Abstract
Background
Angiotensin II type 1 receptor (AT1R)–associated protein (ATRAP; Agtrap gene) promotes AT1R internalization along with suppression of pathological AT1R activation. In this study, we examined whether enhancement of ATRAP in the renal distal tubules affects sodium handling and blood pressure regulation in response to high salt (HS) loading, using ATRAP transgenic mice on a salt‐sensitive C57BL/6J background.
Methods and Results
Renal ATRAP transgenic (rATRAP‐Tg) mice, which exhibit renal tubule–dominant ATRAP enhancement, and their wild‐type littermate C57BL/6J mice on a normal salt diet (0.3% NaCl) at baseline were subjected to dietary HS loading (4% NaCl) for 7 days. In rATRAP‐Tg mice, the dietary HS loading–mediated blood pressure elevation was suppressed compared with wild‐type mice, despite similar baseline blood pressure. Although renal angiotensin II level was comparable in rATRAP‐Tg and wild‐type mice with and without HS loading, urinary sodium excretion in response to HS loading was significantly enhanced in the rATRAP‐Tg mice. In addition, functional transport activity of the amiloride‐sensitive epithelial Na+ channel was significantly decreased under saline volume–expanded conditions in rATRAP‐Tg mice compared with wild‐type mice, without any evident change in epithelial Na+ channel protein expression. Plasma membrane AT1R expression in the kidney of rATRAP‐Tg mice was decreased compared with wild‐type mice.
Conclusions
These results demonstrated that distal tubule–dominant enhancement of ATRAP inhibits pathological renal sodium reabsorption and blood pressure elevation in response to HS loading. The findings suggest that ATRAP‐mediated modulation of sodium handling in renal distal tubules could be a target of interest in salt‐sensitive blood pressure regulation.
Keywords: angiotensin receptors, basic science, gene expression/regulation, hypertension (kidney), receptors, salt‐sensitive, sodium transport (kidney)
Introduction
Blood pressure (BP) is exquisitely regulated so as to maintain circulatory homeostasis in response to various stimuli. This process is critically dependent on sodium balance. In addition, the interaction between genetic and environmental factors, especially the excessive intake of dietary salt typical in many diets and inappropriate sodium retention by the kidney, plays a critical role in pathologically increased BP.1 In the kidney, the distal portion of the renal nephron (ie, containing the distal convoluted tubule, the connecting tubule, and the collecting duct) is the final site at which sodium reabsorption occurs.
Activation of the renal renin–angiotensin system promotes sodium reabsorption in the distal tubules through angiotensin II (Ang II) type 1 receptor (AT1R)–mediated stimulation of the thiazide‐sensitive Na+Cl− cotransporter (NCC) and amiloride‐sensitive epithelial Na+ channel (ENaC).2–4 The AT1R‐associated protein (ATRAP; Agtrap gene) was identified as a molecule that directly binds to the carboxyl‐terminal domain of AT1R in the course of an investigational search for a means to regulate AT1R signaling at local tissue sites.5–11 ATRAP selectively suppresses Ang II–mediated pathological activation of AT1R signaling,11 whereas cardiovascular ATRAP enhancement ameliorates cardiovascular hypertrophy in Ang II–infused mice without any effect on baseline cardiovascular function, including BP.12–13 With respect to the functional role of ATRAP in BP regulation in response to pathological stimuli, systemic ATRAP deficiency provokes the pathological activation of vascular and renal tubular AT1R in response to chronic Ang II infusion, exacerbating hypertension through enhanced vasoconstriction and increased sodium retention.14 This demonstrates the inhibitory role of ATRAP in Ang II–mediated hypertension.
With regard to the role of ATRAP in salt‐mediated BP regulation, we previously showed that sustained recovery of repressed renal ATRAP expression contributed to the long‐term therapeutic effects of prepubertal transient treatment with an AT1R blocker in dietary high salt (HS) loading–mediated hypertension in Dahl Iwai salt‐sensitive rats, a representative animal model of human salt‐sensitive forms of hypertension.15 Little is known, however, about the functionally causal role of ATRAP in HS‐mediated BP regulation. We recently demonstrated that renal distal tubule–dominant ATRAP enhancement in mice on a C57BL/6J background exerted an inhibitory effect on the pathological BP elevation that occurred in response to chronic Ang II infusion.16 Consequently, we hypothesized that renal tubule ATRAP functionally affects BP regulation in response to dietary salt intake. Because C57BL/6J mice are also known as a salt‐sensitive animal model,17–18 we investigated the effects of dietary HS loading on renal sodium handling and BP regulation in the context of renal distal tubule–dominant enhancement of ATRAP, using transgenic mice on a C57BL/6J background.
Materials and Methods
Renal Tubule–Dominant Upregulation of ATRAP in C57BL/6 Mice
Renal ATRAP transgenic (rATRAP‐Tg) mice dominantly expressing hemagglutinin‐tagged ATRAP in the renal distal tubules were generated on a C57BL/6J background, as described previously.16 The mice were housed under a 12/12‐hour light–dark cycle at a temperature of 25°C and fed a normal salt (NS) diet containing 0.3% NaCl (ORIENTAL YEAST Co., Ltd.). This study was performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. All of the animal studies were reviewed and approved by the animal studies committee of Yokohama City University.
Dietary HS Loading and BP Measurement
The rATRAP‐Tg mice and their wild‐type (Wt) littermate mice (n=6 to 8 per group) were fed an HS diet (4% NaCl) during the experimental period of 7 days. Systolic BP was measured indirectly by the tail‐cuff method (MK‐2000; Muromachi Kikai Co) between 9 and 10 pm, as described previously.19 Direct BP measurement in the conscious state was also performed by the radiotelemetric method at baseline and at 7 consecutive days after the start of dietary salt loading, as described.12 Briefly, under anesthesia with isoflurane, an incision was made from the chin to the superior sternum, and the left common carotid artery was surgically exposed. A small incision was made in the artery adjacent to the bifurcation, and the tip of a BP transducer (model TA11PA‐C10; Data Sciences International) was placed in the artery. The catheter was then tied, and the transducer was secured in place under the skin of the right flank with tissue adhesive. All skin wounds were closed with 5‐0 nylon (Sigma Rex). Fourteen days after transplantation, when the circadian rhythm had been restored, hemodynamic measurements were recorded every 5 minutes using Dataquest A.R.T. 4.1 software (Data Sciences International). Baseline telemetric BP values for the NS diet were the average of values from 3 consecutive days.
Plasma Analysis
Arterial blood was collected from the abdominal aorta under anesthesia with an intraperitoneal injection of pentobarbital (50 mg/kg). Whole blood was centrifuged at 500g at 4°C for 15 minutes to separate the plasma. The concentrations of blood urea nitrogen, sodium, potassium, chloride, calcium, and creatinine and the osmolality were measured with an autoanalyzer (Hitachi 7180; Hitachi). Plasma renin activity was measured by radioimmunoassay, essentially as described previously20 Plasma aldosterone concentration was measured with a standard method using an radioimmunoassay kit (SPAC‐S Aldosterone Kit; TFB, Tokyo, Japan) at laboratory of ORIENTAL YEAST Co., Ltd.
Renal Tissue Ang II Analysis
For the analysis of the renal tissue Ang II level, the rATRAP‐Tg and Wt mice were randomly divided into 2 groups for dietary HS loading. Although both the rATRAP‐Tg and Wt mice in the NS group were continuously fed an NS diet (0.3% NaCl) during the 7‐day experimental period, the mice in the HS group were fed an HS diet (4% NaCl) for the same period. At the end of the 7‐day period, the renal tissue Ang II level was determined by radioimmunoassay, as reported previously.21
Metabolic Cage Analysis
Metabolic cage analysis was performed, as described.16 Briefly, the rATRAP‐Tg and Wt mice were acclimated to metabolic cages (Techniplast) for 1 week before the HS‐loading experiments. After an additional 3 days on an NS diet (0.3% NaCl), the mice were fed an HS diet (4% NaCl) for 7 days. Daily body weight (BW), food intake, and water intake were measured, and urine was collected. Mice were given free access to tap water and fed the indicated diet. The cumulative Na+ excretion means the sum of daily sodium excretion during 7 days of dietary HS loading on metabolic cage analysis.
Functional Analysis of Renal Sodium Transporters by Diuretic Test
A diuretic test was performed, essentially as described previously14,22 We prepared a 5‐mg/mL furosemide solution, a 25‐mg/mL hydrochlorothiazide solution, and a 5‐mg/mL amiloride solution in 10% DMSO for intraperitoneal injection. Before injection of the drugs, the rATRAP‐Tg or Wt mice were injected intraperitoneally with saline at 70 μL/g BW to facilitate spontaneous voiding. At 1 hour after the saline injection, furosemide (5 mg/kg BW), hydrochlorothiazide (25 mg/kg BW), or amiloride (5 mg/kg BW) was intraperitoneally injected. The diuretic dose was based on previous studies.22–23 The injection volume was the same (10 μL/g BW) in all groups. Urine was collected every hour by spontaneous voiding or bladder massage, and the urine volume and amount of sodium excretion were measured.
Membranous Protein Extraction and Immunoblot Analysis of Sodium Channels
For the analysis of the renal expression of sodium channels, rATRAP‐Tg and Wt mice were randomly divided into 2 groups for dietary HS loading. Although both the rATRAP‐Tg and Wt mice in the NS group were continuously fed an NS diet (0.3% NaCl) during the 7‐day experimental period, the mice in the HS group were fed an HS diet (4% NaCl) for the same period. At the end of the 7‐day period, membranous proteins were extracted from kidney tissues using a plasma membrane extraction kit (K268‐50; BioVision), and immunoblot analysis was performed, as described previously.16,19,24 Antibodies against phospho‐NCC on Ser71,25 NCC (AB3553; Chemicon), αENaC (PA1‐920A; Affinity Bioreagents), βENaC (sc‐48428; Santa Cruz Biotechnology), and γENaC (ab3468; Abcam) were used.
Analysis of Plasma Membrane AT1R Expression
The plasma membrane was specifically extracted from tissues using a plasma membrane extraction kit (K268‐50; BioVision), as described previously.26 Plasma membranes were incubated with either an anti‐AT1R antibody (sc1173; Santa Cruz Biotechnology) or an anti‐flotillin‐2 monoclonal antibody (3436; Cell Signaling Technology) and subjected to enhanced chemiluminescence. Flotillin‐2 is constitutively localized at the plasma membrane and was used as an internal control protein on the plasma membrane.
Statistical Analysis
Data are expressed as mean±SE. Differences were analyzed as follows. Repeated‐measures ANOVA was used to test for differences over time (Figures 1A, 1B, and 4A through 4F). Two‐factor ANOVA with Bonferroni posttest was used to test for differences in salt loading and light or dark period (Figure 1C and 1D) or differences due to salt loading and genotype (Figures 2A, 2B, 3A through 3C, 5A through 5D, and 6). An unpaired t test was used to test for differences in salt loading within each genotype (Table) or differences in Wt versus transgenic mice (Figures 1E, 1F and 3D, 3E). P values of <0.05 were considered statistically significant.
Figure 1.
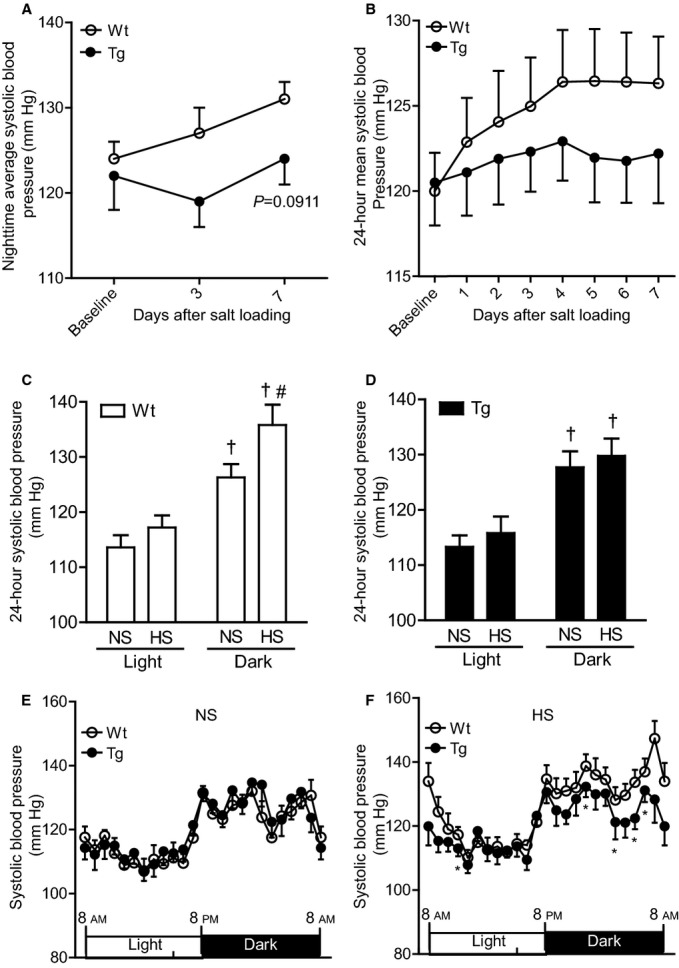
Effects of dietary HS loading on BP in Wt and Tg mice. A, Effects of dietary HS loading on nighttime average systolic BP using the tail‐cuff method. Values are expressed as mean±SE (n=8 in each group). B, Daily and 24‐hour mean systolic BP on telemetry during 7 days of dietary HS (4% NaCl) loading. Values are expressed as mean±SE (n=6 in each group). C and D, Effects of dietary HS loading on systolic BP on telemetry during the light and dark cycles in Wt mice (C) and Tg mice (D). NS designates mice at baseline fed an NS diet (0.3% NaCl); HS designates mice fed an HS diet (4% NaCl) for 7 days. Values are expressed as mean±SE (n=6 in each group). †P<0.01, light vs dark. #P<0.05, NS vs HS. E and F, Circadian systolic BP profile of the Wt and Tg mice on an NS diet (E) and on the seventh day after the start of HS loading (F). Values are expressed as mean±SE (n=6 in each group). *P<0.05, Tg vs Wt mice. BP indicates blood pressure; HS, high salt; NS, normal salt; rATRAP‐Tg, renal angiotensin II type 1 receptor associated protein transgenic; Tg, transgenic; Wt, wild type.
Figure 2.
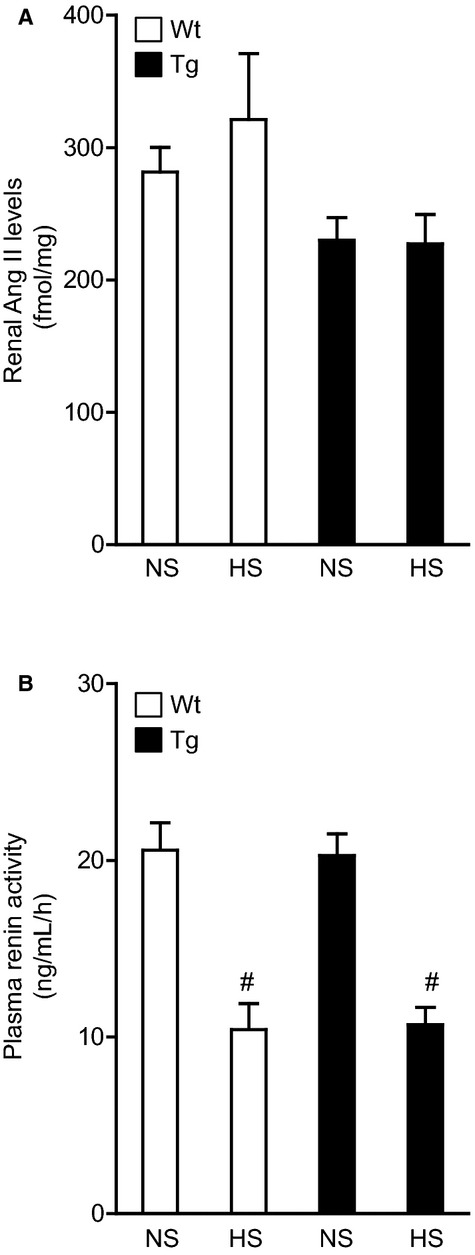
Effects of dietary HS loading on the renal tissue Ang II level and plasma renin activity in Wt and Tg mice. A, Effects of dietary HS loading on renal tissue Ang II level. B, Effects of dietary HS loading on plasma renin activity. NS designates mice fed an NS diet (0.3% NaCl) for 7 days; HS designates mice fed an HS diet (4% NaCl) for 7 days. Values are expressed as mean±SE (n=6 in each group). #P<0.01, NS vs HS. Ang II indicates angiotensin II; HS, high salt; NS, normal salt; Tg, renal angiotensin II type 1 receptor associated protein transgenic; Wt, wild type.
Figure 3.
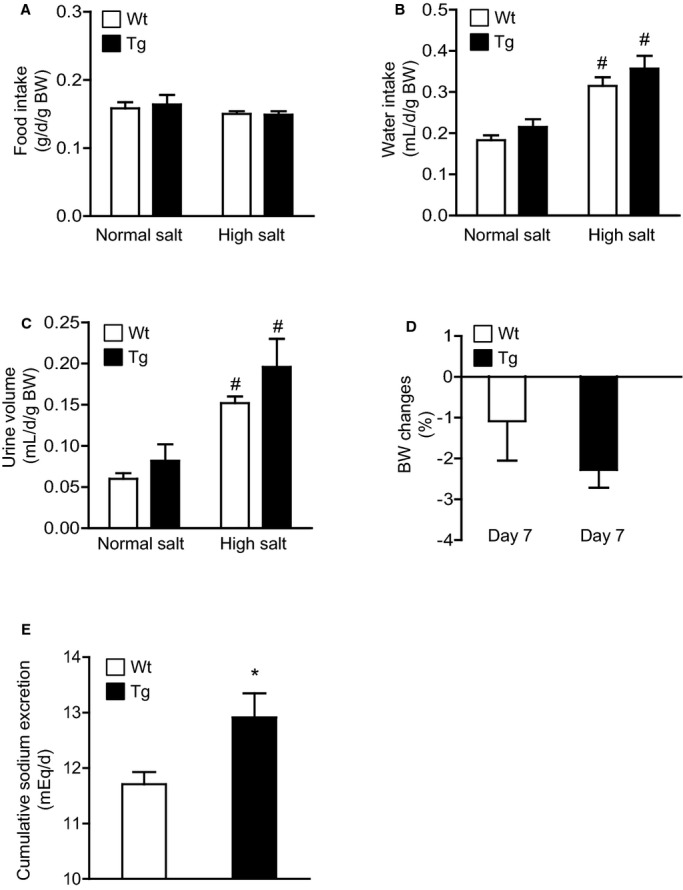
Effects of dietary HS loading on metabolic parameters in Wt and Tg mice. A, Effects of dietary HS loading on food intake. B, Effects of dietary HS loading on water intake. C, Effects of dietary HS loading on urine volume. D, Comparison of the dietary HS loading–mediated BW change on day 7. E, Comparison of cumulative urinary sodium excretion during dietary HS loading. NS designates mice at baseline fed an NS diet (0.3% NaCl); HS designates mice fed an HS diet (4% NaCl) for 7 days. BW changes were calculated as follows: BW change=[(BW at day 7)−(BW at baseline)]/(BW at baseline)×100. Values are expressed as mean±SE (n=6 in each group). #P<0.01, NS vs HS. *P<0.05, Tg vs Wt mice. BW indicates body weight; HS, high salt; NS, normal salt; Tg, renal angiotensin II type 1 receptor associated protein transgenic; Wt, wild type.
Table 1.
Body Weight, Heart Rate, Urine Volume, Creatinine Clearance, and Plasma Parameters of Wt and rATRAP‐Tg Mice
| Variable | Wt | rATRAP‐Tg | ||
|---|---|---|---|---|
| NS (n=8) | HS (n=7) | NS (n=7) | HS (n=7) | |
| Body weight, g | 23.3±0.8 | 23.0±0.9 | 23.0±0.7 | 22.5±0.9 |
| Heart rate, bpm | 535±9 | 524±25 | 522±12 | 524±14 |
| Urine volume, μL/day per gram of body weight | 60±7 | 152±8* | 82±20 | 196±34* |
| Creatinine clearance, μL/min | 385±25 | 356±16 | 428±26 | 405±30 |
| Plasma concentrations | ||||
| Sodium, mEq/L | 151±1.2 | 150±0.8 | 152±1.6 | 150±0.6 |
| Potassium, mEq/L | 5.4±0.2 | 5.3±0.2 | 5.6±0.2 | 5.5±0.2 |
| Chloride, mEq/L | 111±0.8 | 110±0.7 | 110±1.0 | 111±0.4 |
| Calcium, mg/dL | 8.2±0.1 | 8.4±0.1 | 8.3±0.3 | 8.5±0.1 |
| Blood urea nitrogen, mg/dL | 27.5±1.9 | 31.5±1.7 | 25.3±1.8 | 27.4±1.7 |
| Creatinine, mg/dL | 0.08±0.01 | 0.10±0.01* | 0.08±0.01 | 0.09±0.01 |
| Aldosterone, pg/mL | 394±69 | 93±8* | 489±42 | 190±28* |
| Osmolality, mOsm/L | 329±2 | 337±2* | 331±3 | 345±5* |
All values are mean±SEM. Bpm indicates beats per minute; HS, high salt; NS, normal salt; rATRAP‐Tg, renal angiotensin II type 1 receptor associated protein transgenic; Wt, wild type.
P<0.05, vs NS, unpaired t test.
P<0.01, vs NS, unpaired t test.
Results
Renal Tubule–Dominant ATRAP Enhancement Exerts No Evident Effect on Renal Function or Plasma Parameters
At baseline, there were no significant differences in BW, heart rate, urine volume, creatinine clearance, and plasma physiological parameters, including serum osmolality and aldosterone concentration, between the rATRAP‐Tg and Wt mice on the NS diet (Table). HS loading for 7 days significantly increased urine volume and osmolality in the rATRAP‐Tg and Wt mice to similar degrees. In contrast, the plasma aldosterone concentration was similarly decreased in both types of mice after 7 days of HS loading. Although the plasma creatinine concentration was increased only in Wt mice after 7 days of HS loading, 24‐hour creatinine clearance was unaffected by HS loading in both the rATRAP‐Tg and Wt mice. Other plasma parameters were unaffected by HS loading in both the rATRAP‐Tg and Wt mice.
Renal Tubule–Dominant ATRAP Enhancement Inhibits Dietary HS Loading–Induced BP Elevation
To examine the effect of renal tubule–dominant enhancement of ATRAP on dietary HS loading–mediated BP elevation, nighttime indirect systolic BP measurement was performed by the tail‐cuff method in the rATRAP‐Tg and Wt mice at baseline on the NS diet and after 3 and 7 days of HS loading. Although basal nighttime systolic BP was similar in the rATRAP‐Tg and Wt mice, the nighttime systolic BP in response to HS loading for 7 days tended to be lower in rATRAP‐Tg versus Wt mice (repeated‐measures ANOVA, F=3.374, P=0.0911) (Figure 1A).
In addition, the effects of HS loading on systolic BP in the rATRAP‐Tg and Wt mice were carefully compared by direct BP measurement using a radiotelemetric method. Consistent with our previous observation,16 24‐hour mean systolic BP and diurnal systolic BP profiles were similar in the rATRAP‐Tg and Wt mice at baseline on the NS diet (Figure 1B and 1E). In Wt mice, HS loading for 7 days elevated systolic BP in the dark period (HS versus NS; light period, 117.2±2.2 versus 113.6±2.2 mm Hg, not significant; dark period, 135.8±3.7 versus 126.3±2.4 mm Hg, P<0.05) (Figure 1C). This dark period–dominant salt‐sensitive BP elevation in Wt littermate C57BL/6J mice was consistent with a previous observation.17
In the rATRAP‐Tg mice, systolic BP on telemetry was similar before and after dietary HS loading for 7 days in the light and dark periods (HS versus NS; light period, 115.8±3.0 versus 113.3±2.1 mm Hg, not significant; dark period, 129.8±3.1 versus 127.7±2.9 mm Hg, not significant) (Figure 1D). Furthermore, comparison of the circadian systolic BP profile with a light–dark cycle over a 24‐hour period at the end of dietary HS loading (day 7) showed that the systolic BP of the rATRAP‐Tg mice was significantly lower than that of the Wt mice at 11 AM, 0 AM, 3 AM, 5 AM and 6 AM (Figure 1F). These results indicate that renal tubule–dominant enhancement of ATRAP inhibited salt‐sensitive BP elevation in vivo.
Renal Tubule–Dominant ATRAP Enhancement Exerts No Evident Effect on the Renal Tissue Ang II Level in Response to Dietary HS Loading
The paradoxical effect of dietary HS loading has been reported. Studies have reported that expression of renin–angiotensin system components and tissue Ang II level in the kidney are promoted, despite suppression of circulating renin–angiotensin system activity in Dahl salt‐sensitive rats, an established model of salt‐sensitive hypertension.27–30 Consequently, to investigate the mechanisms involved in the inhibition of dietary HS loading–mediated BP elevation in rATRAP‐Tg mice, the renal tissue Ang II level was measured by radioimmunoassay in both the rATRAP‐Tg and Wt mice after the 7‐day dietary salt–loading experiment.
The renal tissue Ang II level was comparable in the HS and NS groups in both the rATRAP‐Tg and Wt mice after salt loading (HS versus NS; rATRAP‐Tg mice, 227.2±22.4 versus 230.1±16.9 fmol/mg, not significant; Wt mice, 321.2±49.9 versus 281.6±18.5 fmol/mg, not significant). Furthermore, there was no significant difference in the renal tissue Ang II level between the rATRAP‐Tg and Wt mice, regardless of the presence or absence of HS loading (Figure 2A). Plasma renin activity was similar in the rATRAP‐Tg and Wt mice at baseline and decreased to a similar degree after 7 days of HS loading (Figure 2B). These results showed that renal distal tubule–dominant enhancement of ATRAP exerts no effect on renal tissue Ang II level in salt‐sensitive BP elevation in mice on a C57BL/6J background.
Renal Tubule–Dominant ATRAP Enhancement Promotes Urinary Sodium Excretion During Dietary HS Loading
Because the rATRAP‐Tg mice exhibited a renal distal tubule–dominant pattern of high ATRAP expression among the tissues analyzed, we next hypothesized that renal tubule–dominant ATRAP enhancement might inhibit dietary HS loading–mediated BP elevation by affecting renal sodium handling. To examine this, we performed metabolic cage analysis and compared urinary sodium excretion levels between the rATRAP‐Tg and Wt mice during the period of HS loading (Figure 3). Food intake during the 7‐day HS‐loading period was similar in the rATRAP‐Tg and Wt mice (Figure 3A). Moreover, water intake and urine volume were similarly increased in both types of mice during the 7‐day HS loading period compared with baseline on an NS diet (Figure 3B and 3C). BW changes were comparable in the rATRAP‐Tg and Wt mice (rATRAP‐Tg versus Wt; −2.28±0.44 versus −1.09±0.97%, not significant) (Figure 3D). In contrast, cumulative sodium excretion during the 7‐day HS loading period was significantly enhanced in the rATRAP‐Tg compared with Wt mice (rATRAP‐Tg versus Wt; 12.91±0.44 versus 11.71±0.22 mEq/day, P=0.03) (Figure 3E), which is consistent with the stimulation of natriuresis as a mechanism underlying the suppression of dietary HS loading–induced BP elevation.
Renal Tubule–Dominant ATRAP Enhancement Functionally Inhibits the Sodium Transporters NCC and ENaC in Response to Dietary HS Loading, Promoting Urinary Sodium Excretion
To investigate the mechanisms involved in the promotion of urinary sodium excretion in response to dietary HS loading in the rATRAP‐Tg mice, we examined the functional activity of the major renal sodium transporters (sodium–potassium–chloride cotransporter 2, or NKCC2; NCC; and ENaC). We performed a diuretic test under saline volume–expanded conditions using potent and specific inhibitors of NKCC2 (furosemide), NCC (hydrochlorothiazide), and ENaC (amiloride) and analyzed the effects of renal tubule–dominant ATRAP enhancement on the activity of these major sodium transporters.
With respect to the effect of intraperitoneally injected furosemide or hydrochlorothiazide, urinary sodium excretion and urine volume were similarly increased in the rATRAP‐Tg and Wt mice under saline volume–expanded conditions (Figure 4A through 4D). In contrast, urinary sodium excretion and urine volume after intraperitoneal injection of amiloride were significantly decreased in rATRAP‐Tg mice compared with Wt mice (urinary sodium excretion, repeated measures ANOVA, F=5.940, P=0.0350; urine volume, repeated‐measures ANOVA, F=9.147, P=0.0128) (Figure 4E and 4F). These results show that the functional transport activity of ENaC, but not NKCC2 and NCC, is decreased under saline volume–expanded conditions so as to increase urinary sodium excretion in rATRAP‐Tg mice.
Figure 4.
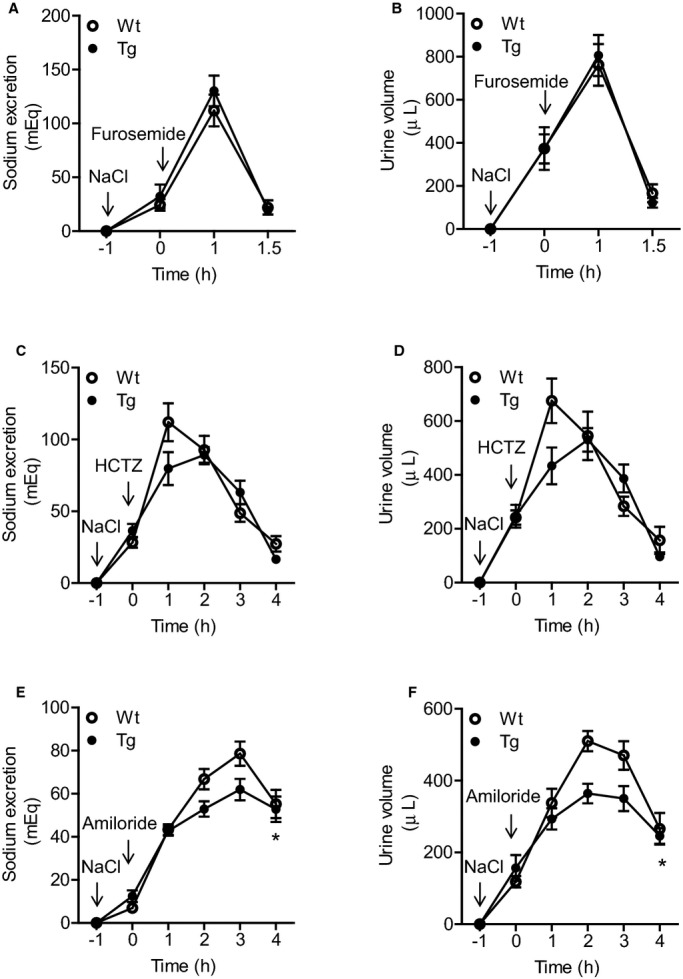
Effects of specific inhibitors of the sodium transporters NKCC2, NCC, and ENaC on natriuresis in Wt and Tg mice. A and B, Effect of furosemide on natriuresis in Wt and Tg mice. The response to furosemide (5 mg/kg) was comparable. C and D, Effect of HCTZ on natriuresis. The response to HCTZ (25 mg/kg) was significantly suppressed in the Tg mice compared with the Wt mice. E and F, Effect of amiloride on natriuresis in Wt and Tg mice. The response to amiloride (5 mg/kg) was also significantly suppressed in the Tg mice. For the experiments, mice were injected intraperitoneally with saline at 70 μL/g BW to facilitate voiding. One hour later, each of the specific sodium transporter inhibitors was injected intraperitoneally (at 0 hour), and urine was collected every hour thereafter. Values are expressed as mean±SE (n=6 in each group). *P<0.05, Tg vs Wt mice. BW indicates body weight; ENaC, epithelial Na+ channel; HCTZ, hydrochlorothiazide; NCC, Na+Cl− cotransporter; NKCC2, sodium–potassium–chloride cotransporter 2; Tg, renal angiotensin II type 1 receptor associated protein transgenic; Wt, wild type.
Renal Tubule–Dominant ATRAP Enhancement Does Not Influence Renal Expression of NCC and ENaC
We next compared renal protein expression of phosphorylated NCC and NCC and the α, β, and γ subunits of ENaC for rATRAP‐Tg and Wt mice. The 7‐day dietary salt loading similarly decreased the renal phosphorylated NCC protein levels in the rATRAP‐Tg and Wt mice (Figure 5A). Moreover, the renal α, β, and γENaC protein levels were unchanged by dietary salt loading in either type of mice (Figure 5B through 5D). These results suggest that suppression of functional activity of renal ENaC in rATRAP‐Tg mice under the salt‐loading condition is not due to regulation of expression of this sodium transporter.
Figure 5.
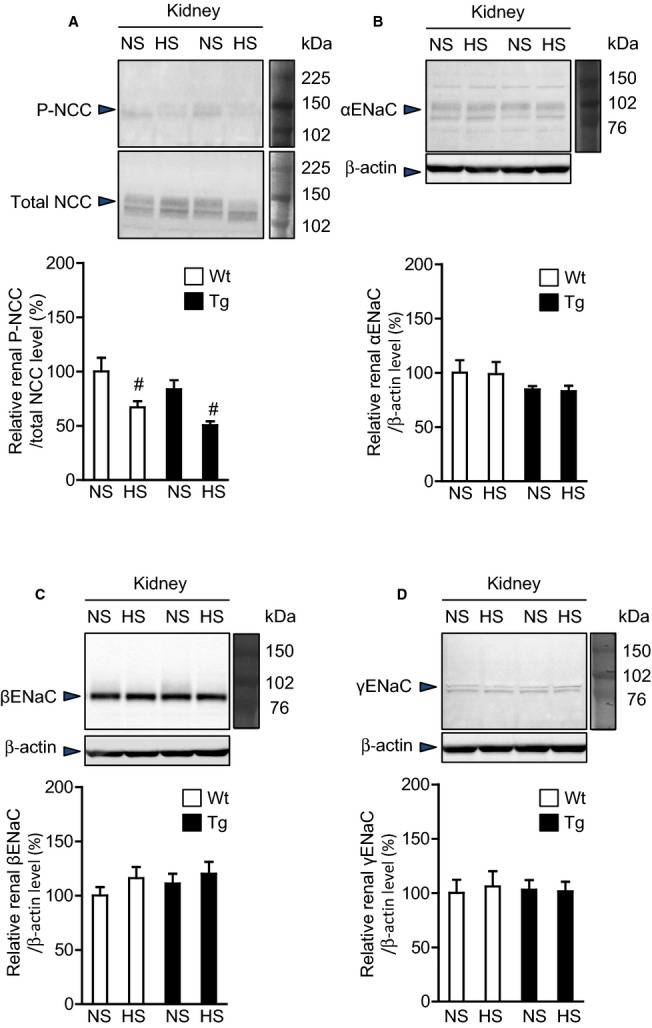
Effects of dietary HS loading on the protein expression of the renal sodium transporters NCC and ENaC in Wt and Tg mice. Effects of dietary HS loading for 7 days on protein expression of the renal sodium transporters NCC (P‐NCC and total NCC) (A), and the α (B), β (C) and γ (D) subunits of ENaC in Wt and Tg mice. NS designates mice fed an NS diet (0.3% NaCl) for 7 days; HS designates mice fed an HS diet (4% NaCl) for 7 days. Values are expressed as mean±SE (n=6 in each group). #P<0.05, NS vs HS. ENaC indicates epithelial Na+ channel; HS, high salt; NCC, Na+Cl− cotransporter; NS, normal salt; P‐NCC, phosphorylated Na+Cl− cotransporter; Tg, renal angiotensin II type 1 receptor associated protein transgenic; Wt, wild type.
Promotion of AT1R Internalization in the Kidney of rATRAP‐Tg Mice
We examined whether the renal tubule–dominant overexpression of ATRAP would promote AT1R internalization. Plasma membrane AT1R protein expression in the kidney of rATRAP‐Tg mice was significantly decreased compared with Wt mice, suggesting promotion of renal AT1R internalization (Figure 6).
Figure 6.
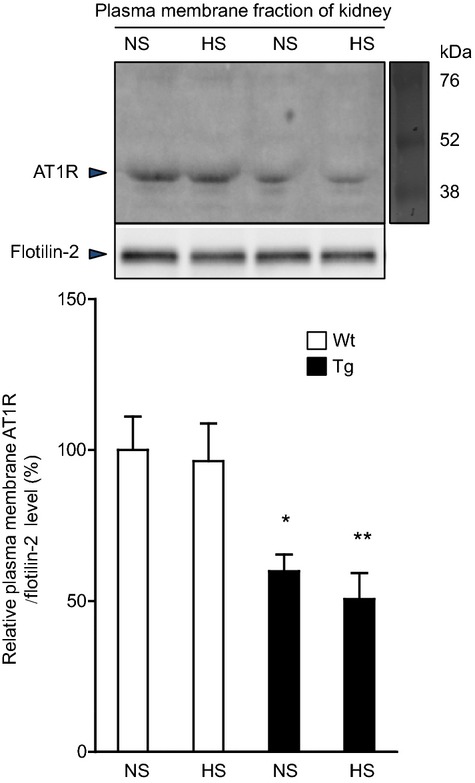
Effects of dietary HS loading on AT1R internalization in the kidneys of Wt and Tg mice. Representative Western blots (top) and quantitative analysis (bottom) of the effects of dietary HS loading for 7 days on plasma membranous AT1R protein expression in the kidney of Wt and Tg mice. NS designates mice fed an NS diet (0.3% NaCl) for 7 days; HS designates mice fed an HS diet (4% NaCl) for 7 days. Values are expressed as the mean±SE (n=6 in each group). *P<0.05, Tg vs Wt mice. **P<0.01, Tg vs Wt mice. AT1R indicates angiotensin II type 1 receptor; HS, high salt; NS, normal salt; Tg, renal angiotensin II type 1 receptor–associated protein transgenic; Wt, wild type.
Discussion
In the present study, renal tubule–dominant enhancement of ATRAP expression in salt‐sensitive C57BL/6J mice exerted the following effects. First, dietary HS loading‐mediated BP elevation was inhibited without a significant effect on baseline BP (Figure 1). Second, no effect on renal tissue Ang II level was evident under dietary HS loading (Figure 2). Third, urinary sodium excretion increased in response to HS loading on metabolic cage analysis (Figure 3). Fourth, functional transport activity of ENaC, a major sodium transporter in the renal distal tubules, was reduced under saline volume–expanded conditions on diuretic testing (Figure 4), without any significant difference in ENaC protein expression (Figure 5). Fifth, the plasma membrane AT1R protein level in the kidney was decreased (Figure 6).
The rATRAP‐Tg mice with renal tubule–dominant ATRAP enhancement did not exhibit any evident alteration in baseline physiological functions, including BP and electrolyte concentration (Table; Figure 1). This finding is consistent with our previously reported observation that alteration of ATRAP expression did not affect physiological cardiovascular function in vivo, as in other types of ATRAP‐transgenic and ‐deficient mice.8,11–14 With respect to the role of AT1R in physiological BP homeostasis, although systemic AT1R deficiency resulted in chronic hypotension at baseline concomitant with mild renal morphologic abnormalities and a defect in the concentration of urine, dietary HS loading was able to restore reduced BP to a level comparable to that in control mice.31–34 Furthermore, renal proximal tubule–specific enhancement of AT1R reportedly provokes significant baseline BP elevation in mice,35 whereas renal proximal tubule–specific deletion of AT1R significantly decreases baseline BP.36 These results indicate that AT1R, particularly renal proximal tubule AT1R and not renal tubule ATRAP, plays a major role in physiological BP homeostasis at baseline.
In contrast to the lack of any evident change in baseline BP in the rATRAP‐Tg mice, dietary HS loading–mediated BP elevation was significantly suppressed in the rATRAP‐Tg mice, concomitant with increased cumulative sodium excretion (Figures 1 and 3). With respect to the mechanisms involved in the suppression of salt‐sensitive BP elevation, stimulated sodium reabsorption in the distal tubules (ie, the post–macula densa segments of the nephron, including the distal convoluted tubule, connecting tubule, and collecting duct) plays a critical role in salt‐sensitive hypertension.37–38 Sodium reabsorption in the distal tubules occurs in the distal convoluted tubule by electroneutral cotransport via the NCC and in the late portion of the distal convoluted tubule (DCT2), connecting tubule, and collecting duct by electrogenic sodium reabsorption through ENaC.37 Consequently, NCC and ENaC activities are considered to play a crucial role in the mechanism that underlies the maintenance of normal fluid volume homeostasis and BP regulation in response to dietary salt intake. Change in the regulation of sodium handling through modification of ENaC activity in the distal tubules is considered to play a role in the inhibition of salt‐sensitive BP elevation, as shown in rATRAP‐Tg mice in this study.
Regarding the distal tubules in renal sodium handling, activation of NCC and ENaC in the distal nephron segments reportedly plays a crucial role in the stimulation of sodium reabsorption by the Ang II–AT1R axis.4,23,39–42 With respect to the relationship between HS loading and the renal renin–angiotensin system, although these rATRAP‐Tg mice did not exhibit any evident change in renal tissue Ang II level under dietary HS loading (Figure 2), dietary HS loading reportedly provokes salt‐induced BP elevation concomitant with activation of the intrarenal renin–angiotensin system in several models of salt‐sensitive hypertension including Dahl salt‐sensitive hypertensive rats and C57BL/6 mice.18,30,43–44
The rATRAP‐Tg mice exhibited a predominantly high expression pattern of the hemagglutinin‐tagged ATRAP transgene in the distal tubules from the distal convoluted tubule to the connecting tubule,16 in which both NCC and ENaC are abundant. The results of the diuretic tests using specific inhibitors of the respective sodium transporters showed that the functional transport activity of ENaC was significantly decreased in rATRAP‐Tg mice compared with Wt mice (Figure 4). These results indicate that inhibition of the functional activity of ENaC is critically involved in suppression of HS‐induced BP elevation in rATRAP‐Tg mice. Considering that protein expression of the ENaC subunits was not altered in the rATRAP‐Tg mouse kidney (Figure 5), posttranslational modifications that alter trafficking or function of the channel, rather than modulation of ENaC expression, may contribute to reduced ENaC function. Further studies are needed to investigate these and other possible mechanisms.
In addition, it was reportedly shown that the phenotype of salt sensitivity was not altered by renal proximal tubule–specific deletion of AT1R, regardless of the decrease in baseline BP.36 Furthermore, the rATRAP‐Tg mice exhibited significant amelioration of Ang II–induced hypertension, despite no evident change in baseline BP, via suppression of upregulation of renal ENaC expression by Ang II stimulation in the absence of any dietary HS loading.16 Collectively, these results indicate that renal distal tubule–dominant ATRAP enhancement exerts an inhibitory effect on dietary HS loading–mediated BP elevation via suppression of the distal tubule AT1R–sodium transporter axis. Indeed, the plasma membrane AT1R level was significantly decreased in the kidney of rATRAP‐Tg mice compared with Wt mice (Figure 6), suggesting that enhancement of renal tubule ATRAP expression beyond baseline promotes AT1R internalization to reduce distal tubule AT1R activity.
Because the present study was performed in rATRAP‐Tg mice and their Wt littermates on a C57BL/6 background, caution must be used in applying these findings to the pathophysiology of salt‐sensitive hypertension in humans. In addition, although the C57BL/6J mouse is known as a salt‐sensitive animal model, these mice reportedly display HS loading–mediated BP increase mainly in the dark period, and the degree of salt‐mediated BP elevation is not as dramatic as in Dahl salt‐sensitive hypertensive rats.17 These features make it somewhat difficult to focus on the inhibitory effect of renal tubule ATRAP on salt‐sensitive BP upregulation. Furthermore, dopamine and Ang II have reportedly been shown to be the 2 key renal factors that maintain water and sodium balance, by counterregulating each other's function.45–46 Recent studies in salt‐sensitive C57BL/6 mice and humans with salt‐sensitive hypertension implicate abnormalities in dopamine receptor regulation.47–48 Consequently, it may be that modulation of renal tubule ATRAP also affects the functional linkage between the intrarenal dopaminergic system and the renin–angiotensin system, with further investigation needed to address this important issue.
Nevertheless, the findings of the present study provide important information for further investigation in vivo into the functional roles of ATRAP in the pathogenesis of salt‐sensitive hypertension in humans. The potential benefit of an ATRAP activation strategy is suggested. Further studies to elucidate the molecular mechanisms of antihypertensive properties may enable clinical applications of ATRAP in the future, such as the use of ATRAP‐activating ligands to inhibit overactivation of tissue AT1R in response to chronic pathological stimuli. In conclusion, the present study shows that renal tubule–dominant enhancement of ATRAP suppressed the functional activity of ENaC in the distal tubules, promoting sodium excretion and contributing to inhibition of dietary salt‐sensitive BP elevation.
Sources of Funding
This work was supported by a Health and Labor Sciences Research grant (to Tamura, Umemura), by Grants‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (Wakui, Tamura, Ohsawa, Kanaoka), and by grants from SENSHIN Medical Research (Wakui, Tamura, Umemura), Banyu Life Science Foundation International (Wakui, Tamura), and Salt Science Research Foundation (No. 1428 to Tamura).
Disclosures
Tamura received research grant or honoraria from Takeda, Daiichi‐Sankyo, Kyowa‐hakko Kirin, Shionogi, Dainippon‐Sumitomo, Novartis, Chu‐gai, Mochida, MSD, Tanabe Mitsubishi, Boehringer Ingelheim, Astellas, Pfizer, AstraZeneca and Sanofi. Umemura received research grant or honoraria from Pfizer, Daiichi‐Sankyo, Boehringer Ingelheim, AstraZeneca, Astellas, Kowa, Shionogi, Novartis, MSD, Dainippon‐Sumitomo, Torii, Takeda, Tanabe Mitsubishi, Kyowa‐hakko Kirin, Byer, Ohtsuka, Chugai, Mochida, Sanwa‐kagaku and Teijin. The remaining authors declare no conflict of interest.
References
- O'Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004; 113:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II‐dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol. 2013; 305:F510-F519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe N, Zietse R, Hoorn EJ. Effects of angiotensin II on kinase‐mediated sodium and potassium transport in the distal nephron. Curr Opin Nephrol Hypertens. 2013; 22:120-126. [DOI] [PubMed] [Google Scholar]
- Zaika O, Mamenko M, Staruschenko A, Pochynyuk O. Direct activation of ENaC by angiotensin II: recent advances and new insights. Curr Hypertens Rep. 2013; 15:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviet L, Lehtonen JY, Tamura K, Griese DP, Horiuchi M, Dzau VJ. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem. 1999; 274:17058-17062. [DOI] [PubMed] [Google Scholar]
- Lopez‐Ilasaca M, Liu X, Tamura K, Dzau VJ. The angiotensin II type I receptor‐associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell. 2003; 14:5038-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Iwai M, Horiuchi M. Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol. 2007; 27:2532-2539. [DOI] [PubMed] [Google Scholar]
- Tamura K, Tanaka Y, Tsurumi Y, Azuma K, Shigenaga A, Wakui H, Masuda S, Matsuda M. The role of angiotensin AT1 receptor‐associated protein in renin‐angiotensin system regulation and function. Curr Hypertens Rep. 2007; 9:121-127. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci (Lond). 2012; 123:193-203. [DOI] [PubMed] [Google Scholar]
- Castrop H. Angiotensin receptor‐associated proteins: local modulators of the renin‐angiotensin system. Pflugers Arch. 2013; 465:111-119. [DOI] [PubMed] [Google Scholar]
- Tamura K, Wakui H, Maeda A, Dejima T, Ohsawa M, Azushima K, Kanaoka T, Haku S, Uneda K, Masuda SI, Azuma K, Shigenaga AI, Koide Y, Tsurumi‐Ikeya Y, Matsuda M, Toya Y, Tokita Y, Yamashita A, Umemura S. The physiology and pathophysiology of a novel angiotensin receptor‐binding protein ATRAP/Agtrap. Curr Pharm Des. 2013; 19:3043-3048. [DOI] [PubMed] [Google Scholar]
- Wakui H, Tamura K, Tanaka Y, Matsuda M, Bai Y, Dejima T, Masuda S, Shigenaga A, Maeda A, Mogi M, Ichihara N, Kobayashi Y, Hirawa N, Ishigami T, Toya Y, Yabana M, Horiuchi M, Minamisawa S, Umemura S. Cardiac‐specific activation of angiotensin II type 1 receptor‐associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II‐infused mice. Hypertension. 2010; 55:1157-1164. [DOI] [PubMed] [Google Scholar]
- Wakui H, Dejima T, Tamura K, Uneda K, Azuma K, Maeda A, Ohsawa M, Kanaoka T, Azushima K, Kobayashi R, Matsuda M, Yamashita A, Umemura S. Activation of angiotensin II type 1 receptor‐associated protein exerts an inhibitory effect on vascular hypertrophy and oxidative stress in angiotensin II‐mediated hypertension. Cardiovasc Res. 2013; 100:511-519. [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Tamura K, Wakui H, Maeda A, Dejima T, Kanaoka T, Azushima K, Uneda K, Tsurumi‐Ikeya Y, Kobayashi R, Matsuda M, Uchida S, Toya Y, Kobori H, Nishiyama A, Yamashita A, Ishikawa Y, Umemura S. Deletion of the angiotensin II type 1 receptor‐associated protein enhances renal sodium reabsorption and exacerbates angiotensin II‐mediated hypertension. Kidney Int. 2014; 86:570-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejima T, Tamura K, Wakui H, Maeda A, Ohsawa M, Kanaoka T, Haku S, Kengo A, Masuda S, Shigenaga A, Azuma K, Matsuda M, Yabana M, Hirose T, Uchino K, Kimura K, Nagashima Y, Umemura S. Prepubertal angiotensin blockade exerts long‐term therapeutic effect through sustained ATRAP activation in salt‐sensitive hypertensive rats. J Hypertens. 2011; 29:1919-1929. [DOI] [PubMed] [Google Scholar]
- Wakui H, Tamura K, Masuda S, Tsurumi‐Ikeya Y, Fujita M, Maeda A, Ohsawa M, Azushima K, Uneda K, Matsuda M, Kitamura K, Uchida S, Toya Y, Kobori H, Nagahama K, Yamashita A, Umemura S. Enhanced angiotensin receptor‐associated protein in renal tubule suppresses angiotensin‐dependent hypertension. Hypertension. 2013; 61:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SH, Wyss JM. Long‐term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000; 35:E1-E5. [DOI] [PubMed] [Google Scholar]
- Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002; 39:1007-1014. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Tamura K, Tanaka Y, Koide Y, Sakai M, Yabana M, Noda Y, Hashimoto T, Kihara M, Hirawa N, Toya Y, Kiuchi Y, Iwai M, Horiuchi M, Umemura S. Interacting molecule of AT1 receptor, ATRAP, is colocalized with AT1 receptor in the mouse renal tubules. Kidney Int. 2006; 69:488-494. [DOI] [PubMed] [Google Scholar]
- Tamura K, Yokoyama N, Sumida Y, Fujita T, Chiba E, Tamura N, Kobayashi S, Kihara M, Murakami K, Horiuchi M, Umemura S. Tissue‐specific changes of type 1 angiotensin II receptor and angiotensin‐converting enzyme mRNA in angiotensinogen gene‐knockout mice. J Endocrinol. 1999; 160:401-408. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002; 39:129-134. [DOI] [PubMed] [Google Scholar]
- Nomura N, Tajima M, Sugawara N, Morimoto T, Kondo Y, Ohno M, Uchida K, Mutig K, Bachmann S, Soleimani M, Ohta E, Ohta A, Sohara E, Okado T, Rai T, Jentsch TJ, Sasaki S, Uchida S. Generation and analyses of R8L barttin knockin mouse. Am J Physiol Renal Physiol. 2011; 301:F297-F307. [DOI] [PubMed] [Google Scholar]
- Castaneda‐Bueno M, Cervantes‐Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4‐dependent process. Proc Natl Acad Sci USA. 2012; 109:7929-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ND, Wistrand PJ, Isenberg H, Askmark H, Jeffery S, Hopkinson D, Edwards Y. Induction of carbonic anhydrase III mRNA and protein by denervation of rat muscle. Biochem J. 1988; 256:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007; 5:331-344. [DOI] [PubMed] [Google Scholar]
- Wakui H, Tamura K, Matsuda M, Bai Y, Dejima T, Shigenaga A, Masuda S, Azuma K, Maeda A, Hirose T, Ishigami T, Toya Y, Yabana M, Minamisawa S, Umemura S. Intrarenal suppression of angiotensin II type 1 receptor binding molecule in angiotensin II‐infused mice. Am J Physiol Renal Physiol. 2010; 299:F991-F1003. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt‐sensitive rats on high salt diet. Hypertension. 2003; 41:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen‐activated protein activity in Dahl salt‐sensitive rats. Kidney Int. 2004; 65:972-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Leenen FH. Prevention of salt induced hypertension and fibrosis by angiotensin converting enzyme inhibitors in Dahl S rats. Br J Pharmacol. 2007; 152:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Yoneda T, Demura M, Karashima S, Usukura M, Yamagishi M, Takeda Y. Effect of mineralocorticoid receptor blockade on the renal renin‐angiotensin system in Dahl salt‐sensitive hypertensive rats. J Hypertens. 2009; 27:800-805. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Delnomdedieu M, Best CF, Li P, Morris M, Callahan MF, Johnson GA, Smithies O, Coffman TM. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol Renal Physiol. 2000; 278:F75-F82. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension. 2000; 35:550-554. [DOI] [PubMed] [Google Scholar]
- Mangrum AJ, Gomez RA, Norwood VF. Effects of AT(1A) receptor deletion on blood pressure and sodium excretion during altered dietary salt intake. Am J Physiol Renal Physiol. 2002; 283:F447-F453. [DOI] [PubMed] [Google Scholar]
- Sato K, Kihara M, Hashimoto T, Matsushita K, Koide Y, Tamura K, Hirawa N, Toya Y, Fukamizu A, Umemura S. Alterations in renal endothelial nitric oxide synthase expression by salt diet in angiotensin type‐1a receptor gene knockout mice. J Am Soc Nephrol. 2004; 15:1756-1763. [DOI] [PubMed] [Google Scholar]
- Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011; 301:R1067-R1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley SB, Riquier‐Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011; 13:469-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000; 80:277-313. [DOI] [PubMed] [Google Scholar]
- Ronzaud C, Loffing‐Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler‐Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O. Renal tubular NEDD4‐2 deficiency causes NCC‐mediated salt‐dependent hypertension. J Clin Invest. 2013; 123:657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II‐infused mice. Hypertension. 2009; 54:120-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide‐sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011; 79:66-76. [DOI] [PubMed] [Google Scholar]
- Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone‐independent epithelial Na+ channel activation. Hypertension. 2013; 62:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes AD, van der Lubbe N, Zietse R, Loffing J, Hoorn EJ. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch. 2014; 466:107-118. [DOI] [PubMed] [Google Scholar]
- Tamura K, Chiba E, Yokoyama N, Sumida Y, Yabana M, Tamura N, Takasaki I, Takagi N, Ishii M, Horiuchi M, Umemura S. Renin‐angiotensin system and fibronectin gene expression in Dahl Iwai salt‐sensitive and salt‐resistant rats. J Hypertens. 1999; 17:81-89. [DOI] [PubMed] [Google Scholar]
- Ushio‐Yamana H, Minegishi S, Ishigami T, Araki N, Umemura M, Tamura K, Maeda E, Kakizoe Y, Kitamura K, Umemura S. Renin angiotensin antagonists normalize aberrant activation of epithelial sodium channels in sodium‐sensitive hypertension. Nephron Exp Nephrol. 2012; 122:95-102. [DOI] [PubMed] [Google Scholar]
- Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006; 2:637-650. [DOI] [PubMed] [Google Scholar]
- Chugh G, Pokkunuri I, Asghar M. Renal dopamine and angiotensin II receptor signaling in age‐related hypertension. Am J Physiol Renal Physiol. 2013; 304:F1-F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009; 297:R1660-R1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep. 2012; 14:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]


