Abstract
1) Objective
The mechanism responsible for increased fetal hemoglobin (HbF) levels following decitabine treatment remains controversial. These experiments were performed to evaluate the role of transcriptional versus translational mechanisms in the ability of decitabine to increase HbF levels in vivo.
2) Methods
Three normal, nonanemic baboons were treated with decitabine subcutaneously (0.5mg/kg/d) for 10 days. The effect of decitabine on globin chain synthesis and globin mRNA levels was measured in pre- and post-treatment bone marrow (BM) aspirates by biosynthetic radiolabelling with [3H] leucine followed by separation of globin chains by HPLC, and real time PCR, respectively. The effect on DNA methylation of the ε- and γ-globin gene promoters was determined by bisulfite sequence analysis.
3) Results
Decitabine treatment of normal, nonanemic baboons induced similar increases in the γ/γ+β chain synthetic ratio and the γ/total β-like globin RNA ratio and also increased expression of ε-globin transcripts. Increased expression of ε- and γ-globin was associated with decreased DNA methylation of the ε- and γ-globin gene promoters.
4) Conclusion
Decitabine increases HbF in vivo by transcriptional activation of the γ-globin gene.
Introduction
Increased fetal hemoglobin (HbF) alleviates the symptoms associated with sickle cell disease and β-thalassemia increases the life span of patients (1, 2). The ability of decitabine (5-aza-2′-deoxycytidine) to increase HbF levels (>20% HbF) and F cell numbers (>80%) in patients with sickle cell disease suggests that this drug may be a useful pharmacological agent in the treatment of this disease (3).
Although the ability of pharmacological inhibitors of DNA methyltransferase (DNA MTase) such as decitabine and 5-azacytidine to increase HbF has been known for nearly thirty years, the mechanism remains controversial. Evidence from multiple studies has implicated DNA methylation of the γ-globin gene promoter in repression of γ-globin gene expression. These include 1) a correlation between DNA hypomethylation of the γ-globin promoter and γ-globin gene expression during development (4, 5, 6), 2) phylogenetic footprinting studies showing acquisition of CpG residues in the 5′ γ-globin promoter region coincident with the recruitment of the γ-globin gene to a fetal-stage expression pattern during the evolution of simian primates (7), and 3) the ability of DNA MTase inhibitors to increase HbF levels in experimental primates and patients with β-thalassemia and sickle cell disease (3, 8-14). The mechanism responsible for DNA methyltransferase (DNA MTase) inhibitors to increase HbF was therefore hypothesized to involve a reduction in the level of DNA methylation of the γ-globin gene promoter, thus alleviating DNA methylation-mediated gene repression and allowing high levels of γ-globin transcription.
The effects of DNA MTase inhibitors are not limited to DNA methylation, however, and other mechanisms have been proposed to explain the ability of DNA MTase inhibitors to increase HbF. These drugs can alter the kinetics of erythroid differentiation by inducing terminal erythroid differentiation in a population of more primitive progenitors capable of expressing higher levels of HbF (15). Transcription factors such as CREB and ATF2 that bind to sequences near the γ-globin gene promoter (20) can be activated by DNA damage and stress signaling pathways following incorporation of DNA MTase inhibitors into DNA (16-19). While stress signaling has been proposed as a general mechanism for HbF induction by a variety of agents (21), the dual mechanism model states that drugs such as histone deacetylase inhibitors and DNA MTase inhibitors increase γ-globin transcription by alleviating the repressive chromatin state of the γ-globin promoter to allowing access to transcription factors activated by stress signaling pathways (22). Recent data from cell culture systems has suggested the hypothesis that decitabine increases γ-globin expression by post-transcriptional and/or translational mechanisms associated with activation of stress-signal transduction pathways rather than through direct transcriptional activation (21, 23, 24). The objective of our studies was to investigate the role of transcriptional and translational control mechanisms in the ability of decitabine to increase HbF in vivo in order to address differences between these proposed mechanisms.
Materials and Methods
Animals
Three normal, unbled baboons (P. anubis) were treated with decitabine (0.5mg/kg/d; sc) for ten days. Pre-treatment BM aspirates were obtained prior to treatment and post-treatment BM aspirates were obtained on the day following the last decitabine injection for analysis of globin chain synthesis and globin mRNA levels. All baboon treatments were performed with approval of the Animal Care Committee of the University of Illinois at Chicago.
Purification of BM Erythroid Precursor Cells
Low-density mononuclear cells were enriched from BM aspirates by Percoll gradient sedimentation. Erythroid cells were purified by magnetic column separation using a mouse monoclonal antibody to baboon erythrocytes (BD Bioscience) in combination with anti-mouse IgG1 microbeads (Miltenyi) as previously described (6). Purity of cell purifications were evaluated by microscopic examination of Wright's stained cytospin preparations.
Measurement of Globin Chain Synthesis
Globin chain synthesis in pre-treatment and post-treatment BM erythroid cells was determined by biosynthetic radiolabelling of globin chains in the presence of [3H] leucine (25). Purified BM erythroid precursor cells (1 × 106) were incubated overnight at 37°C in 1ml leucine-free αMEM (Invitrogen) containing 20% dialyzed fetal bovine serum and 50μCi L-[4, 5-3H] leucine. Cells were lysed by multiple freeze-thaw cycles in dry ice methanol baths. Globin chain separation was achieved by HPLC using a LithoCART 250-4 column (VWR) and a Spectra System HPLC (Thermo-Finnegan with acetonitrile-methanol gradients (26). Quantitation of radioactivity in collected fractions was determined by liquid scintillation counting using a Packard Tricarb 1600TR liquid scintillation analyzer. Results are expressed as γ/γ+β chain ratios.
Real Time PCR Analysis of Globin mRNA
RNA was purified from BM erythroid precursor cells using the RNeasy Mini kit (Qiagen) according to manufacturer's instructions. RNA was treated with DNase I (Ambion) and used to prepare cDNA using kits according to the instructions of the manufacturers (Fermentas). Custom designed primer-probe combinations from Applied Biosystems were used for the analysis of ε- γ-β- and α-globin gene expression. The sequences of these are also listed in Supplemental Data. Absolute numbers of globin transcripts were determined by extrapolation from standard curves prepared from the cloned amplicons. Results were expressed as a ratio of total β-like (ε+γ+β)-globin mRNA. Statistical significance was assessed using a two-tailed T test for either paired or unpaired observations.
Bisulfite Sequence Analysis
Genomic DNA was extracted using the QIAmp DNA Blood Mini Kit (Qiagen) according to manufacturer's instructions. Bisulfite conversion was performed using the EpiTect Bisulfite kit (Qiagen). PCR amplification of the baboon ε- and γ-globin promoter regions from bisulfite converted DNA was performed as previously described (6). The PCR products were cloned in the pCR4-TOPO vector using a TOPO-TA cloning kit (Invitrogen) and E.coli TOP10 cells for transformation. Clones were selected on LB plates containing ampicillin and minilysate DNA from individual clones was sequenced using an ABI PRISM 3100 genetic analyzer at the University of Illinois at Chicago Core Genomic Facility. At least ten clones were sequenced for each sample.
Results
Effect of decitabine on globin chain synthesis
Three normal nonanemic baboons were treated with decitabine (0.5mg/kg/d; 10d; sc). The effect on globin chain synthesis was measured by biosynthetic radiolabelling of purified erythroid precursor cells isolated from pre-and post-treatment BM aspirates. The globin chains in radiolabelled lysates were separated by HPLC and radioactivity in the separated fractions determined. The γ/γ+β chain ratio was low in the pre-treatment samples (0.04±0.05; mean±SD; Table 1). One animal, PA 7470, did exhibit an unexpectedly high γ/γ+β ratio (0.10) in the pretreatment sample, while γ/γ+β chain ratios in the other two animals reflected the normal low level of HbF synthesis. The γ/γ+β chain ratio was increased in all three animals following the ten day course of decitabine treatment (0.41±0.15; mean±SD; Table 1).
Table 1. Effect of decitabine on globin chain synthesis in vivo.
| Globin chain synthesis (γ/γ+β) | ||
|---|---|---|
|
| ||
| Animal | Pre | Post |
| PA 7482 | 0.01 | 0.51 |
| PA7470 | 0.10 | 0.49 |
| PA 7472 | 0.01 | 0.24 |
|
| ||
| Mean±SD | 0.04±0.01 | 0.41±0.15 |
Effect of decitabine on globin gene transcription
The effect of decitabine on globin gene expression was determined by real time PCR analysis of RNA isolated from pre- and post-treatment purified BM erythroid precursor cells. The ratio of ε-globin RNA / total β-like globin RNA was increased following decitabine treatment (.014±.0007; mean ±SD; p<.01, Table 2) compared to pre-treatment (.0006±.0002). Likewise, the γ-globin / total β-like globin ratio was increased following decitabine treatment (0.378±.173; p<.05, Table 2) compared to pre-treatment (0.065±.081), while the β-globin / total β-like globin RNA ratio was decreased following decitabine treatment (0.505±.054; p<.01, Table 2) compared to the pre-treatment level (0.934±.081). No significant difference in the ratio of α-globin/ total β-like globin RNA was observed between the pre-treatment and post-treatment samples.
Table 2. Effect of decitabine on globin mRNA expression in vivo.
| Globin mRNA expression (mRNA/total β-like globin mRNA) |
||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Animal | ε | γ | β | |||
| Pre | Post | Pre | Post | Pre | Post | |
| PA 7482 | .0008 | .0141 | .026 | .526 | .972 | .457 |
| PA 7470 | .0005 | .0135 | .158 | .421 | .841 | .564 |
| PA 7472 | .0005 | .0149 | .011 | .187 | .988 | .493 |
|
| ||||||
| Mean±SD | .0006±.0002 | .014±.0007 | .065±.081 | .378±.173 | .934±.081 | .505±.054 |
The similarity of the γ/γ+β chain ratio (0.41±0.15) and the γ-globin / total β-like globin RNA ratio (0.378±.173) of the three animals following decitabine treatment shows that increased γ-globin chain synthesis results entirely from increased levels of γ-globin mRNA.
Effect of decitabine on DNA methylation of the γ-globin gene promoter
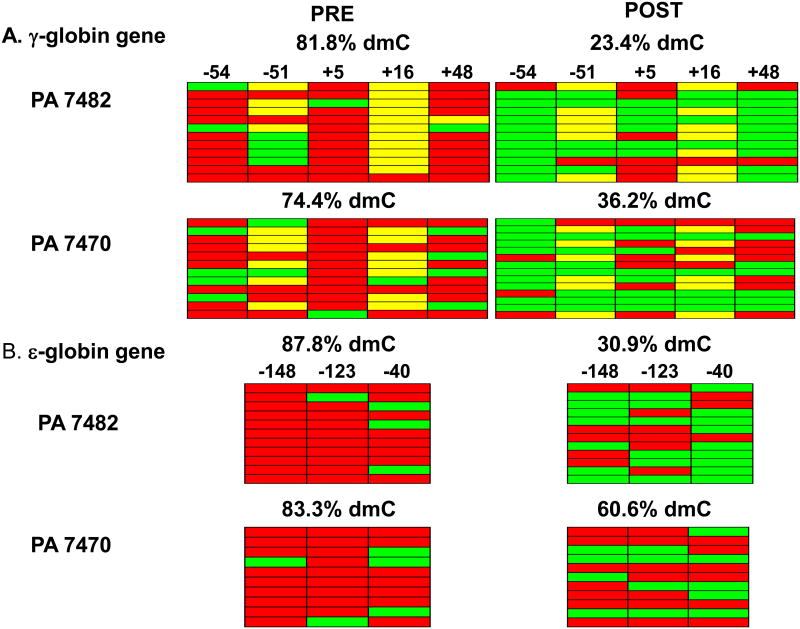
The effect of decitabine on DNA methylation of both the ε- and γ-globin gene promoter regions was analyzed by bisulfite sequencing. Three CpG residues in the 5′ ε-globin promoter region and five CpG sites in the 5′ γ-globin promoter region were analyzed in pre and post-treatment purified BM erythroid precursor cells in 2 of the 3 animals treated. Decreased levels of DNA methylation of the γ-globin promoter were observed in post-treatment samples (23.4, 36.2 % dmC) compared to pretreatment samples (81.8, 74.4% dmC), respectively (Figure 1A). Similar decreases in DNA methylation of the ε-globin gene promoter were also observed in post-treatment (38.9, 60.6% dmC) compared to pre-treatment samples (87.8, 83.3% dmC, respectively (Figure 1B).
Figure 1.
Effect of decitabine on DNA methylation of ε- and γ-globin promoter regions. Results of bisulfite sequence analysis of DNA methylation of CpG residues within the 5′ ε- and γ-globin promoter regions in the BM erythroid precursor cells isolated from two baboons pre- and post-treatment. Each row depicts the results of sequence analysis of a PCR amplicon cloned in pCR4. Methylated residues (Red), unmethylated residues (green), polymorphic sites in the baboon γ-globin promoter where the CpG site is absent (yellow).
Discussion
Decitabine treatment of nonanemic baboons produced similar increases in the γ/ γ+β synthetic chain ratio and the γ-globin/total β-like globin RNA ratio. Therefore, we conclude that decitabine increases HbF levels in vivo by increasing the level of γ-globin mRNA. Our data does not support a role for additional translational effects. These results are consistent with a previous report showing that 5-azacytdine increased γ-globin mRNA 4-6 fold in the BM cells of 8 of 9 patients (27). Previous analysis of the effect of decitabine on association of RNA polymerase II, histone acetyl H3, and H3 trimethyl (lys4) throughout the β-globin gene locus clearly showed that decitabine increased association of RNA polymerase II, acetyl histone H3, and histone H3 trimethyl (lys4) with the γ-globin gene promoter (28). Taken together, these results allow us to conclude that increased γ-globin gene transcription is the primary mechanism responsible for increased HbF levels by decitabine in vivo.
The exact mechanism whereby decitabine increases γ-globin transcription remains unclear. According to the dual mechanism model, activation of stress-signal transduction pathways plays a major role in increased γ-globin transcription (20, 22). Effects of the drug on the kinetics of erythroid differentiation could also play a role in the increased level of γ-globin transcription. Because the capacity to reactivate γ-globin is dependent on the differentiation state of erythroid progenitors, increased γ-globin transcription could thus result from induction of terminal differentiation in more primitive erythroid progenitors that contain a mix of positive and negative transcriptional regulatory proteins favoring γ- over β-globin transcription (29).
The large increases in γ-globin expression in these normal, nonanemic baboons are only slightly less than HbF levels observed in bled baboons treated with decitabine. Therefore, our results also clearly show that erythropoietic stress is not required to reactivate high levels of HbF synthesis in response to decitabine, confirming a previous report from this laboratory (8). Significant increases in HbF have been observed in cancer patients treated with azacytidine and decitabine confirm that erythropoietic stress is not required to achieve increased HbF synthesis in man (30, 31).
Supplementary Material
Acknowledgments
This work was supported by funds from the NIH Basic Translational Research Program in Sickle Cell Disease U54 HL090513 and University of Illinois Comprehensive Sickle Cell Center.
Footnotes
Support and Financial Disclosure Statement: No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.Saunthararajah Y, Hillery CA, Lavelle D, Molokie R, Dorn L, Bressler L, Gavazova S, Chen YH, Hoffman R, DeSimone J. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102:3865–3870. doi: 10.1182/blood-2003-05-1738. [DOI] [PubMed] [Google Scholar]
- 4.van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- 5.Mavilio F, Gaimpaolo A, Care' A, Migliaccio G, Calandrini M, Russo G, Pagliardi GL, Mastroberardino G, Marinucci M, Peschle C. Molecular mechanisms of human hemoglobin switching: Selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci USA. 1983;80:6907–6911. doi: 10.1073/pnas.80.22.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. Developmental changes in DNA methylation and covalent histone modifications of chromatin associated with the ε-, γ-, and β-globin gene promoters in Papio anubis. Blood Cell Mol Dis. 2006;36:269–278. doi: 10.1016/j.bcmd.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Tagle DA, Koop BF, Goodman M, et al. Embryonic and gamma globin genes of a prosimian primate (Galago crassicaudatus). Nucleotide and amino acid sequences, developmental regulation, and phylogenetic footprints. J Mol Biol. 1988;203:439–455. doi: 10.1016/0022-2836(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 8.DeSimone J, Heller P, Hall L, Zwiers D. 5-azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;67:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. The effect of 5-aza-2′-deoxycytidine (Decitabine) on covalent histone modifications of chromatin associated with the ε-, γ-, and β-globin genes in baboon (P. Anubis) Exp Hematol. 2006;34:339–347. doi: 10.1016/j.exphem.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Ley TJ, DeSimone J, Anagnou N, Keller GH, Humphries RK, Turner PH, Young NS, Keller P, Nienhuis AW. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 11.Ley TJ, DeSimone J, Noguchi CT, Turner PH, Schechter AN, Heller P, Nienhuis AW. 5-azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983;62:370–380. [PubMed] [Google Scholar]
- 12.Lowrey CH, Nienhuis AW. Brief report: treatment with azacitidine of patients with end-stage beta-thalassemia. N Engl J Med. 1993;329:845–848. doi: 10.1056/NEJM199309163291205. [DOI] [PubMed] [Google Scholar]
- 13.Koshy M, Dorn L, Bressler L, Molokie R, Lavelle D, Talischy N, Hoffman R, van Overveld W, DeSimone J. 2-Deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96:2379–2384. [PubMed] [Google Scholar]
- 14.DeSimone J, Koshy M, Dorn L, Lavelle D, Bressler L, Molokie R, Talischy N. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood. 2002;99:3905–3908. doi: 10.1182/blood.v99.11.3905. [DOI] [PubMed] [Google Scholar]
- 15.Torealba-de Ron AT, Papayannopoulou Th, Knapp MS, Fu MF, Knitter G, Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984;63:201–210. [PubMed] [Google Scholar]
- 16.Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W, Dai Z, Tong T, Villalona-Calero MA, Plass C, Otterson GA. 5-aza-2′-deoxycytidine activates the p53/p21/Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem. 2003;279:15161–15166. doi: 10.1074/jbc.M311703200. [DOI] [PubMed] [Google Scholar]
- 17.Jiemjit A, Fandy TE, Carraway H, Bailey KA, Baylin S, Herman JG, Gore SD. p21 (WAF1/CIP1) induction of 5-azacytosine nucleosides requires DNA damage. Oncogene. 2008;27:3615–3623. doi: 10.1038/sj.onc.1211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Zhao Y, Li L, McNutt MA, Wu L, Lu S, Yu Y, Zhou W, Feng J, Chai G, Yang Y, Zhu WG. An ATM- and Rad3-related (ATR) signaling pathway and a phosphorylation-acetylation cascade are involved in activation of p53/p21Waf1/Cip1 in response to 5-aza-2′-deoxycytidine treatment. J Biol Chem. 2008;283:2564–2574. doi: 10.1074/jbc.M702454200. [DOI] [PubMed] [Google Scholar]
- 19.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. 2008;28:752–771. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangerman J, Lee MS, Yao X, Oteng E, Hsiao CH, Li W, Zein S, Ofori-Acquah SF, Pace BS. Mechanism for fetal hemoglobin induction by histone deacetylase inhibitors involves gamma-globin activation by CREB1 and ATF-2. Blood. 2006;108:3590–3599. doi: 10.1182/blood-2006-01-023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, Lowrey CH. A cell stress signaling model of fetal hemoglobin induction; what doesn't kill red blood cells may make them stronger. Exp Hematol. 2008;36:1057–1072. doi: 10.1016/j.exphem.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Pace BS, Zein S. Understanding mechanisms of gamma-globin gene regulation to develop strategies for pharmacological fetal hemoglobin induction. Dev Dyn. 2006;235:1727–1737. doi: 10.1002/dvdy.20802. [DOI] [PubMed] [Google Scholar]
- 23.Mabaera R, Greene MR, Richardson CA, Conine SJ, Kozul CD, Lowrey CH. Neither DNA hypomethylation nor changes in the kinetics of erythroid differentiation explain 5-azacytidine's ability to induce human fetal hemoglobin. Blood. 2008;111:411–420. doi: 10.1182/blood-2007-06-093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabaera R, Lowrey CH. 5-azacytidine induces human fetal hemoglobin production through activation of the p38 MAPK and integrated stress response signaling pathways. Blood. 2008;112:489A. [Google Scholar]
- 25.DeSimone J, Heller P, Adams JG. Hemopoietic stress and fetal hemoglobin synthesis: comparative studies in vivo and in vitro. Blood. 1979;54:1176–1181. [PubMed] [Google Scholar]
- 26.Leone L, Monteleone M, Gabutti V, Amione C. Reversed-phase high-performance liquid chromatography of human haemoglobin chains. J Chromatog. 1985;321:407–419. doi: 10.1016/s0021-9673(01)90459-5. [DOI] [PubMed] [Google Scholar]
- 27.Humphries RK, Dover G, Young NS, Moore JG, Charache S, Ley T, Nienhuis AW. 5-azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J Clin Invest. 1985;75:547–557. doi: 10.1172/JCI111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin J, Singh M, Banzon V, Vaitkus K, Ibanez V, Kouznetsova T, Mahmud N, DeSimone J, Lavelle D. Transcriptional activation of the γ-globin gene in baboons treated with decitabine and in cultured erythroid progenitor cells involves different mechanisms. Exp Hematol. 2009;37:1131–1142. doi: 10.1016/j.exphem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papayannopoulou Th, Kalmantis T, Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci USA. 1979;76:6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appleton K, Mackay HJ, Judson I, Plumb JA, McCormick C, Strathdee G, Lee C, Barrett S, Reade S, Jadayel D, Tang A, Bellenger K, MacKay L, Setanoians A, Schatzlein A, Twelves C, Kaye SB, Brown R. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol. 2007;25:4603–4609. doi: 10.1200/JCO.2007.10.8688. [DOI] [PubMed] [Google Scholar]
- 31.Sonpavde G, Aparicio AM, Zhan F, North B, Delaune R, Garbo LE, Rousey SR, Weinstein RE, Xiao L, Boehm KA, Asmar L, Fleming MT, Galsky MD, Berry WR, Von Hoff DD. Azacitidine favorably modulates PSA kinetics correlating with plasma DNA LINE-1 hypomethylation in men with chemonaive castration-resistant prostate cancer. Uro Oncol. 2010 doi: 10.1016/j.urolonc.2009.09.015. epub. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.