Abstract
Background
In the ROCKET AF (Rivaroxaban–Once‐daily, oral, direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) trial, marked regional differences in control of warfarin anticoagulation, measured as the average individual patient time in the therapeutic range (iTTR) of the international normalized ratio (INR), were associated with longer inter‐INR test intervals. The standard Rosendaal approach can produce biased low estimates of TTR after an appropriate dose change if the follow‐up INR test interval is prolonged. We explored the effect of alternative calculations of TTR that more immediately account for dose changes on regional differences in mean iTTR in the ROCKET AF trial.
Methods and Results
We used an INR imputation method that accounts for dose change. We compared group mean iTTR values between our dose change–based method with the standard Rosendaal method and determined that the differences between approaches depended on the balance of dose changes that produced in‐range INRs (“corrections”) versus INRs that were out of range in the opposite direction (“overshoots”). In ROCKET AF, the overall mean iTTR of 55.2% (Rosendaal) increased up to 3.1% by using the dose change–based approach, depending on assumptions. However, large inter‐regional differences in anticoagulation control persisted.
Conclusions
TTR, the standard measure of control of warfarin anticoagulation, depends on imputing daily INR values for the vast majority of follow‐up days. Our TTR calculation method may better reflect the impact of warfarin dose changes than the Rosendaal approach. In the ROCKET AF trial, this dose change–based approach led to a modest increase in overall mean iTTR but did not materially affect the large inter‐regional differences previously reported.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique identifier: NCT00403767.
Keywords: anticoagulants, arrhythmia, embolism, prevention, risk factors
Introduction
The time in the therapeutic range (TTR) of the international normalized ratio (INR) calculated by using the Rosendaal linear interpolation technique has become a widely applied measure of the quality of vitamin K antagonist (VKA) anticoagulant treatment.1–5 Atrial fibrillation (AF) is the most prevalent category of patients treated with VKAs, and for these patients, the standard therapeutic INR range is 2 to 3.6–9 Observational studies have reported strong associations between group means of individual patient TTRs (iTTRs) and reduced risks of ischemic stroke and major bleeding in patients with AF.10–11 Commentaries and guidelines on AF have provided TTR‐based guidance, based on the Rosendaal technique, for deciding whether to continue prescribing VKAs, such as warfarin, or switch to a novel anticoagulant.7–8,12 However, the generalizability of these guidelines throughout the world is uncertain. We and others observed striking variation in average group iTTRs across geographic regions in global clinical trials.13–14 We also found wide variation in the inter‐INR test intervals across regions.13
The Rosendaal method imputes daily INR values between INR tests by using a linear interpolation approach.1 A patient's iTTR is the proportion of days with measured or imputed INR values in a defined therapeutic range. In the face of variation in inter‐INR test intervals, the Rosendaal method may provide a biased measure of group differences in average iTTR. In particular, linear interpolation may not account for dose changes that are not followed shortly thereafter by repeat INR testing.
We hypothesized that if a change in warfarin dose was implemented soon after an out‐of‐range INR test, then regions with longer periods between the out‐of‐range test and the next INR test would have artifactually low mean iTTR levels calculated by using the Rosendaal method because the change in INR due to the dose change would not be fully reflected in the linearly interpolated daily INR value. In this study of patients in the warfarin arm of the ROCKET AF (Rivaroxaban–Once‐daily, oral, direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) clinical trial, we compare the regional mean iTTR values calculated by using the traditional Rosendaal method with an alternative method of calculating TTR that more immediately accounts for changes in dose of warfarin.
Methods
The design, conduct, and main results of the ROCKET AF trial have been presented previously.15–16 In brief, rivaroxaban (20 mg daily, or 15 mg daily in patients with creatinine clearance of 30 to 49 mL/min) was compared with adjusted‐dose warfarin (INR point target of 2.5, range 2.0 to 3.0) for the prevention of stroke or systemic embolism. Patients with electrocardiographically documented nonvalvular AF at a predominantly high risk of stroke were recruited at 1178 participating centers in 45 countries. Elevated risk was indicated by a history of stroke, transient ischemic attack (TIA), or systemic embolism, or ≥2 of the following: heart failure or left ventricular ejection fraction ≤35%, hypertension, age ≥75 years, or diabetes mellitus (CHADS2 score [which estimates risk based on the presence of congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or TIA] ≥2). The proportion of patients without prior ischemic stroke, TIA, or systemic embolism and only 2 risk factors was limited to 10% of the cohort by region; the remainder either required prior thromboembolism or had ≥3 risk factors.15 Trial investigators were chosen on the basis of performance in clinical trials and access to large clinical practices. Warfarin dosing was managed by local physicians based on INR values generated at study visits using a standard fingerstick point‐of‐care device (HemoSense, now Alere Inc).17 The device displayed a code that, when entered into an interactive voice response system, along with the patient's identifying number, provided the INR test result. While physicians were reminded about the INR target of the trial and the need for monthly INR tests even when patients’ anticoagulation status was stable, the study did not provide specific treatment algorithms for anticoagulation management. As with our prior analysis of regional iTTR patterns, we grouped the countries involved in the ROCKET AF trial into the following regions: East Asia (China, Hong Kong, Korea, Malaysia, Philippines, Singapore, Thailand, and Taiwan); India; Eastern Europe (Bulgaria, Czech Republic, Greece, Hungary, Lithuania, Poland, Romania, Russia, Turkey, and Ukraine); Western Europe and similar (Australia, Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Great Britain, Israel, Italy, Netherlands, Norway, New Zealand, and Sweden); South Africa; Latin America (Argentina, Brazil, Chile, Colombia, Mexico, Peru, and Venezuela); and Canada/United States. These regional groupings were modified from those used in the primary trial report to provide more cultural and ethnic homogeneity.
Statistical Analysis
The current analysis was restricted to patients in the warfarin arm of the ROCKET AF trial and who, in fact, received ≥1 dose and who had ≥2 INR measurements while taking warfarin. We did not include INR measurements made during drug interruptions or after permanent discontinuation. As a result, 150 of the original 7133 individuals randomly assigned to warfarin were not included in the current analysis. All study‐related INR tests were recorded.
Calculation of TTR
In the ROCKET AF trial, INR values were obtained during the initiation of warfarin at the start of the trial and after temporary interruptions. We did not include INR values obtained during temporary interruptions of ≥7 days or any time after permanent discontinuation. Only 0.18% of inter‐INR test intervals were >8 weeks, but these were included in our calculations of TTR. If a patient had >1 INR test on a given day, the average value was used for that day.
We compared the Rosendaal linear interpolation method1 of calculating TTR with a method that accounted for intervening dose changes (see later). The ROCKET AF trial did not record complete dosing information. Instead, dosing information was limited to the daily doses on the 3 days preceding each INR test. Because warfarin dosing can vary day to day (eg, alternating 2 doses each day, or using 1 dose on Monday, Wednesday, and Friday of each week and another dose on the remaining days), the limited data available from ROCKET AF could lead to uncertainty regarding whether a dose change had actually been made. We addressed this uncertainty via 2 different approaches to inferring whether a dose change had been made:
Approach 1: Determining whether a change in warfarin dose was made using INR test values and dosing information (“specific” approach). A patient was assumed to have had a dose change immediately after an INR test (INR 1) if all of the following criteria were met:
The INR 1 value was out of the target range (for target range, we use both INR <2.0 or >3.0 and INR <1.8 or >3.2).
The mean dose for the 3 days before the subsequent INR test (INR 2) was different in the expected direction than the mean dose for the 3 days before INR 1 (for a high value, the new dose would be expected to be lower; for a low value, the new dose would be expected to be higher).
At least 1 dose before INR 2 was different in the expected direction from any of the 3 daily doses before INR 1 (expected as defined earlier).
Approach 2: Determining whether a change in warfarin dose was made using only INR test values. A patient was assumed to have had a dose change immediately after INR 1 if the INR value was out of the target range (“sensitive” approach).
Calculation of iTTR in the Face of Dose Changes
With each of these approaches, we started with an INR value (INR 1) that was out of range (<2.0 or >3.0). If we concluded that the warfarin dose was changed in response to the out‐of‐range value, we assumed that the dose was changed immediately and that it would take 5 days for the full effect of the dose change to be realized.18–19 Therefore, linear interpolation between INR 1 and the subsequent INR value (INR 2) did not apply. Instead, we assumed that INR 2's value was reached 5 days after the date of INR 1 (the date of the dose change). Between the date of INR 1 and 5 days later, we used linear interpolation between the values of INR 1 and INR 2, and for subsequent days up to the date of INR 2, we used the value of INR 2. We repeated this analysis with an expanded definition of the therapeutic range—ie, INR <1.8 and >3.2—because physicians may not change warfarin dose unless the INR value is out of the target range by some margin. If criteria for assuming that a dose change had occurred were not met (or if the INR measurements were ≤5 days apart), linear interpolation between INR 1 and INR 2 (Rosendaal approach) was used. Figure 1 illustrates the possible transitions between sequential pairs of INR tests and the differences in imputed INR values for the days between tests as calculated by using the Rosendaal method and our inferred dose change–based approach. The dose change–based approach will differ from the Rosendaal approach only when the first INR of a pair is out of range. If the second INR value is in range (a “correction,” point E to point F in Figure 1) then the dose change–based approach will impute more days in range than will the Rosendaal approach. If the second INR is out of range in the opposite direction (an “overshoot,” point D to point E in Figure 1), the dose change–based approach will impute more days out of range. The longer the intertest interval, the larger will be these effects. Finally, if the second INR test is out of range in the same direction as the first INR test (point C to point D in Figure 1), all days in the intertest interval will be counted as out of range with both calculation approaches.
Figure 1.
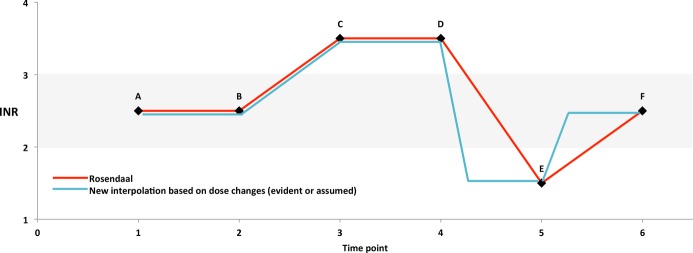
Schematic diagram comparing imputation of international normalized ratio (INR) values between pairs of INR tests using the Rosendaal linear interpolation approach (in red) versus a dose change–based approach (in blue). In this diagram, the target INR range is 2.0 to 3.0 and is highlighted in gray. Points A to F represent INR test results. Points A and B are both in range, and points C and D are both out of range. Since point B is in range, there will be no dose change between B and C. As a result, the 2 imputation approaches do not differ between points A through D. At point D, the INR is above range and a dose change is made resulting in the below‐range INR at point E (an “overshoot”). The imputation of INR values will differ by algorithm as illustrated (see Methods), with the result that the individual patient time in the therapeutic range (iTTR) (time in the gray range) will be lower using the dose change‐based algorithm. The path from point E to point F illustrates an out‐of‐range to in‐range transition (a “correction”). For such transitions, the dose change–based algorithm will impute a larger iTTR. Across a group of individuals, the difference in mean iTTR according to the 2 imputation approaches will depend on the net effect of corrections versus overshoots.
For each patient, the difference between each alternative calculation of iTTR and the Rosendaal‐based iTTR was computed. Analysis of variance models were used to determine whether this quantity differed from zero. Geographic region was included in each model to account for variation in iTTR among regions, as well as to determine whether the difference between iTTRs differed across regions. Additional models were used to determine whether the iTTR differences differed across quartiles of center median iTTR and across quartiles of center median intertest interval. These models also contained region.
The ROCKET AF trial was supported by research grants from Janssen Research & Development and Bayer HealthCare AG. The Duke Clinical Research Institute in Durham, NC, coordinated the trial and performed the statistical analyses for this manuscript independent of the sponsors. All appropriate national regulatory agencies and ethics/institutional review boards at each participating center approved the study, and all subjects gave informed consent. An international, multispecialty executive committee designed the study and takes responsibility for the accuracy and completeness of all data and subsequent analyses.
Results
We included 6983 patients with AF assigned to and taking warfarin in the ROCKET AF trial. Their characteristics were described in detail in a previous publication.13 In brief, their mean age was 71 years, 61% were male, 81% had persistent AF, and 44% were experienced warfarin users. By design, ROCKET AF patients were at very high stroke risk, with 52% having a history of stroke or TIA, 62% having heart failure, 91% having hypertension, and 39% having diabetes. For the current analysis, patients were followed for a mean of 1.6 years, during which 183 004 individual INR tests were accumulated; 48.9% were out of range, using the criteria of <2.0 or >3.0, and 24.8% were out of range using the criteria of <1.8 or >3.2. Follow‐up INR tests were obtained at a median (IQR) of 24 (9 to 28) days after the out‐of‐range INR value (ie, INR <2 or >3).
Table 1 displays the average iTTR according to the Rosendaal and alternative calculation approaches. Table 2 simplifies the comparisons by displaying the difference in mean value compared with the Rosendaal approach. With use of the Rosendaal approach, the overall mean iTTR was 55.2%. By using the dose change–based approaches, the calculated mean iTTR increased. The increase was modest for the “sensitive” approach (Table 2) that assumed a dose change was made after each out‐of‐range INR value―3.1% for an INR target range of 2.0 to 3.0 and 2.4% for an INR target range of 1.8 to 3.2. The increase was minimal for the “specific” approach (Table 2)―ie, 0.7% for an INR target range of 2.0 to 3.0 and 0.8% for an INR target range of 1.8 to 3.2. The same effect of different approaches to calculating mean iTTR was seen across all 7 geographic regions (Table 2).
Table 1.
iTTR Summary Statistics for Each Method of INR Imputation, Overall and by Geographic Region
| Region | iTTR Statistic | Imputation Method: | ||||
|---|---|---|---|---|---|---|
| 1.a. Assuming Dose Change After Any OOR <2 or >3 | 1.b. Assuming Dose Change After Any OOR <1.8 or >3.2 | 2.a. Using Dose Data With OOR <2 or >3 | 2.b. Using Dose Data With OOR <1.8 or >3.2 | 3. Rosendaal | ||
| All regions (N=6983) | Mean (SD) | 58.3 (22.2) | 57.6 (21.7) | 55.9 (21.7) | 56.0 (21.6) | 55.2 (21.3) |
| Median (IQR) | 62.0 (46.3, 74.6) | 61.1 (46.0, 73.5) | 58.9 (43.3, 71.8) | 58.9 (43.9, 71.8) | 57.9 (43.0, 70.6) | |
| East Asia (n=727) | Mean (SD) | 54.0 (22.3) | 53.3 (22.0) | 51.3 (22.0) | 51.5 (22.0) | 50.4 (21.4) |
| Median (IQR) | 58.2 (40.6, 70.0) | 56.8 (39.3, 68.9) | 55.0 (37.4, 67.0) | 55.3 (38.2, 67.0) | 53.1 (37.6, 64.8) | |
| India (n=130) | Mean (SD) | 37.4 (26.0) | 37.3 (25.6) | 36.2 (24.9) | 36.4 (24.7) | 35.9 (23.3) |
| Median (IQR) | 36.2 (15.9, 56.1) | 36.9 (15.0, 57.4) | 34.9 (15.0, 54.5) | 35.6 (15.0, 54.5) | 36.7 (18.6, 50.7) | |
| Eastern Europe (n=2663) | Mean (SD) | 52.6 (22.5) | 52.1 (22.0) | 50.5 (21.9) | 50.7 (21.7) | 49.7 (21.2) |
| Median (IQR) | 55.8 (38.9, 68.8) | 55.2 (39.2, 67.8) | 52.9 (37.0, 66.2) | 53.2 (37.6, 66.3) | 51.9 (36.8, 64.7) | |
| Western Europe and similar (n=1088) | Mean (SD) | 67.0 (18.6) | 65.9 (18.3) | 64.1 (18.6) | 64.1 (18.5) | 63.2 (18.5) |
| Median (IQR) | 70.5 (58.4, 79.3) | 69.0 (57.3, 77.9) | 66.6 (55.2, 77.0) | 66.6 (55.2, 76.6) | 66.1 (53.7, 75.9) | |
| South Africa (n=124) | Mean (SD) | 58.8 (22.6) | 57.8 (22.1) | 55.1 (22.6) | 55.0 (22.5) | 54.8 (22.1) |
| Median (IQR) | 63.6 (47.8, 74.8) | 61.3 (46.6, 74.2) | 58.3 (42.7, 72.9) | 58.2 (42.9, 72.9) | 57.3 (42.9, 70.6) | |
| Latin America (n=924) | Mean (SD) | 58.0 (21.3) | 57.5 (20.8) | 55.8 (20.5) | 55.9 (20.3) | 55.2 (20.0) |
| Median (IQR) | 61.2 (47.5, 73.6) | 60.5 (47.2, 72.9) | 58.5 (45.0, 70.5) | 58.8 (45.0, 70.7) | 58.0 (44.5, 69.3) | |
| Canada/USA (n=1327) | Mean (SD) | 67.1 (18.4) | 66.2 (18.1) | 64.7 (18.3) | 64.6 (18.2) | 64.1 (18.2) |
| Median (IQR) | 69.9 (58.0, 80.0) | 68.9 (57.4, 78.7) | 66.9 (55.6, 77.3) | 66.9 (55.4, 77.4) | 66.3 (54.8, 77.0) | |
iTTR is given as percent. INR indicates international normalized ratio; iTTR, individual patient time in the therapeutic range; OOR, out of range.
Table 2.
Differences Between Mean iTTR From Each New Method and the Rosendaal Method of INR Imputation, Overall and by Geographic Region
| Region | Imputation Method: | |||
|---|---|---|---|---|
| 1.a. Assuming Dose Change After Any OOR <2 or >3 | 1.b. Assuming Dose Change After Any OOR <1.8 or >3.2 | 2.a. Using Dose Data With OOR <2 or >3 | 2.b. Using Dose Data With OOR <1.8 or >3.2 | |
| All regions (N=6983) | 3.1 (5.2) | 2.4 (4.4) | 0.7 (3.7) | 0.8 (3.3) |
| P value (overall) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| East Asia (n=727) | 3.7 (5.5) | 2.9 (4.7) | 0.9 (3.8) | 1.1 (3.5) |
| India (n=130) | 1.5 (6.7) | 1.5 (6.0) | 0.3 (5.4) | 0.5 (4.8) |
| Eastern Europe (n=2663) | 2.9 (5.8) | 2.4 (4.9) | 0.8 (4.5) | 1.0 (4.0) |
| Western Europe and similar (n=1088) | 3.8 (4.4) | 2.7 (3.7) | 0.9 (2.4) | 0.8 (2.1) |
| South Africa (n=124) | 4.0 (5.2) | 3.0 (5.0) | 0.3 (2.8) | 0.2 (2.7) |
| Latin America (n=924) | 2.8 (5.4) | 2.3 (4.6) | 0.6 (3.7) | 0.7 (3.4) |
| Canada/USA (n=1327) | 2.9 (3.8) | 2.1 (3.2) | 0.5 (2.2) | 0.5 (2.0) |
| P value (region) | <0.0001 | <0.0001 | 0.052 | <0.0001 |
iTTR is given as percent. Each table cell contains mean (SD) new iTTR–Rosendaal iTTR. P value (overall) is for the test of no difference between each iTTR and the Rosendaal‐based iTTR, adjusted for region. P value (region) is for the test that the difference between each iTTR and the Rosendaal‐based iTTR differs across regions. INR indicates international normalized ratio; iTTR, individual patient time in the therapeutic range; OOR, out of range.
There was no clear association across geographic regions between gain in mean iTTR and inter‐INR test interval. Of the 5 largest regions, Eastern Europe, East Asia, and Latin America had the longest mean intertest interval (22.7, 23.7, and 21.3 days, respectively), and Canada/US and Western Europe/similar had the shortest (14.9 and 16.7 days, respectively). Yet, Western Europe/similar and East Asia had the largest increases in iTTR when using the alternative dose change–based approaches, while Canada/US and Latin America had the smallest. The magnitude of the regional differences in mean iTTR was much larger than the net change in difference resulting from the alternative calculations of iTTR. As a consequence, the relationship of mean iTTR across regions was hardly affected by the alternative calculations of iTTR (Figure 2).
Figure 2.
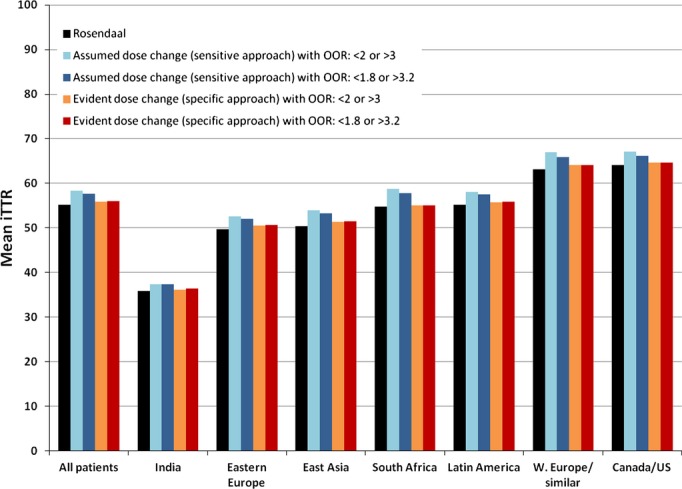
The mean individual patient time in the therapeutic range (iTTR) as calculated by the Rosendaal and dose change–based algorithms, stratified by geographic region. iTTR is measured as percent. Dose changes were inferred using 2 different approaches, the “assumed” or sensitive approach, and the “evident” or specific approach (see Methods). In addition, dose changes were assumed to be triggered at 2 different sets of thresholds: <2.0 or >3.0 and <1.8 or >3.2 (see Methods). OOR indicates outside of range.
Across all regions, approximately two‐thirds of INR time spent out of range was spent below range (Table 3). The change in mean iTTR by using alternative calculations was roughly proportionally distributed above and below range. For regions with the largest percentage of INR time that was below range, there was a greater effect of alternative iTTR calculations on reducing the percentage time below range.
Table 3.
Percent Time Above and Below Therapeutic Range Using iTTR Values Resulting From Different Methods of INR Imputation, Overall and by Geographic Region
| Region | INR | Imputation Method: | ||||
|---|---|---|---|---|---|---|
| 1.a. Assuming Dose Change After Any OOR <2 or >3 | 1.b. Assuming Dose Change After Any OOR <1.8 or >3.2 | 2.a. Using Dose Data With OOR <2 or >3 | 2.b. Using Dose Data With OOR <1.8 or >3.2 | 3. Rosendaal | ||
| All regions (N=6983) | <2 | 27.2 (21.3) | 28.0 (21.4) | 29.0 (21.9) | 29.0 (21.8) | 29.1 (21.9) |
| >3 | 14.5 (12.6) | 14.4 (12.5) | 15.1 (13.0) | 15.0 (12.9) | 15.7 (13.1) | |
| East Asia (n=727) | <2 | 34.0 (22.2) | 35.1 (22.4) | 36.7 (23.1) | 36.6 (23.0) | 37.1 (23.0) |
| >3 | 11.9 (12.2) | 11.7 (12.1) | 12.0 (12.7) | 12.0 (12.6) | 12.5 (12.8) | |
| India (n=130) | <2 | 43.0 (26.1) | 43.3 (26.1) | 44.3 (25.8) | 44.3 (25.8) | 44.1 (25.9) |
| >3 | 19.5 (21.5) | 19.3 (21.5) | 19.6 (21.5) | 19.3 (21.4) | 20.0 (21.3) | |
| Eastern Europe (n=2663) | <2 | 33.3 (22.4) | 34.0 (22.2) | 35.2 (22.8) | 35.1 (22.7) | 35.2 (22.8) |
| >3 | 14.1 (12.2) | 13.9 (12.1) | 14.4 (12.5) | 14.1 (12.3) | 15.1 (12.6) | |
| Western Europe and similar (n=1088) | <2 | 18.4 (16.9) | 19.2 (17.1) | 19.9 (17.7) | 20.1 (17.7) | 20.2 (17.9) |
| >3 | 14.6 (12.2) | 14.8 (12.3) | 15.9 (13.0) | 15.9 (13.0) | 16.5 (13.2) | |
| South Africa (n=124) | <2 | 22.0 (21.3) | 22.9 (21.3) | 23.9 (23.1) | 24.0 (23.1) | 23.9 (23.2) |
| >3 | 19.2 (17.5) | 19.4 (17.5) | 21.0 (18.5) | 21.0 (18.3) | 21.3 (18.1) | |
| Latin America (n=924) | <2 | 26.0 (19.6) | 26.7 (19.6) | 27.4 (19.9) | 27.6 (19.9) | 27.4 (20.0) |
| >3 | 16.0 (12.6) | 15.7 (12.5) | 16.7 (12.8) | 16.5 (12.6) | 17.4 (12.9) | |
| Canada/USA (n=1327) | <2 | 18.3 (15.6) | 19.1 (15.8) | 19.8 (16.1) | 19.8 (16.1) | 19.9 (16.2) |
| >3 | 14.6 (11.6) | 14.7 (11.6) | 15.6 (12.2) | 15.5 (12.1) | 16.0 (12.3) | |
Each table cell contains the mean (SD) for time spent in the range shown in the INR column. INR indicates international normalized ratio; iTTR, individual percent of patient time in the therapeutic range; OOR, out of range.
In Tables 4 and 5, the analysis is arrayed according to quartiles of center intertest intervals following an out‐of‐range INR test result. The increase in calculated mean iTTR based on alternative calculation approaches was least in the quartile with the shortest intertest intervals and greatest in the upper 3 quartiles (Table 5). But in all quartiles, the effect was modest, at most. Overall, 49% of INR test pairs began with an out‐of‐range value. This was composed of 17% where the second INR test remained out of range in the same direction, 11% where the second value was out of range in the opposite direction (“overshoot”), and 21% where the second INR value was in range (“correction”). Only in the latter 2 categories would the dose‐change approach differ from the Rosendaal approach and the changes would be in the opposite direction (Figure 1). The net effect of these 2 categories was 10% of INR pairs leading to an increased number of days counted as in range using the dose change–based approach. The intertest interval was 1 day longer for the “overshoot” than for the “correction” test pairs. The consequence of this distribution of test pair transitions and intertest intervals was the relatively small effect of using the dose change–based approach. This effect was smaller still when we categorized the presence of a dose change based on our dosing information. In this last circumstance, there were 7% of test pairs that were “overshoots” versus 6% that were “corrections,” largely canceling out each other.
Table 4.
iTTR Summary Statistics for Each Method of INR Interpolation, Stratified by Quartile of Median Center ITI After an OOR (<2 or >3) INR Test
| Quartile of Median* Center ITI | INR Interpolation Method: | ||||
|---|---|---|---|---|---|
| 1.a. Assuming Dose Change After Any OOR <2 or >3 | 1.b. Assuming Dose Change After Any OOR <1.8 or >3.2 | 2.a. Using Dose Data With OOR <2 or >3 | 2.b. Using Dose Data With OOR <1.8 or >3.2 | 3. Rosendaal | |
| 1. 4.6 to 16.8 days (n=1743) | 65.9 (19.9) | 65.2 (19.6) | 64.1 (19.8) | 64.1 (19.7) | 63.4 (19.8) |
| 2. 16.8 to 22.2 days (n=1747) | 60.4 (21.6) | 59.5 (21.1) | 57.8 (20.9) | 57.9 (20.8) | 56.9 (20.6) |
| 3. 22.2 to 24.7 days (n=1743) | 55.6 (21.8) | 54.9 (21.3) | 53.0 (21.1) | 53.1 (20.9) | 52.2 (20.4) |
| 4. 24.8 to 34.9 days (n=1744) | 51.3 (22.5) | 50.8 (22.1) | 48.8 (21.9) | 49.1 (21.8) | 48.2 (21.1) |
Each table cell contains mean (SD) iTTR (as percent). Six patients are not included in this summary; these 6 came from centers with 1 trial patient each, and there were no INR tests subsequent to an OOR value. INR indicates international normalized ratio; ITI, intertest interval; iTTR, individual patient time in the therapeutic range; OOR, out of range.
Centers were ordered by ITI and included in quartiles up to one‐fourth of study patients.
Table 5.
Differences Between iTTR From Each New Method and iTTR From Rosendaal Method of INR Interpolation, Stratified by Quartile of Median Center ITI After an OOR (<2 or >3) INR Test
| Quartile of Median Center ITI | INR Interpolation Method: | |||
|---|---|---|---|---|
| 1.a. Assuming Dose Change After Any OOR <2 or >3 | 1.b. Assuming Dose Change After Any OOR <1.8 or >3.2 | 2.a. Using Dose Data With OOR <2 or >3 | 2.b. Using Dose Data With OOR <1.8 or >3.2 | |
| 1. 4.6 to 16.8 days (n=1743) | 2.5 (3.5) | 1.8 (3.0) | 0.7 (2.1) | 0.7 (1.9) |
| 2. 16.8 to 22.2 days (n=1747) | 3.5 (5.2) | 2.6 (4.4) | 0.8 (3.4) | 0.9 (3.1) |
| 3. 22.2 to 24.7 days (n=1743) | 3.4 (5.8) | 2.7 (4.9) | 0.8 (4.2) | 0.9 (3.8) |
| 4. 24.8 to 34.9 days (n=1744) | 3.1 (6.0) | 2.6 (5.1) | 0.6 (4.5) | 0.9 (4.0) |
| P value | <0.0001 | <0.0001 | 0.030 | 0.32 |
Each table cell contains mean (SD) new iTTR–Rosendaal iTTR. A positive value indicates that the new method produces a higher TTR, and a negative value, a lower TTR. P value is for the test that the difference between each iTTR and the Rosendaal‐based iTTR differs across quartiles. INR indicates international normalized ratio; ITI, intertest interval; iTTR, individual percent of patient time in the therapeutic range; OOR, out of range.
Discussion
Although the Rosendaal linear interpolation of time in different INR ranges was originally developed to determine the optimal intensity of oral anticoagulant therapy, it has become a widely used measure of the quality of VKA anticoagulant treatment.1–5 It reflects physician practice, patient adherence, cultural factors such as diet, and resource and geographic factors posing barriers to ready follow‐up of out‐of‐range INR tests. Several large global studies have demonstrated striking differences in mean iTTR across geographic regions.13–14 Indeed, in the ROCKET AF trial, variation in mean iTTR across geographic region was far more important than variation attributable to patients’ clinical features.13 In ROCKET AF, poorer regional mean iTTR levels were associated with longer INR intertest intervals, for both out‐of‐range and in‐range test values. We hypothesized that dose changes were made shortly after out‐of‐range tests in all regions but that local barriers led to delayed follow‐up INR tests in selected regions. Our concern was that the Rosendaal calculation of TTR would not appropriately account for the effect of dose change and would result in biased low measures of average iTTR, particularly for regions with longer duration between dose change and subsequent INR test. We addressed this concern by constructing a measure of TTR that incorporated the pharmacodynamic effect of dose changes in warfarin.3 Because we did not have explicit recording of dose changes, we inferred dose changes by using 2 methods: (1) assuming a dose change occurred after each out‐of‐range INR value and (2) assuming a dose change occurred based on the warfarin dose information that was collected in the study. The first method likely overestimated and the second method likely underestimated the occurrence of dose changes. Further, we included 2 sets of thresholds for changing dose: INR <2.0 or >3.0 and INR <1.8 and >3.2. Our results are clear. Even when assuming the highest frequency of dose changes (ie, assuming a dose change whenever an INR test result was either <2.0 or >3.0), the calculated group mean iTTR levels were only modestly different (ie, higher) than those calculated via the Rosendaal method, and the pattern of large regional differences in mean iTTR persisted.
The optimal INR range for AF (ie, 2.0 to 3.0) is empirically well supported.20–21 As a consequence, the TTR is a logical measure of quality of warfarin treatment. Ideally, TTR would be calculated from daily INR test results. Because INR tests are obtained much less frequently, estimating TTR is an exercise in imputing missing INR values between consecutive measures. The Rosendaal linear interpolation approach is a reasonable imputation method. It will work best when INR intertest intervals are short and when no major perturbation to warfarin effect occurs mid‐interval. When follow‐up INR tests are obtained weeks after a warfarin dose change, an INR imputation method that accounts for dose change, such as we have used, should be more accurate. As we demonstrate here, a dose change–based algorithm will count more days out of range for INR overshoots and more days in range for INR corrections than will the Rosendaal approach. The longer the postdose interval, the larger was the difference. In ROCKET AF, the corrections were moderately more frequent than overshoots, and the intertest intervals after an out‐of‐range result were relatively short, such that the dose change–based TTR was not markedly different from the Rosendaal TTR.
Our analysis benefited from a very large prospectively collected global data set. Its most obvious limitation was the lack of explicit recording of changes in warfarin dose. However, the warfarin doses for the 3 days preceding each INR test were recorded, and we were able to identify cases where dose clearly was changed. Even when we simply assumed that a change in dose was made after each out‐of‐range INR test result, the results were consistent. We note that for our dose change–based approach to calculating TTR, the assumed time to stable INR will have to be adjusted according to pharmacodynamic properties if the VKA used is not warfarin.22
In conclusion, we assessed the impact of a dose change‐based calculation of TTR versus the standard Rosendaal approach, both in the abstract and in the warfarin arm of the global ROCKET AF trial. We identified test result transitions where the 2 methods will differ. In the ROCKET AF trial, the net impact of the dose change‐based calculations was a small increase in mean iTTR. This increase had little effect on the large regional differences in mean iTTR observed in the trial.
Acknowledgments
We acknowledge the editorial assistance of Elizabeth Cook and Peter Hoffmann of the Duke Clinical Research Institute.
Sources of Funding
The ROCKET AF trial was supported by research grants from Janssen Research & Development, (Raritan, NJ, USA) and Bayer HealthCare AG (Leverkusen, Germany). Dr Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital.
Disclosures
Dr Singer received consulting/advisory board fees from Bayer HealthCare, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, and Pfizer and research grants from Bristol‐Myers Squibb and Pfizer. Dr Patel received consulting fees/honoraria from Bayer Healthcare, Ortho‐McNeil‐Janssen, Medscape (theheart.org), and Ikaria and research grants from Janssen, Maquet, AstraZeneca, National Heart, Lung, and Blood Institute, and Genzyme. Dr Piccini received consulting fees/honoraria from Medtronic and Janssen and research grants from ARCA biopharma, GE Healthcare, Johnson & Johnson, ResMed, and Boston Scientific. Dr Hankey received honoraria for consulting and speaking at sponsored scientific symposia from Bayer and Medscape (theHeart.org). Dr Breithardt received honoraria from Bayer HealthCare and Bristol‐Myers Squibb/Pfizer, and consulting/advisory board fees from Bayer HealthCare, Bristol‐Myers Squibb/Pfizer, and Sanofi‐Aventis. Dr Halperin received consulting fees from Bayer HealthCare AG, Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, Ortho‐McNeil‐Janssen, Pfizer, Sanofi‐Aventis, AstraZeneca, Biotronik, Boston Scientific, Janssen, and Medtronic. Dr Becker received consulting fees/honoraria from Portola, Daiichi‐Sankyo, Bristol‐Myers Squibb, and Boehringer Ingelheim, and research grants from AstraZeneca and Johnson & Johnson. Dr Nessel is an employee of Janssen Research & Development. Dr Mahaffey received consulting fees from AstraZeneca, Bayer, Bristol‐Myers Squibb, Forest, Johnson & Johnson, and WebMD. Dr Fox received consulting fees/honoraria from Boehringer Ingelheim, Sanofi‐Aventis, Astra Zeneca, Johnson & Johnson/Bayer, and Janssen, and research grants from Eli Lilly. Dr Califf received research grants from Amylin, Bristol‐Myers Squibb, Eli Lilly & Company, Janssen Research & Development, Merck, and Novartis; consulting fees from Amgen, Medscape (theheart.org), and Novartis; and has equity in N30 Pharma and Portola. The other authors have no disclosures.
References
- Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236-239. [PubMed] [Google Scholar]
- Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk‐adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA). Circ Cardiovasc Qual Outcomes. 2011; 4:22-29. [DOI] [PubMed] [Google Scholar]
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141suppl 2:e44S-e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014; 129:1407-1414. [DOI] [PubMed] [Google Scholar]
- Wieloch M, Sjalander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo‐embolic complications from the national quality registry AuriculA. Eur Heart J. 2011; 32:2282-2289. [DOI] [PubMed] [Google Scholar]
- Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012; 5:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64:e1-e76.10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof PESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012; 33:2719-2747. [DOI] [PubMed] [Google Scholar]
- You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012; 141suppl:e531S-e575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, Albers GW. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007; 167:239-245. [DOI] [PubMed] [Google Scholar]
- Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010; 376:975-983. [DOI] [PubMed] [Google Scholar]
- Singer DE, Go AS. A new era in stroke prevention for atrial fibrillation: comment on “current trial‐associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation”. Arch Intern Med. 2012; 172:631-633. [DOI] [PubMed] [Google Scholar]
- Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, Halperin JL, Becker RC, Breithardt G, Hankey GJ, Hacke W, Nessel CC, Patel MR, Califf RM, Fox KAA. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013; 2:e00006710.1161/JAHA.112.000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008; 118:2029-2037. [DOI] [PubMed] [Google Scholar]
- ROCKET AF Study Investigators. Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010; 159:340-347. [DOI] [PubMed] [Google Scholar]
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883-891. [DOI] [PubMed] [Google Scholar]
- Taborski U, Braun SL, Voller H. Analytical performance of the new coagulation monitoring system INRatio™ for the determination of INR compared with the coagulation monitor Coaguchek® S and an established laboratory method. J Thromb Thrombolysis. 2004; 18:103-107. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001; 119:8S-21S. [DOI] [PubMed] [Google Scholar]
- Wessler S, Gitel SN. Warfarin: from bedside to bench. N Engl J Med. 1984; 311:645-652. [DOI] [PubMed] [Google Scholar]
- Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation? The ATRIA study. Circ Cardiovasc Qual Outcomes. 2009; 2:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjusted‐dose warfarin versus low‐intensity fixed‐dose warfarin plus aspirin for high‐risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996; 348:633-638. [PubMed] [Google Scholar]
- van Leeuwen Y, Rosendaal FR, van der Meer FJM. The relationship between maintenance dosages of three vitamin K antagonists: acenocoumarol, warfarin and phenprocoumon. Thromb Res. 2008; 123:225-230. [DOI] [PubMed] [Google Scholar]


