Pennsylvania; Richmond, VA: 2008. [Google Scholar]
- 29.White-Traut R, Rankin K, Lucas R, Shapiro N, Medoff-Cooper B. Evaluating sucking maturation under two pressure thresholds. Early Hum Dev. 2013:89. doi: 10.1016/j.earlhumdev.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson EC, Hobel CJ. POPRAS: A guide to using the prenatal, intrapartum, postpartum record. South Bay Regional Perinatal Project Professional Staff Association; Torrence, CA: 1978. [Google Scholar]
- 31.Medoff-Cooper B. Multi-system approach to the assessment of successful feeding. Acta Paediatrica. 2000;89:393–4. doi: 10.1080/080352500750028050. [DOI] [PubMed] [Google Scholar]
- 32.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatrica. 2008;97:61–7. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gewolb IH, Vice FL, Schwietzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Developmental Medicine and Child Neurology. 2001;43:22–7. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- 34.Medoff-Cooper B, McGrath J, Shults J. Feeding patterns of full term and preterm infants at forty weeks post-conceptional age. Developemental and Behavioral Pediatrics. 2002;23:231–6. doi: 10.1097/00004703-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 35.White-Traut R, Norr K. An Ecological Model for Premature Infant Feeding. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2009;38:478–90. doi: 10.1111/j.1552-6909.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medoff-Cooper B. International Congress of Infant Studies. Lawrence Erlbaum Associates; Toronto, Canada: 2002. Feeding behaviors as an index of developmental outcomes. [Google Scholar]