Abstract
Background
Lignans in plant foods are metabolized by gut bacteria to the enterolignans, enterodiol (END) and enterolactone (ENL). Enterolignans have biologic activities important to the prevention of cancer and chronic diseases. We examined the composition of the gut microbial community (GMC) as a contributor to human enterolignan exposure.
Methods
We evaluated the association between the GMC in stool, urinary enterolignan excretion, and diet from a 3-day food record in 115 premenopausal (ages 40–45 y) women in the United States. Urinary enterolignans were measured using GC/MS. The GMC was evaluated using 454 pyrosequencing of the 16S rRNA gene. Sequences were aligned in SILVA (www.arb-silva.de). Operational taxonomic units (OTU) were identified at 97% sequence similarity. Taxonomic classification was performed and alpha and beta diversity in relationship to ENL production were assessed. Multivariate analysis and regression were used to model the association between enterolignan excretion and the GMC. Bacteria associated with ENL production were identified using univariate analysis and ridge regression.
Results
After adjusting for dietary fiber intake and adiposity, we found a significant positive association between ENL excretion and either the GMC (p=0.0007), or the diversity of the GMC (p=0.01). The GMC associated with high ENL production was distinct (UNIFRAC, p<0.003, MRPP) and enriched in Moryella spp., Acetanaerobacterium spp., Fastidiosipila spp., and Streptobacillus spp.
Conclusion
Diversity and composition of the GMC are associated with increased human exposure to enterolignans.
Impact
Differences in gut microbial diversity and composition explain variation in gut metabolic processes that impact environmental exposures and influences human health.
Keywords: gut microbiome, phytochemical, lignan, diversity
Introduction
Epidemiologic studies have shown that the consumption of foods of plant origin is associated with lower risk of several cancers (1). In particular, the intake of lignans, which are polyphenolic compounds concentrated in woody portions of plants, seed coats, and the bran layer of grains, has been inversely associated with risk of breast (2–7) and colon cancer (8, 9). Lignans are converted by the gut microbiota to enterolignans, which are bioactive chemicals found in measurable quantities in plasma and urine. Evidence from in vitro and in vivo studies suggest that enterolignans possess a variety of biologic activities relevant to human health, including weak estrogenic and anti-estrogenic properties, inhibition of enzymes involved in hormone metabolism, and anti-tumor activities (10). High inter-individual variation in excretion, circulating concentrations, and extent of metabolism of enterolignans exists (11). Dietary factors account for a modest amount of the variation in enterolignan excretion; and often unaccounted for sources of variation include gastrointestinal transit time, sex, and the composition of the gut microbiome (12, 13). We hypothesize that variation in the composition of the microbiome influences the exposure of the host to lignan metabolites and that this may ultimately influence health outcomes.
Several biochemical steps are required to transform plant lignans into enterolignans and each step is likely catalyzed by consortia of bacteria that share metabolic intermediates (14). To date, no one bacteria has been identified that can completely metabolize the plant lignan, secoisolarisiresinol diglucoside (SDG) to ENL. For example, isolated Eggerthella lenta cannot reduce SECO; however, it can dehydroxylate 2, 3-bis-(3, 4-dihydroxy-benzyl) butane-1, 4-diol to END, one of the intermediary steps in ENL production (12, 15). END can then be converted to ENL by different bacteria (16, 17). Several more bacterial groups likely play similarly unique and complex biochemical roles in the transformation of plant lignans to enterolignans (16). Hence, the complexity and diversity of the GMC is essential for maximizing conversion of plant lignans into enterolignans and likely influences human exposure to these bacterial compounds. The objective of this study was to evaluate the association between GMC and urinary enterolignan excretion in a well-characterized group of premenopausal women.
Material and Methods
Research design and study participants
This observational study was conducted in premenopausal women who were part of a larger study designed to evaluate the relationship between bacterial metabolic phenotypes, diet, and biomarkers of sex steroid hormone status (18). Of the 203 women in the parent study, 120 collected a fecal sample. Of the 120 women that donated fecal samples, 116 filled out a 3-day food record (3-DFR) and 101 of the samples were taken within 1 month of the 3DFR. One woman who provided a stool sample and 3DFR did not have an ENL measurement. Although all participants were premenopausal, with normal menstrual cycles, we did not collect urines in conjunction with time in menstrual cycle. Previous work by Lampe et al (19) showed no difference in enterodiol or enterolactone by phase of cycle in a carefully controlled study of flaxseed supplementation. The aims of this study are addressed in this subset of women. The women were recruited from Group Health, a large integrated health plan in Western Washington, and were eligible to participate if they were 40–45 years and had undergone a screening mammogram in the last 10 months (18). Women were excluded if they had more than one prescription for hormone therapy (i.e., oral contraceptives) within 18 months of the sampling date; had any history of breast cancer; had breast implants; had a hysterectomy or oophorectomy; used tamoxifen or raloxifene; had any diagnosis of gastrointestinal disorders or gastrointestinal surgeries 10 years before their mammogram; or if they had prescriptions for antibiotics, bisphosphonates, or corticosteroids within 3 months of their sampling date. All study parameters were approved by the FHCRC and Group Health IRB and all participants provided written informed consent.
Specimen and data collection
Participants completed a health and demographics questionnaire and recorded all food and drink consumed for 3 consecutive days (18). Completed food record booklets were submitted and dietary intake was analyzed for nutrient content. Body composition (% adiposity) was measured using dual energy X-ray absorptiometry (DXA; Hologic Delphi, Hologic Inc. Bedford, MA). The fecal sample was collected in RNAlater (Ambion, Austin, TX) using a method described previously (20). A protocol was developed to allow participants to collect samples in the privacy of their own home. The stool sample was collected in a plastic tub and a portion scooped directly into a collection tube containing approximately 5 mls of RNAlater and glass beads. The sample was shaken vigorously to enhance dispersal of the stool in the preservative. Specimens were immediately brought to FHCRC where they were frozen at −80 °C until further analysis. Morning first void urines were collected from the participants and frozen at −80 °C upon receipt until further analysis.
Urinary lignan analysis
The first-void urine samples were analyzed for the enterolignans, END and ENL, by GC-MS, with deuterated internal standards (21). All enterolignan measurements were adjusted for creatinine concentration to account for urine dilution. The lowest level of quantification (LOQ) for END and ENL in 2 ml urine was 70 μg/l.
Gut microbial community analysis
DNA extraction
DNA was extracted from stool that had been stored in RNAlater at −80°C (20). The 16S rRNA gene was amplified and sequenced using 454 pyrosequencing primers 27f and 519r (V1–V3) (22) for amplicon pyrosequencing (bTEFAP) (23–27) at Research and Testing (Shallowater, TX) using Roche 454 FLX titanium instruments and reagents and following manufacturer’s guidelines. Sequences have been deposited in the Sequence Read Archive of NCBI under accession number SRP028900.
16S rRNA gene sequencing and curation
Sequences were compiled and processed using MOTHUR (v.1.28.0) (28). Sequences were converted to standard FASTA format from .sff files. Sequences were removed if they were < 300 bp, had homopolymers > 8 bp, more than one mismatch to the forward primer, more than 1 mismatch to the barcode, or ambiguous bases. Sequences were denoised (29), and aligned to the Silva 16S rRNA gene reference alignment (www.arb-silva.de) using the NAST algorithm (28, 30, 31). Sequences that did not align to the appropriate 16S rRNA gene region were removed. Low abundance sequences were merged to the high abundant sequences using the pre.cluster option in MOTHUR to minimize the effect of pyrosequencing errors in overestimating microbial diversity (32). Potentially chimeric sequences were removed using ChimeraSlayer (33, 34).
Analysis of the microbiome
Sequences were clustered into OTUs at 97% similarity based on the average neighbor-joining algorithm. The sequences were classified using the naive Bayesian Classifier trained against a RDP training set as implemented in MOTHUR (27). Classified sequences were assigned to phylum and genus-level phylotypes (35) to characterize the community structure. To characterize the alpha diversity, we used OTUs rarefied to 1265 sequences per sample since uneven sampling depth biases diversity estimates. Diversity of the microbial community within an individual (alpha diversity), was calculated from OTUs (at 3% divergence) using the inverse Simpson’s index (36). Similarity in the GMC between individuals (beta diversity) was calculated using the Theta YC (ΘYC) distance metric which accounts for shared and unshared OTU’s between (37), and weighted and unweighted UniFrac (38, 39). The UniFrac approaches create a distance metric based upon a combined phylogenetic tree and compares which branches the two individuals have in common. We used a relaxed Neighbor-Joining algorithm implemented in Clearcut (40) to generate the phylogenetic trees.
Statistical analysis
Anthropometrics, demographics, dietary and lifestyle factors
The association of ENL and END excretion with anthropometric measurements, demographics, and dietary and lifestyle factors was calculated using linear regression.
Microbiome data cleaning
Bacterial taxa were removed if they represented less than 0.08% of the total sequences in a sample based on empirical data (background was 4 sequences/10,000 and 2x background) and appeared in 20 % or more of the subjects as also established in the literature (41–43). The number of sequences in each genera was converted to relative percent of the total sequence abundance per individual for multivariate analysis.
Multivariate analysis
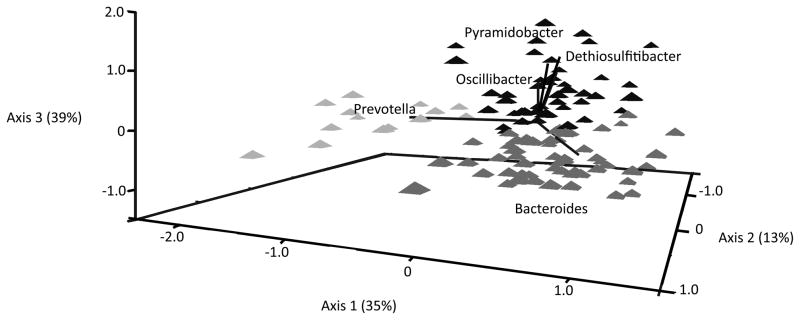
Distance estimates between each pair of samples were calculated using the Jensen-Shannon Divergence (44, 45). Data dimension reduction was performed using non-metric multidimensional scaling (NMS) on the matrix of JSD distances generated between each pair of samples (44, 45). A joint plot was used to visualize the relative strength and magnitude of the association between each genera and the NMS axes (r2> 0.49) (46) (Figure 1). To model the variation in ENL excretion and the microbiome, the NMS axes used to describe the microbial community were included in separate models of the GMC (Axis 1, Axis 2, or Axis 3) and anthropometric, dietary, and lifestyle factors. Three models incorporating each NMS axes separately and using urinary ENL as the response variable were investigated.
Figure 1.
The characterization of the gut microbial community in a cross-sectional analysis of premenopausal women. The first three axes of the NMS analysis of the gut microbial community accounts for up to 85% of the variation in the data. The vectors radiating from the centroid and overlain on the NMS plot represent the relative association of the genera of bacteria and the axes (r2>0. 49) and the magnitude of the association.
 Cluster 1 (Bacteroides);
Cluster 1 (Bacteroides);
 Cluster 2 (Prevotella,); ▲ Cluster 3 (Dethiosulfitibacter, Pyramidobacter, Oscillibacter)
Cluster 2 (Prevotella,); ▲ Cluster 3 (Dethiosulfitibacter, Pyramidobacter, Oscillibacter)
Identification of bacterial enterotypes
To investigate whether the gut microbiome of our study participants clustered by enterotype, the Jensen-Shannon Divergence (JSD) (44) was computed on pairs of samples followed by clustering using partitioning around medoids (PAM) clustering. Optimal numbers of clusters were determined using the Calinski-Harabasz (CH) index (47).
Identification of bacterial genera associated with ENL production
To reveal which, if any, taxa were associated with ENL production, we used two regression approaches. The first approach was a univariate regression model where each taxon was considered individually. The second approach was a penalized regression approach where all of the taxa were considered simultaneously (48) (see Supplementary Data). OTUs associated with ENL production using both regression approaches were considered the most parsimonious.
Microbial diversity and ENL production
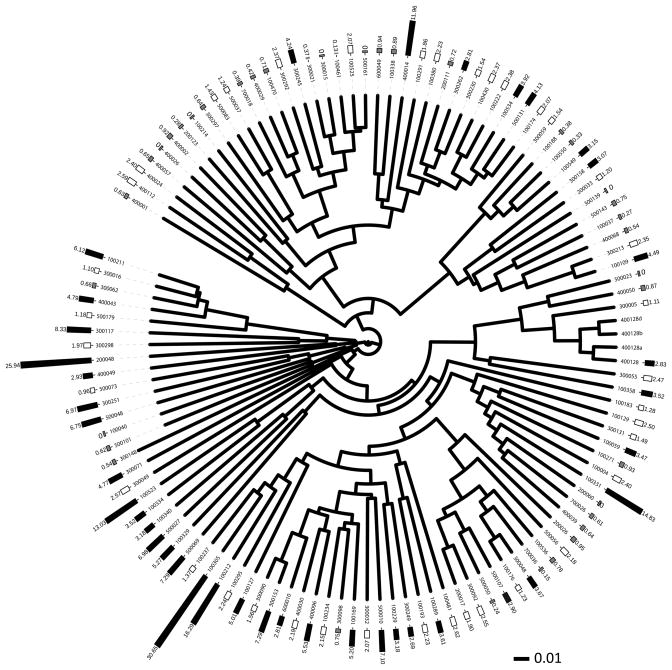
ANOVA was used to test the null hypothesis that there was no statistical difference in the alpha diversity of the GMC between high or low ENL excreters based on tertiles of excretion. Multiple response permutation procedure (MRPP) was used to test the null hypothesis that there was no statistical difference in the composition of the GMC between high or low ENL excreters based on tertiles of excretion. Differences in the GMC by tertiles of ENL excretion were visualized by cluster analysis from a Θyc based distance matrix and clustered using Unweighted Pair Group Method with Arithmetic Mean (UPGMA) (Figure 2).
Figure 2.
The composition of the gut microbial community is significantly different between high and low enterolactone (ENL) excreters (MRPP; P<0.001). Cluster analysis of beta diversity (Θyc) of the microbiome in tertiles of ENL excretion. Bars are the amount of urinary ENL (μg/mg creatinine) color –coded for tertile. (Low = Light gray, Medium = white and High = black). The three samples without bars are technical replicates.
Statistical analyses were implemented in PC-ORD version 6.14, vegan (49), and R version 3.1 and visualized using iTOL 2.1 (50).
Results
Anthropometrics, demographics, diet, and phenotype
The mean age of participants (n=115) was 42 years, and the majority were white, with at least some college education (Table 1). All women had urinary ENL concentrations above the LOQ (>70 μg/l); mean (± SD) urinary ENL was 3.08 ± 4.44 μg/mg creatinine. 58 participants had urinary END concentrations above the detection limit (>70 μg/l); mean END in these women was 0.23 ± 0.53 μg/mg creatinine (Table 1). Between the highest and lowest tertiles of ENL excretion, there were significant decrease in BMI (p<0.003) and adiposity (p<0.004), and a significant increase in dietary fiber intake (p=0.0008), education (p=0.03), and self-reported frequency of diarrhea (p=0.03) (Table 1). Significant increases in dietary fiber intake (p<0.002), ENL excretion (p=0.003), and education (p=0.0004) were observed between participants above and below the LOQ for urinary END (data not shown).
Table 1.
Study participant anthropometrics, demographics, and diet and lifestyle factors by tertiles of ENL excretion.
| 1st tertile n = 38 |
2nd tertile n = 38 |
3rd tertile n = 39 |
p.value | |
|---|---|---|---|---|
| Continuous Descriptive Statistics, Mean (SD) | ||||
| Urinary ENL (μg/mg creatinine) (ENL) | 0.46 (0.37) | 1.86 (0.91) | 5.66 (4.52) | NA |
| Urinary END (μg/mg creatinine) (END) | 0.06 (0.13) | 0.09 (0.13) | 0.56 (1.33) | 0.35 |
| Age (y) | 42.49 (1.41) | 42.05 (1.35) | 42.59 (1.32) | 0.78 |
| BMI (kg/m2) | 27.10 (4.18) | 25.61 (4.56) | 24.82 (5.2) | 0.003 |
| Adiposity (% fat) | 35.33 (5.68) | 34.82 (7.19) | 30.85 (7.07) | 0.004 |
| Energy (kcal/d) | 1937.22 | 1894.27 | 1956.14 | 0.84 |
| Carbohydrate (g/d) | 230.18 (67.32) | 220.59 | 245.65 (62.93) | 0.38 |
| Protein (g/d) | 78.02 (19.54) | 76.57 (15.81) | 79.07 (19.73) | 0.81 |
| Fat (g/d) | 77.09 (25.22) | 76.44 (19.85) | 72.24 (21.2) | 0.32 |
| Dietary fiber (g/d) | 18.51 (6.4) | 18.12 (6.1) | 24.40 (8.87) | 0.0008 |
| Bowel Movements (n/week) | 8.76 (4.83) | 7.14 (4.11) | 7.22 (2.5) | 0.59 |
| Categorical Descriptive Statistics, n(%) | ||||
| Education, n (%) | ||||
| ≤ 12 y | 1 (2.7%) | 4 (11.11%) | 1 (2.7%) | |
| 13–16 y | 26 (70.27%) | 20 (55.56%) | 15 (40.54%) | |
| 17+ y | 10 (27.03%) | 12 (33.33%) | 21 (56.76%) | 0.03 |
| Residence at Birth, n (%) | ||||
| Rural | 9 (25.71%) | 6 (18.75%) | 13 (34.21%) | |
| Not Rural | 26 (74.29%) | 26 (81.25%) | 25 (65.79%) | 0.34 |
| Ethnicity, n (%) | ||||
| White | 28 (82.35%) | 29 (90.62%) | 34 (94.44%) | |
| Asian | 4 (11.76%) | 2 (6.25%) | 0 (0%) | |
| Other | 2 (5.88%) | 1 (3.12%) | 2 (5.56%) | 0.29 |
| Breast Fed as an infant, n (%) | ||||
| Yes | 17 (58.62%) | 12 (42.86%) | 20 (58.82%) | |
| No | 12 (41.38%) | 16 (57.14%) | 14 (41.18%) | 0.37 |
| Self-Reported IBS, n (%) | ||||
| Yes | 2 (5.71%) | 3 (9.09%) | 3 (7.89%) | |
| No | 33 (94.29%) | 30 (90.91%) | 35 (92.11%) | 0.90 |
| Diarrhea, n (%) | ||||
| Yes | 15 (42.86%) | 12 (36.36%) | 6 (15.79%) | |
| No | 20 (57.14%) | 21 (63.64%) | 32 (84.21%) | 0.03 |
| Laxative Use, n (%) | ||||
| Yes | 3 (8.57%) | 0 (0%) | 1 (2.63%) | |
| No | 32 (91.43%) | 33 (100%) | 37 (97.37%) | 0.21 |
| Constipation, n (%) | ||||
| Yes | 7 (20%) | 12 (36.36%) | 10 (26.32%) | |
| No | 28 (80%) | 21 (63.64%) | 28 (73.68%) | 0.35 |
Enterolactone, ENL; enterodiol, END, irritable bowel syndrome, IBS.
Evaluation of gut microbial community
Using 454 pyrosequencing of the V1–V3 region, a total of 1.4 million raw sequences were processed. The resulting pool of 644,956 sequences, which averaged 5201 ± 2741 sequences per participant, was analyzed. The sequences were, on average, 367 ± 29 bp long. The trimmed sequences represented a total of 342 bacterial genera (phylotypes), of which, 133 OTUs met the microbiome data cleaning criterion (see Methods). We used this multivariate matrix containing 133 OTU’s for statistical analysis.
Bacteria were distributed across phyla: Firmicutes (68%), Bacteroidetes (27%), Proteobacteria (2%), Synergistes (1.0%), Actinobacteria (0.5%), Fusobacterium (0.4%), Verrucomicrobia (0.02%), Ternicutes (0.1%), and Lentisphaerae (0.05%). Good’s coverage was 0.996 ± 0.003.
NMS on the matrix of JSD distances was used to describe the microbiome associated with variation in ENL excretion (44, 51, 52). The final solution for NMS analysis of the GMC patterns had a stable stress value of 13.32, after 400 iterations using a random seed of 2564. The 3 axes cumulatively accounted for 87 % of the variation in the GMC measured using 16S rRNA gene data; Axes 1, 2, and 3 accounted for 35, 13, and 39 %, respectively (Figure 1). Bacteroides was negatively correlated with Axis 3 (r = −0.79), Prevotella was negatively correlated with Axis 1 (r=−0.82), and Oscillibacter (r= 0.94), Dethiosulfatibacter (r=0.86), and Pyramidobacter (r= 75) were positively correlated with Axis 3 (Figure 1). We also identified three clusters in the microbial community (Figure 1). Each cluster was subsequently observed to be associated with different dominant microbial genera: 1) Bacteroides, 2) Prevotella, or 3) a combination of Pyramidobacter, Dethiosulfatibacter, and Oscillibacter (Supplementary Figure S1, S2, and S3). The distribution of the relative percent of these groups showed discrete grouping for some of the dominant genera (Supplementary Figure S3). The NMS axes were used in regression models to relate the gut microbial community to ENL excretion and dietary intake.
We fit a linear regression model for the association of ENL excretion with NMS axis 1 (JSD), adiposity, and fiber (tertiled, using the third tertile as reference) and adjusted for calories (n=101). ENL excretion was significantly positively associated with GMC described by NMS axis 1 (p=0.0007), and fiber adjusted for energy intake (p=0.02), and significantly negatively associated with % adiposity (p=0.02). ENL production was also significantly positively associated with microbial alpha diversity (p=0.01), and fiber intake adjusted for energy intake (p=0.01). In contrast, there was no significant association between END excretion and the GMC composition or diversity (data not shown).
To reveal individual OTU that were associated with ENL, we additionally considered two regression approaches. Using either univariate or Ridge regression, we identified four bacterial genera, Moryella (53), Acetanaerobacterium (54), Fastidiosipila (55), and Streptobacillus (56, 57) that were significantly increased in the high ENL excreter tertile (see Supplementary Data). Genera associated with ENL excretion represented between 0.03 and 0.7 % of the total microbiome (Table 2; see Supplemental Material for more details).
Table 2.
List of genera whose abundances exhibit an association with ENL excretion in 115 women. The list includes OTU that exhibit an association with ENL by way of a confidence interval that does not include zero in either a univariate model (upper part of table) or a multivariate model (lower part of table); four OTUs appear in both. Underlined genera were common to both methods.
| N1 | OTU2 | Low | High | |
|---|---|---|---|---|
| Univariate Estimates | ||||
| Acetanaerobacterium | 100 | 0.1850 | 0.0006 | 0.0063 |
| Acetitomaculum | 29 | 0.0190 | 0.0006 | 0.0069 |
| Acetivibrio | 99 | 0.3990 | 0.0004 | 0.0063 |
| Bacteroides | 115 | 13.7820 | −0.0064 | −0.0002 |
| Cerasibacillus | 43 | 0.1610 | 0.0006 | 0.0221 |
| Dethiosulfatibacter | 76 | 1.1360 | 0.0039 | 0.0113 |
| Ethanoligenens | 64 | 0.0660 | 0.0016 | 0.0101 |
| Eubacterium | 84 | 0.1010 | 0.0016 | 0.0103 |
| Fastidiosipila | 109 | 0.3420 | 0.0007 | 0.0099 |
| Holdemania | 74 | 0.0440 | −0.0200 | −0.0012 |
| Moryella | 68 | 0.0320 | 0.0055 | 0.0147 |
| Pseudobutyrivibrio | 115 | 9.6760 | −0.0071 | −0.0021 |
| Pyramidobacter | 81 | 0.7040 | 0.0018 | 0.0117 |
| Reichenbachiella | 77 | 0.5940 | 0.0002 | 0.0067 |
| Ruminococcus | 96 | 0.2900 | 0.0010 | 0.0083 |
| Sarcina | 27 | 0.0370 | 0.0011 | 0.0064 |
| Sedimentibacter | 56 | 0.0380 | 0.0029 | 0.0101 |
| Streptobacillus | 70 | 0.3260 | 0.0000 | 0.0137 |
| Synergistes | 26 | 0.1000 | 0.0006 | 0.0044 |
| Victivallis | 38 | 0.1010 | 0.0015 | 0.0140 |
| Ridge Regression | ||||
| Acetanaerobacterium | 100 | 0.185 | 0.0009 | 0.0076 |
| Butyricimonas | 61 | 0.112 | 0.0019 | 0.0076 |
| Clostridium | 77 | 0.072 | 0.0006 | 0.0066 |
| Coprococcus | 115 | 10.836 | 0.0093 | 0.0518 |
| Fastidiosipila | 109 | 0.342 | 0.0001 | 0.0101 |
| Moryella | 68 | 0.032 | 0.0001 | 0.0010 |
| Oscillibacter | 115 | 2.934 | 0.0015 | 0.0388 |
| Prevotella | 54 | 0.639 | 0.0002 | 0.0421 |
| Sharpea | 29 | 0.161 | 0.0016 | 0.0060 |
| Streptobacillus | 70 | 0.326 | 0.0036 | 0.0174 |
Number of subjects for whom a non-zero OTU abundance was observed
Median of the relative abundance of each OTU (*100)
Bacterial diversity was positively associated with high ENL excretion. The bacterial alpha diversity, a measure of the variation of the composition of the GMC within a person, was significantly different between women in ENL excreter groups (Inverse Simpson’s Index, ANOVA, F=12.90, n=115, p<0.0001). More specifically, there was a significant difference in the alpha diversity between low and high (Tukey’s, q=6.5, p<0.0001) excreters. Beta diversity, a comparison of bacterial diversity between subjects, was significantly different in women in the low ENL group as compared to those the high ENL group using weighted Unifrac, which normalizes for the number of sequences in an OTU cluster (MRPP; A=0.014, p=0.003; 999 permutations), unweighted Unifrac (MRPP; A=0.005, p=0.001; 999 permutations) or using the Θyc (MRPP, A=0.02, p<0. 001; 999 permutations) as visualized in Figure 2.
Discussion
In this cross-sectional study, we evaluated differences in GMC in relation to lignan metabolizing-phenotypes. We found that GMC differed by tertile of ENL but not END excretion. GMC diversity increased with greater ENL excretion. We identified components of the microbiome associated with excretion of ENL. These data suggest that the environmental exposures from dietary intake can be altered by the metabolic capacity of the more minor components of the gut microbiome to influence health outcomes.
Pharmacokinetic studies have shown a wide variation in enterolignan excretion, in both magnitude and time of excretion (13). These excretion patterns have been shown to vary within different human populations. In our study, we found a wide range of excretion in both ENL and END (Table 1). END, an intermediate compound in the conversion of some plant lignans to ENL, was 10 fold lower than ENL in our study participants. Enterolignan excretion (END+ENL) ranged from 0 to 30 μg/mg creatinine. These ranges encompass or are higher than other study populations consuming a predominantly western diet (58–60).
The metabolism of lignans in the gut occurs by a consortia of microorganisms through a series of reactions (61–65) and can result in a measurable amount of bacterial metabolites in host systemic circulation. There was a significant difference in the composition of the microbiome between the highest and lowest tertiles of ENL excretion (Figure 2). Genera representing median values between 0.03% and 0.34% of the microbiome were associated with ENL production (Table 2). While each bacterial population may represent a minor component of the microbiome, when considered across the sum of the entire metabolic transformation from plant lignans to enterolignans, they had a measurable impact on ENL excretion. These findings are in keeping with those of Clavel et al. (12) who reported that in vitro lignan degradation was associated with minor components (<1%) of the gut microbiome.
In humans, the microbiome plays an essential role in the catabolism of dietary fibers since the human genome does not encode the range of enzymes required to degrade the biochemical structural diversity found in plant materials. We found that microbial diversity was significantly different (Simpson Index, p<0.05) between high and low ENL excreters and was positively associated with fiber intake. The association between ENL excretion and microbial diversity may reflect the complexity of the microbial metabolism involved in ENL metabolism since there are several transformations involved in the production of enterolignans from lignans and different bacteria species can catalyze each step (15–17, 66–68). In a recent study comparing human populations consuming either a western diet or a rural sub-Saharan traditional diet, dietary fiber intake was also associated with gut microbial diversity (69). The association between intake of dietary fiber as plant material and gut microbiome diversity may not be apparent because most studies of the human microbiome have been conducted in human populations consuming a western diet, which is traditionally low in fiber. Furthermore, microbial functional gene diversity may be associated with long term dietary patterns that include a high fruit and vegetable intake and therefore a higher fiber intake (70). In addition to being associated with a beneficial phenotype, high microbial diversity associated with dietary fiber intake may provide a key to maintaining resilience of the host to infection and other environmental impacts. For example, dietary patterns that were associated with decreased microbiome structural and functional diversity have been associated with obesity (70), reduced cognitive function in the elderly (41), Clostridium difficile-associated disease (CDAD) (71), and irritable bowel disease (IBD) (26).
Dietary intake of lignans is a major factor that influences variation in urinary ENL. A high plant-food diet, rich in whole grains, nuts, seeds and fruits, and vegetables, has been associated with higher production of enterolignans (72). We found that dietary fiber intake was significantly associated with ENL production (Table 1). This has been observed in previous studies and supports the findings that lignan content and dietary fiber content of foods are often highly correlated (73–75). In-vitro incubations have also shown that the type of fiber may influence ENL production (62). Insoluble fiber includes lignan-rich categories, such as seed coats and bran layers. Higher rates of ENL production were associated with insoluble fiber in other cross-sectional studies (76).
Obesity in adults has been linked to the gut microbiome and the altered functional potential of the obesidogenic microbiome influences myriad negative health outcomes (77). Consistent with previous studies (78, 79), we found that adiposity was inversely associated with ENL production even after controlling for fiber intake. Kilkkinen et al (79) found an inverse association between ENL production and BMI. More specifically they found that normal weight women produced significantly higher ENL than their underweight or obese counterparts. Frankenfeld (11) found that overweight and obese individuals were less likely to excrete high levels of ENL. This association was potentially stronger in women than in men. In our study, the inverse association between adiposity and ENL production could reflect the lower intake of high-fiber foods (Table 1) and therefore a lower intake of plant lignans by women with higher percent body fat.
Although ENL is produced by bacterial consortia, identification of the bacteria involved in ENL production has previously been based on isolation of a pure culture of the organism associated with a specific part of the metabolic pathway (16, 67, 80). We found four bacterial genera (Moryella, Streptobacillus, Fastidiosipila, and Acetanaerobacterium) associated with the high ENL producers (Table 2). Although they have not been identified before in ENL production (81), the bacteria we identified are closely related to genera that have been previously associated with the bacterial metabolism of lignans. For example, the initial production of secoisolariciresinol from secoisolariciresinol diglucoside is associated with glucosidase activity. Bacteria related to the genera Streptobacillus produce extracellular enzymes involved in glucose cleavage from complex molecules (56, 57) as do the genera Fastidiosipilia (55). Once these sugars are made available, members of the genera Acetanaerobacterium and Moryella are able to ferment glucose to acetate or butyrate (53, 54). Also, Clostridia closely related to Oscillibacter are involved in anaerobic ring cleavage and ring cleavage of methoxylated compounds (82, 83).
Diet can influence the gut microbiome in humans (70, 77, 84–87). Enterotypes dominated by Bacteroides (Enterotype 1) or Prevotella (Enterotype 2) have been associated with diets rich in fats and carbohydrates (52, 88, 89). In our study of healthy women, the bacterial composition of groups 1 and 2 is similar to previous findings (52) although the composition of a third group of bacteria varied from other published report (Figure 1; Supplementary Figure S2) (90–92). Enterotype groupings have been identified in several studies, but studies have found either fewer than three enterotypes (87) or no pattern (93) when considering adults consuming western diets. A classification system based upon functional genes instead of the dominant members of the gut microbiome may be more appropriate since enterotypes do not necessarily reflect the complexity of metabolism catalyzed by microbial consortia involved in phytochemical metabolism that influences health (52, 94).
This study has several strengths. This study was conducted in a sample of premenopausal women selected originally for a study of isoflavone metabolism and hormonal factors (18). As a result, factors that could affect GMC (e.g., antibiotic use) were considered in participant selection. Dietary intake was measured using a3DFR rather than relying on a food frequency questionnaire. Body fat was measured using DXA, providing a more accurate measure of adiposity. High throughput sequencing was used to characterize the GMC, which given that ENL is produced by metabolic consortia of bacteria, was able to capture the complexity of the GMC-ENL association.
Our study has some weaknesses. Most of the women recruited in this study were white, well-educated and were recruited to achieve a wide range of breast density. Therefore, the findings may not be applicable to the general population. However, despite this, the ranges of enterolignan excretion encompass or were slightly higher to those found in predominantly white populations and data associated with lignan metabolism by gender is equivocal (59, 60, 73, 74, 95, 96). Dietary data were not collected at the same time as the urine for enterolignan phenotyping or as the stool sample. However, to optimize sample size and minimize lag time in sampling we excluded participants whose 3DFR was taken greater than one month apart from stool samples. Our study is also limited by the fact that participants were consuming their habitual diets which contributed to variation in the amounts and types of plant lignans consumed and therefore also contributed to the variation in END and ENL excretion.
The gut microbiome can influence the magnitude and flux of dietary metabolites to which the host is exposed. Major bacterial parameters to consider in enterolignan bioavailability include how dietary fiber influences diversity, community composition, and functional activity of the microbiome that appears to be altered in the metabolic phenotypes studied here. We observed differences in GMC in relation to ENL excretion in a group of premenopausal women. Bacterial diversity and community structure were significantly associated with ENL excretion and we identified several bacterial groups newly associated with in-vivo ENL production. Future studies of the microbial response to diet will further our understanding of how environmental exposures may be altered by the gut microbiome and influence health outcomes.
Supplementary Material
Acknowledgments
Supported by US NIH grants R01 CA097366 and U01 CA162077, Kellogg Corporate Citizens Fund, and Fred Hutchinson Cancer Research Center.
Grant Support
This work was supported by NIH grants R01 CA97366 (J. W. Lampe, PI), U01 CA63731 (E. White, PI), U54 CA116847 (A. McTiernan, PI), and R03 CA115209 (J. W. Lampe, PI).
Footnotes
Potential conflicts of Interests: None
References
- 1.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 2.Buck K, Zaineddin aK, Vrieling A, Heinz J, Linseisen J, Flesch-Janys D, et al. Estimated enterolignans, lignan-rich foods, and fibre in relation to survival after postmenopausal breast cancer. Br J Cancer. 2011;105:1151–7. doi: 10.1038/bjc.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. 2010;92:141–53. doi: 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmini P, Rubagotti A, Boccardo F. Serum enterolactone levels and mortality outcome in women with early breast cancer: a retrospective cohort study. Breast Cancer Res Treat. 2012;132:661–8. doi: 10.1007/s10549-011-1881-8. [DOI] [PubMed] [Google Scholar]
- 5.McCann SE, Hootman KC, Weaver AM, Thompson LU, Morrison C, Hwang H, et al. Dietary intakes of total and specific lignans are associated with clinical breast tumor characteristics. J Nutr. 2012;142:91–8. doi: 10.3945/jn.111.147264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touré A, Xueming X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr Rev Food Sci Food Saf. 2010;9:261–9. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Velentzis LS, Cantwell MM, Cardwell C, Keshtgar MR, Leathem aJ, Woodside JV. Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer. 2009;100:1492–8. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotterchio M, Boucher Ba, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–53. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuijsten A, Arts IC, Hollman PC, van’t Veer P, Kampman E. Plasma enterolignans are associated with lower colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1132–6. doi: 10.1158/1055-9965.EPI-05-0991. [DOI] [PubMed] [Google Scholar]
- 10.Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer. 2005;51:117–31. doi: 10.1207/s15327914nc5102_1. [DOI] [PubMed] [Google Scholar]
- 11.Frankenfeld CL. Relationship of obesity and high urinary enterolignan concentrations in 6806 children and adults: analysis of National Health and Nutrition Examination Survey data. Eur J Clin Nutr. 2013;67:887–9. doi: 10.1038/ejcn.2013.107. [DOI] [PubMed] [Google Scholar]
- 12.Clavel T, Henderson G, Engst W, Dore J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol Ecol. 2006;55:471–8. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuijsten A, Arts IC, Vree TB, Hollman PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. 2005;135:795–801. doi: 10.1093/jn/135.4.795. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wähälä K, Deyama T, et al. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem. 2001;49:3178–86. doi: 10.1021/jf010038a. [DOI] [PubMed] [Google Scholar]
- 15.Jin J-S, Zhao Y-F, Nakamura N, Akao T, Kakiuchi N, Min B-S, et al. Enantioselective dehydroxylation of enterodiol and enterolactone precursors by human intestinal bacteria. Biol Pharm Bull. 2007;30:2113–9. doi: 10.1248/bpb.30.2113. [DOI] [PubMed] [Google Scholar]
- 16.Clavel T, Lippman R, Gavini F, Dore J, Blaut M. Clostridium saccharogumia sp nov and Lactonifactor longoviformis gen. nov., sp nov., two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside. Syst Appl Microbiol. 2007;30:16–26. doi: 10.1016/j.syapm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Jin JS, Hattori M. Human Intestinal Bacterium, Strain END-2 is responsible for demethylation as well as lactonization during plant lignan metabolism. Biol Pharm Bull. 2010;33:1443–7. doi: 10.1248/bpb.33.1443. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson C, Newton KM, Bowles EJ, Yong M, Lampe JW. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am J Clin Nutr. 2008;87:679–87. doi: 10.1093/ajcn/87.3.679. [DOI] [PubMed] [Google Scholar]
- 19.Lampe JW, Martini MC, Kurzer MS, Adlercreutz H, Slavin JL. Urinary lignan and isoflavonoid excretion in premenopausal women consuming flaxseed powder. Am J Clin Nutr. 1994;60:122–8. doi: 10.1093/ajcn/60.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Hullar MA, Schwarz Y, Lampe JW. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J Nutr. 2009;139:1685–91. doi: 10.3945/jn.109.108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson C, Lampe JW, Scholes D, Chen C, Wähälä K, Schwartz SM. Lignan and isoflavone excretion in relation to uterine fibroids: a case-control study of young to middle-aged women in the United States. Am J Clin Nutr. 2006;84:587–93. doi: 10.1093/ajcn/84.3.587. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Hullar MA, Lampe JW. Optimization of terminal restriction fragment polymorphism (TRFLP) analysis of human gut microbiota. J Microbiol Methods. 2007;68:303–11. doi: 10.1016/j.mimet.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowd SE, Sun Y, Wolcott RD, Domingo A, Carroll JA. Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog Dis. 2008;5:459–72. doi: 10.1089/fpd.2008.0107. [DOI] [PubMed] [Google Scholar]
- 24.Callaway TR, Dowd SE, Wolcott RD, Sun Y, McReynolds JL, Edrington TS, et al. Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult Sci. 2009;88:298–302. doi: 10.3382/ps.2008-00222. [DOI] [PubMed] [Google Scholar]
- 25.Wickham ME, Lupp C, Vazquez A, Mascarenhas M, Coburn B, Coombes BK, et al. Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 2007;9:400–7. doi: 10.1016/j.micinf.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterol. 2010;139:1844–54. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-Source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quince C, Lanzen A, Davenport RJ, Turnbaugh PJ. Removing Noise From Pyrosequenced Amplicons. BMC Bioinformatics. 2011:12. doi: 10.1186/1471-2105-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schloss PD. Secondary structure improves OTU assignments of 16S rRNA gene sequences. ISME J. 2013;7:457–60. doi: 10.1038/ismej.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–96. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol. 2010;12:1889–98. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M, Morrison M, Yu Z. Evaluation of different partial 16S rRNA gene sequence regions for phylogenetic analysis of microbiomes. J Microbiol Methods. 2011;84:81–7. doi: 10.1016/j.mimet.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Magurran A. Measuring biological diversity. Oxford, UK: Blackwell; 2004. [Google Scholar]
- 37.Yue JC, Clayton MK. A similarity measure based on species proportions. Commun Stat-Theor M. 2005;34:2123–31. [Google Scholar]
- 38.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–35. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73:1576–85. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheneman L, Evans J, Foster JA. Clearcut: a fast implementation of relaxed neighbor joining. Bioinformatics. 2006;22:2823–4. doi: 10.1093/bioinformatics/btl478. [DOI] [PubMed] [Google Scholar]
- 41.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108 (Suppl 1):4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet. 2012;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu ZJ, et al. Advancing Our Understanding of the Human Microbiome Using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Endres DM, Schindelin JE. A new metric for probability distributions. IEEE Trans Info Theory. 2003;49:1858–60. [Google Scholar]
- 45.Kruskal JB. Nonmetric multidimensional scaling:a numerical method. Psycometrika. 1964;29:115–29. [Google Scholar]
- 46.McCune B, Grace JB. Analysis of ecological communities. 1. Glendenen Beach, OR: MjM Software; 2002. [Google Scholar]
- 47.Calinski TaH, Jl A dendrite method for cluster analysis. Comm Stat. 1974;3:1–27. [Google Scholar]
- 48.Brumback BA, Ruppert D, Wand MP. Variable selection and function estimation in additive nonparametric regression using a data-based prior - Comment. J Am Stat Assoc. 1999;94:794–7. [Google Scholar]
- 49.Dixon P. VEGAN, a package of R functions for community ecology. Journal of Vegetation Science. 2003;14:927–30. [Google Scholar]
- 50.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39:W475–8. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCune B, Grace JB. Analysis of Ecological Communities. Gleneden Beach, OR: MjM Software Design; 2002. [Google Scholar]
- 52.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlier JP, K’Ouas G, Han XY. Moryella indoligenes gen. nov., sp. nov., an anaerobic bacterium isolated from clinical specimens. Int J Syst Evol Microbiol. 2007;57:725–9. doi: 10.1099/ijs.0.64705-0. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Dong X. Acetanaerobacterium elongatum gen. nov., sp. nov., from paper mill waste water. Int J Syst Evol Microbiol. 2004;54:2257–62. doi: 10.1099/ijs.0.63212-0. [DOI] [PubMed] [Google Scholar]
- 55.Falsen E, Collins MD, Welinder-Olsson C, Song Y, Finegold SM, Lawson PA. Fastidiosipila sanguinis gen. nov., sp. nov., a new Gram-positive, coccus-shaped organism from human blood. Int J Syst Evol Microbiol. 2005;55:853–8. doi: 10.1099/ijs.0.63327-0. [DOI] [PubMed] [Google Scholar]
- 56.Larcia LLH, Estacio RC, Dalmacio LMM. Bacterial diversity in Philippine fermented mustard (burong mustasa) as revealed by 16S rRNA gene analysis. Beneficial Microbes. 2011;2:263–71. doi: 10.3920/BM2011.0019. [DOI] [PubMed] [Google Scholar]
- 57.Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13–22. doi: 10.1128/CMR.00016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eichholzer M, Richard A, Nicastro HL, Platz EA, Linseisen J, Rohrmann S. Urinary lignans and inflammatory markers in the US National Health and Nutrition Examination Survey (NHANES) 1999–2004 and 2005–2008. Cancer Causes Control. 2014;25:395–403. doi: 10.1007/s10552-014-0340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, et al. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care. 2014;37:1287–95. doi: 10.2337/dc13-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, et al. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila) 2009;2:887–94. doi: 10.1158/1940-6207.CAPR-09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Dore J, et al. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005;71:6077–85. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clavel T, Dore J, Blaut M. Bioavailability of lignans in human subjects. Nutr Res Rev. 2006;19:187–96. doi: 10.1017/S0954422407249704. [DOI] [PubMed] [Google Scholar]
- 63.Lampe JW, Atkinson C, Hullar MA. Assessing exposure to lignans and their metabolites in humans. J AOAC Int. 2006;89:1174–81. [PubMed] [Google Scholar]
- 64.Eeckhaut E, Struijs K, Possemiers S, Vincken JP, Keukeleire DD, Verstraete W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J Agric Food Chem. 2008;56:4806–12. doi: 10.1021/jf800101s. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Meselhy MR, LI Y, Qin G, Hattori M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem Pharmol Bull. 2000;48:1606–10. doi: 10.1248/cpb.48.1606. [DOI] [PubMed] [Google Scholar]
- 66.Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12:140–7. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Jin J-S, Kakiuchi N, Hattori M. Enantioselective oxidation of enterodiol to enterolactone by human intestinal bacteria. Biol Pharm Bull. 2007;30:2204–6. doi: 10.1248/bpb.30.2204. [DOI] [PubMed] [Google Scholar]
- 68.Jin JS, Hattori M. Further studies on a human intestinal Bacterium Ruminococcus sp END-1 for transformation of plant lignans to mammalian lignans. J Agric Food Chem. 2009;57:7537–42. doi: 10.1021/jf900902p. [DOI] [PubMed] [Google Scholar]
- 69.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 71.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 72.Adlercreutz H, Fotsis T, Bannwart C, Wähälä K, Mäkelä T, Brunow G, et al. Determination of urinary lignans and phytoestrogen metabolites, potential antiestrogens and anticarcinogens, in urine of women on various habitual diets. J Steroid Biochem. 1986;25:791–7. doi: 10.1016/0022-4731(86)90310-9. [DOI] [PubMed] [Google Scholar]
- 73.Horner NK, Kristal AR, Prunty J, Skor HE, Potter JD, Lampe JW. Dietary determinants of plasma enterolactone. Cancer Epidemiol Biomarkers Prev. 2002;11:121–6. [PubMed] [Google Scholar]
- 74.Lampe JW, Gustafson DR, Hutchins AM, Martini MC, Li S, Wähälä K, et al. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol Biomarkers Prev. 1999;8:699–707. [PubMed] [Google Scholar]
- 75.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133:956S–64S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 76.Aura A-M, Myllymäki O, Bailey M, Penalvo JL, Adlercreutz H, Poutanen K. Interrelationships between carbohydrate type, phenolic acids and initial pH on in vitro conversion of enterolactone from rye lignans. In: Salovaara H, Gates F, Tenkanen M, editors. Dietary Fibre: Components and Functions. Wageningen, The Netherlands: Wageningen Academic Publishers; 2007. pp. 235–45. [Google Scholar]
- 77.Hullar MA, Lampe JW. The gut microbiome and obesity. Nestle Nutr Inst Workshop Ser. 2012;73:67–79. doi: 10.1159/000341288. [DOI] [PubMed] [Google Scholar]
- 78.Frankenfeld CL, Lampe JW, Shannon J, Gao DL, Li W, Ray RM, et al. Fruit and vegetable intakes in relation to plasma nutrient concentrations in women in Shanghai, China. Public Health Nutr. 2012;15:167–75. doi: 10.1017/S1368980011001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilkkinen A, Valsta LM, Virtamo J, Stumpf K, Adlercreutz H, Pietinen P. Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J Nutr. 2003;133:1830–3. doi: 10.1093/jn/133.6.1830. [DOI] [PubMed] [Google Scholar]
- 80.Roncaglia L, Amaretti A, Raimondi S, Leonardi A, Rossi M. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl Microbiol Biotechnol. 2011;92:159–68. doi: 10.1007/s00253-011-3338-8. [DOI] [PubMed] [Google Scholar]
- 81.Yoder S, Lancaster S, Hullar MAJ, Lampe JW. Gut microbial metabolism of plant lignans: influence on human health. In: Del Rio D, Tuohy K, editors. Diet-Microbe Interactions in the Gut. Elsevier; 2014. [Google Scholar]
- 82.Lee GH, Kumar S, Lee JH, Chang DH, Kim DS, Choi SH, et al. Genome sequence of Oscillibacter ruminantium strain GH1, isolated from rumen of Korean native cattle. J Bacteriol. 2012;194:6362. doi: 10.1128/JB.01677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee GH, Rhee MS, Chang DH, Lee J, Kim S, Yoon MH, et al. Oscillibacter ruminantium sp. nov., isolated from the rumen of Korean native cattle. Int J Syst Evol Microbiol. 2013;63:1942–6. doi: 10.1099/ijs.0.041749-0. [DOI] [PubMed] [Google Scholar]
- 84.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–8. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–8. doi: 10.1097/MOG.0b013e3282f323f3. [DOI] [PubMed] [Google Scholar]
- 86.Wu GD, Bewtra M, Hoffmann C, Chen YY, Keilbaugh SA, Bittinger K, et al. Controlled feeding experiments demonstrate the impact of diet on the composition of the human gut microbiome. Gastroenterology. 2011;140:S47-S. [Google Scholar]
- 87.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011:334. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Claesson MJ, O’Toole PW. Evaluating the latest high-throughput molecular techniques for the exploration of microbial gut communities. Gut Microbes. 2010;1:277–8. doi: 10.4161/gmic.1.4.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108 (Suppl 1):4592–8. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One. 2012;7:e34242. doi: 10.1371/journal.pone.0034242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajilic-Stojanovic M, Heilig HG, Tims S, Zoetendal EG, de Vos WM. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 2012;15:1146–59. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 95.Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hämäläinen E, Hasegawa T, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 96.Milder IEJ. Lignan intake in the Netherlands and its relation with mortality. Helsinki, Finland: Wageningen Universiteit; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.