Abstract
Prenatal ethanol exposure disrupts social behavior in humans and rodents. One system particularly important for social behavior is the somatosensory system. Prenatal ethanol exposure alters the structure and function of this area. Docosahexaenoic acid (DHA), an omega 3 polyunsaturated fatty acid, is necessary for normal brain development and brains from ethanol-exposed animals are DHA deficient. Thus, we determined whether postnatal DHA supplementation ameliorated behavioral deficits induced by prenatal ethanol exposure. Timed pregnant Long-Evans rats were assigned to one of three groups: ad libitum access to an ethanol-containing liquid diet, pair fed an isocaloric isonutritive non-alcohol liquid diet, or ad libitum access to chow and water. Pups were assigned to one of two postnatal treatment groups; gavaged intragastrically once per day between postnatal day (P)11 and P20 with DHA (10 g/kg in artificial rat milk) or artificial rat milk. A third group was left untreated. Isolation-induced ultrasonic vocalizations (iUSVs) were recorded on P14. Social behavior and play-induced USVs were tested on P28 or P42. Somatosensory performance was tested with a gap crossing test around P33 or on P42. Anxiety was tested on elevated plus maze around P35. Animals exposed to ethanol prenatally vocalized less, play fought less, and crossed a significantly shorter gap than control-treated animals. Administration of DHA ameliorated these ethanol-induced deficits such that the ethanol-exposed animals given DHA were no longer significantly different to control-treated animals. Thus, DHA administration may have therapeutic value to reverse some of ethanol’s damaging effects.
Keywords: fetal alcohol syndrome, omega 3 polyunsaturated fatty acid, DHA, social interaction
1. INTRODUCTION
Humans with fetal alcohol spectrum disorders (FASD) have difficulties with social interaction. These include deficits in relationships, coping skills, and use of play and leisure time [1, 2]. Other problems include poor social skills, difficulties understanding social cues, and inappropriate social behavior (e.g.,[3, 4]). Such deficits may continue through adolescence and into adulthood [5]. Rats exposed to alcohol during development also have altered social behaviors (e.g., [6–9]), including altered social interaction.
Social behavior is dependent on a number of sensory systems, and in the rodent the somatosensory system appears to be particularly important. If the parietal cortex (the region containing the somatosensory cortex) is removed, play behavior is altered [10]. Similarly, anesthetizing the nape of the neck, i.e., altering input to the somatosensory system, also alters play behaviors [8, 11]. Intriguingly, ethanol-exposed animals that show increased social behaviors have reduced neuronal activation in somatosensory cortex following social interaction [8].
The somatosensory system is a particular target of prenatal ethanol exposure. Somatosensory cortex is 30% smaller and has ∼30% fewer cells in adult animals that were exposed to ethanol prenatally, whereas all of cortex shows only a 10% decrease in volume [12]. Somatosensory cortex also shows decreased activity in ethanol-exposed animals [13, 14]. Although the thalamic component of the somatosensory system (the ventrobasal nucleus of the thalamus) does not show an effect of ethanol on cell number in the adolescent animal [15, 16], the axonal terminal fields in both somatosensory cortex and thalamus are affected by prenatal ethanol exposure [17–19]. This may contribute to the decreased cortical and thalamic activity [8, 13, 14, 20].
Docosahexaenoic acid (DHA) is an omega 3 polyunsaturated fatty acid (PUFA) that plays key roles in neuronal development and function; it is highly enriched in synaptic membranes in brain and has a role in neuronal development and synaptic function [21–23]. DHA is concentrated and retained in the nervous system and is known to have roles in neuroprotection, memory, and vision [23]. In culture, it can increase neurite outgrowth of hippocampal cells in a dose-dependent manner [22, 24–26]. Loss of DHA in membranes correlates with a decline in structural and functional integrity of the tissue (e.g., [27]), and deficits in DHA are linked with behavioral abnormalities in rodents (e.g., [28–32]).
DHA can be synthesized from alpha-linolenic acid (ALA) and/or supplied by the diet. It is accrued in the brain at a rapid rate during the third trimester of pregnancy and the first two to three years after birth (e.g., [33, 34]). Although breast milk naturally contains DHA, maternal diet can alter DHA levels (e.g., [35]). Supplementing maternal diet or infant formula with DHA or the omega 6 PUFA arachidonic acid results in improved problem solving abilities in infants (e.g., [36–38]) and higher Mental Development Index scores [39]. In contrast, deficiencies in dietary omega 3 fatty acids result in loss of DHA in rat brain and impaired spatial learning [30].
Dietary supplements that have been used to mitigate ethanol-induced damage in rodents and have achieved some success include antioxidants such as taurine or vitamin E [40, 41], choline (e.g., [42–46]), zinc [47], omega 3 PUFA [48–51], and a synthetic diet containing fish oil (which is high in omega 3 and omega 6 PUFA [52, 53]). All have been shown to be partly effective with the outcome depending on timing and dose of ethanol, timing and dose of supplement, and outcome measured. Intriguingly, administration of choline to rats exposed to alcohol in the early postnatal period (postnatal day (P) 4 - P9) can ameliorate some of the deficits that result and is most effective when the choline is given between P11 and P20 [44]. This suggests that this time period may be a critical window of opportunity.
Previous studies that looked at omega 3 PUFA either gave supplementation at the same time as administering ethanol [48–51] or fed animals exposed to ethanol prenatally a synthetic diet containing fish oil from just prior to birth through young adulthood [52, 53]. Supplementation with omega 3 PUFA at the time of ethanol exposure normalizes the ethanol-induced effects on brain and body weights, as well as on delayed eye-opening, and the auditory startle reflex, although it fails to remediate ethanol’s effects on motor development or reversal learning [48–50]. Long-term supplementation with fish oil reduced ethanol-induced oxidative stress in the brain and restored long term potentiation in the hippocampus [52, 53].
Prenatal ethanol exposure reduces DHA levels in brain [54, 55]. Increasing the amount of DHA in the diet can increase DHA in some areas of the brain [56]. Thus, we hypothesize that DHA reduction may underlie (or contribute to) the neurobehavioral problems observed in FASD, and we predict that postnatal supplementation with DHA will ameliorate neurobehavioral problems in an animal model of FASD.
2. METHODS
2.1. Animals
Timed pregnant Long-Evans rats (Harlan Laboratories, Frederick MD) were received on gestational day (G)3. The first day on which a sperm-positive plug was identified was designated G1. Rats were housed at the University of Maryland School of Medicine in an AAALAC accredited facility. Rooms were maintained on a 12-hour light/dark cycle (lights on 07:00 to 19:00) and rooms were temperature-controlled (22°C). Procedures were performed with approval of the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore and were in accordance with guidelines for animal care established by the National Institutes of Health.
Dams were housed singly and were randomly assigned to one of three groups; they were fed a liquid diet (L10251 and L10252, Research Diets, New Brunswick NJ) containing ethanol (Et), were pair-fed an isocaloric liquid diet (PF), or received ad libitum access to chow (Ch). All animals had ad libitum access to water. Et-fed animals were weaned onto the diet and received 11.5% ethanol-derived calories (EDC; final ethanol concentration 2.1% v/v) on G6 and G7, 23.5% EDC (3.8% v/v) on G8-G10, and 35% EDC (6.3% v/v) on G11-G21. Prior studies show that blood alcohol concentrations of 100–150 mg/dl are achieved using this model (e.g., [57, 58]). Dams were weighed three times per week.
Within 24 h of birth each litter was culled to 10, maintaining 6–8 males and 2–4 females per litter, and was surrogate-fostered to an untreated dam. Average body weights for male pups were recorded on postnatal day (P) 1. On P11, male pups were randomly assigned to one of three postnatal treatment conditions (artificial rat milk (Milk), DHA 10 g/kg in artificial rat milk (DHA), and non-treated control (NTC)), resulting in a total of nine treatment groups for the study (n=10–14 per treatment group; Table 1). DHA or Milk treatments were administered once daily by intragastric gavage from P11-P20. Composition of artificial rat milk is described elsewhere [59]. Pups were given 0.01 ml milk per g of bodyweight. DHA was 99% pure and was obtained from Sigma (SIG-D2534-1G; Sigma, St Louis MO). Animals were weaned on P21 and housed in same sex pairs or triads with littermates.
Table 1.
Number of pups in each group
| P28 Social Interaction |
P33 Gap Crossing |
Elevated Plus Maze |
P42 Social Interaction |
P42 Gap Crossing |
|
|---|---|---|---|---|---|
| Ch-NTC | 12 | 13 | 9 | 13 | 14 |
| Ch-Milk | 14 | 14 | 8 | 12 | 13 |
| Ch-DHA | 12 | 14 | 8 | 11 | 12 |
| PF-NTC | 12 | 13 | 8 | 11 | 12 |
| PF-Milk | 14 | 14 | 8 | 11 | 12 |
| PF-DHA | 13 | 14 | 7 | 11 | 13 |
| Et-NTC | 13 | 14 | 8 | 12 | 13 |
| Et-Milk | 12 | 13 | 8 | 12 | 13 |
| Et-DHA | 10 | 11 | 6 | 11 | 11 |
Rats from any given litter were assigned to different postnatal treatment groups such that only one animal per litter was in a group. Two cohorts were examined; cohort 1 underwent isolation ultrasonic vocalization (iUSV) testing on P14, social interaction on P28, gap crossing on one day between P33-P35, and a subset of animals underwent elevated plus maze testing on one day between P35-P38. Cohort 2 animals underwent iUSV testing on P14, and social interaction testing then gap crossing on P42. A subset of videos from the social interaction test was lost due to file corruption hence the n shown in Table 1 differs among the tests, but for all animals the first test after iUSV was social interaction which was then followed by gap crossing.
2.2. Isolation USV testing
On P14, each of the experimental pups was isolated for 6 min prior to being gavaged. Isolation USVs (iUSVs; 40 kHz) were recorded using an ultrasound microphone (Condenser Microphone 116H; Avisoft Bioacoustics, Berlin, Germany) and examined using Avisoft Bioacoustics™ equipment and sonographic software (Avisoft-RECORDER Version 4.0 and Avisoft SAS Lab Pro; Avisoft Bioacoustics). The sampling rate was 250.0 kHz and data were recorded in a 16-bit format. The latency to the first vocalization and the total number of 40 kHz USV calls were assessed.
2.3. Social interaction testing
Animals underwent social interaction testing during early adolescence (on P28) or in mid adolescence (P42). Testing was performed in dim light. All animals were isolated for 30 min. Play partners (offspring of Ch-fed dams) were housed in a new cage for 30 min. Experimental subjects (offspring of Ch-fed, PF, and Et-fed dams) had a 20 min period of isolation in a new cage followed by 10 min habituation to the social interaction apparatus. After this, an untreated play partner matched for weight, age, and sex was introduced into the social interaction test box (30 cm x 20 cm x 20 cm). This box included a clear partition in the middle with a semicircular hole (7 cm x 5 cm), which allowed the animals to move between compartments. Animals were video-taped for the 10 min habituation and for 10 min with the play partner. ANY-maze software was used to track distance traveled by the experimental animal during the habituation phase. Social behavior was scored by a trained observer blinded to experimental conditions. Behavioral measures included play fighting (counts of nape attacks, tags, and pins), social investigation (time spent sniffing the partner), chasing (time spent chasing the play partner) and social motivation (coefficient (%) = (crossing the partition towards the partner – crossing the partition away from partner) / total partition crosses [60–64].
2.4. USV during Social Interaction
USVs were recorded during social interaction as described above; the latency to the first vocalization and the total number of 22 kHz (aversive) and 50 kHz USV (hedonic) calls were assessed.
2.5. Gap crossing testing
Somatosensory performance was examined around P33 (age range P33–35) or on P42 using a gap crossing task in which rats traversed a gap to an escape box (after Lee et al., [65]). Animals were placed on a 15 cm wide x 60 cm long platform that was 31 cm above the floor of a brightly lit box. Initial starting distance to a darkened escape box was increased by 1 cm after each successful trial until they failed to cross the distance within 120 s. Two failures ended the test and the distance of the gap successfully achieved was recorded. Animals that underwent this test around P33 had previously undergone social interaction testing on P28. Animals tested on P42 underwent social interaction on P42 prior to this test.
2.6. Elevated plus maze
On P35 (age range P35–38), anxiety was assessed in a subset of animals (n=7–9 per group) using an elevated plus apparatus (Stoelting, Wood Dale, IL). Activity was tracked for 10 min using ANY-maze software. The number of entries and time spend in the closed and open arms of the maze were measured. Preference for the closed arm was calculated as a ratio: [(time (s) in closed arm - time in open arm)/total time (s)]*100. Distance traveled and speed were also assessed.
2.7. Statistical analysis
All scoring was performed by an investigator who was blinded to the treatment group. The mean (± the standard error of the mean) was calculated for each measure for each treatment group. For social interaction data, a three way analysis of variance (ANOVA) was run to determine the overall effect of prenatal exposure, postnatal treatment, and age. For each measure a 3 (prenatal exposure: Ch, PF, Et) x 3 (postnatal treatment: NTC, Milk, DHA) x 2 (age: P28, P42) factorial design was used. Data was analyzed separately for each behavioral measure using a corresponding univariate ANOVA to determine the source of any significant interactions or main effects. Where ANOVA identified significant (p<0.05) differences, data were probed using post hoc Tukey comparisons. For non parametric data or data that failed normality testing by the Shapiro-Wilk test, ANOVA was run on ranks using the Kruskal-Wallis test, where significance was identified post hoc Mann Whitney U tests were applied. An alpha of equal to or less than 0.05 was set as the statistical significance criterion. Trends are reported where 0.05≥ p ≤0.06. Statistics were run using Sigma Plot 12.3 software (Systat Software Inc., San Jose CA).
Effect sizes were assessed using IBM SPSS 22 statistical software (IBM Corp., Armonk, NY). Partial-eta values () are also reported to describe what percentage of the variance in outcome was accounted for by main and interactive effects by the between subjects factors, with small, medium, and large effects as follows: 0.010–0.089, 0.090–0.249, and 0.250+. Cohen’s d (d) was applied to significant post hoc comparisons, quantifying the magnitude of the difference between the groups. Criterion for small, medium, and large effects was as follows: ± 0.20–0.49, ± 0.50–0.79, and ± 0.80+.
3. RESULTS
3.1. Dam and litter outcomes
There was no significant difference among the three prenatal exposure groups on the average daily weight gain of the dams using Kruskal-Wallis one way ANOVA on ranks (H2=3.234, p=0.199; Table 2). There was also was no difference among the three prenatal exposure groups in the average number of pups in each litter (H2=0.942, p=0.636), the proportion of males (F2,59=0.071, p=0.931), or the average pup weight on P1 (H2=2.171, p=0.338; Table 2).
Table 2.
Dam and Litter Outcomes
| Prenatal Treatment (n) |
Average dam wt gain (g/day) |
# pups | % male | Average pup wt on P1 (g) |
|---|---|---|---|---|
| Chow (25) | 6.26 ± 0.43 | 9.64 ± 0.35 | 50.01 ± 3.17 | 5.94 ± 0.11 |
| Pair Fed (17) | 7.09 ± 0.39 | 10.12 ± 0.53 | 48.23 ± 3.05 | 5.89 ± 0.16 |
| Ethanol (20) | 6.22 ± 0.20 | 9.50 ± 0.37 | 48.99 ± 3.69 | 5.70 ± 0.14 |
Data shown are mean ± standard error of the mean. n is the number of litters in each prenatal treatment group.
The daily weight of male pups between P11 and P20 was recorded (Table 3). On average, pups gained ∼2 g per day. A two way ANOVA with repeated measures showed a main effect of age (F10,2270=3038.069, p<0.001, =0.930) and a main effect of prenatal exposure (F2,218=15.017, p<0.001, =0.117). Post hoc analysis showed that weight of all pups increased with age, and pups from Ch-fed dams were significantly heavier than pups from PF dams (p=0.009, d=−0.552) or Et-fed dams (p<0.001, d=−1.069).
Table 3.
Pup body weights from P11–20
| P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ch-NTC$ | 23.84 ± 0.97 | 25.83 ± 1.00 | 27.49 ±1.03 | 29.68 ± 1.12 | 31.58 ± 1.19 | 33.77 ± 1.24 | 35.68 ± 1.40 | 37.88 ± 1.33 | 39.98 ± 1.24 | 42.96 ± 1.32 |
| Ch-Milk$ | 22.87 ± 1.19 | 24.36 ± 1.14 | 26.27 ± 1.19 | 28.37 ± 1.17 | 30.53 ± 1.26 | 32.47 ± 1.32 | 34.16 ± 1.34 | 36.66 ± 1.41 | 39.42 ± 1.31 | 42.00 ± 1.28 |
| Ch-DHA$ | 24.26 ± 0.87 | 25.32 ± 0.99 | 27.04 ± 0.98 | 28.80 ± 1.24 | 30.81 ± 1.18 | 33.01 ± 1.20 | 34.74 ± 1.43 | 36.87 ± 1.55 | 39.48 ± 1.49 | 42.48 ± 1.45 |
| PF-NTC | 21.43 ± 0.83 | 23.65 ± 1.11 | 25.18 ± 0.85 | 27.77 ± 1.17 | 29.30 ± 0.86 | 31.15 ± 0.90 | 33.43 ± 0.91 | 35.75 ± 0.91 | 38.23 ± 0.99 | 40.57 ± 0.96 |
| PF-Milk | 21.96 ± 0.72 | 23.68 ± 0.74 | 25.46 ± 0.82 | 27.64 ± 0.83 | 29.43 ± 0.79 | 31.21 ± 0.88 | 33.07 ± 0.78 | 35.32 ± 0.83 | 37.46 ± 0.91 | 39.82 ± 0.95 |
| PF-DHA | 20.80 ± 0.76 | 22.09 ± 0.74 | 24.03 ± 0.80 | 26.02 ± 0.85 | 27.90 ± 0.83 | 29.75 ± 0.86 | 31.63 ± 0.82 | 33.68 ± 0.79 | 35.85 ± 0.91 | 38.20 ± 0.98 |
| Et-NTC | 21.89 ± 0.86 | 23.41 ± 0.86 | 25.01 ± 0.87 | 27.12 ± 0.88 | 29.32 ± 0.97 | 31.10 ± 1.02 | 33.07 ± 1.20 | 35.24 ± 1.40 | 37.21 ± 1.54 | 39.71 ± 1.47 |
| Et-Milk | 21.99 ± 0.96 | 23.20 ± 1.03 | 25.40 ± 1.02 | 26.97 ± 1.01 | 28.72 ± 1.01 | 30.90 ±1.25 | 32.99 ± 1.28 | 34.90 ± 1.30 | 36.84 ± 1.61 | 39.35 ± 1.33 |
| Et-DHA | 21.98 ± 1.43 | 22.98 ± 1.48 | 24.30 ± 1.56 | 26.35 ± 1.65 | 28.38 ± 1.81 | 30.28 ± 1.91 | 32.15 ± 2.05 | 34.13 ± 2.27 | 36.57 ± 2.19 | 38.53 ± 2.28 |
Data shown are mean ± standard error of the mean.
significantly different to PF- and Et-fed pups (p<0.05).
Body weights taken from 28-day-old animals in the first cohort show an effect of prenatal exposure (F2,113= 3.173, p=0.046, =0.53; Table 4). At this age Ch-fed offspring outweighed Et-exposed pups (p=0.041, d=−0.510), but not PF-exposed animals (p=0.657, d=0.233). No significant differences in body weight were noted between the PF- and Et-exposed groups (p=0.253, d=−0.325). Examination of body weights of the second cohort at P42 showed no significant differences among the groups.
Table 4.
Pup N and body weights on P28 for cohort 1 and on P42 for cohort 2.
| N | P28 | N | P42 | |
|---|---|---|---|---|
| Ch-NTC | 13 | 73.70 ± 1.79 | 14 | 169.36 ± 6.13 |
| Ch-Milk | 14 | 75.50 ± 1.35 | 13 | 167.76 ± 6.92 |
| Ch-DHA | 14 | 76.07 ± 1.89 | 12 | 169.25 ± 8.34 |
| PF-NTC | 13 | 74.76 ± 2.21 | 12 | 164.50 ± 6.67 |
| PF-Milk | 14 | 71.61 ± 1.43 | 12 | 167.33 ± 6.59 |
| PF-DHA | 14 | 74.64 ± 1.79 | 13 | 167.62 ± 4.95 |
| Et-NTC | 14 | 72.50 ± 2.83 & | 13 | 161.77 ± 5.58 |
| Et-Milk | 13 | 70.08 ± 3.42 & | 13 | 163.61 ± 5.10 |
| Et-DHA | 11 | 69.36 ± 2.44 & | 11 | 153.09 ± 5.15 |
Data shown are mean ± standard error of the mean.
significantly different to Ch-fed pups at same age (p<0.05).
3.2. Social Behavior
3.2.1. Play fighting
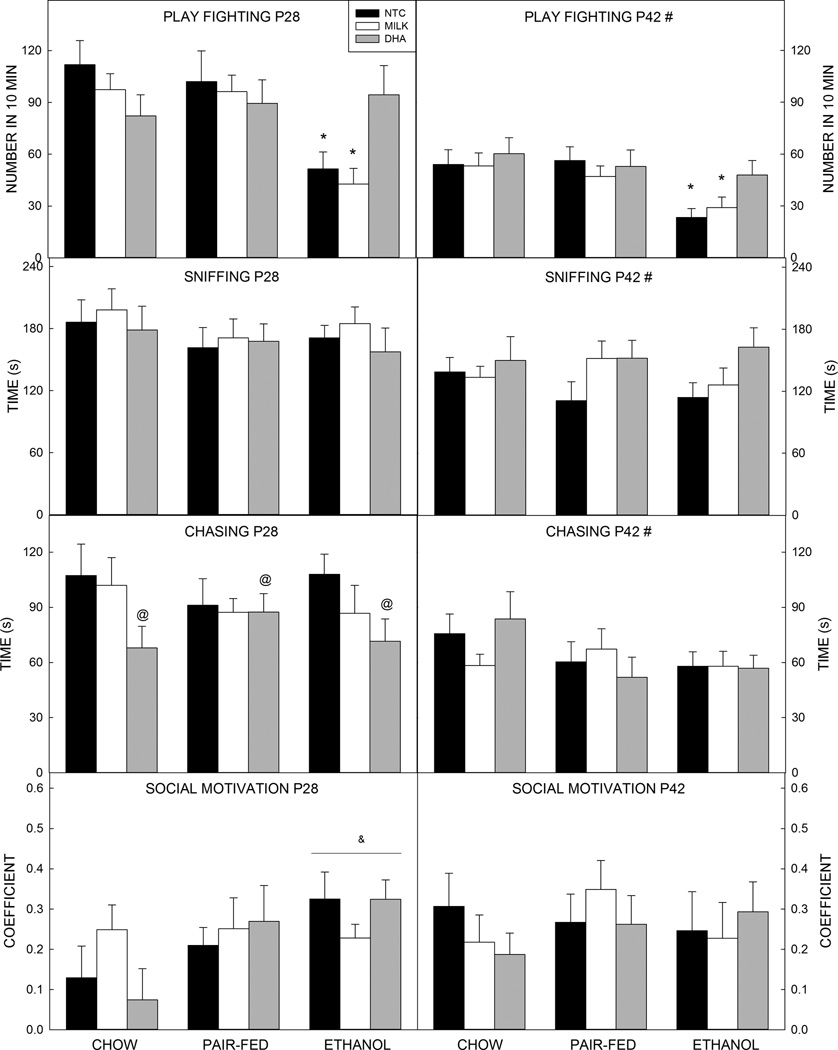
Statistical analysis revealed a significant medium sized effect of prenatal exposure (F2,198= 12.647, p<0.001, =0.113) and age (F1,198=58.286, p<0.001, =0.227, d= 0.980), and a small interaction between prenatal exposure and postnatal treatment (F4,198=3.189, p=0.014, =0.061) on the amount of play fighting performed in 10 min (Figure 1). Overall, animals exposed to ethanol prenatally play fought less than offspring of PF- (p<0.001, d=−0.677) or Ch-fed dams (p<0.001, d=−0.750), and younger animals play fought more than older animals (p<0.001, d=−0.981). Probing the interaction revealed that Et-exposed animals that were untreated or that received milk in the postnatal period play fought significantly less than all other groups (vs. PF groups, p values ranged from p=0.002 to p<0.001, d=−1.123 to −1.320; vs. Ch groups, p<0.001, d=−1.040 to −1.062). Et-exposed animals given DHA played significantly more than Et-NTC (p=0.004, d=0.826) and Et-Milk (p=0.005, d=0.913). Et-DHA animals were not significantly different from any of the control groups.
Figure 1. Social behavior outcomes.
(A) Prenatal exposure to ethanol decreased play fighting in both younger and older animals. DHA administration improved this behavior back to control levels. Younger animals play fought significantly more than older animals. (B) Time spent sniffing was not significantly affected by prenatal ethanol exposure, but was higher in younger animals than older animals. (C) Younger animals also spent more time chasing than older animals. At 28 days of age, DHA-treated animals spent more time chasing than NTC pups. No effects were seen in 42-day-old animals. (D) Social motivation was not significantly affected by prenatal ethanol exposure or postnatal treatment.
Data are mean of 10–14 animals per group (see Table 2). T–bars are the standard error of the mean. *significantly (p<0.05) different to Ch (all), PF (all), and Et-DHA. # significantly (p<0.05) different to P28. & significantly (p<0.05) different to Ch (all) at same age. @ all DHA significantly (p<0.05) different to all NTC.
3.2.2. Sniffing
There was a small but significant effect of age (F1,198=19.299, p<0.001, =0.089, d=0.622); younger animals spent more time sniffing than older animals (Figure 1). There were no effects of prenatal or postnatal treatments.
3.2.3. Chasing
Chasing behavior showed a significant medium sized effect of age (three way ANOVA F1,198=23.520, p<0.001, =0.106, d=0.679); overall, younger animals spent more time chasing than older animals (Figure 1).
A two way ANOVA run on data from 28-day-old animals showed that postnatal treatment approached significance (F2,103=3.062, p=0.051, =0.056); follow-up one way ANOVA showed that NTC pups chased more than the DHA pups (p=0.040, d=−0.592), but DHA pups did not differ significantly in time spent chasing a play partner when compared to milk-treated pups (p=0.468, d= −0.379).
No significant effect of prenatal or postnatal exposure was identified in the 42-day-old pups.
3.2.4. Social Motivation
The coefficient of social motivation was positive for all groups at both ages tested, showing social preference under all conditions (Figure 1). No significant effects of prenatal exposure or postnatal treatment were seen.
3.3. Activity
There was no effect of prenatal exposure or postnatal treatment on activity during habituation (distance (m) or speed (m/s); Tables 5 and 6). There was a trend towards a main effect of age on distance traveled (F1,198=3.637, p=0.058, =0.018, d=−0.251); older animals travel further than their younger counterparts. Speed was not affected by age.
Table 5.
Activity at P28 and ∼P35
| Prenatal Treatment |
Habituation (distance, m) |
Habituation (speed, m/s) |
Social interaction activity (# crosses) |
Elevated plus maze (distance, m) |
Elevated plus maze (speed, m/s) |
|---|---|---|---|---|---|
| Ch-NTC | 14.40 ± 2.10 | 0.023± 0.004 | 31.33 ± 4.32 | 18.91 ± 2.42 | 0.032 ± 0.004 |
| Ch-Milk | 14.36 ± 1.10 | 0.029 ± 0.003 | 32.29 ± 3.10 | 23.22 ± 2.43 | 0.039 ± 0.004 |
| Ch-DHA | 15.85 ± 1.37 | 0.045 ± 0.018 | 32.08 ± 5.70 | 25.07 ± 2.34@ | 0.042 ± 0.004@ |
| PF-NTC | 15.32 ± 0.80 | 0.031 ± 0.006 | 36.17 ± 5.28 | 13.34 ± 3.95 | 0.022 ± 0.007 |
| PF-Milk | 15.46 ± 1.57 | 0.042 ± 0.010 | 40.00 ± 3.50 | 23.77 ± 3.45 | 0.040 ± 0.006 |
| PF-DHA | 15.11 ± 1.29 | 0.048 ± 0.013 | 31.61 ± 3.79 | 25.24 ± 3.62@ | 0.042 ± 0.006@ |
| Et-NTC | 15.80 ± 1.32 | 0.047 ± 0.014 | 33.38 ± 2.46 | 20.12 ± 2.20 | 0.033 ± 0.004 |
| Et-Milk | 14.52 ± 1.31 | 0.054 ± 0.024 | 30.50 ± 3.56 | 19.97 ± 1.42 | 0.025 ± 0.005 |
| Et-DHA | 14.68 ± 1.40 | 0.049 ± 0.023 | 34.70 ± 5.50 | 24.72 ± 2.76@ | 0.041 ± 0.005@ |
Data shown are mean ± standard error of the mean. n is shown in Table 2.
all DHA significantly (p<0.05) different to all NTC.
Table 6.
Activity at P42
| Prenatal Treatment |
Habituation (distance, m) |
Habituation (speed, m/s) |
Social interaction (# crosses) |
|---|---|---|---|
| Ch-NTC | 16.40 ± 2.10 | 0.032 ± 0.003 | 31.91 ± 4.39 |
| Ch-Milk | 16.84 ± 1.44 | 0.039 ± 0.003 | 29.82 ± 3.41 |
| Ch-DHA | 17.98 ± 1.44 | 0.055 ± 0.002 | 33.82 ± 4.43 |
| PF-NTC | 17.79 ± 2.53 | 0.048 ± 0.007 | 30.73 ± 4.63 |
| PF-Milk | 15.82 ± 0.91 | 0.046 ± 0.003 | 26.82 ± 1.93 |
| PF-DHA | 16.70 ± 1.52 | 0.042 ± 0.002 | 28.09 ± 2.89 |
| Et-NTC | 18.43 ± 1.22 | 0.048 ± 0.004 | 26.18 ± 3.14 |
| Et-Milk | 19.21 ± 1.69 | 0.055 ± 0.004 | 27.83 ± 2.63 |
| Et-DHA | 15.29 ± 2.17 | 0.051 ± 0.003 | 26.82 ± 4.05 |
Data shown are mean ± standard error of the mean. n is shown in Table 2.
Activity during the social interaction test (total number of crosses between compartments) differed only as a function of age (F1,198=6.678, p=0.023, =0.010, d=−0.364) with older animals crossing between compartments more than younger animals.
3.4. Gap Crossing
Analysis of the gap crossing data showed a significant and large effect of prenatal exposure (F2,215=43.878, p<0.001, =0.285), a medium sized effect on postnatal treatment (F2,215=13.518, p<0.001, =0.110), and a small prenatal exposure by postnatal treatment interaction (F4,215=4.974, p<0.001, =0.087; Figure 2) on gap crossing outcomes. Post hoc analysis revealed that offspring of Et-exposed dams crossed shorter gaps than offspring of PF-or Ch-fed dams (p<0.001 for both, d=−1.117 and −1.291, respectively), and that animals given DHA crossed longer gaps than animals given milk or not treated (9.3 cm vs. ∼7.5 cm; p<0.001 for both, effect sizes d=0.668 and 0.567, respectively. The interaction revealed that within the Et-exposed group, Et-DHA treated animals crossed significantly larger gaps than Et-NTC or Et-Milk animals (Et-Milk vs. Et-DHA p<0.001, d= 1.568, Et-NTC vs. Et-DHA p<0.001, d= 1.325). There was no difference in the size of the gap crossed by Et-DHA animals compared with any of the control groups.
Figure 2. Gap crossing.
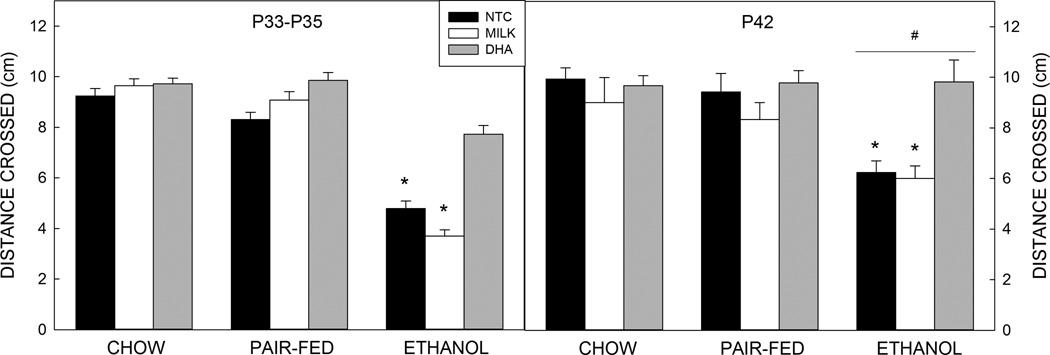
At both ages, ethanol-exposed animals crossed a shorter gap than pups from pair-fed or chow-fed dams. Older ethanol-exposed animals crossed a longer gap than younger ethanol-exposed animals. Postnatal treatment with DHA improved the distance crossed for all ethanol-exposed animals.
Data are mean of 11–14 animals per group (see Table 2). T–bars are the standard error of the mean. *significantly (p<0.05) different to Ch (all), PF (all), and Et-DHA. # significantly (p<0.05) different to P28.
Gap crossing data also showed a small main effect of age (F2,215=5.274, p=0.023, =0.026, d= −0.225) and a prenatal exposure x age interaction (F2,215=4.478, p=0.015, =0.038). Post hoc analysis showed that the older offspring of Et-fed dams crossed longer gaps than Et-exposed younger animals (p<0.001, d=−0.744), this was apparent across all three postnatal treatment groups.
3.5. Elevated Plus Maze
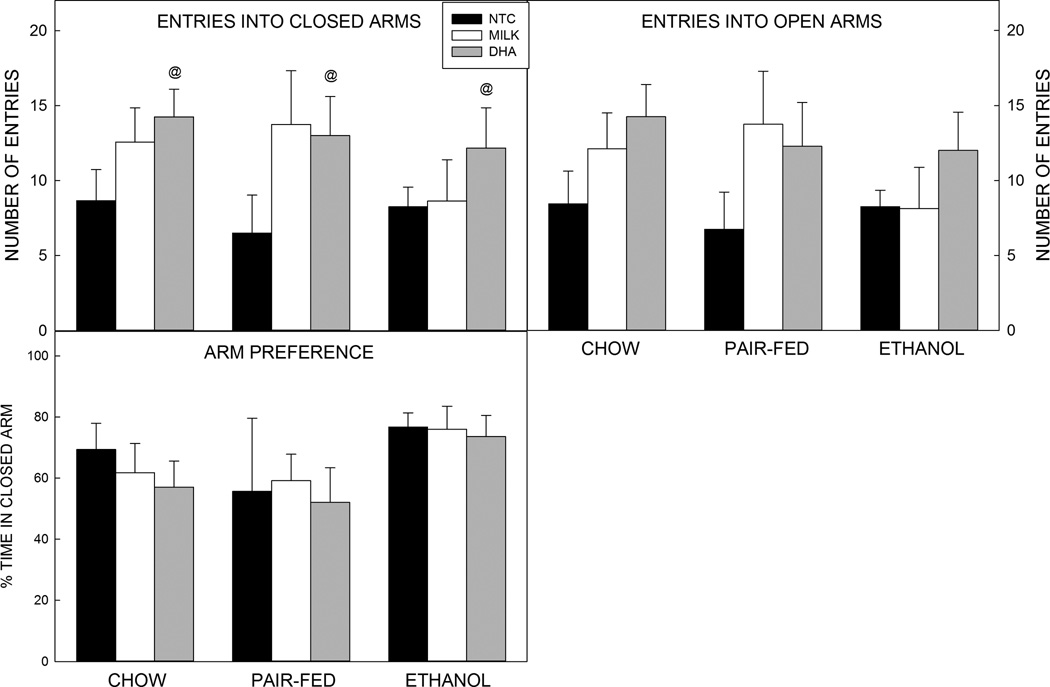
There was a significant medium sized effect of postnatal treatment on entries into the closed arm of the maze (F2,62=3.695, p=0.030, =0.106; Figure 3), distance traveled in the elevated plus maze (F2,62=4.218, p=0.019, =0.120; Table 3), and in speed (F2,62=3.834, p=0.027, =0.110; Table 3). Post hoc analyses showed that DHA treated pups entered the closed arm more often (p=0.035. d=0.932), travelled a longer distance (p=0.016 d=0.859, and were faster than the postnatal NTC group (p=0.025 d=0.814). No significant differences were seen between DHA and milk-treated rats in closed arm entries (p=0.729, d=0.217), distance traveled (p=0.425, d=0.398), or speed (p=0.164, d=0.427) or when comparing milk-treated animals with NTC animals (closed arm entries: p=0.131, d=0.540; distance traveled: p=0.215, d=0.491; speed: p=0.632, d<0.001).
Figure 3. Elevated plus maze.
Prenatal exposure to ethanol did not alter behavior on the elevated plus maze. Postnatal treatment with DHA increased the number of entries into the closed arm compared with NTC pups. Arm preference was calculated as a ratio for the closed arm: [(time (s) in closed arm -time in open arm)/total time (s)]*100.
Data are mean of 6–9 animals per group (see Table 2). T–bars are the standard error of the mean. @ all DHA significantly (p<0.05) different to all NTC.
There was a trend for postnatal treatment to alter the number of entries into the open arm of the maze (F2,62=3.107, p=0.052, =0.091). DHA-treated animals entered open arms more often than NTC animals (p=0.045, d=0.842). Animals that received milk were not different compared with DHA-treated animals (p=0.728, d=0.213) or NTC animals (p=0.192 d=0.494). No significant effects were found for time spent in either arm or for closed arm preference as calculated by the preference ratio (Figure 3).
The differences between DHA-treated animals and NTC animals but not milk-treated animals may result from the combined effects of artificial rat milk and DHA supplementation or gavage stress and DHA.
3.6. Ultrasonic Vocalization
3.6.1. iUSV
Analysis of the iUSV data showed a medium sized main effect of prenatal exposure on latency to first vocalization upon isolation (F2,227=16.248, p<0.0001, =0.125; Figure 4). Post hoc analysis revealed that pups from Et-fed dams took longer to vocalize than pups from either PF- or Ch-fed dams (p<0.001 for both groups, with effects sizes of d=0.653 and 0.721, respectively). Examination of the total number of 40 kHz calls revealed a main effect of prenatal exposure (F2,227=19.359, p<0.001, =0.146;) and a small prenatal exposure by postnatal treatment interaction (F4,218=4.489, p=0.002, =0.073). Post hoc analysis revealed that offspring of Et-exposed dams vocalized less than offspring of PF- or Ch-fed dams (p<0.001 for both, d=−0.818 and −0.960, respectively). Breakdown of the interaction revealed that within the Et-exposed group, Et-DHA treated pups vocalized more than their Et-NTC or Et-Milk treated cohorts (p<0.001 for both groups, d=1.577 and 1.490, respectively). No difference in the number of 40 kHz calls was detected between the Et-DHA animals compared with any of the control groups.
Figure 4. Isolation-induced ultrasonic vocalizations.
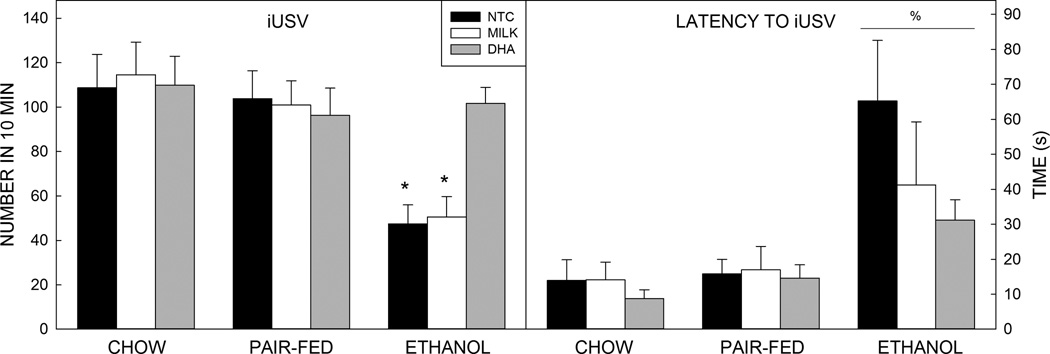
Prenatal exposure to ethanol increased the time until the first vocalization (latency) and the number of vocalizations. Within the ethanol-exposed group, treatment with DHA increased the number of vocalizations but did not alter latency to call.
Data are mean of 10–14 animals per group. T–bars are the standard error of the mean. *significantly (p<0.05) different to Ch (all), PF (all), and Et-DHA. % significantly (p<0.05) different to Ch (all) and PF (all).
3.6.2. USV during social interaction
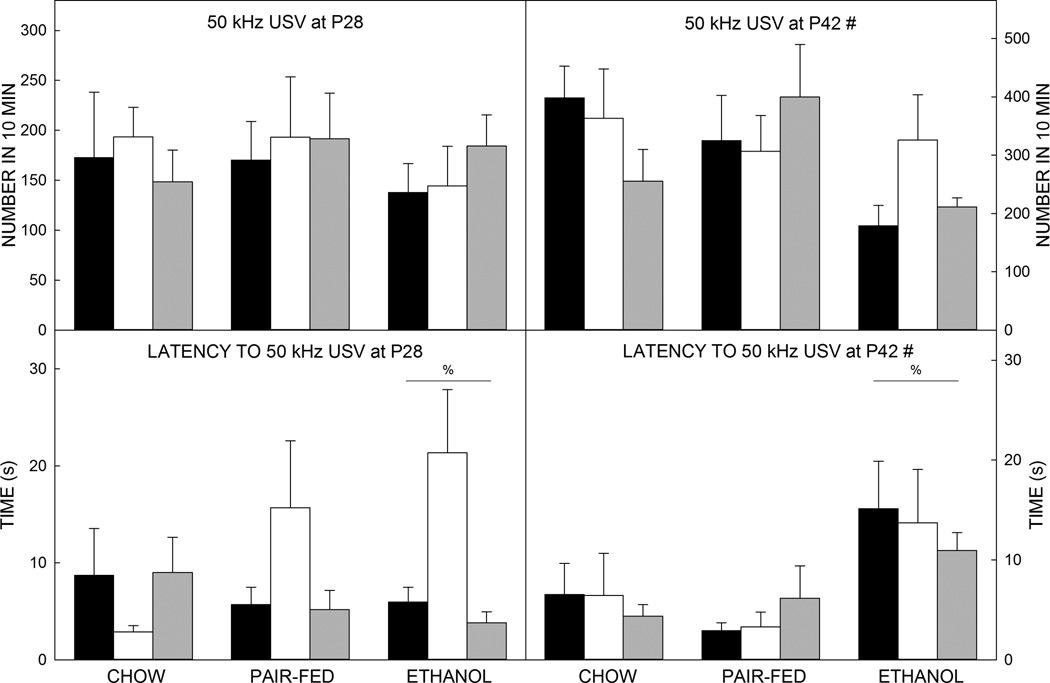
The social ultrasonic vocalization data revealed a small main effect of prenatal exposure on latency to emit 50 kHz USVs upon introduction of a social partner (F2,209=3.755, p=0.025, =0.038; Figure 5). Post hoc assessments showed social pairs with an Et-exposed animal took significantly longer to start vocalizing compared to pairs with a Ch-exposed animal (p=0.044, d=0.394) or PF-animal (p=0.043, d=0.348). There was also a main effect of age on the number of 50 kHz USVs emitted by the social pairs (F1,209=24.371, p<0.001, =0.117, d=−0.566); older social pairs called in the appetitive range more often than the younger cohort.
Figure 5. Play-induced 50 kHz ultrasonic vocalizations.
Older animals emitted more 50 kHz USVs than younger animals (top). Prenatal exposure to ethanol increased the latency until the first 50 kHz vocalization (bottom).
Data are mean of 10–14 animals per group. T–bars are the standard error of the mean. % significantly (p<0.05) different to Ch (all) and PF (all). # significantly (p<0.05) different to P28.
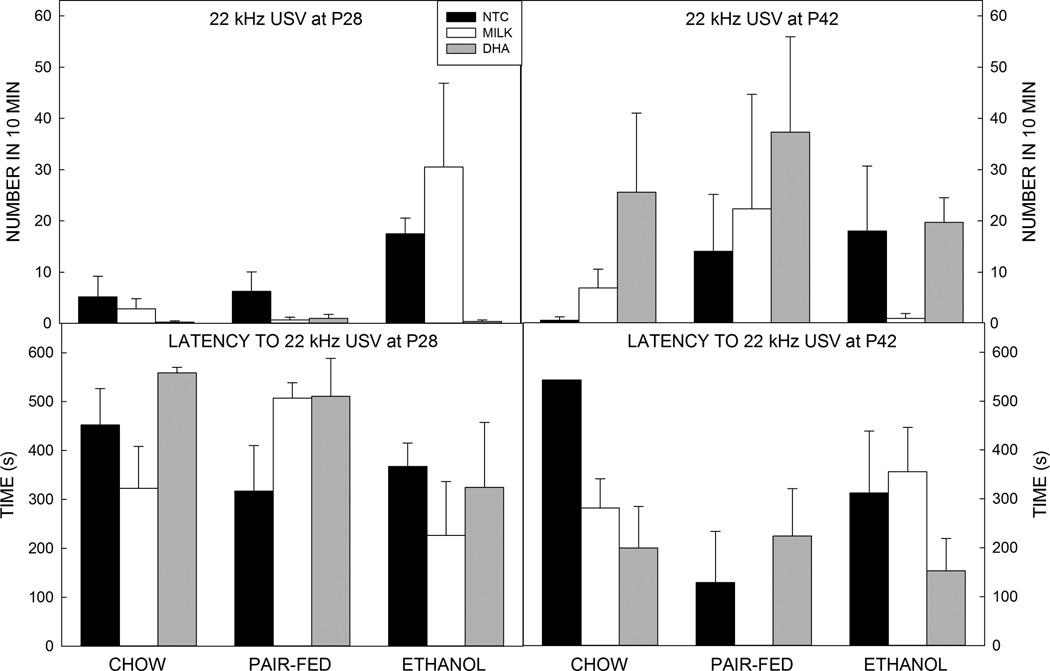
Assessment of the number of 22 kHz USVs showed that the three way ANOVA identified a significant interaction between age and postnatal treatment (F2,209=3.520, p=0.031, =0.032). Post hoc testing showed that DHA-treated animals emitted more 22 kHz USVs at P42 than P28 (p=0.002, d=−0.678) (Figure 5). Of the pairs that did emit a 22 kHz USV there was a significant main effect of age on latency (F1,46=8.561, p=0.005, =0.157, d=0.740), showing that older animals emitted aversive calls earlier than the younger pairings.
Figure 5. Play-induced 50 kHz ultrasonic vocalizations.
There were no effects of treatments or age on the number of 22 kHz USVs (top) or on the latency to emit a 22 kHz USV (bottom).
Data are mean of 10–14 animals per group. T–bars are the standard error of the mean.
4. DISCUSSION
Prenatal exposure to ethanol causes changes in rat social behavior and ultrasonic vocalizations and reduces the size of a gap crossed. Treatment with DHA improves the ethanol-induced changes in isolation-induced USVs, play fighting, and gap crossing such that the Et-DHA treated animals are no different to control animals. This improvement occurs in the absence of observed significant negative effects of DHA treatment on control animals and it persists at least three weeks after the last DHA treatment.
Humans with FASD may have abnormal social behaviors (e.g.,[1–4]) and sensorimotor performance, including integration of information from the somatosensory system [66, 67]. Two of the behaviors examined, social play and gap crossing, utilize the somatosensory system [65, 68, 69], which is reported to be a target of the ethanol exposure paradigm used in these studies [12–14].
The ethanol-induced behavior deficits were seen at two different ages during the adolescent period and are consistent with prior reports in the literature: rats exposed to ethanol during the prenatal period show decreased juvenile play [6] and reduced isolation ultrasonic vocalizations (USVs) [70]. In contrast with other reports, there was no increase in locomotor activity [71–73], although this was not directly tested in an open field test. Nor were there increases in investigation [74] or alterations on the elevated plus maze.
Social behavior outcomes showed a medium sized effect of prenatal ethanol exposure on play fighting, but differences in other behaviors were mostly between the ages. The decreased play fighting is similar to that seen previously [75] but is at odds with reports from others [e.g., 9], however, this may be partly due to the higher peak blood alcohol concentration and/or the shorter isolation period (30 min vs, 24 h) used in the present study.
In the gap crossing test, animals exposed to ethanol prenatally cross approximately half the distance that control animals do. This outcome is similar to those described in an earlier study [75] and is the only outcome that showed a large effect of prenatal ethanol exposure. The gap crossing deficit occurred in the absence of any change in performance on the elevated plus maze or the length of whiskers, suggesting that it is not due to anxiety or loss of motivation (see below), or shortened whiskers.
Anxiety has been shown to be induced with prenatal ethanol exposure in some animal models [76–79], although not by others [80]. To determine whether anxiety or motivation could underlie the outcome of the gap crossing task, we performed an elevated plus maze test [81–83]. Our results showed no effect of prenatal ethanol exposure on outcomes of this task; Et-exposed animals spent ∼80% of their time in the closed arms and had an approximately equivalent number of entries into closed and open arms as animals from Ch- or PF-fed dams. Together, these data suggest that Et-exposed animals were not any more anxious than offspring of PF or Ch dams and that they were motivated to move out of the open arms.
Age played a role in behavioral changes noted; young animals showed more tagging, pinning, sniffing and chasing compared to their older counterparts, but older animals crossed a longer distance in the gap crossing test. Despite these age-related changes, the effects of ethanol were seen to persist into mid-adolescence. This is consistent with a recent report showing no appreciable improvement in ethanol-induced behavioral changes through young adulthood [84]. Intriguingly, the effects of the DHA also persist into mid-adolescence, i.e., they are still apparent at P42, three weeks after the last supplementation.
Prenatal ethanol exposure has been reported to both decrease USV calls made during isolation [70] and to have no effect [85], however Marino et al., [86] showed that pups vocalize more after prolonged exposure to ethanol during gestation and the early postnatal period. The increased latency can also be seen after neonatal ethanol exposure alone [87, 88]. Typically, these calls are thought to be important for maternal retrieval (e.g. [89]), and to elicit milk let down and nurturing from the dam [90–92]. The ethanol-induced increased latency to call and decreased number of calls may shape maternal care of these animals, which can alter social behavior [93, 94]. The DHA-induced improvement in isolation-induced USVs may improve maternal care and this may contribute to the improvements seen in behavior.
Prenatal exposure to ethanol also subtly increased the time it took for the pairs to emit 50 kHz USVs made during social play. Typically, these are thought to be hedonic signals [95, 96]. DHA treatment did not alter the ethanol-induced changes in vocalization in the 50 kHz range during social play. This, combined with the finding that DHA improves the ethanol-induced social play deficits, suggests that hedonic USVs and social play are not necessarily intertwined (see also [97]).
While the behaviors investigated are somatosensory-dependent (see above), they also rely on other brain regions. Indeed, the frontal cortices and amygdala are also implicated in social behavior and have been shown to be affected by prenatal ethanol exposure [9, 74]. Other as yet unidentified regions may also be important. Additionally, both social behavior and gap crossing require a motor output and some degree of motivation to participate in the task. Since the effects of both ethanol and DHA are likely to be on multiple parts of the brain, it is difficult to dissect exact contributions of specific parts of the brain to these outcomes. As noted above, however, it is clear that the somatosensory system is negatively affected by prenatal ethanol exposure, thus, it makes a useful system for understanding mechanisms of both ethanol-induced brain damage and how DHA might act to improve outcomes.
Although it has not directly been shown that the somatosensory cortex has lower levels of DHA after prenatal ethanol exposure, this has been noted in whole brain homogenates [54] and in hippocampus [55]. Intriguingly, increasing dietary DHA levels has been shown to increase DHA in parietal cortex, which contains somatosensory cortex [56], thus, it is possible that the dietary DHA is transported to somatosensory cortex, as well as other areas of the brain. Prenatal ethanol exposure results in ∼30% fewer cells in somatosensory cortex of adult animals [12], decreased activity in this area [8, 13, 14, 20], and smaller axonal terminal fields in both somatosensory cortex and thalamus (e.g., [17–19]). Potential mechanisms by which DHA may improve outcomes include increasing cell survival [98–100] and/or improving process outgrowth and synaptogenesis [22, 25, 101, 102]. Prenatal ethanol exposure also significantly alters membrane phospholipid species, particularly phosphatidylserine, in brain [49, 55, 103, 104]. More than 35% of the fatty acids in phosphatidylserine are DHA [105], thus, increasing dietary DHA may support membrane integrity.
The timing of DHA administration encompasses a developmental period similar to around birth to the first six months of life in humans [106], a time critical for accumulation of DHA in humans [107]. This time was also identified by Ryan et al [44] as the critical time for intervention with choline in animal models of developmental ethanol exposure, and it aligns with timing in humans at which a baby is either breast-fed (breast milk being high in fatty acids) or could be fed a formula supplemented with DHA. It is possible that there are other times at which DHA supplementation would be equally effective.
Limitations of this study include the high dose of DHA used and the use of only male subjects. The dose of DHA used in this study was very high. Indeed, it is considerably higher than the amount currently available in infant formula and even a small baby would need significant supplementation. There is anecdotal evidence that the DHA in formula may cause gastric distress in infants. The only noted effects of DHA in this study that were independent of prenatal exposure were decreased time spent chasing at P28, increased activity in the elevated plus maze, and mild diarrhea, however, it should be noted that the diarrhea did not alter body weight and it resolved after the DHA exposure was completed. That said, current work is examining lower doses and is also looking at effects in females.
In summary, DHA was able to mitigate some ethanol-induced behavioral deficits in our rat pups, which appeared to persist for at least three weeks after administration ceased. We did see mild effects of the high dose DHA that were independent of prenatal exposure but these were limited to decreased chasing in young animals and increased activity in the elevated plus maze. Future testing with different timing and dosing, as well as the inclusion of females, would shed more light on the long term efficacy of rescue treatment with DHA for FASD affected individuals.
Highlights.
Chronic prenatal exposure to ethanol significantly reduced isolation-induced ultrasonic vocalizations, play fighting, and gap crossing in adolescent male rats
Postnatal treatment with docosahexaenoic acid (DHA) reversed the effects of chronic prenatal ethanol exposure on behavior
The improvement afforded by DHA was still apparent three weeks after the treatment ended
Acknowledgments
The authors thank Celina Tran and Nathan Nguyen for technical assistance. This research was supported by the National Institute of Alcohol Abuse and Alcoholism (AA018693 and AA022413 to SMM).
ABBREVIATIONS
- ALA
alpha-linolenic acid
- ANOVA
analysis of variance
- Ch
chow-fed
- DHA
docosahexaenoic acid
- EDC
ethanol-derived calories
- Et
ethanol-fed
- FASD
fetal alcohol spectrum disorder
- G
gestational day
- g/kg
grams per kilogram body weight
- kHz
kilohertz
- mg/dl
milligrams per deciliter
- NTC
non-treated control
- iUSV
isolation-induced ultrasonic vocalization
- P
postnatal day
- PF
pair-fed
- PUFA
polyunsaturated fatty acid
- sem
standard error of the mean
- USV
ultrasonic vocalization
- v/v
volume in volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–533. [PubMed] [Google Scholar]
- 2.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- 4.Whaley SE, O'Connor And MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res. 2001;25:1018–1024. [PubMed] [Google Scholar]
- 5.Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- 6.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- 7.Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 8.Charles Lawrence R, Cale Bonner H, Newsom RJ, Kelly SJ. Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behav Brain Res. 2008;188:209–218. doi: 10.1016/j.bbr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panksepp J, Normansell L, Cox JF, Siviy SM. Effects of neonatal decortication on the social play of juvenile rats. Physiol Behav. 1994;56:429–443. doi: 10.1016/0031-9384(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 11.Siviy SM, Panksepp J. Sensory modulation of juvenile play in rats. Dev Psychobiol. 1987;20:39–55. doi: 10.1002/dev.420200108. [DOI] [PubMed] [Google Scholar]
- 12.Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- 13.Miller MW, Dow-Edwards DL. Vibrissal stimulation affects glucose utilization in the trigeminal/somatosensory system of normal rats and rats prenatally exposed to ethanol. J Comp Neurol. 1993;335:283–284. doi: 10.1002/cne.903350211. [DOI] [PubMed] [Google Scholar]
- 14.Miller MW, Dow-Edwards DL. Structural and metabolic alterations in rat cerebral cortex induced by prenatal exposure to ethanol. Brain Res. 1988;474:316–326. doi: 10.1016/0006-8993(88)90445-3. [DOI] [PubMed] [Google Scholar]
- 15.Mooney SM, Miller MW. Effects of prenatal exposure to ethanol on systems matching: the number of neurons in the ventrobasal thalamic nucleus of the mature rat. Brain Res Dev Brain Res. 1999;117:121–125. doi: 10.1016/s0165-3806(99)00111-x. [DOI] [PubMed] [Google Scholar]
- 16.Mooney SM, Miller MW. Prenatal exposure to ethanol affects postnatal neurogenesis in thalamus. Exp Neurol. 2010;223:566–573. doi: 10.1016/j.expneurol.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minciacchi D, Granato A, Santarelli M, Sbriccoli A. Modifications of thalamo-cortical circuitry in rats prenatally exposed to ethanol. Neuroreport. 1993;4:415–418. doi: 10.1097/00001756-199304000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Granato A, Santarelli M, Sbriccoli A, Minciacchi D. Multifaceted alterations of the thalamo-cortico-thalamic loop in adult rats prenatally exposed to ethanol. Anat Embryol (Berl) 1995;191:11–23. doi: 10.1007/BF00215293. [DOI] [PubMed] [Google Scholar]
- 19.Santarelli M, Granato A, Sbriccoli A, Gobbi G, Janiri L, Minciacchi D. Alterations of the thalamo-cortical system in rats prenatally exposed to ethanol are prevented by concurrent administration of acetyl-L-carnitine. Brain Res. 1995;698:241–247. doi: 10.1016/0006-8993(95)00997-5. [DOI] [PubMed] [Google Scholar]
- 20.Vingan RD, Dow-Edwards DL, Riley EP. Cerebral metabolic alterations in rats following prenatal alcohol exposure: a deoxyglucose study. Alcohol Clin Exp Res. 1986;10:22–26. doi: 10.1111/j.1530-0277.1986.tb05607.x. [DOI] [PubMed] [Google Scholar]
- 21.Wainwright PE. Do essential fatty acids play a role in brain and behavioral development? Neurosci Biobehav Rev. 1992;16:193–205. doi: 10.1016/s0149-7634(05)80180-0. [DOI] [PubMed] [Google Scholar]
- 22.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44:216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuya H, Watanabe T, Sugioka Y, Inagaki Y, Okazaki I. Effect of ethanol and docosahexaenoic acid on nerve growth factor-induced neurite formation and neuron specific growth-associated protein gene expression in PC12 cells. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37:513–522. [PubMed] [Google Scholar]
- 25.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 26.Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem. 2005;16:538–546. doi: 10.1016/j.jnutbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.McNamara RK. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins Leukot Essent Fatty Acids. 2013;88:33–42. doi: 10.1016/j.plefa.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 29.Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot Essent Fatty Acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N., Jr An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behav Neurosci. 2009;123:196–205. doi: 10.1037/a0013801. [DOI] [PubMed] [Google Scholar]
- 31.Fedorova I, Alvheim AR, Hussein N, Salem N., Jr Deficit in prepulse inhibition in mice caused by dietary n-3 fatty acid deficiency. Behav Neurosci. 2009;123:1218–1225. doi: 10.1037/a0017446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, Pitta CF, Mello e Souza >T, Wofchuk S, Elisabetsky E, Vinade L, Perry ML, Souza DO. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int. 2010;56:753–759. doi: 10.1016/j.neuint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Martinez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71:2528–2533. doi: 10.1046/j.1471-4159.1998.71062528.x. [DOI] [PubMed] [Google Scholar]
- 34.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 35.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 36.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352:688–691. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- 37.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 38.Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr. 2007;85:1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- 39.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 40.Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol. 2003;25:1–9. doi: 10.1016/s0892-0362(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 41.Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000;24:512–518. [PubMed] [Google Scholar]
- 42.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22:1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summers BL, Henry CM, Rofe AM, Coyle P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behav Brain Res. 2008;186:230–238. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Wainwright PE, Huang YS, Mills DE, Ward GR, Ward RP, McCutcheon D. Interactive effects of prenatal ethanol and N-3 fatty acid supplementation on brain development in mice. Lipids. 1989;24:989–997. doi: 10.1007/BF02544067. [DOI] [PubMed] [Google Scholar]
- 49.Wainwright PE, Huang YS, Simmons V, Mills DE, Ward RP, Ward GR, Winfield D, McCutcheon D. Effects of prenatal ethanol and long-chain n-3 fatty acid supplementation on development in mice. 2. Fatty acid composition of brain membrane phospholipids. Alcohol Clin Exp Res. 1990;14:413–420. doi: 10.1111/j.1530-0277.1990.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 50.Wainwright PE, Ward GR, Winfield D, Huang YS, Mills DE, Ward RP, McCutcheon D. Effects of prenatal ethanol and long-chain n-3 fatty acid supplementation on development in mice. 1. Body and brain growth, sensorimotor development, and water T-maze reversal learning. Alcohol Clin Exp Res. 1990;14:405–412. doi: 10.1111/j.1530-0277.1990.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 51.Furuya H, Aikawa H, Yoshida T, Okazaki I. The use of docosahexaenoic acid supplementation to ameliorate the hyperactivity of rat pups induced by in utero ethanol exposure. Environ Health Prev Med. 2000;5:103–110. doi: 10.1265/ehpm.2000.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patten AR, Sickmann HM, Dyer RA, Innis SM, Christie BR. Omega-3 Fatty Acids can Reverse the Long-Term Deficits in Hippocampal Synaptic Plasticity Caused by Prenatal Ethanol Exposure. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 53.Patten AR, Brocardo PS, Christie BR. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J Nutr Biochem. 2013;24:760–769. doi: 10.1016/j.jnutbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Burdge GC, Postle AD. Effect of maternal ethanol consumption during pregnancy on the phospholipid molecular species composition of fetal guinea-pig brain, liver and plasma. Biochim Biophys Acta. 1995;1256:346–352. doi: 10.1016/0005-2760(95)00044-d. [DOI] [PubMed] [Google Scholar]
- 55.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 56.Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- 57.Miller MW. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: effect of in utero exposure to ethanol. Brain Res. 1992;595:17–24. doi: 10.1016/0006-8993(92)91447-m. [DOI] [PubMed] [Google Scholar]
- 58.Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci. 2007;121:1293–1305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 60.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 61.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci. 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen OS, Varlinskaya EI, Wilson CA, Glatt SJ, Mooney SM. Acute prenatal exposure to a moderate dose of valproic acid increases social behavior and alters gene expression in rats. Int J Dev Neurosci. 2013;31:740–750. doi: 10.1016/j.ijdevneu.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varlinskaya EI, Mooney SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav Brain Res. 2014;261:106–109. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee LJ, Chen WJ, Chuang YW, Wang YC. Neonatal whisker trimming causes long-lasting changes in structure and function of the somatosensory system. Exp Neurol. 2009;219:524–532. doi: 10.1016/j.expneurol.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Jirikowic TL, McCoy SW, Lubetzky-Vilnai A, Price R, Ciol MA, Kartin D, Hsu LY, Gendler B, Astley SJ. Sensory control of balance: a comparison of children with fetal alcohol spectrum disorders to children with typical development. J Popul Ther Clin Pharmacol. 2013;20:e212–e228. [PMC free article] [PubMed] [Google Scholar]
- 67.Williams L, Jackson CP, Choe N, Pelland L, Scott SH, Reynolds JN. Sensory-motor deficits in children with fetal alcohol spectrum disorder assessed using a robotic virtual reality platform. Alcohol Clin Exp Res. 2014;38:116–125. doi: 10.1111/acer.12225. [DOI] [PubMed] [Google Scholar]
- 68.Hutson KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- 69.Harris JA, Petersen RS, Diamond ME. Distribution of tactile learning and its neural basis. Proc Natl Acad Sci U S A. 1999;96:7587–7591. doi: 10.1073/pnas.96.13.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kehoe P, Shoemaker W. Opioid-dependent behaviors in infant rats: effects of prenatal exposure to ethanol. Pharmacol Biochem Behav. 1991;39:389–394. doi: 10.1016/0091-3057(91)90197-a. [DOI] [PubMed] [Google Scholar]
- 71.Lehotzky K, Ungvary G, Szeberenyi JM, Kiss A. Development of the central nervous system functions in rat pups prenatally exposed to alcohol (study on the behavioural teratology of ethanol in CFY rat pups) Acta Physiol Hung. 1988;72:171–180. [PubMed] [Google Scholar]
- 72.Gibson MA, Butters NS, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on locomotor activity, and hippocampal weight, neurons, and nitric oxide synthase activity of the young postnatal guinea pig. Neurotoxicol Teratol. 2000;22:183–192. doi: 10.1016/s0892-0362(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 73.Becker HC, Randall CL. Effects of prenatal ethanol exposure in C57BL mice on locomotor activity and passive avoidance behavior. Psychopharmacology (Berl) 1989;97:40–44. doi: 10.1007/BF00443410. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res. 2010;214:66–74. doi: 10.1016/j.bbr.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wellmann KA, Mooney SM. Unilateral whisker clipping exacerbates ethanol-induced social and somatosensory behavioral deficits in a sex- and age-dependent manner. Physiol Behav. 2014 doi: 10.1016/j.physbeh.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cullen CL, Burne TH, Lavidis NA, Moritz KM. Low dose prenatal ethanol exposure induces anxiety-like behaviour and alters dendritic morphology in the basolateral amygdala of rat offspring. PLoS One. 2013;8:e54924. doi: 10.1371/journal.pone.0054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohta K, Sakata-Haga H, Fukui Y. Alteration in anxiety-related behaviors and reduction of serotonergic neurons in raphe nuclei in adult rats prenatally exposed to ethanol. Congenit Anom (Kyoto) 2010;50:105–114. doi: 10.1111/j.1741-4520.2010.00269.x. [DOI] [PubMed] [Google Scholar]
- 78.Ohta K, Sakata-Haga H, Fukui Y. Prenatal ethanol exposure impairs passive avoidance acquisition and enhances unconditioned freezing in rat offspring. Behav Brain Res. 2012;234:255–258. doi: 10.1016/j.bbr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Sliwowska JH, Song HJ, Bodnar T, Weinberg J. Prenatal alcohol exposure results in long-term serotonin neuron deficits in female rats: modulatory role of ovarian steroids. Alcohol Clin Exp Res. 2014;38:152–160. doi: 10.1111/acer.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998;22:685–696. [PubMed] [Google Scholar]
- 81.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 82.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 83.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 84.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimmerberg B, Drucker PC, Weider JM. Differential behavioral effects of the neuroactive steroid allopregnanolone on neonatal rats prenatally exposed to alcohol. Pharmacol Biochem Behav. 1995;51:463–468. doi: 10.1016/0091-3057(95)00008-k. [DOI] [PubMed] [Google Scholar]
- 86.Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- 87.Rubin MA, Wellmann KA, Lewis B, Overgaauw BJ, Littleton JM, Barron S. Difluoromethylornithine (DFMO) reduces deficits in isolation-induced ultrasonic vocalizations and balance following neonatal ethanol exposure in rats. Pharmacol Biochem Behav. 2009;92:44–50. doi: 10.1016/j.pbb.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wellmann K, Lewis B, Barron S. Agmatine reduces ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol. Neurotoxicol Teratol. 2010;32:158–163. doi: 10.1016/j.ntt.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofer MA, Shair H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Dev Psychobiol. 1978;11:495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- 90.Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–387. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- 91.Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus) Anim Behav. 1972;20:175–185. doi: 10.1016/s0003-3472(72)80189-1. [DOI] [PubMed] [Google Scholar]
- 92.Brouette-Lahlou I, Vernet-Maury E, Vigouroux M. Role of pups' ultrasonic calls in a particular maternal behavior in Wistar rat: pups' anogenital licking. Behav Brain Res. 1992;50:147–154. doi: 10.1016/s0166-4328(05)80296-7. [DOI] [PubMed] [Google Scholar]
- 93.van Hasselt FN, Tieskens JM, Trezza V, Krugers HJ, Vanderschuren LJ, Joels M. Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiol Behav. 2012;106:701–706. doi: 10.1016/j.physbeh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Edelmann MN, Demers CH, Auger AP. Maternal touch moderates sex differences in juvenile social play behavior. PLoS One. 2013;8:e57396. doi: 10.1371/journal.pone.0057396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 96.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 97.Willey AR, Spear LP. Effects of ethanol on social approach and 50 kHz ultrasonic vocalization production in adolescent male Sprague-Dawley rats. Dev Psychobiol. 2014;56:857–863. doi: 10.1002/dev.21143. [DOI] [PubMed] [Google Scholar]
- 98.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J Biol Chem. 2000;275:35215–35223. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 99.Akbar M, Kim HY. Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J Neurochem. 2002;82:655–665. doi: 10.1046/j.1471-4159.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 100.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin RE, Bazan NG. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem. 1992;59:318–325. doi: 10.1111/j.1471-4159.1992.tb08906.x. [DOI] [PubMed] [Google Scholar]
- 103.Miller RR, Jr, Ugolini AM, Nothdorf RA, Searcy KJ, Taylor CL, Spidle DL. Ethanol alters brain phospholipid levels which correlate with altered brain morphology. Comp Biochem Physiol B Biochem Mol Biol. 1997;116:407–417. doi: 10.1016/s0305-0491(96)00259-3. [DOI] [PubMed] [Google Scholar]
- 104.Kim HY. Biochemical and biological functions of docosahexaenoic acid in the nervous system: modulation by ethanol. Chem Phys Lipids. 2008;153:34–46. doi: 10.1016/j.chemphyslip.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hitzemann R. Developmental changes in the fatty acids of synaptic membrane phospholipids: effect of protein malnutrition. Neurochem Res. 1981;6:935–947. doi: 10.1007/BF00965025. [DOI] [PubMed] [Google Scholar]
- 106.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guesnet P, Alessandri JM. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) - Implications for dietary recommendations. Biochimie. 2011;93:7–12. doi: 10.1016/j.biochi.2010.05.005. [DOI] [PubMed] [Google Scholar]