Abstract
Oxidative stress has long been implicated in cardiovascular disease, but more recently, the role of reactive oxygen species in normal physiological signaling has been elucidated. Signaling pathways modulated by reactive oxygen species (ROS) are complex and compartmentalized, and we are only beginning to identify the molecular modifications of specific targets. Here we review the current literature regarding ROS signaling in the cardiovascular system, focusing on the role of ROS in normal physiology and how dysregulation of signaling circuits contributes to cardiovascular diseases including atherosclerosis, ischemia-reperfusion injury, cardiomyopathy and heart failure. In particular, we consider how ROS modulate signaling pathways related to phenotypic modulation, migration and adhesion, contractility, proliferation and hypertrophy, angiogenesis, endoplasmic reticulum stress, apoptosis and senescence. Understanding the specific targets of ROS may guide the development of the next generation of ROS-modifying therapies to reduce morbidity and mortality associated with oxidative stress.
Keywords: Oxidative stress, reactive oxygen species, signal transduction, signaling pathways, cardiovascular pathophysiology
Introduction
For decades oxidative stress was defined as a cellular imbalance between oxidants and reductants. Now it is clear that differences in subcellular and tissue compartmentalization of reactive oxygen species (ROS) contribute to stress responses.1 Recent research has shown that ROS signaling pathways are complex, compartmentalized and in many cases essential for normal cardiovascular physiology. In addition, ROS signaling and oxidative stress have been implicated systemically or acutely in a variety of cardiovascular diseases and conditions including atherosclerosis, ischemia-reperfusion injury, diabetic vascular disease, arrhythmia, myocardial infarction (MI), hypertrophy, cardiomyopathy and heart failure. The differences between normal redox signaling required for cell survival and function and excess or inappropriate activation of redox circuits during oxidative stress are important to understand. Attempts at treating diseases with antioxidants prophylactically have been largely ineffective and in some cases harmful;2, 3 there is a definite need for improvement in timing, targeting and a reduction in off-target effects. In this review, we will summarize the current literature regarding ROS signaling in the cardiovascular system focusing on the role of ROS in normal physiology and how dysregulation of signaling circuits contributes to cardiovascular disease. A detailed understanding of these pathways allows the development of more targeted therapies to reduce morbidity and mortality associated with oxidative stress.
Key ROS in signaling and oxidative stress
ROS can be loosely defined as reactive molecules containing oxygen. A number of ROS including superoxide (O2•−), hydrogen peroxide (H2O2), peroxynitrite (OONO−) and the hydroxyl radical (HO•) are all produced in biological systems. O2•− and H2O2 are produced enzymatically, and are involved in both reversible, physiological signaling processes and the pathologies associated with oxidative stress. Other ROS such as OONO− and HO• are not considered signaling molecules due to their highly reactive nature and irreversible modifications, but can nonetheless contribute to oxidative stress and tissue damage pathologically. Additionally, the reactive nitrogen species nitric oxide (NO) is sometimes considered to be a ROS. For the purpose of this review, we will only discuss NO in the greater context of O2•− and H2O2-mediated signaling.
Superoxide
O2•− is produced biologically by a number of enzymes including NADPH oxidases (Nox), xanthine oxidase (XO), lipoxygenase, myeloperoxidase, uncoupled endothelial nitric oxide synthase (eNOS), and the mitochondrial respiratory chain via a one-electron reduction of molecular oxygen. O2•− can spontaneously dismutate to H2O2 (rate constant = 8 × 104 M−1 s−1) or be converted to H2O2 by the enzyme superoxide dismutase (SOD) (rate constant = 2 × 109 M−1 s−1) (Figure 1).4 Although much of the O2•− produced is rapidly converted to H2O2, which is believed to mediate downstream signaling, some direct modifications such as oxidation of (FeS)4 clusters5 and heme groups6 are directly attributable to O2•− (Figure 1).
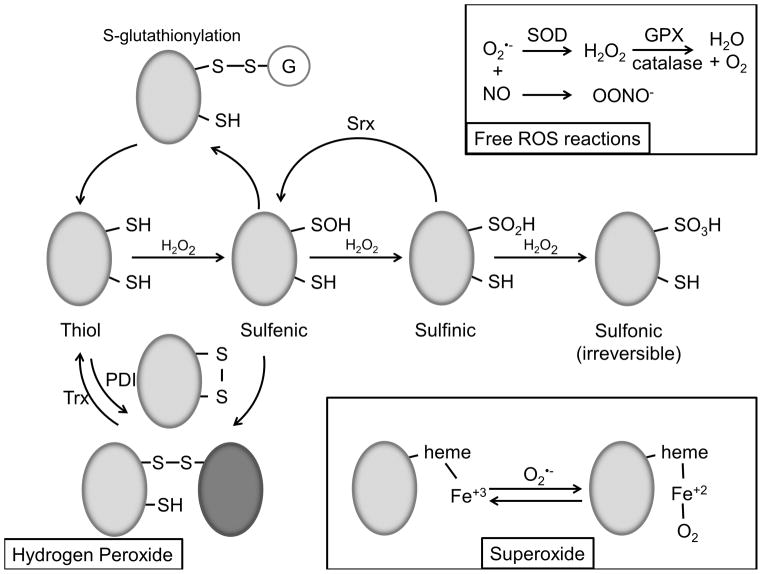
Figure 1. Oxidative modifications.
(Hydrogen Peroxide). Low pKa protein thiols react with H2O2 to produce various oxidative modifications. Initial oxidation results in formation of the sulfenic acid functional group, which is subject to s-glutathionylation and disulfide bond formation. Disulfide bonds are also created by PDI and reversed by Trx. Subsequent oxidation of sulfenic acid forms sulfinic acid, which may be reversible by Srx, and sulfonic acid, which is believed to be irreversible. (Superoxide). O2•− reversibly reacts with heme Fe3+ to form Fe2+ + O2. (Free ROS reactions). Free O2•− in the cell is converted to H2O2 by SOD and H2O + O2 by GPX and catalase. Free O2•− can also react with NO to form OONO−.
Hydrogen peroxide
H2O2 is primarily formed by the dismutation of O2•− by SOD (Figure 1). In the case of Nox4, H2O2 may be produced directly before O2•− leaves the enzyme.7 H2O2 is of particular interest in signaling due to higher stability compared to oxygen radicals and the ability to cross biological membranes. H2O2 reacts with low pKa protein thiols such as those on cysteine and methionine to form disulfide bonds (-SSR) or sulfenic acid (-SOH), which can influence protein function (Figure 1). These modifications are reversible by antioxidant mechanisms such as glutathione peroxidase and peroxiredoxin.8 Further oxidation by H2O2 can form sulifinic acid (-SO2H), which has been demonstrated to be reversible in peroxiredoxin by the enzyme sulfiredoxin (Srx),9 and sulfonic acid (-SO3H), which is not readily reversible and not believed to be involved in signaling. It is important to note that not only does H2O2 act upon adjacent targets, but because it is relatively stable, can also serve paracrine functions, as between endothelial and smooth muscle cells to regulate vasomotor tone.
Hydroxyl radical
HO• highly reactive, with a biological half-life of approximately 10−9 seconds.10 It is formed by the decomposition of H2O2, which is catalyzed by free metals in Fenton chemistry or by radiation-excited molecular oxygen reacting with H2O. Due to its nonselective and often irreversible reactivity, HO• can be damaging to many biological molecules including amino acids/proteins, carbohydrates, lipids and DNA. Certain biological scavengers such as glutathione can react with HO• to prevent damage. Generally, HO• is not considered a signaling molecule except as an intermediate due to its nonselective and reactive nature and will not be thoroughly discussed in this review. It should be noted however, that HO• plays a role in the oxidative damage associated with oxidative stress.11
Biological sources of ROS
NADPH oxidases (Nox)
Nox proteins constitute a family of membrane-associated, multiunit enzymes that catalyze the reduction of molecular oxygen using NADPH as an electron donor. The Nox family is composed of seven members, Nox1-5 and Duox1/2, with varying binding partners and activities. Only Nox1, Nox2, Nox4 and Nox5 are present in cardiovascular tissues.12 Nox proteins primarily produce O2•− by a single electron reduction; however, in the case of Nox4, H2O2 is mainly produced. Although Nox signaling is essential for normal physiology, upregulated and overactive Nox enzymes contribute to oxidative stress and cardiovascular disease.
Xanthine oxidase (XO)
XO is the oxidized form of xanthine dehydrogenase, which forms by sulfhydryl oxidation or proteolytic modification. XO catalyzes the conversion of hypoxanthine to xanthine and xanthine to uric acid. O2•− is formed as a byproduct of this reaction. XO is also capable of producing H2O2, especially in low oxygen conditions, permitting additional paracrine signaling by this enzyme.13 In cases of oxidative stress, the conversion of xanthine dehydrogenase to XO occurs, which produces additional ROS in a feed forward mechanism. XO is believed to be a major contributor to the large amounts of O2•− produced after ischemia/reperfusion injury.14 Inhibitors of XO are effective at attenuating the contribution of O2•− to this injury.15
Lipoxygenase (Lox)
Enzymes of the Lox family catalyze the oxidation of polyunsaturated fatty acids, including those involved in the biosynthesis of inflammatory leukotriene molecules.16 Loxes have been implicated in lipid oxidation in atherosclerosis. Increased expression of 5-lipoxygenase (5-LO) is found in atherosclerotic plaques and abdominal aortic aneurisms. Reduced expression of 5-LO17 or knockout of 12/15-LO18 reduced atherosclerosis in a LDL-R null model.
Myeloperoxidase (MPO)
MPO is heme-containing peroxidase expressed in neutrophils and monocytes that forms hypohalous acids from H2O2 and halides (Cl−, Br−, I−) or pseudohalide (SCN−). The hypohalous acids are strong oxidants believed to mediate the anti-microbial phagocyte respiratory burst. Excessive or off-target MPO activity is believed to produce ROS that contribute to lipid oxidation in atherosclerosis.19 In addition, MPO may be transferred from neutrophils to endothelial cells via β2-integrin mediated cell-cell contact.20 This intimal localization of MPO could contribute to the formation of atherosclerotic plaques. MPO derived oxidants have also been implicated in left ventricular remodeling after MI.21
Nitric oxide synthase (NOS)
Uncoupling of eNOS by reduced availability of substrates and cofactors such as BH4 and/or L-arginine results in increased O2•− production and reduced NO.22 Peroxynitrite (ONOO−), formed by the reaction of NO with O2•− at a rate believed to be higher than O2•− dismutation by SOD, is a strong oxidant that can pass through membrane anion channels, but is not expected to diffuse freely in an outward direction, thus limiting its potential paracrine effects. Peroxynitrite is, however, believed to contribute to eNOS uncoupling,22 and oxidation of BH4 increases ROS production.23 A number of factors have been linked to reduced bioavailability of NOS cofactors including smoking, hypertension, diabetes, ischemia-reperfusion injury, coronary artery disease and oxidative stress.24 The endothelial localization makes uncoupled eNOS both a contributor to endothelial dysfunction and a prime target of therapeutics to limit its detrimental effects.25
Mitochondria
Another source of O2•− in cardiovascular cells is from the mitochondria as a byproduct of respiration.26 In particular, complexes I27 and III28 of the mitochondrial respiratory chain exhibit “electron leak” during respiration that produces O2•−. In the case of complex I, O2•− is only released into the matrix, whereas O2•− is released on both sides of the inner mitochondrial membrane from Complex III.28 Antioxidants in the mitochondria such as SOD2 and glutathione rapidly degrade or sequester O2•− to reduce reactivity. Perhaps due to high concentrations of mitochondria in cardiac tissue, reduced mitochondrial antioxidant capacity results in cardiac dysfunction.29
Antioxidants
A large network of proteins and signaling pathways regulate the breakdown of ROS. From a systems perspective, overproduction of ROS in the cell can misregulate thiol redox circuits by changing the redox state of thioredoxins, glutathione and other cysteine pools.30 Ultimately, overproduction or high levels of exposure of a cell to exogenous ROS leads to a state of oxidative stress, which can result in DNA damage, reduced growth, metabolic problems and cell death. Thus, antioxidant systems are critically important to temporally and spatially regulate ROS-mediated signaling.
In addition to the control of redox potential by glutathione- and thioredoxin-based redox circuits, antioxidant enzymes such as superoxide dismutase (SOD), catalase and glutathione peroxidase (Gpx) rapidly break down ROS to less reactive or non-reactive products. These proteins are key in preventing damage from oxidative stress. In response to oxidative stress, cells activate transcription of protective antioxidant genes via redox-sensitive transcription factors such as nuclear factor (erythroid 2–related) factor 2 (Nrf2).
Nrf2 binds to the promoters of genes containing the cis-acting antioxidant response element (ARE) transcriptional element.31 Many ARE genes are involved in detoxification such as glutathione-S-transferases and NAD(P)H dehydrogenase.32 Numerous antioxidant genes contain AREs including catalase,33 SOD,33 Gpx,34 peroxiredoxin (Prx),35 and thioredoxin (Trx)36 (see Ma31 for more detail). Nrf2 is retained in the cytoplasm by binding to Keap1 (INrf2), which scaffolds to actin filaments. Keap1 also acts as an adaptor for the E3 ubiquitin ligase Cul3 and targets Nrf2 for proteasomal degradation by ubiquitination.37 Oxidation of Keap1 results in disruption of the complex and translocation of Nrf2 to the nucleus.38
Superoxide dismutases
SODs rapidly convert O2•− to H2O2. Most mammalian cells contain three forms of SOD (SOD1, SOD2 and SOD3) with differing localizations. SOD1, a Cu/Zn SOD, is expressed in the cytoplasm, and regulates angiogenesis and vasomotor tone. Genetic deletion of SOD1 leads to increased O2•− and ONOO−, resulting in increased vasoconstrictor responses and impaired endothelium-dependent relaxation, but no major change in blood pressure.39 Ischemia-induced vascular permeability is increased in these mice, but neovascularization is impaired.40, 41 However, transgenic overexpression of SOD1 reduces AngII-induced hypertension.42 SOD2 is also known as mitochondrial manganese SOD due to its localization. Deletion of SOD2 induces perinatal lethality due to cardiomyopathy, while SOD2+/− mice develop age-related hypertension43 and accelerated atherosclerosis on an ApoE−/− background.44 SOD3 (also Cu/Zn containing), which is secreted and then tethered to the outer plasma membrane, is particularly important in the cardiovascular system due to high expression in blood vessels, lung and heart.45 Manipulation of SOD3 has no effect on basal blood pressure, but SOD3 deficiency enhances blood pressure in response to angiotensin II (AngII) infusion.46 These mice also show defective neovascularization,47 but the role of SOD3 in atherosclerosis remains unclear.48
Catalase
Catalase catalyzes the decomposition of H2O2 to water and oxygen. Catalase is a tetramer containing four heme groups. The heme-iron is initially oxidized by H2O2 to form a high-valence intermediate known as compound I. Further reaction with H2O2 reduces compound I, forming molecular oxygen and water.49 Although catalase is highly abundant in many tissues, catalase null mice develop normally.50 Peroxiredoxins likely provide the necessary redundancy in H2O2 breakdown to allow for normal development. Overexpression of catalase protects against aneurysm formation, but inhibits collateralization.51, 52
Glutathione peroxidase
Gpx catalyzes the decomposition of H2O2 and lipid hydroperoxides to water or corresponding alcohols using reduced glutathione (GSH).53 Gpx exhibits higher expression in cardiovascular tissues than catalase, and due to its ability to reduce H2O2 and lipid peroxides, Gpx is believed to play a more critical protective antioxidant role in the cardiovascular system than catalase.54 Indeed, studies of Gpx knockout mice exhibit impaired angiogenesis,55 endothelial dysfunction,54, 56 increased susceptibility to ischemia-reperfusion injury57 and various other cardiovascular phenotypes that are believed to be mediated by increased ROS.58 A recent review discusses Gpx in more detail.59
Peroxiredoxins
Peroxiredoxins are a class of six antioxidant enzymes that are typically classified by characteristics of their H2O2-sensitive catalytic cysteines as 2-Cys (Prx1-4), atypical 2-Cys (Prx5) and 1-Cys (Prx6). Prx enzymes are highly expressed in cardiovascular tissues and in circulating erythrocytes, presumably to protect hemoglobin from oxidation leading to anemia. Prx isoforms 3–6 are downregulated in failing myocardium.60 Prx4 has been proposed to be a biomarker of oxidative stress based on evidence that patients with increased Prx4 have elevated CVD risk and mortality.61 Prx6 exhibits both glutathione peroxidase and phospholipase A2 activities, and is necessary for activation of Nox2 in endothelial cells and neutrophils.62
Thioredoxin
The thioredoxins are ubiquitously expressed antioxidants with a highly conserved CGPC catalytic motif that reduce substrate proteins by cysteine thiol-disulfide exchange.63 In mammals two thioredoxins are present, Trx1 in the cytosol and Trx2 in the mitochondria. Mice with transgenic Trx overexpression exhibit reduced ROS related cardiovascular toxicity.64 Altered Trx levels have been identified in atherosclerosis.65
Compartmentalization of ROS signaling
As with any regulated signaling process, compartmentalization of redox signaling plays a key role in defining the cellular response generated by the initial ROS-based signal. The subcellular localization of ROS-producing enzymes and antioxidants is tightly regulated. ROS detection in real time by novel methods such as the H2O2 sensor HyPer confirms spatially regulated subcellular localization of ROS production in response to certain stimuli. For example, by targeting HyPer to the endoplasmic reticulum (ER), Wu et al.66 demonstrated localized H2O2 production by Nox4 in response to tunicamycin and HIV-1 Tat. Datla et al.67 showed dynamic ROS production localized to focal adhesions in a model of nocodazole-induced focal adhesion turnover. Finally, Nox4-derived ROS have been detected in the perinuclear space.68
Both ROS producing enzymes (Nox169/Nox270) and antioxidant enzymes (SOD171) are associated with a pool of redox endosomes in non-phagocytic cells including VSMCs, fibroblasts, and potentially cardiomyocytes.68 These ‘redoxosomes’ are formed in response to cytokine signaling or other external stimuli, mediate downstream signaling by acting upon spatially restricted molecular targets and often require anion channels to help neutralize charge and facilitate transfer of the ‘signal’ across membranes.68 In addition, endosomes can mediate cross-talk between spatially distinct signaling circuits. Because the localization of ROS production likely plays a large role in which signaling pathways are activated, targeting antioxidants to particular compartments to disrupt pathological signaling would likely be more effective than the ubiquitous prophylactic antioxidant treatments that have been attempted in the past.
Role of ROS in fundamental cellular responses
ROS contribute to the cardiovascular diseases by a number of mechanisms (see ROS and oxidative stress in cardiovascular disease). Many of these processes are also important during development, in minute-to-minute regulation of blood flow, and in physiological adaptations to environmental stresses. In the following sections, we will discuss signaling pathways that are modified by ROS organized by functional response: cell migration/adhesion, cell proliferation/hypertrophy, ER stress/autophagy, and apoptosis/senescence. Depending on the source and severity of ROS production, as well as the cardiovascular cell type, ROS can contribute to some or all of these pathways simultaneously.
Phenotypic modulation
One of the most fundamental roles of ROS in the vasculature is in phenotypic regulation of VSMCs. These cells are normally differentiated and contractile, but in response to certain environmental cues can undergo phenotypic modulation to become synthetic, proliferative and migratory. Nox4 expression and activity are important for maintaining the differentiated phenotype.72 Regulation of smooth muscle-specific gene expression in VSMCs by Nox4 involves activation of p38MAPK, which regulates the activity of the SMC transcription factors serum response factor (SRF) and myocardin-related transcription factor (MRTF-A).73, 74 Actin itself is also directly oxidized by the flavoprotein oxidoreductase MICAL-2 in the nucleus, which promotes G-actin disassembly and increases nuclear translocation of the MRTF-A.75 In contrast, Nox1 expression and activity is associated with a reduction in differentiation markers and increased migration and growth.72, 76
ROS produced by Nox proteins have also been implicated in cardiac cell differentiation. Nadworny et al.77 induced the differentiation of c-kit+ cardiac precursor cells (CPCs) into mature cells and observed upregulation of Nox2 and Nox4 during differentiation. Furthermore, silencing of Nox2 and Nox4 increased expression of CPC genes such as c-kit and Flk-1, and decreased the expression of differentiation genes. Nox4 activation of c-jun upregulates the cardiac differentiation transcription factor GATA-4 in pluripotent embryonal carcinoma cells.78
Cell migration and adhesion
VSMC, endothelial cell and fibroblast migration play important roles in vessel development and repair. ROS regulate multiple steps in the migratory process including tyrosine kinase signaling,79, integrin engagement,80 focal adhesion formation81, actin dynamics,82 and small GTPase signaling.83 The amount and location of ROS production are critical; for example, while ROS are necessary for collateral formation, excess ROS can actually inhibit collateral formation.84 Overactivation of migratory signaling in pro-disease conditions, either by excessive extracellular oxidative stress or by hyperactivation of the normal healing response, can lead to inappropriate vascular cell migration, macrophage infiltration and lesion formation.
Cell migration is a multistep process involving the creation and dissolution of focal adhesions coordinated with cytoskeleton contraction to mediate movement (Figure 2). The cell first establishes polarity by sensing a gradient of a migratory stimulus. For example, after vascular injury, PDGF and PDGF receptor expression increase,85 providing a stimulus for VSMC migration. The PDGF receptor dimerizes and autophosphorylates, providing binding sites for phospholipase C (PLC), Src and phosphoinositide 3-kinase (PI3K). PI3K promotes the formation of PIP3, which activates Rho-GEFs to stimulate Rho-GTPase family members such as Rho, Rac and cdc42. Rac activates several Nox family members, notably Nox1 and Nox2, which can further increase ROS signaling, depending on the cell type. Activation of Rho by direct oxidation of a redox-sensitive motif has also been identified by Aghajanian et al.83 Rac and Rho are critical mediators of actin polymerization and the formation of focal adhesions, respectively, which are essential for protrusion and attachment and reattachment as the cell moves forward. Activation of the PDGF receptor is limited by the low molecular weight protein tyrosine phosphatase (LMW-PTP), but in the presence of ROS, LMW-PTP is inactivated by direct oxidation on Cys12 and Cys17 to form an inactivating disulfide bond,86 thus further amplifying the signal.
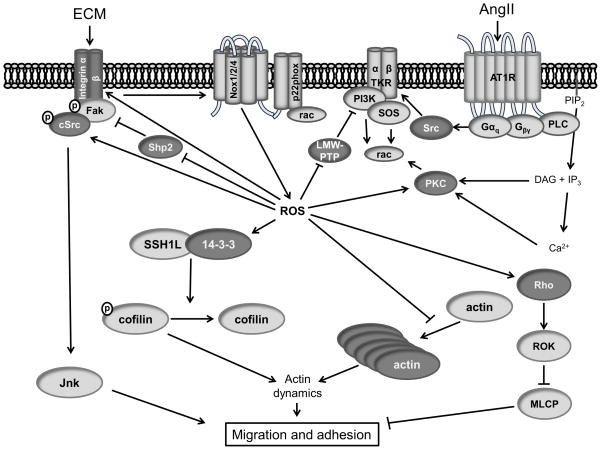
Figure 2. Redox signaling pathways in migration and adhesion.
(Dark Gray) Redox modified proteins. (Illustration Credit: Ben Smith).
After sensing the migratory stimulus, the leading edge of the migrating cell forms as lamellipodia extend in the direction of movement. Nox1 dependent activation of SSH1L and oxidation of 14-3-3 in PDGF-stimulated VSMCs results in the dephosphorylation and subsequent activation of the actin filament disassembly protein cofilin (Figure 2).87 This activation of cofilin results in actin depolymerization, a necessary step in the formation of new actin filaments that result in protrusion of lamellipodia and increased cell migration.88
Integrin binding and engagement in newly formed lamellipodia is essential for cell adhesion and migration. Recently, integrins have been demonstrated to be directly modified by ROS. Rezende et al.89 identified redox-sensitive cysteines in the integrin α7 subunit that influence integrin binding to laminin. Integrin engagement has been reported to induce an oxidative burst, which may play a role in cell adhesion and spreading; however, the mechanism is not well understood.90 Integrins sense and bind extracellular matrix proteins and in turn phosphorylate focal adhesion kinase (FAK), which recruits SH2- and SH3-domain-containing proteins such as Src, PI3K, Grb7, PLCY, and other focal adhesion proteins such as paxillin and vinculin into focal complexes that mature into stronger focal adhesions via a Rho-dependent pathway. Continuous focal adhesion turnover is essential for effective migration, as both too much turnover and too little turnover seem to inhibit cell migration.91
Phosphatases appear to be major redox targets during migration. Chiarugi et al.92 demonstrated that increased ROS inhibit the activity of a phosphatase for FAK, which is necessary for subsequent focal adhesion formation and spreading. They showed that oxidation of the LMW-PTP mentioned above during cell adhesion inhibits its ability to bind and dephosphorylate FAK. Others have implicated the redox sensitive phosphatase Shp2 in FAK activation.93 Shp2 is inactivated by direct oxidation, which ultimately increases FAK activity (Figure 2).94 In addition to FAK, LMW-PTP targets the PDGF receptor and p190RhoGAP, a guanine nucleotide exchange factor (GEF) for Rho.86 The tyrosine phosphatase PTP-PEST regulates paxillin and the Rho GEF Vav2.95 PTP1B, which binds to integrins and the p130cas scaffold, is also sensitive to oxidation.96
The focal adhesion complex is tethered to the cell’s actin cytoskeleton. Actin itself can be modified by ROS, either enhancing or inhibiting polymerization, depending on the type and amount of oxidant.97 In actively migrating endothelial cells, elevated levels of ROS are detected, and treatment with the SOD mimetic MnTMPyP abolishes actin monomer incorporation at the barbed end of growing actin filaments.98 However, the same concentration of H2O2 that causes fragmentation of F-actin in fibroblasts leads to reorganization of F-actin into stress fibers in endothelial cells, suggesting that oxidation can be environment- or cell type-dependent.99
The force that is necessary to move the cell forward towards the leading edge is generated by actin myosin interactions. The myosin light chain is regulated primarily by calcium-calmodulin, but can be influenced by the activity of the redox-sensitive GTPase Rho and the potentially redox-sensitive cdc42.100 In pulmonary smooth muscle, ROS activation of Rho results in deactivation of myosin light chain phosphatase via ROCK, which promotes contraction.101
In addition to redox regulation of migratory signaling, matrix degradation, a necessary step in migration, is also regulated by ROS. Matrix metalloproteinases (MMPs) that degrade extracellular matrix proteins are regulated by ROS transcriptionally102 by activation of Akt/NFκB103 and Erk1/2,104 and directly by a cysteine switch mechanism.105, 106 Although activation of MMPs may be protective in the case of mild atherosclerosis, in more severe plaques MMP activation can result in plaque instability and rupture by breaking down extracellular matrix.
Integrin-associated matricellular protein receptors such as CD47 and CD36 have recently been shown to be involved in pathological ROS production.107 Activation of CD47 and CD36 by thrombospondin-1 (TSP1) inhibits NO signaling108 in VSMCs and may contribute to eNOS uncoupling.107 Additionally, knockout studies of CD36 have implicated CD36 activity in the regulation of Nrf2 nuclear export and degradation,109 which ultimately results in higher levels of oxidative stress due to reduced antioxidant expression. Activation of CD47 by TSP1 induces Nox1-mediated ROS production via PLC,110 further supporting regulation of ROS signaling by matricellular proteins.
Vascular tone and cardiac contraction
As with most other vascular functions, ROS contribute to homeostatic regulation of vascular tone, but also are associated with hypertension when produced in excess. Similarly, ROS have been implicated in inotropic dysfunction in cardiomyopathy/heart failure.
A key regulator of vascular tone is NO produced in the endothelium by eNOS (Figure 3A). NO activates guanylate cyclase by binding to its associated heme, which increases cGMP production resulting in protein kinase G (PKG) activation and ultimately smooth muscle cell relaxation.111 Oxidative modification of the eNOS substrate BH4 by peroxynitirite, uncouples the enzyme, switching production from NO to O2•−23 and contributing to DOCA-salt hypertension in mice.23 eNOS itself is redox sensitive by reversible S-glutathionylation on several reactive cysteine residues including C382, C689 and C908 in an elevated GSSG:GSH environment.112 Crabtree et al.113 demonstrated that both of these mechanisms of eNOS uncoupling are additive but functionally independent. Although these two mechanisms result in decreased NO production in response to increased ROS, ROS can also paradoxically increase eNOS function. Cai et al.114 demonstrated that H2O2 produced by Nox in response to AngII stimulation increases NO production by eNOS, at least in part by increasing eNOS expression. This induction of eNOS is unlikely to be sufficient to compensate for oxidative stress, however. Tong et al.115 demonstrated that upregulation of Nox4 in arterial smooth muscle oxidizes SERCA, ultimately reducing cellular response to NO. Stimulation of murine endothelial cells with AngII results in increased O2•− production by Nox2 and the mitochondria, resulting in AngII-induced hypertension.116 In fact, a number of transgenic animal models with altered vascular Nox expression have altered blood pressure responses.117, 118
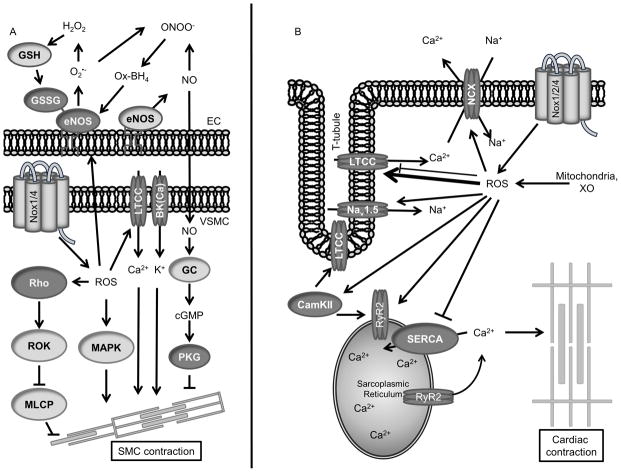
Figure 3. Redox signaling pathways in contraction.
(Left) VSMC contraction proteins and their relationship to EC-derived NO. (Right) Cardiac contraction proteins. (Dark Gray) Redox modified proteins.
H2O2 can act as a vasodilator, as first identified by Wolin’s group in rat cremasteric arterioles.119 It was soon demonstrated that H2O2 acts through the cGMP pathway, but it has only been recently appreciated that PKG itself can be activated by oxidation via disulfide bond formation,111 explaining in part why H2O2 causes direct vasodilation in some vascular beds. cGMP depletion sensitizes PKG to oxidation, especially in resistance arteries, suggesting a compensatory mechanism of vasodilation when NO is depleted.111 In human coronary arteries, H2O2 causes dimerization and activation of PKG, leading to subsequent opening of smooth muscle BK(Ca) channels and hyperpolarization.120 Similarly, chronic hypoxia is accompanied by increased H2O2 and PKG activation, which may in turn partially counteract pulmonary hypertension.121
Elevated H2O2 also plays a role in constriction by multiple pathways. Nearly 30 years ago, Heinle122 demonstrated that direct application of H2O2 induces vasoconstriction of the carotid artery. Since then, it has been shown that AngII-induced contraction produces H2O2 by Nox activation, which mediates vascular constriction by activating ERK1/2123 and p38 MAPK124, 125 in smooth muscle cells (Figure 3A). Additionally, ROS mediate activation of RhoA by reversible oxidation of reactive cysteines C16/C19.83 Rho activation increases Rho kinase activity, leading to contraction by inhibiting myosin light chain phosphatase (MLCP).126 It should be noted that in pulmonary circulation, hypoxia induces vasoconstriction.127 Although the exact mechanisms have yet to be fully defined, differences in ROS production by Nox proteins127 and altered extracellular SOD levels128 in response to hypoxia in pulmonary versus coronary cells may help to explain the difference.
Alternative pathways such as elevation of intracellular Ca2+ due to redox modification of L-type Ca2+ channels (LTCC) in vascular smooth muscle129 may play a role in chronic constriction and hypertension (Figure 3A), but additional work is necessary to better understand the role of these channels. Regulation of large-conductance Ca2+ activated K+ channel (BK(Ca)) has also been implicated in redox control of vascular tone.120 Ultimately, the regulation of vessel tone by ROS is likely dependent on concentration and localization. Moreover, the acute effect of a short term elevation of ROS may activate different signaling pathways related to contractility compared to chronic elevation of ROS, which can activate antioxidant response element (ARE) genes and other compensatory antioxidant mechanisms.
ROS regulation of cardiac contractility occurs at multiple levels (Figure 3B). Excessive ROS production by overactive Nox, XO or mitochondria results in cardiomyopathy due to direct modification of ion channels and transporters as well as altered intracellular contractility signaling. Cardiac contraction begins with the propagation of an action potential by the rapid activation of voltage-gated sodium channels such as Nav1.5 and voltage-dependent LTCCs. Oxidation of redox sensitive methionine residues on Nav1.5 impairs the inactivation of the channel resulting in elevated intracellular Na+.130 LTCC activation increases intracellular Ca2+ resulting in Ca2+-induced Ca2+ release by the ryanodine receptor (RyR2). Although the α1c pore-forming subunit of LTCC has been demonstrated to be sensitive to oxidation which reduces the peak Ca2+ current carried by the channel,131 ROS-mediated activation of CaMKII,132 PKA133 and PKC134 seem to ultimately result in a net increase in Ca2+ current by LTCC. Moreover, activation of CaMKII itself by Ca2+/Calmodulin is augmented by oxidation of M281/282.135 Ultimately, the sarcoplasmic reticulum (SR) calcium channel RyR2 is activated by the influx in Ca2+ by LTCC to release SR Ca2+ into the cytosol. Key cysteine residues in the RyR are oxidized to form activating disulfide bonds.136–138, resulting in increased cytosolic Ca2+ and ultimately heightened cardiac contractility.
During diastole, Ca2+ efflux and reuptake by the SR occurs (Figure 3B). The sodium/calcium exchanger (NCX) mediates Ca2+ efflux by removing one Ca2+ for three imported Na+ ions. There have been conflicting reports about ROS activating or inhibiting ion flux via NCX.139, 140 Additional work is necessary to identify ROS-sensitive coactivators or related post-translational modifications. An analysis of potentially redox-sensitive cysteines failed to identify directly modified residues.141 The reuptake of Ca2+ by the SR after contraction is regulated by the Ca2+-ATPase SERCA. SERCA contains the highly reactive cysteine C674, which reduces SERCA activity when H2O2 induces its S-glutathionylation.142, 143 However, S-glutathionylation of C674 also occurs in response to NO, which increases SERCA activity to promote relaxation.144 Additional work is necessary to clarify the role of S-glutathionylation of C674 and whether other still-to-be-identified modifications may contribute to SERCA activity.
Proliferation and hypertrophy
Like migration, cell proliferation is an important process in development as well as wound healing and repair. In cases of oxidative stress in the vasculature, excessive cell proliferation can contribute to a growing atherosclerotic plaque or a narrowing artery due to restenosis after percutaneous coronary intervention (PCI).145 In VSMCs, many of the same factors that induce a migratory phenotype, such as PDGF, also activate proliferation pathways, and in the case of AngII, hypertrophy (Figure 4). In mice with VSMC-specific overexpression the p22phox subunit of NADPH oxidase146 or Nox1,117 increased H2O2 and increased aortic hypertrophy is observed in response to AngII. In the heart, cardiac remodeling and hypertrophy associated with oxidative stress contribute to reduced output and progression to heart failure.147
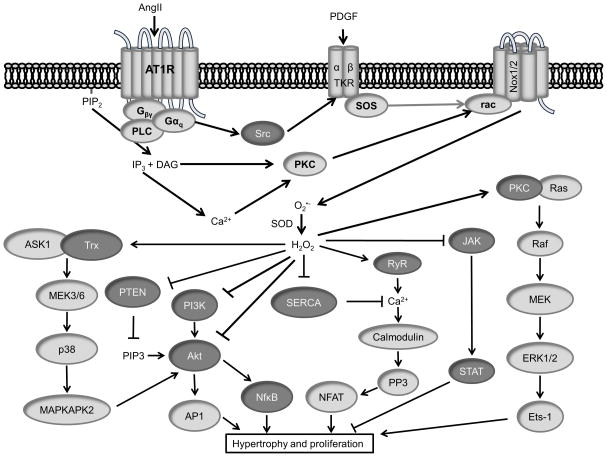
Figure 4. Redox signaling pathways in hypertrophy and proliferation.
(Dark Gray) Redox modified proteins. (illustration credit: Ben Smith).
ROS play a regulatory role in many proliferation pathways and ROS production is tightly regulated during the cell cycle (see reference 148 for review). Low levels of ROS regulate the activation of tyrosine kinase receptors by inactivating inhibitory protein tyrosine phosphatases.96 High levels of ROS inactivate growth factor signaling mediators and activate cell cycle arrest proteins. However, redox regulation of phosphatases may have different consequences in migration and proliferation. Similar to their role in migration, ROS-mediated inactivation of active site cysteines in PTP1B and Shp1/2 results in increased proliferation.96, 148, 149 Redox regulation of LMW-PTP, in contrast, mediates growth inhibition.150
Growth factor stimulation also activates the redox-sensitive PI3K cascade (Figure 4). Src is activated in part by ROS mediating disulfide bond formation between C245 and C487.151 Src activates PI3K, which phosphorylates the phospholipid PIP2 to its active form PIP3. This is reversed by the PIP3 phosphatase PTEN, which is inactivated by oxidation in cases of growth factor stimulation152 and oxidative stress153. PI3K itself was identified as a target of oxidation by a high throughput method,154 but the functional consequence of such a modification is unknown. Since PIP3 is an activator of PH domain proteins such as PDK1 and Akt, oxidation of PTEN ultimately activates Akt. Akt is also oxidized on Cys310, which may regulate Akt binding to PDK1.155 The redox sensitive p38MAPK and MAPKAPK-2 additionally complex with and activate Akt in response to AngII-stimulated ROS in VSMCs.156 Ultimately, Akt activates the transcription factors AP1 and NFκB to promote cell cycle progression.
A number of proliferation-related transcription factors are directly modified by oxidation. C-Jun and STAT3 are both negatively regulated by S-glutathionylation.157, 158 S-glutathionylation was also observed in p53; however, it is not known how this modification may affect cardiovascular signaling.159
Cardiac hypertrophy is usually considered as physiological (adaptive) or pathophysiological (progressing to heart failure). ROS play a role in both types of hypertrophy, and can be either protective, as is the case for Nox4-induced preservation of capillary density during chronic pressure overload,160 or injurious, as with Nox2-mediated activation of ERK, ASK-1, and NF-κB signaling pathways during AngII-induced hypertrophy. It is likely that redox signaling, as opposed to oxidative stress, plays a role in compensatory hypertrophy, while chronic stress conditions lead to a more generalized activation of ROS sensitive pathways. For example, treatment with antioxidants in neonatal rat cardiomyocytes reduces TNFα- and AngII-induced hypertrophy.161 ROS have also been implicated in α-adrenergic receptor-mediated hypertrophy in rat ventricular myoctes.162 Oxidative stress is reported to directly oxidize cysteine residues on Ras,163 activating downstream signaling to PI3K164, Raf165, MEK1/2166 and ERK1/2167. This increased Ras activity in response to α-adrenergic receptor stimulation is blocked by overexpression of thioredoxin-1.165 ASK1, which is a redox sensitive kinase due to Trx binding in its reduced form,168 and Akt169 activate NFκB which transcribes inflammatory genes involved in the hypertrophic response. Oxidative stress can also mediate JNK activation and PKC activation of ETS-1, which contributes to the increase in MMP activity and reduction in collagen synthesis170 that is observed in myocardial remodeling. The exact molecular target of ROS in these signaling pathways is often unclear and awaits further redox proteomic analysis.
ROS can also contribute to hypertrophy by regulating cardiac calcium channels and transporters, similar to their role in cardiac contraction.142 Sustained calcium release due to oxidative stress contributes to cardiac remodeling.171 Hypoxia has been implicated in both mitochondrial172- and Nox173-dependent cytosolic calcium release. Ca2+ channel blockers such as amlodipine174 and benidipine175 and intracellular calcium chelators such as BAPTA-AM176 reduce oxidative stress, potentially by preventing the crosstalk between mitochondrial ROS production and subsequent activation of Nox enzymes.177 Additionally, the redox sensitive deactivation and degradation of SERCA reduces calcium reuptake into the ER resulting in sustained calcium elevation in the cytosol.178
Sustained elevation of intracellular calcium in cardiac muscle results in myocardial dysfunction and hypertrophy. One mechanism is via calmodulin-dependent activation of the serine/threonine phosphatase calcineurin (PP3). PP3 dephosphorylates the transcription factor NFAT, which translocates to the nucleus to transcribe hypertrophy-promoting genes.179 NFAT activity and localization were demonstrated to be redox sensitive by Kalivendi et al.180 in a model of doxorubicin-mediated oxidative stress. The authors showed that treatment with antioxidants inhibits Dox-mediated nuclear translocation of NFAT in cardiac cells. Similar results were found after intermittent hypoxia, where the SOD mimetic tempol prevents the activation of NFAT.181
Angiogenesis
Neovascularization and angiogenesis are ROS-sensitive processes that combine many of the mechanisms described above including proliferation, migration and adhesion. Angiogenesis is induced by the activation of proangiogenic factors such as vascular endothelial growth factor (VEGF) and angiopoietin-1 in endothelial cells. Angiogenesis is particularly relevant in cancer biology, since vascularization is required for tumors to grow greater than a few millimeters in diameter.
In tumors, hypoxia induces VEGF expression by activating HIF1α in a ROS-dependent manner. HIF1α stability and subsequent activity can be regulated by redox-regulation of prolyl hydroxylase 2 (PHD2).182 HIF1α activation results in production of angiopoetin,183 which activates Tie2 and increases VEGF expression, in turn activating the redox-sensitive VEGFR2. Oxidation of VEGFR2 by disulfide linkage between C1199 and C1206 is inactivating, and is reversed by peroxiredoxin II.184 VEGFR2 is also negatively regulated by dephosphorylation by PTP1B,185 which is inactivated by ROS.186 In this way, ROS can both activate and inhibit the activity of VEGFR2 in certain contexts. Activation of VEGFR2 results in increased ROS production by NADPH oxidase via Rac1 activation187 and IQGAP1.188
Osteopontin (OPN) was recently identified by Lyle et al.189 as an important player in the redox regulation of angiogenesis. The authors found that increased translation of OPN occurs in response to the redox-regulated phosphorylation of 4E-BP1 at S65. Concomitantly, prolonged exposure to H2O2 increases OPN transcription. When ROS levels are reduced in vivo, OPN is not upregulated and neovascularization is impaired, supporting OPN’s role in ischemic neovascularization.190
Elevated ROS production in angiogenesis also results in the activation of the transcription factors Sp1, Ref-1, NFκB, p53, AP-1 and ETS-1, although the precise redox modifications remain unclear (reviewed by Ushio-Fukai et al.191). These transcription factors upregulate gene expression of proteins involved in migration, adhesion, survival and proliferation, all of which promote neovascularization and angiogenesis.
Nox4 has also been implicated in angiogenesis due to eNOS activation in endothelial cells.192 Transgenic mice with an endothelial-specific overexpression of Nox4 recovered from hind limb ischemia more rapidly and exhibited enhanced capillary formation.192 When the Nox4 transgenic mice were crossed with eNOS null mice, increased angiogenesis was not observed, suggesting that the H2O2 produced by Nox4 may be responsible for regulated eNOS expression, as proposed by Cai et al.114 Conversely, in global Nox4 knockout mice angiogenesis is impaired.193 Although the mechanisms by which Nox4 induces angiogenesis have not been fully worked out, overexpression of Nox4 in vitro was found to increase receptor tyrosine kinase activation and subsequently Erk phosphorylation.194
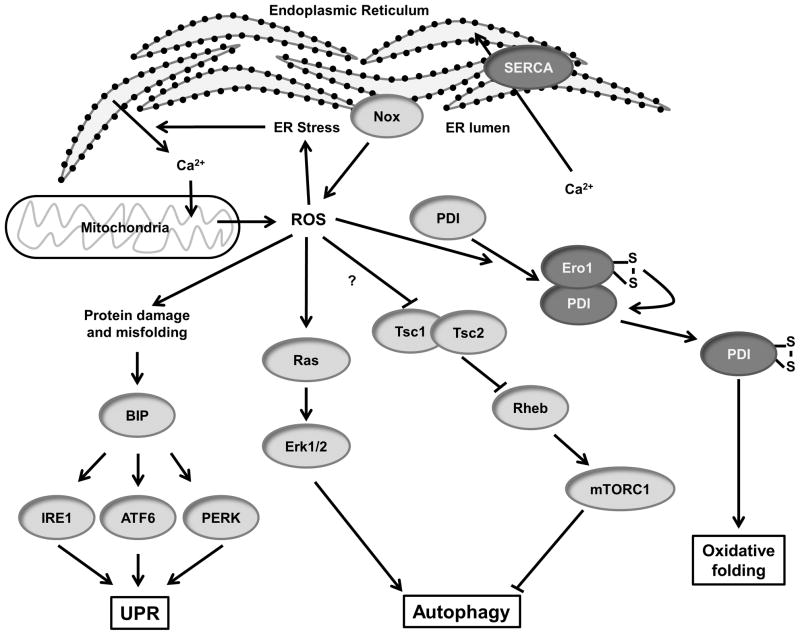
ER stress and autophagy
The endoplasmic reticulum (ER) is involved in multiple cellular processes including calcium homeostasis, protein transport/secretion and protein folding/post-translational modification. There is a certain amount of error that occurs in this process, which results in the production of unfolded and misfolded proteins that can contribute to ER stress. To counteract this, the unfolded protein response (UPR) can regulate the rate of secretion of proteins as well as recognize and degrade misfolded proteins and protein aggregates via the proteasome and autophagy. ER stress generally increases cellular ROS generation,195 in part by increasing calcium release, which increases ROS production by the mitochondria.196 This completes a positive feedback loop as oxidative stress contributes to ER stress and is directly involved in protein secretion, folding and degradation.66 In this section we will discuss the influence of ROS on ER stress and proteasome- and autophagy-related pathways.
The ER lumen is an oxidative environment compared to the cytosol, with a high ratio of GSSG:GSH.197 A recent study by Wu et al.,66 however, did not find increased free H2O2 in the ER compared to the cytosol using the redox probe HyPer, suggesting that much of the observed redox potential is protein-bound. The oxidative ER environment is conducive to the formation of disulfide bonds. A key class of thiol oxidoreductase chaperone proteins in the ER, protein disulfide isomerases (PDIs), catalyze the formation and breakage of disulfide bonds between cysteine residues of proteins in a process known as oxidative folding. PDI has emerged as a key redox-sensitive player in protein folding and ER stress. PDI is composed of four thioredoxin domains, and contains redox-sensitive cysteines whose oxidation state can influence the protein binding and activity of PDI. In its reduced form, PDI acts as an isomerase, whereas oxidation of PDI enables it to form disulfide bridges.198 Reduced PDI can bind another ER oxidoreductase, ER oxidoreductin 1 (Ero1), which is capable of oxidizing PDI and produces H2O2 in the ER as a consequence (Figure 5).199 Ero1 is believed to be essential for PDI activity by generating internal disulfide bonds and transferring them via PDI to target proteins.200 Recent evidence suggests that the inhibition of PDI in cells can contribute to ER stress and apoptosis. Toldo et al.201 found that overexpression of PDI protects against myocardial damage in an acute MI mouse model. The authors suggest that PDI activity is anti-apoptotic in part by increasing the activity of SOD1.
Figure 5. Redox signaling in ER stress and autophagy.
(Dark Gray) Redox modified proteins.
Oxidative stress in the ER can result in the misfolding of proteins, which are recognized by the chaperone BiP (Figure 5). Binding to misfolded proteins causes BiP to dissociate from unfolded protein response (UPR) transmembrane proteins, including protein kinase-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6) and inositol requiring protein-1 (IRE1), leading to their activation. Notably, in human aortic smooth muscle cells, activation of IRE1 by 7-ketocholesterol increases Nox4 expression.202 Additional information about ROS and the UPR can be found in a recent review by Santos et al.203
Nox proteins have also been implicated in ROS production during ER stress.195 Overexpression of PDI in VSMCs results in increased ROS, Nox1 mRNA and Nox4 expression in response to AngII.204 Additionally, PDI was found to associate with Nox subunits p22phox, Nox1 and Nox4, potentially regulating trafficking and activity of the enzymes.205 Moreover, Nox4 has been shown to localize to the ER and to activate PTP1B, which regulates epidermal growth factor receptor trafficking.206
In addition to mediating the physiological activation of PTP1B, ER-resident Nox4 also plays a role in ER stress. In endothelial cells, ER stress induced by HIV-1 Tat and tunicamycin activates Nox4-derived ROS production in the ER.66 This results in Ras and Erk1/2 activation, which induces autophagy. Autophagy involves the breakdown of excess or dysfunctional cellular components by lysosomes. Autophagy can be activated by ROS-mediated protein misfolding, as above, or via the absence of growth factors to maintain cellular energy levels, which results in inactivation of mammalian target of rapamycin (mTor). mTor activity is redox sensitive via the Tsc1/2-Rheb GTPase pathway (Figure 5). Yoshida et al.207 demonstrated that the thiol cross-linking agent PAO activates mTorc1. The authors propose that inactivating cross-linking of cysteine residues in Tsc1/2 could be responsible for the observed regulation of mTorc, but mutational analysis has not been performed yet to verify this assumption. Thus, ROS signaling pathways are implicated in both activation and inhibition of autophagy depending on the ER stress stimulus.
There is compelling evidence that autophagy plays a role in cardiovascular disease, and in many cases this leads to cell death. In the case of myocardial infarction and ischemia/repurfusion injury, cardiomyocytes undergo oxidative stress which results in autophagy-mediated cell death.208 Although autophagy-induced cell death in cardiomyocytes in this case is detrimental, there are scenarios in which this cell death can be beneficial. The drug Everolimus, for example, induces macrophages to undergo autophagy-mediated cell death in atherosclerotic plaques.209 Autophagy can also reduce cardiac hypertrophy in the cases of heart failure.210
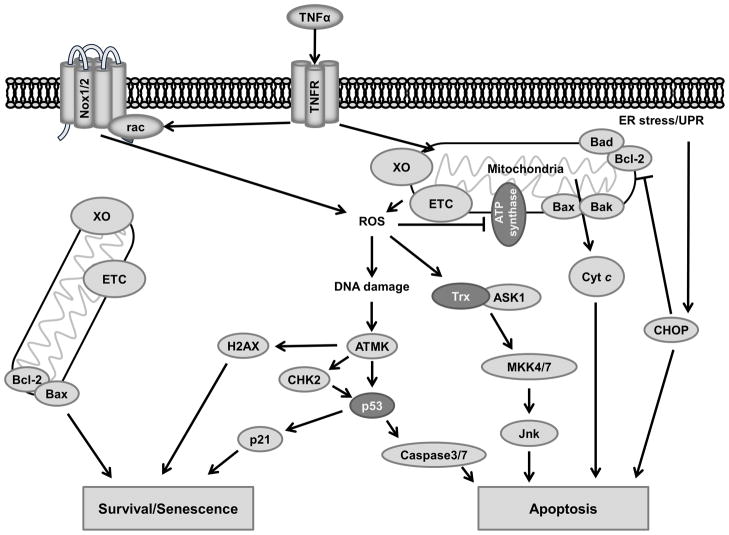
Apoptosis and senescence
Apoptosis, or programmed cell death, is process whereby cells shrink and self-phagocytize with minimal inflammation occurring in the surrounding tissue. This occurs naturally during development, but pathologically occurs in response to DNA damage and certain types of cell stress. In some contexts apoptosis can be protective, such as preventing cells that may become malignant from taking hold. However, in the cardiovascular system apoptosis can result in tissue damage and reduced cardiac function. Much of the damage to the heart that occurs after myocardial infarction is due to apoptosis of tissue that was oxygen deprived.211 During reperfusion of these oxygen-deprived tissues, large amounts of damaging ROS are produced enzymatically by many sources including XO, the electron transport chain (ETC) and Nox.212 Apoptosis also contributes to cardiomyopathy due to remodeling in chronic hypertension.213 Apoptosis signaling is closely related to senescence, with the expression and activity of key regulators such as p53 determining cell fate in cases of cellular stress.
Senescence is an irreversible halt in replication that occurs due to damage or aging. Apoptosis and senescence are both consequences of oxidative stress in cardiovascular cells including cardiomyocytes,214 endothelial cells,215 and VSMCs.216 Although the two endpoints are different, many of the signaling pathways that contribute to senescence or death are related. The ultimate outcome of oxidative damage is controlled by the level of damage and whether the cell is able to repair itself.
Cultured cardiomyocytes undergo apoptosis after exposure to as little as 10 μM of H2O2, which induces the tumor suppressor transcription factor p53 and Bad expression (Figure 6), as well as cytochrome C release.217 Recent evidence by Del Re and colleagues218 implicates oxidative stress-mediated oxidation and activation of K-Ras in this response, which activates Bax via Mst1 and BCL-xL to promote apoptosis. Additionally, p53 is a redox-sensitive component of the DNA damage response.219 Briefly, DNA breaks and certain types of oxidative modifications are sensed by repair proteins and damage sensors. If the amount of damage passes a threshold, ATM kinase is activated which phosphorylates downstream tumor suppressors such as CHK2, H2AX and p53. p53 is a key determinate of whether cells enter apoptosis or senescence. p53 activity promotes the expression of p21, a master regulator of senescence in oxidative stress.220 p53 also modulates the expression of caspase proteases that play an essential role in apoptosis.221
Figure 6. Redox signaling in apoptosis and senescence.
(Dark Gray) Redox modified proteins.
Although DNA damage is a major cause of apoptosis, other cellular signals can likewise result in apoptosis. As noted above, increased oxidative stress can result in heightened protein misfolding and aggregation leading to ER stress. In ER stress, the serine/threonine protein kinase IRE1 is activated, which activates the UPR and, in cases of severe stress, initiates apoptosis by degrading pro-survival miRNAs,222 as well as activating Jnk and Bax, thus initiating apoptosis by promoting cytochrome C release from the mitochondria via the mitochondrial apoptosis-induced channel (MAC) pore formation. Activation of the UPR also activates CEBP-homologous protein (CHOP), which inactivates Bcl-2 and promotes apoptosis by multiple mechanisms.223 Treatment with the antioxidant N-acetyl cysteine (NAC) prevents MAPK activation upstream of Jnk, indicating that ROS is involved.224 One potential mechanism is via ROS-mediated activation of Trx/Ask1 (Figure 6). Activation of Trx/Ask1 promotes Jnk activation via MKK4/7.225 Additionally, ROS control the expression and activity of Bax/Bad regulator Bcl-2 and Bad itself by regulating ubiquitination and phosphorylation.226, 227 Mice lacking pro-apoptosis genes such as Bax exhibit less myocardial cell death after ROS-producing reperfusion injury.228 However, simply eliminating apoptosis genes does not always prevent cell death. In Bax/Bak double knockout cells, DNA damage induces a ROS-mediated necrosis process induced by p53 and cathepsin.229
Cytokine signaling by TNFα has a demonstrated role in apoptosis via the ROS-dependent activation of Jnk.230 Kamata et al.231 showed that treatment with antioxidants prevents TNFα-mediated cell death by reducing oxidation of the MAP kinase phosphatases (MKP). ROS activation of NFκB conversely acts to inhibit Jnk activity and reduce ROS production to promote cell survival.232
To protect against apoptosis caused by ROS, protective genes such as PTEN and Nrf2 are activated. Nrf2 induces the expression of the cell survival gene Bcl-2 to inhibit p53 and prevent apoptosis.233 Nrf2 knockdown during hypoxia was demonstrated to reduce cell survival, further supporting Nrf2’s protective role.234 Nrf2 also regulates mitochondria function, promotes the expression of antioxidant enzymes and reduces the GSSG:GSH ratio to reduce additional oxidative damage.31, 235
ROS and oxidative stress in cardiovascular disease
As noted above, ROS are key players in normal cardiovascular physiology and signaling, but many redox sensitive pathways are also activated during development of disease. Moreover, certain catastrophic events such as mechanical injury or the disruption of blood flow result in increased local ROS production and/or reduced capacity of the cells to degrade or disperse these reactive molecules. Oxidative stress in turn influences signaling pathways that contribute to altered cell migration, proliferation/senescence, apoptosis, ER stress, and autophagy, which ultimately contribute to cardiovascular disease.
Exposure of endothelial and smooth muscle cells to circulating hormones and growth factors stimulate localized production of ROS that modify specific signaling molecules or cellular GSH/GSSG ratios that regulate entire circuits. AngII, for instance, is well known to induce hypertrophy in part due to localized production of ROS. Activation of the AT1 receptor by AngII rapidly induces ROS production by activation of NADPH oxidase169, 236 and due to positive feedback, additional ROS is produced in the mitochondria237. Platelet-derived growth factor (PDGF) is a potent migratory stimulus for vascular smooth muscle cells (VSMCs) that activates ROS production by activating Rac.238, 239
When normal signaling goes awry, or when ROS are produced in excess, the result is cardiovascular pathology. For example, high blood pressure is associated with impaired vasorelaxation and increased ROS production by NADPH oxidases.240 One key mechanism by which ROS contributes to vessel tone is via the inactivation of NO by O2•−. O2•− produced by Nox can react with NO to form OONO−, preventing the vasodilatory effect of NO.241 ROS also contribute to hypertension by regulating signaling in the renal and central nervous systems.242
In atherosclerosis, lesions form in areas of oscillatory or disturbed blood flow. This may be in part due to sensing of stretch or disturbed flow by baroreceptors and subsequent CNS feedback, but cells in culture also exhibit increased ROS production in response to mechanical stimuli, suggesting that a redox-coupled mechanosensor is present in the cells themselves. Cyclic stretch243 and shear stress244 from disturbed flow is associated with Nox1 activation and vascular remodeling.
One of the most rapid and severe inducers of oxidative stress in the cardiovascular system is reperfusion after a period of ischemia. Ischemia can result from microvascular injury, embolism, occlusion due to atherosclerosis, myocardial infarction/stroke or during surgical procedures such as transplants. Upon the restoration of blood flow, vascular cells produce more ROS and less NO. In cases of prolonged ischemia, xanthine dehydrogenase begins to function in reverse as an oxidase, producing excess ROS.14 Due to the rapid and severe influx of ROS and inflammation that occurs in ischemia/reperfusion injury, much of the surrounding tissue undergoes apoptosis if left untreated. Reperfusion injury is believed to be a major contributor to death associated with MI. Experimental efforts targeting antioxidants and scavengers to affected areas during reperfusion to reduce oxidative damage show promise;245 however, with the exception of the scavenger edaravone, which has limited use in the treatment of ischemic stroke,246 there is little translation of this research to the clinic.
Diabetic vascular disease is another area in which strong support exists for a role of ROS (for details, see a recent review by Giacco et al.247). ROS production by elevated glucose occurs by multiple mechanisms. Glucose can react with proteins to form an Amadori product, which is oxidized to form an advanced glycation end-product (AGE).248 AGE activates the RAGE receptor to stimulate intracellular ROS production via Nox1.249 Mitochondrial ROS also play a major role in diabetic vascular disease. Diabetic oxidative stress results in cardiac and vascular dysfunction including altered vascular tone, inflammation, and proliferation. Diabetics exhibit increased rates of atherosclerosis and cardiomyopathies, likely in part due to hyperglycemia-induced oxidative stress.247
Oxidative stress is also believed to be both a cause and a consequence of aging. ROS produced in the mitochondria as a consequence of respiration can damage mitochondria and cellular components over time, which ultimately results in more ROS production and oxidative stress.250 A study of rat cardiac mitochondria found increased oxidative stress markers and antioxidant enzyme activity in aged (24 mo) rat cardiac interfibrillar mitochondria compared to tissue isolated from young (6 mo) rats.251 Additionally, mice with a mitochondrial targeted overexpression of catalase exhibit prolonged lifespan and reduced cardiac aging.252
Conclusions
Given the fact that ROS act as signaling molecules in so many cellular processes, it can be difficult to separate pathological oxidative stress from normal physiological signaling. Whereas once oxidative stress was defined as an oxidative overload in an entire cell or tissue, recent work supports the idea of localized redox imbalance within a cell having pathological signaling consequences. Such is the case in growth factor induction of ROS signaling in fibrosis and atherosclerosis, which causes pathological cell migration and extracellular matrix deposition. The development of therapeutics to target oxidative stress pathways must therefore focus on subcellular ROS or downstream molecular signals to avoid undesirable off-target effects. Before such therapies can be developed we must have a better understanding of the ROS-sensitive signaling pathways in the cardiovascular system. Future work should focus on the identification of specific molecular targets that regulate redox circuits which modify cellular functions; dissecting the role of compartmentalization of ROS-generating and ROS-catabolizing enzymes; and understanding the whole body consequences of inhibiting specific sources of ROS in order to predict side effects of targeted therapies.
Acknowledgments
Sources of funding
The authors’ work is supported by National Institutes of Health grants HL38206, HL095070 and HL058863.
Non-standard Abbreviations and Acronyms
- AGE
Advanced glycation end-product
- AngII
Angiotensin II
- ARE
Antioxidant response element
- BiP
Binding immunoglobulin protein
- CPCs
c-kit+ cardiac precursor cells
- CHOP
CEBP-homologous protein
- Cyt c
Cytochrome c
- ETC
Electron transport chain
- eNOS
Endothelial nitric oxide synthase
- ER
Endoplasmic reticulum
- FAK
Focal adhesion kinase
- Gpx
Glutathione peroxidase
- H2O2
Hydrogen peroxide
- HO•
Hydroxyl radical
- LMW-PTP
Low molecular weight protein tyrosine phosphatase
- LTCC
L-type Ca2+ channels
- MPO
Myeloperoxidase
- MI
Myocardial infarction
- Nox
NADPH oxidases
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid 2–related factor 2
- OONO−
Peroxynitrite
- Prx
Peroxiredoxin
- PDGF
Platelet derived growth factor
- PDI
Protein disulfide isomerase
- ROS
Reactive oxygen species
- RyR2
Ryanodine receptor
- NCX
Sodium/calcium exchanger
- O2•−
Superoxide
- SOD
Superoxide dismutase
- Trx
Thioredoxin
- TSP1
Thrombospondin-1
- UPR
Unfolded protein response
- VEGF
Vascular endothelial growth factor
- VSMCs
Vascular smooth muscle cells
- XO
Xanthine Oxidase
Footnotes
Disclosures
None.
References
- 1.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab. 2010;12 (Suppl 2):116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371:177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 4.Fridovich I. Superoxide radical: An endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 5.Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 6.Butler J, Jayson GG, Swallow AJ. The reaction between the superoxide anion radical and cytochrome c. Biochim Biophys Acta. 1975;408:215–222. doi: 10.1016/0005-2728(75)90124-3. [DOI] [PubMed] [Google Scholar]
- 7.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The e-loop is involved in hydrogen peroxide formation by the NADPH oxidase nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-cys peroxiredoxin requires glutathionylation mediated by heterodimerization with pi gst. Proc Natl Acad Sci U S A. 2004;101:3780–3785. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo HA, Kang SW, Kim HK, Yang KS, Chae HZ, Rhee SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 10.Pryor WA. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C, Mackey MM, Diaz AA, Cox DP. Hydroxyl radical is produced via the fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 12.Brandes RP, Weissmann N, Schroder K. Redox-mediated signal transduction by cardiovascular nox NADPH oxidases. J Mol Cell Cardiol. 2014;73C:70–79. doi: 10.1016/j.yjmcc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Doehner W, Landmesser U. Xanthine oxidase and uric acid in cardiovascular disease: Clinical impact and therapeutic options. Semin Nephrol. 2011;31:433–440. doi: 10.1016/j.semnephrol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Andreou A, Feussner I. Lipoxygenases - structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Mehrabian M, Allayee H, Wong J, Shi W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 18.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-lipoxygenase gene disruption attenuates atherogenesis in ldl receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- 19.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerke U, Rolle S, Purfurst B, Luft FC, Nauseef WM, Kettritz R. Beta2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cells. J Biol Chem. 2013;288:12910–12919. doi: 10.1074/jbc.M112.434613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 22.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr A, Hussein D, Nandi M. The regulation of vascular tetrahydrobiopterin bioavailability. Vascul Pharmacol. 2013;58:219–230. doi: 10.1016/j.vph.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (enos) expression and preventing enos uncoupling. Br J Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:Ubiquinone oxidoreductase (complex i) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller FL, Liu Y, Van Remmen H. Complex iii releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 30.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen T, Nioi P, Pickett CB. The nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 34.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R. The gi-gpx gene is a target for nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of nrf2 and is up-regulated by hypoxia/reoxygenation: Implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 36.Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of the thioredoxin gene by nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor keap1 functions as an adaptor for cul3-based e3 ligase to regulate proteasomal degradation of nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velichkova M, Hasson T. Keap1 regulates the oxidation-sensitive shuttling of nrf2 into and out of the nucleus via a crm1-dependent nuclear export mechanism. Mol Cell Biol. 2005;25:4501–4513. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in cuznsod-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of cuzn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groleau J, Dussault S, Haddad P, Turgeon J, Menard C, Chan JS, Rivard A. Essential role of copper-zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:2173–2181. doi: 10.1161/ATVBAHA.110.212530. [DOI] [PubMed] [Google Scholar]
- 42.Wang HD, Johns DG, Xu S, Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H1697–1702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial sod deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol (1985) 2007;102:255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 44.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 45.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 47.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular sod in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 48.Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alfonso-Prieto M, Biarnes X, Vidossich P, Rovira C. The molecular mechanism of the catalase reaction. J Am Chem Soc. 2009;131:11751–11761. doi: 10.1021/ja9018572. [DOI] [PubMed] [Google Scholar]
- 50.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 51.Hodara R, Weiss D, Joseph G, Velasquez-Castano JC, Landazuri N, Han JW, Yoon YS, Taylor WR. Overexpression of catalase in myeloid cells causes impaired postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2011;31:2203–2209. doi: 10.1161/ATVBAHA.111.233247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parastatidis I, Weiss D, Joseph G, Taylor WR. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2013;33:2389–2396. doi: 10.1161/ATVBAHA.113.302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M. Glutathione peroxidase family - an evolutionary overview. FEBS J. 2008;275:3959–3970. doi: 10.1111/j.1742-4658.2008.06542.x. [DOI] [PubMed] [Google Scholar]
- 54.Forgione MA, Cap A, Liao R, Moldovan NI, Eberhardt RT, Lim CC, Jones J, Goldschmidt-Clermont PJ, Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 55.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forgione MA, Weiss N, Heydrick S, Cap A, Klings ES, Bierl C, Eberhardt RT, Farber HW, Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida T, Maulik N, Engelman RM, Ho YS, Magnenat JL, Rousou JA, Flack JE, 3rd, Deaton D, Das DK. Glutathione peroxidase knockout mice are susceptible to myocardial ischemia reperfusion injury. Circulation. 1997;96:II-216–220. [PubMed] [Google Scholar]
- 58.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Brixius K, Schwinger RH, Hoyer F, Napp A, Renner R, Bolck B, Kumin A, Fischer U, Mehlhorn U, Werner S, Bloch W. Isoform-specific downregulation of peroxiredoxin in human failing myocardium. Life Sci. 2007;81:823–831. doi: 10.1016/j.lfs.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 61.Abbasi A, Corpeleijn E, Postmus D, Gansevoort RT, de Jong PE, Gans RO, Struck J, Schulte J, Hillege HL, van der Harst P, Peelen LM, Beulens JW, Stolk RP, Navis G, Bakker SJ. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J Am Heart Assoc. 2012;1:e002956. doi: 10.1161/JAHA.112.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, Fisher AB. Peroxiredoxin 6 phosphorylation and subsequent phospholipase a2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–11706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collet JF, Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxid Redox Signal. 2010;13:1205–1216. doi: 10.1089/ars.2010.3114. [DOI] [PubMed] [Google Scholar]
- 64.Takagi Y, Gon Y, Todaka T, Nozaki K, Nishiyama A, Sono H, Hashimoto N, Kikuchi H, Yodoi J. Expression of thioredoxin is enhanced in atherosclerotic plaques and during neointima formation in rat arteries. Lab Invest. 1998;78:957–966. [PubMed] [Google Scholar]
- 65.Shioji K, Nakamura H, Masutani H, Yodoi J. Redox regulation by thioredoxin in cardiovascular diseases. Antioxid Redox Signal. 2003;5:795–802. doi: 10.1089/152308603770380106. [DOI] [PubMed] [Google Scholar]
- 66.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived h2o2 mediates endoplasmic reticulum signaling through local ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307:H945–957. doi: 10.1152/ajpheart.00918.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spencer NY, Engelhardt JF. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry. 2014;53:1551–1564. doi: 10.1021/bi401719r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal nox2 facilitates redox-dependent induction of nf-kappab by tnf-alpha. Antioxid Redox Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by nox4-produced h2o2. Am J Physiol Cell Physiol. 2009;296:C711–723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- 74.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK. NADPH oxidase 4 mediates TGF-beta-induced smooth muscle alpha-actin via p38mapk and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lundquist MR, Storaska AJ, Liu TC, Larsen SD, Evans T, Neubig RR, Jaffrey SR. Redox modification of nuclear actin by mical-2 regulates srf signaling. Cell. 2014;156:563–576. doi: 10.1016/j.cell.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nadworny AS, Guruju MR, Poor D, Doran RM, Sharma RV, Kotlikoff MI, Davisson RL. Nox2 and nox4 influence neonatal c-kit(+) cardiac precursor cell status and differentiation. Am J Physiol Heart Circ Physiol. 2013;305:H829–842. doi: 10.1152/ajpheart.00761.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray TV, Smyrnias I, Shah AM, Brewer AC. NADPH oxidase 4 regulates cardiomyocyte differentiation via redox activation of c-jun protein and the cis-regulation of gata-4 gene transcription. J Biol Chem. 2013;288:15745–15759. doi: 10.1074/jbc.M112.439844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003;5:781–788. doi: 10.1089/152308603770380089. [DOI] [PubMed] [Google Scholar]
- 80.Zeller KS, Riaz A, Sarve H, Li J, Tengholm A, Johansson S. The role of mechanical force and ros in integrin-dependent signals. PLoS One. 2013;8:e64897. doi: 10.1371/journal.pone.0064897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang S, Yip R, Polena S, Sharma M, Rao S, Griciene P, Gintautas J, Jerome H. Reactive oxygen species increased focal adhesion kinase production in pulmonary microvascular endothelial cells. Proc West Pharmacol Soc. 2004;47:54–56. [PubMed] [Google Scholar]
- 82.Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- 83.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of rhoa by reactive oxygen species requires a redox-sensitive motif. PLoS One. 2009;4:e8045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dodd T, Wiggins L, Hutcheson R, Smith E, Musiyenko A, Hysell B, Russell JC, Rocic P. Impaired coronary collateral growth in the metabolic syndrome is in part mediated by matrix metalloproteinase 12-dependent production of endostatin and angiostatin. Arterioscler Thromb Vasc Biol. 2013;33:1339–1349. doi: 10.1161/ATVBAHA.113.301533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 86.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 87.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, San Martin A. Platelet-derived growth factor (pdgf) regulates slingshot phosphatase activity via nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem. 2011;286:35430–35437. doi: 10.1074/jbc.M111.268284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by lim kinase and slingshot-1l phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res. 2008;102:432–438. doi: 10.1161/CIRCRESAHA.107.158923. [DOI] [PubMed] [Google Scholar]
- 89.de Rezende FF, Martins Lima A, Niland S, Wittig I, Heide H, Schroder K, Eble JA. Integrin alpha7beta1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic Biol Med. 2012;53:521–531. doi: 10.1016/j.freeradbiomed.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 90.Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: Two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- 91.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 92.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a fak tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell-matrix adhesion. J Biol Chem. 2006;281:15593–15596. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- 94.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 95.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1b in a431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 97.San Martin A, Griendling KK. NADPH oxidases: Progress and opportunities. Antioxid Redox Signal. 2014;20:2692–2694. doi: 10.1089/ars.2014.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 99.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 100.Heo J, Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active rho gtpases. J Biol Chem. 2005;280:31003–31010. doi: 10.1074/jbc.M504768200. [DOI] [PubMed] [Google Scholar]
- 101.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate rhoa/rho kinase-induced ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nelson KK, Subbaram S, Connor KM, Dasgupta J, Ha XF, Meng TC, Tonks NK, Melendez JA. Redox-dependent matrix metalloproteinase-1 expression is regulated by jnk through ets and ap-1 promoter motifs. J Biol Chem. 2006;281:14100–14110. doi: 10.1074/jbc.M601820200. [DOI] [PubMed] [Google Scholar]
- 103.Lee SJ, Seo KW, Yun MR, Bae SS, Lee WS, Hong KW, Kim CD. 4-hydroxynonenal enhances mmp-2 production in vascular smooth muscle cells via mitochondrial ros-mediated activation of the Akt/nf-kappab signaling pathways. Free Radic Biol Med. 2008;45:1487–1492. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 104.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 105.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and cd47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via cd36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. Cd36 participates in a signaling pathway that regulates ros formation in murine vsmcs. J Clin Invest. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ. Thrombospondin-1 regulates blood flow via cd47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. Cgmp-dependent activation of protein kinase g precludes disulfide activation: Implications for blood pressure control. Hypertension. 2012;60:1301–1308. doi: 10.1161/HYPERTENSIONAHA.112.198754. [DOI] [PubMed] [Google Scholar]
- 112.Chen CA, De Pascali F, Basye A, Hemann C, Zweier JL. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry. 2013;52:6712–6723. doi: 10.1021/bi400404s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem. 2013;288:561–569. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC, Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem. 2002;277:48311–48317. doi: 10.1074/jbc.M208884200. [DOI] [PubMed] [Google Scholar]
- 115.Tong X, Hou X, Jourd’heuil D, Weisbrod RM, Cohen RA. Upregulation of nox4 by TGF{beta}1 oxidizes serca and inhibits no in arterial smooth muscle of the prediabetic zucker rat. Circ Res. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal. 2014;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 118.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wolin MS, Rodenburg JM, Messina EJ, Kaley G. Oxygen metabolites and vasodilator mechanisms in rat cremasteric arterioles. Am J Physiol. 1987;252:H1159–1163. doi: 10.1152/ajpheart.1987.252.6.H1159. [DOI] [PubMed] [Google Scholar]
- 120.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2o2-induced dilation in human coronary arterioles: Role of protein kinase g dimerization and large-conductance ca2+-activated k+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel D, Alhawaj R, Wolin MS. Exposure of mice to chronic hypoxia attenuates pulmonary arterial contractile responses to acute hypoxia by increases in extracellular hydrogen peroxide. Am J Physiol Regul Integr Comp Physiol. 2014;307:R426–433. doi: 10.1152/ajpregu.00257.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heinle H. Vasoconstriction of carotid artery induced by hydroperoxides. Arch Int Physiol Biochim. 1984;92:267–271. doi: 10.3109/13813458409071166. [DOI] [PubMed] [Google Scholar]
- 123.Touyz RM, Deschepper C, Park JB, He G, Chen X, Neves MFT, Virdis A, Schiffrin EL. Inhibition of mitogen-activated protein/extracellular signal-regulated kinase improves endothelial function and attenuates ang ii-induced contractility of mesenteric resistance arteries from spontaneously hypertensive rats. J Hypertens. 2002;20:1127–1134. doi: 10.1097/00004872-200206000-00024. [DOI] [PubMed] [Google Scholar]
- 124.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. P38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 125.Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. P38 map kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H741–751. doi: 10.1152/ajpheart.2000.279.2.H741. [DOI] [PubMed] [Google Scholar]
- 126.Jin L, Ying Z, Webb RC. Activation of rho/rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 127.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H13–21. doi: 10.1152/ajpheart.00629.2004. [DOI] [PubMed] [Google Scholar]
- 128.Ahmad M, Zhao X, Kelly MR, Kandhi S, Perez O, Abraham NG, Wolin MS. Heme oxygenase-1 induction modulates hypoxic pulmonary vasoconstriction through upregulation of ecsod. Am J Physiol Heart Circ Physiol. 2009;297:H1453–1461. doi: 10.1152/ajpheart.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amberg GC, Earley S, Glapa SA. Local regulation of arterial l-type calcium channels by reactive oxygen species. Circ Res. 2010;107:1002–1010. doi: 10.1161/CIRCRESAHA.110.217018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. 2008;456:1085–1095. doi: 10.1007/s00424-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fearon IM, Palmer AC, Balmforth AJ, Ball SG, Varadi G, Peers C. Modulation of recombinant human cardiac l-type ca2+ channel alpha1c subunits by redox agents and hypoxia. J Physiol. 1999;514 (Pt 3):629–637. doi: 10.1111/j.1469-7793.1999.629ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Noh CI, Lee SH, Ho WK. L-type ca(2+) channel facilitation mediated by h(2)o(2)-induced activation of camkii in rat ventricular myocytes. J Mol Cell Cardiol. 2010;48:773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 133.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac l-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 134.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for cav1.2 phosphorylation by protein kinase c isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 135.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of camkii by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abramson JJ, Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21:283–294. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- 137.Liu G, Abramson JJ, Zable AC, Pessah IN. Direct evidence for the existence and functional role of hyperreactive sulfhydryls on the ryanodine receptor-triadin complex selectively labeled by the coumarin maleimide 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin. Mol Pharmacol. 1994;45:189–200. [PubMed] [Google Scholar]
- 138.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hinata M, Matsuoka I, Iwamoto T, Watanabe Y, Kimura J. Mechanism of na+/ca2+ exchanger activation by hydrogen peroxide in guinea-pig ventricular myocytes. J Pharmacol Sci. 2007;103:283–292. doi: 10.1254/jphs.fp0060015. [DOI] [PubMed] [Google Scholar]
- 140.Liu T, O’Rourke B. Regulation of the na+/ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J Biol Chem. 2013;288:31984–31992. doi: 10.1074/jbc.M113.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Santacruz-Toloza L, Ottolia M, Nicoll DA, Philipson KD. Functional analysis of a disulfide bond in the cardiac na(+)-ca(2+) exchanger. J Biol Chem. 2000;275:182–188. doi: 10.1074/jbc.275.1.182. [DOI] [PubMed] [Google Scholar]
- 142.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 143.Qin F, Siwik DA, Lancel S, Zhang J, Kuster GM, Luptak I, Wang L, Tong X, Kang YJ, Cohen RA, Colucci WS. Hydrogen peroxide-mediated serca cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates serca in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 147.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiu J, Dawes IW. Redox control of cell proliferation. Trends Cell Biol. 2012;22:592–601. doi: 10.1016/j.tcb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 149.Chen CY, Willard D, Rudolph J. Redox regulation of sh2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48:1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 150.Chiarugi P. The redox regulation of lmw-ptp during cell proliferation or growth inhibition. IUBMB Life. 2001;52:55–59. doi: 10.1080/15216540252774775. [DOI] [PubMed] [Google Scholar]
- 151.Giannoni E, Chiarugi P. Redox circuitries driving src regulation. Antioxid Redox Signal. 2014;20:2011–2025. doi: 10.1089/ars.2013.5525. [DOI] [PubMed] [Google Scholar]
- 152.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor pten in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fata JE, Debnath S, Jenkins EC, Jr, Fournier MV. Nongenomic mechanisms of pten regulation. Int J Cell Biol. 2012;2012:379685. doi: 10.1155/2012/379685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol. 2009;4:783–799. doi: 10.1021/cb900105q. [DOI] [PubMed] [Google Scholar]
- 155.Antico Arciuch VG, Galli S, Franco MC, Lam PY, Cadenas E, Carreras MC, Poderoso JJ. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS One. 2009;4:e7523. doi: 10.1371/journal.pone.0007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 mapk and mapkapk-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494–499. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- 157.Butturini E, Darra E, Chiavegato G, Cellini B, Cozzolino F, Monti M, Pucci P, Dell’Orco D, Mariotto S. S-glutathionylation at cys328 and cys542 impairs STAT3 phosphorylation. ACS Chem Biol. 2014 doi: 10.1021/cb500407d. [DOI] [PubMed] [Google Scholar]
- 158.Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-jun DNA binding by reversible s-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 159.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 162.Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, Colucci WS. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:131–139. doi: 10.1006/jmcc.2000.1285. [DOI] [PubMed] [Google Scholar]
- 163.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 164.Tu VC, Bahl JJ, Chen QM. Signals of oxidant-induced cardiomyocyte hypertrophy: Key activation of p70 s6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther. 2002;300:1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
- 165.Kuster GM, Pimentel DR, Adachi T, Ido Y, Brenner DA, Cohen RA, Liao R, Siwik DA, Colucci WS. Alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 166.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by h2o2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 167.Wei S, Rothstein EC, Fliegel L, Dell’Italia LJ, Lucchesi PA. Differential map kinase activation and na(+)/h(+) exchanger phosphorylation by h(2)o(2) in rat cardiac myocytes. Am J Physiol Cell Physiol. 2001;281:C1542–1550. doi: 10.1152/ajpcell.2001.281.5.C1542. [DOI] [PubMed] [Google Scholar]
- 168.Zhao X, Zhang Y, Li X, Wang R, Jiao X. Variations of thioredoxin system contributes to increased susceptibility to apoptosis in cardiomyocytes of type 2 diabetic rats. Acta Biochim Biophys Sin (Shanghai) 2014;46:318–329. doi: 10.1093/abbs/gmu006. [DOI] [PubMed] [Google Scholar]
- 169.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase b by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 170.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 171.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liao B, Zheng YM, Yadav VR, Korde AS, Wang YX. Hypoxia induces intracellular ca2+ release by causing reactive oxygen species-mediated dissociation of fk506-binding protein 12.6 from ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal. 2011;14:37–47. doi: 10.1089/ars.2009.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ros]i and [ca2+]i through the mitochondrial ros-pkcepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ganafa AA, Walton M, Eatman D, Abukhalaf IK, Bayorh MA. Amlodipine attenuates oxidative stress-induced hypertension. Am J Hypertens. 2004;17:743–748. doi: 10.1016/j.amjhyper.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 175.Yasunari K, Maeda K, Nakamura M, Watanabe T, Yoshikawa J. Benidipine, a long-acting calcium channel blocker, inhibits oxidative stress in polymorphonuclear cells in patients with essential hypertension. Hypertens Res. 2005;28:107–112. doi: 10.1291/hypres.28.107. [DOI] [PubMed] [Google Scholar]
- 176.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and pkr activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum ca(2+) atpase in diabetic pig aorta. Free Radic Biol Med. 2008;45:756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Molkentin JD. Calcineurin-nfat signaling regulates the cardiac hypertrophic response in coordination with the mapks. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 180.Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated t-lymphocytes and fas ligand transcription: Role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Friedman JK, Nitta CH, Henderson KM, Codianni SJ, Sanchez L, Ramiro-Diaz JM, Howard TA, Giermakowska W, Kanagy NL, Gonzalez Bosc LV. Intermittent hypoxia-induced increases in reactive oxygen species activate nfatc3 increasing endothelin-1 vasoconstrictor reactivity. Vascul Pharmacol. 2014;60:17–24. doi: 10.1016/j.vph.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Niecknig H, Tug S, Reyes BD, Kirsch M, Fandrey J, Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of hif-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 183.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a hif binding site located in its first intron and by the central factors gata-2 and ets-1. J Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 184.Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, Kim J, Kang SW. Peroxiredoxin ii is an essential antioxidant enzyme that prevents the oxidative inactivation of vegf receptor-2 in vascular endothelial cells. Mol Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 185.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1b in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1b. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 187.Garrett TA, Van Buul JD, Burridge K. Vegf-induced rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. Iqgap1 mediates ve-cadherin-based cell-cell contacts and vegf signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 189.Lyle AN, Remus EW, Fan AE, Lassegue B, Walter GA, Kiyosue A, Griendling KK, Taylor WR. Hydrogen peroxide regulates osteopontin expression through activation of transcriptional and translational pathways. J Biol Chem. 2014;289:275–285. doi: 10.1074/jbc.M113.489641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lyle AN, Joseph G, Fan AE, Weiss D, Landazuri N, Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2012;32:1383–1391. doi: 10.1161/ATVBAHA.112.248922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 194.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 195.Laurindo FR, Pescatore LA, de Fernandes DC. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic Biol Med. 2012;52:1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 196.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: Folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 197.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 198.Rutkevich LA, Cohen-Doyle MF, Brockmeier U, Williams DB. Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol Biol Cell. 2010;21:3093–3105. doi: 10.1091/mbc.E10-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laurindo FR, Araujo TL, Abrahao TB. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid Redox Signal. 2014;20:2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gross E, Kastner DB, Kaiser CA, Fass D. Structure of ero1p, source of disulfide bonds for oxidative protein folding in the cell. Cell. 2004;117:601–610. doi: 10.1016/s0092-8674(04)00418-0. [DOI] [PubMed] [Google Scholar]
- 201.Toldo S, Severino A, Abbate A, Baldi A. The role of pdi as a survival factor in cardiomyocyte ischemia. Methods Enzymol. 2011;489:47–65. doi: 10.1016/B978-0-12-385116-1.00003-0. [DOI] [PubMed] [Google Scholar]
- 202.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 204.Fernandes DC, Manoel AH, Wosniak J, Jr, Laurindo FR. Protein disulfide isomerase overexpression in vascular smooth muscle cells induces spontaneous preemptive NADPH oxidase activation and nox1 mrna expression: Effects of nitrosothiol exposure. Arch Biochem Biophys. 2009;484:197–204. doi: 10.1016/j.abb.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 205.Janiszewski M, Lopes LR, Carmo AO, Pedro MA, Brandes RP, Santos CX, Laurindo FR. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 206.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ros signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, Okada M, Guan KL, Inoki K. Redox regulates mammalian target of rapamycin complex 1 (mtorc1) activity by modulating the tsc1/tsc2-rheb gtpase pathway. J Biol Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 209.Martinet W, Verheye S, De Meyer GR. Everolimus-induced mtor inhibition selectively depletes macrophages in atherosclerotic plaques by autophagy. Autophagy. 2007;3:241–244. doi: 10.4161/auto.3711. [DOI] [PubMed] [Google Scholar]
- 210.Hariharan N, Ikeda Y, Hong C, Alcendor RR, Usui S, Gao S, Maejima Y, Sadoshima J. Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One. 2013;8:e51632. doi: 10.1371/journal.pone.0051632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: Role of the terminal complement components and inhibition by anti-c5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 212.Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Curr Pharm Biotechnol. 2012;13:97–114. doi: 10.2174/138920112798868782. [DOI] [PubMed] [Google Scholar]
- 213.Gonzalez A, Fortuno MA, Querejeta R, Ravassa S, Lopez B, Lopez N, Diez J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–562. doi: 10.1016/s0008-6363(03)00498-x. [DOI] [PubMed] [Google Scholar]
- 214.Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell. 2008;7:125–136. doi: 10.1111/j.1474-9726.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 215.Erusalimsky JD. Vascular endothelial senescence: From mechanisms to pathophysiology. J Appl Physiol (1985) 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mistry Y, Poolman T, Williams B, Herbert KE. A role for mitochondrial oxidants in stress-induced premature senescence of human vascular smooth muscle cells. Redox Biol. 2013;1:411–417. doi: 10.1016/j.redox.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 218.Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of bcl-xl. Mol Cell. 2014;54:639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 220.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor PM, Anderson CW, Appella E. A p53-independent pathway for activation of waf1/cip1 expression following oxidative stress. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 221.Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. P53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 222.Chen Y, Brandizzi F. Ire1: Er stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee JE, Park JH, Shin IC, Koh HC. Reactive oxygen species regulated mitochondria-mediated apoptosis in pc12 cells exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2012;263:148–162. doi: 10.1016/j.taap.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 225.Dhanasekaran DN, Reddy EP. Jnk signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ros) control the expression of bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004;95:644–650. doi: 10.1111/j.1349-7006.2004.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 229.Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H, Sasagawa S, Hsieh JJ, Cheng EH. The p53-cathepsin axis cooperates with ros to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci U S A. 2009;106:1093–1098. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shen HM, Pervaiz S. Tnf receptor superfamily-induced cell death: Redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 231.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote tnfalpha-induced death and sustained jnk activation by inhibiting map kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 232.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and nf-kappab signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kolamunne RT, Dias IH, Vernallis AB, Grant MM, Griffiths HR. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox Biol. 2013;1:418–426. doi: 10.1016/j.redox.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brewer AC, Murray TV, Arno M, Zhang M, Anilkumar NP, Mann GE, Shah AM. Nox4 regulates nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med. 2011;51:205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. P22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 237.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvement of the small gtpase rac in activation of the superoxide-producing NADPH oxidase nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 239.Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, et al. Pdgf stimulates an increase in gtp-rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 240.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, Antenucci G, Mascio G, Bettarini U, Landolfi A, Iorio L, Maffei A, Lembo G. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betapix/rac-1 pathway. Hypertension. 2009;54:1028–1034. doi: 10.1161/HYPERTENSIONAHA.109.136572. [DOI] [PubMed] [Google Scholar]
- 241.Lynch SM, Frei B, Morrow JD, Roberts LJ, 2nd, Xu A, Jackson T, Reyna R, Klevay LM, Vita JA, Keaney JF., Jr Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol. 1997;17:2975–2981. doi: 10.1161/01.atv.17.11.2975. [DOI] [PubMed] [Google Scholar]
- 242.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11:1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Heo KS, Fujiwara K, Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J. 2011;75:2722–2730. doi: 10.1253/circj.cj-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 246.Feng S, Yang Q, Liu M, Li W, Yuan W, Zhang S, Wu B, Li J. Edaravone for acute ischaemic stroke. Cochrane Database Syst Rev. 2011:CD007230. doi: 10.1002/14651858.CD007230.pub2. [DOI] [PubMed] [Google Scholar]
- 247.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.San Martin A, Foncea R, Laurindo FR, Ebensperger R, Griendling KK, Leighton F. Nox1-based NADPH oxidase-derived superoxide is required for vsmc activation by advanced glycation end-products. Free Radic Biol Med. 2007;42:1671–1679. doi: 10.1016/j.freeradbiomed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 250.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 251.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: Implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 252.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]