Abstract
Background
Maintenance of the structure and mechanical properties of the thoracic aorta contributes to aortic function and is dependent on the composition of the extracellular matrix and the cellular content within the aortic wall. Age‐related alterations in the aorta include changes in cellular content and composition of the extracellular matrix; however, the precise roles of these age‐related changes in altering aortic mechanical function are not well understood.
Methods and Results
Thoracic aortic rings from the descending segment were harvested from C57BL/6 mice aged 6 and 21 months. Thoracic aortic diameter and wall thickness were higher in the old mice. Cellular density was reduced in the medial layer of aortas from the old mice; concomitantly, collagen content was higher in old mice, but elastin content was similar between young and old mice. Stress relaxation, an index of compliance, was reduced in aortas from old mice and correlated with collagen fraction. Contractility of the aortic rings following potassium stimulation was reduced in old versus young mice. Furthermore, collagen gel contraction by aortic smooth muscle cells was reduced with age.
Conclusions
These results demonstrate that numerous age‐related structural changes occurred in the thoracic aorta and were related to alterations in mechanical properties. Aortic contractility decreased with age, likely because of a reduction in medial cell number in addition to a smooth muscle contractile deficit. Together, these unique findings provide evidence that the age‐related changes in structure and mechanical function coalesce to provide an aortic substrate that may be predisposed to aortopathies.
Keywords: aging, aorta, compliance, contractility
Introduction
The thoracic aorta is a large elastic artery with functionality beyond a static, passive conduit.1 Functions of the aorta include regulating systolic blood pressure by accepting the ejection bolus through expansion and maintaining diastolic blood pressure by dynamic, elastic recoil.1–2 Consequently, normal functioning of the aorta requires consistent passive and active mechanical properties, which include compliance and contractility.3 Vessel compliance is generally considered to be related to the composition of the extracellular matrix (ECM).1 Two ECM proteins in particular, collagen and elastin, provide the aortic wall with tensile strength and elastic recoil and contribute to maintaining compliance properties of the aorta.4 Aortic contractility is reflected by the contractile force generated primarily by vascular smooth muscle cells (SMCs) and provides the active tension component within the aorta.5 Together, the extracellular and cellular components regulate mechanical properties within the aorta and, when altered, could contribute to vascular pathophysiology.6
Aging is associated with changes in human aortic geometry.7–8 Redheuil and colleagues reported an age‐dependent increase in the diameter and thickness of the human thoracic aorta.9 Furthermore, aging was associated with fibrosis in a number of organs including the aorta.10–11 In particular, an increase in collagen content has been reported to occur in aortas from older subjects.12 Although elastin content has been reported to be similar in aortas from young and older human subjects, the abundance of elastin relative to other ECM components is reduced in the aortas from old subjects.12 The precise roles of these age‐related changes in altering aortic mechanical function are not well understood. Accordingly, this study used young and old mice to test the hypothesis that aging is accompanied by ECM and cellular changes that directly affect the compliance and contractility of the thoracic aorta.
Methods
Overview
The goals of this study were to determine the effects of age on the geometry, the cellular and ECM composition, and the relationship between ECM composition and mechanical characteristics of the thoracic aorta.
Animals
All procedures were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (2012) and approved by the institutional animal care and use committee of the Medical University of South Carolina. C57BL6 wild‐type male and female mice (N=103, 50 male, 53 female) aged 6 (n=55) and 21 (n=48) months were obtained from the National Institute on Aging mouse colony at Charles River Laboratories International (Wilmington, MA, USA). Equal numbers of male and female mice were used in each study. Old mice were heavier than young mice (33.5±0.9 versus 28.5±0.8 g; old, n=38; young, n=42). Tibia length was measured with a digital caliper after incubating 48 hours in 2 mol/L potassium hydroxide and was similar between the old and young groups (18.2±0.1 mm versus 18.0±0.2 mm; old (n=9) versus young (n=19), respectively).
In Vivo Aortic Diameter Measurement
Anesthetized mice (2% isoflurane) were intubated and mechanically ventilated.13 A left posterolateral thoracotomy was performed at the sixth intercostal space. Images of the exposed descending thoracic aorta were captured on a calibrated video microscopy system (PAXcam using PAX‐it software version 7.6; MIS Inc) and then were used to measure external aortic diameter, as described previously.13
Histology and Quantitative Analysis
Descending thoracic aortas from young (n=10) and old (n=10) mice were harvested from the level of the eighth rib to the diaphragm and fixed in 10% formalin for 48 hours and then transferred to 70% ethanol for storage at 4°C. Fixed aortic segments were embedded in paraffin and sectioned at 2‐μm thickness. Sequential sections were stained with picrosirius red for collagen, Verhoeff‐Van Gieson for elastin, and hematoxylin and eosin to visualize cell nuclei for quantification. Aortic tissue sections were visualized on a Zeiss Axioskop 2 microscope (Carl Zeiss MicroImaging), and digital images were acquired (Axiocam MRc camera and AxioVision software, version 4.8). All quantitative image analysis was performed using SigmaScan Pro version 5.0 (Systat Software Inc) and Adobe Photoshop CS5 (Adobe). For each study, analysis of histology sections was performed using at least 3 randomly chosen high‐power fields from 3 different sections from each young (n=10) and old (n=10) mouse. Aortic medial thickness was measured from the internal elastic lamina to the adventitial border. Aortic lumen perimeter was calculated by summation of the edge lengths of a digital overlay filling the vessel lumen. Collagen and elastin volume fraction in the medial layer was determined as the ratio of the area stained by picrosirius red and Verhoeff‐Van Gieson stain, respectively, divided by the total area of tissue within each high‐power field. The volume fraction of thin (green–yellow birefringence) and thick (red–orange birefringence) collagen fibers was measured in picrosirius red–stained sections illuminated by polarized light. Cell density (in cells/mm2) in the medial aortic wall was calculated by counting the number of nuclei in 0.0023‐mm2 areas of medial tissue from 3 sections from young (n=10) and old (n=10) mice and dividing by the total area of tissue examined.
Aortic Ring Preparation and Measurement of Passive Tension
Descending thoracic aortas from young (n=25) and old (n=24) mice were harvested and immediately placed in cold Krebs‐Hanseleit buffer (118 mmol/L NaCl, 4.6 mmol/L KCl, 1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 2.5 mmol/L CaCl2, 0.5 mmol/L Na2‐EDTA, 11 mmol/L glucose, 1.2 mmol/L MgSO4, pH 7.4). The endothelium was removed by gently rubbing the intimal surface to mitigate any potential endothelial effects on medial contractility.14 The aorta was divided into 3‐mm‐long segments (typically 2 segments per mouse) that were each mounted on parallel wires in a water‐jacketed tissue bath system (25 mL; Radnoti LLC) maintained at 37°C and connected to an isometric force transducer (Radnoti).15 The vessels were allowed to equilibrate for 30 minutes in the absence of tension and washed every 15 minutes with warm Krebs‐Hanseleit solution aerated with 95% O2 and 5% CO2.
Tension was applied by gradually separating the parallel wires, as described previously.16 The Krebs‐Hanseleit solution was supplemented with EGTA (5 mmol/L) to minimize calcium‐induced SMC contraction. Stress relaxation was quantified by sequentially stretching vessel segments in 0.1‐g increments (from 0.2 to 1.2 g of applied tension) and measuring residual tension after 3 minutes to calculate the percentage of relaxation. Measurements were recorded using Biobench software (National Instruments). Stress relaxation values were normalized to wall thickness for comparison of differently sized segments.
Determination of Aortic Contractile Properties
To determine contractile properties, the aortic rings harvested from young (n=14) and old (n=8) mice were equilibrated with an applied tension of 0.4 g for 30 minutes in standard Krebs‐Hanseleit buffer without EGTA. Contraction was stimulated by adding KCl (final concentration of 100 mmol/L in the bath), and the peak contractile force (in grams) generated over the ensuing 8 minutes was recorded. The rings were washed 3 times with fresh Krebs‐Hanseleit solution to remove residual KCl. These steps were repeated at 0.1‐g increments up to 1.2 g of applied tension, as described previously.15 To control for differences in ring tissue mass between young and old mice, the peak contractile force was normalized to aortic ring volume, determined from aortic diameter and wall thickness.
Smooth Muscle Cell Isolation
Primary SMCs were isolated using an outgrowth procedure in which minced pieces of aorta from young and old mice (6 young, 6 old) were plated in SMC‐specific growth medium (C22062; PromoCell) and allowed to extravasate for 2 weeks. After 2 weeks, the pieces of aorta were removed, and the SMCs were maintained in culture. Cells in passages 3 to 7 were used for all in vitro experiments.
Collagen Gel Contraction Assay
Equal numbers of SMCs (50 000) were embedded in disks of rat tail collagen type I (1 mg/mL in SMC growth medium; Gibco) polymerized in a 24‐well nontissue culture‐treated plate in the presence of broad‐spectrum matrix metalloproteinase inhibitor GM6001 (25 μmol/L final concentration; Millipore). Disk area was measured from digital images collected at 0, 3, 6, 9, 12, 18, and 24 hours. Contraction was expressed as a percentage of baseline area at time 0.
Statistical Analysis
All results are presented as mean±SEM. The Student t test was used to compare measurements of aortic geometry, histology, and peak active tension between young and old groups. Repeated‐measures ANOVA was used to compare passive tension and collagen gel contraction between experimental groups. If ANOVA revealed significant differences, post hoc mean separation was performed using Tukey's wholly significant difference test. The relationships between the abundance of ECM components and aortic mechanical properties were established using linear least‐squares regression analysis. All statistical analyses were performed using Stata statistical software (version 8, intercooled; StataCorp). P values of <0.05 were considered to be statistically significant.
Results
Thoracic Aortic Diameter and Medial Thickness With Aging
Thoracic aortic diameter, measured in vivo, was significantly greater in the old mice than in young mice (Figure 1A). The aortic medial layer, measured from histological sections (as shown in representative photomicrographs in Figure 1B), was significantly thicker in old mice than in young mice. In addition, lumen perimeter was greater in old versus young mice (Figure 1C). Together, these results suggest that an age‐dependent increase in aortic diameter was the result of both dilation and increased wall thickness.
Figure 1.
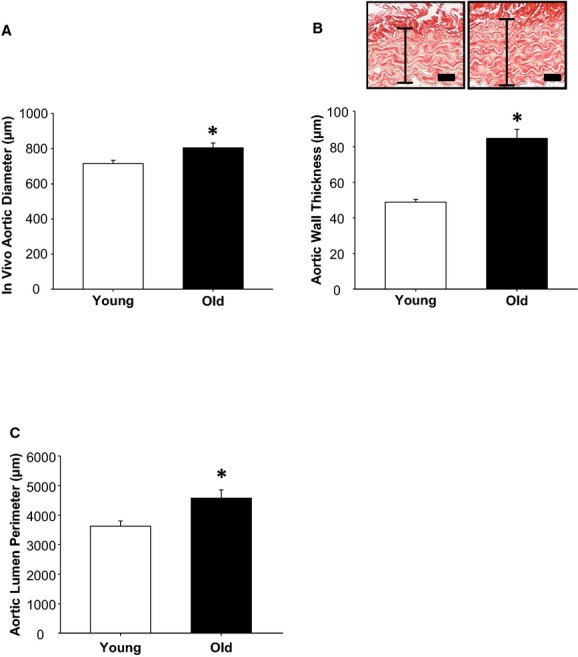
Thoracic aortic diameter, wall thickness, and lumen perimeter increase with age. A, In vivo aortic diameter (in micrometers) was increased in old mice (n=14) compared with young mice (n=12). B, Representative histology showing full thickness of thoracic aortas from young mice (left) and old mice (right) stained with picrosirius red. Scale bar=20 μm. Aortic wall thickness was greater in old (n=10) versus young (n=10) mice. C, Aortic lumen perimeter was increased in old mice (n=8) relative to young mice (n=8). *P<0.05 vs young.
Medial Extracellular Matrix and Cellular Changes With Aging
Representative picrosirius red–stained sections in bright‐field illumination are shown in Figure 2A (top panel). Quantification of the amount of staining from these sections revealed that collagen volume fraction was increased in the old group, as summarized in Figure 2A (bottom panel). To assess collagen fiber characteristics, picrosirius red–stained sections were illuminated in polarized light (Figure 2B, top panel). The thin fibers show birefringence in the green–yellow spectrum, whereas the thick fibers are birefringent in the orange–red spectrum.17 The medial volume fraction of thin collagen fibers and thick collagen fibers were both increased in the old group compared with the young group (Figure 2B, bottom panel). To examine the elastic architecture, aortic sections from young and old mice were stained with Verhoeff‐Van Gieson (Figure 2C, top panel). The medial elastin volume fraction was not different between young and old mice (Figure 2C, bottom panel).
Figure 2.
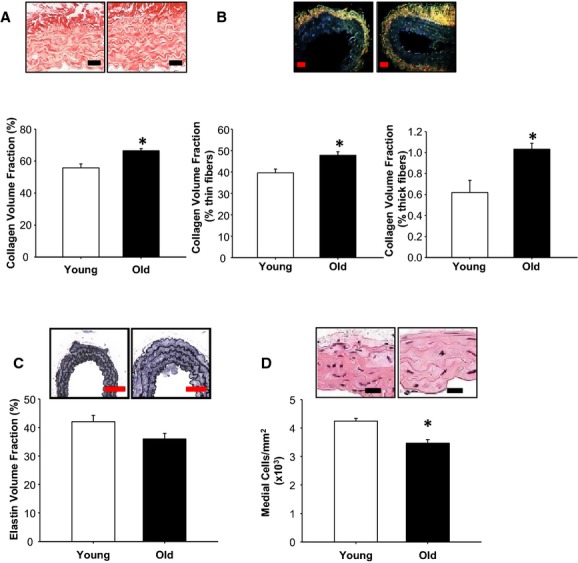
Thoracic aortic structure and composition change with age. The structure and composition of the aortic medial layer were compared between young (n=10) and old (n=10) mice using histological staining. A, Representative images of collagen stained with picrosirius red viewed under bright‐field light in young (left) and old (right) mice. Scale bar=25 μm. Collagen volume fraction was increased in old mice relative to young mice. B, Representative images of collagen stained with picrosirius red viewed under polarized light in young (left) and old (right) mice. Scale bar=25 μm. The volume fraction of thin collagen fibers (green–yellow birefringence) was increased in old mice relative to young mice (left graph). Similarly, the volume fraction of thick collagen fibers (red–orange birefringence) was increased in old mice relative to young mice (right graph). C, Representative images of elastin stained with Verhoeff‐Van Gieson in young (left) and old (right) mice. Scale bar=75 μm. Elastin volume fraction was not different between young and old mice. D, Representative images of aortic sections stained with hematoxylin and eosin (red/pink indicates cytoplasmic and extracellular proteins, black/blue indicates nuclei) in young (left) and old (right) mice. Scale bar=15 μm. The total number of nuclei, equivalent to medial cellularity, was reduced in old mice relative to young mice. *P<0.05 vs young.
To determine cellular density, hematoxylin and eosin–stained aortas from young and old mice were examined and nuclei were counted (Figure 2D, top panel). The density of cells in the medial layer was significantly lower in the aortas from old mice compared with young mice (Figure 2D, bottom panel).
Thoracic Aortic Compliance Declines With Age, Correlates With Medial Collagen Volume Fraction but Not With Medial Elastin Volume Fraction
Alterations in the relative abundance of medial ECM proteins may be expected to alter the passive mechanical properties of the thoracic aorta. Accordingly, stress relaxation was measured in aortic rings of young and old mice as a direct indicator of aortic compliance. The results demonstrated that aortic rings from old mice were less compliant than rings from young mice, indicated by the downward shift in the relationship between applied tension and stress relaxation (Figure 3A). As one may expect, stress relaxation was negatively correlated with collagen volume fraction (r=−0.6206, P<0.05) (Figure 3B). There was no correlation between stress relaxation and elastin volume fraction (r=0.2824, P was not significant) (Figure 3C).
Figure 3.
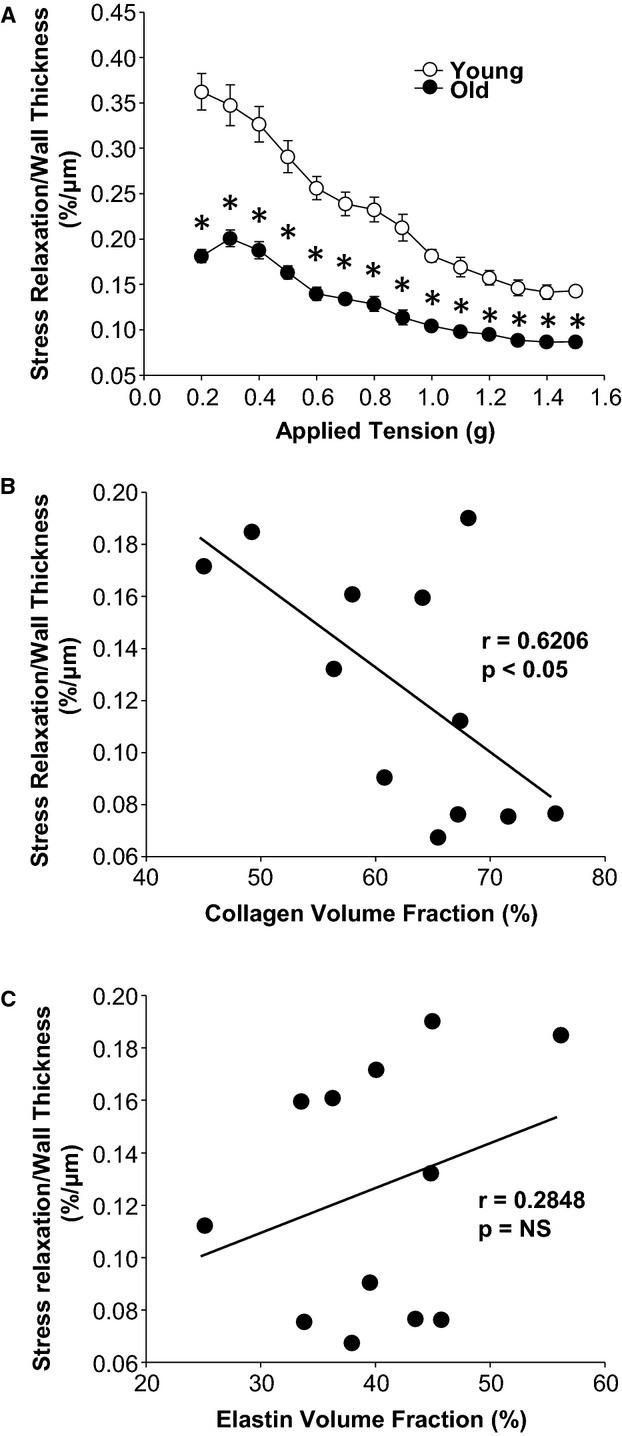
Mechanical properties of young and old aortas. A, Stress relaxation normalized to wall thickness was decreased in old mice (n=24) relative to young mice (n=24). B, Stress relaxation normalized to wall thickness is negatively correlated with collagen volume fraction; correlation coefficient, R, and P value of linear regression are shown. C, Stress relaxation normalized to wall thickness was not related to elastin volume fraction; correlation coefficient, R, and P value of linear regression are shown. *P<0.05 vs young.
Thoracic Aortic Contractility With Aging
Peak contractile force was used to compare thoracic aortic contractility between young and old mice.15 Peak contractile force normalized to aortic tissue volume (Figure 4) was reduced in aortic segments from old mice compared with young mice.
Figure 4.
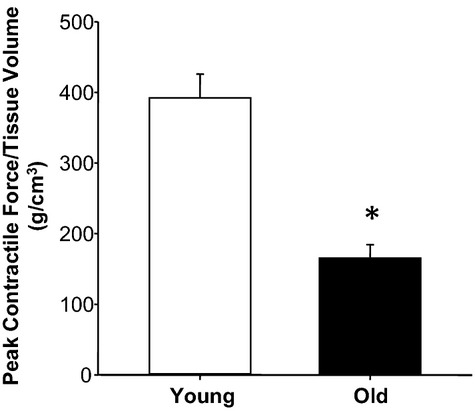
Aortic contractility decreases with age. Peak contractile force normalized to tissue volume was reduced in old mice (n=8) relative to young mice (n=14). *P<0.05 vs young.
Smooth Muscle Cell Contraction of Collagen With Aging
The collagen gel contraction assay was used to determine whether the reduced peak contractile force observed in aortas from old mice was due to age‐related changes in the contractile phenotype of aortic SMCs (ASMCs) or just due to a decrease in overall medial cellularity. Isolated primary SMCs from young and old mice were examined in the presence of a global matrix metalloproteinase inhibitor. Collagen disks seeded with AMSCs isolated from young and old mice displayed a time‐dependent reduction in area over the 24‐hour duration of the assay (Figure 5). Gel contraction mediated by ASMCs from old mice was significantly reduced compared with ASMCs from young mice (repeated‐measures ANOVA, F=133.875, P<0.05). In comparison, the reduction in disk area at and after the 6‐hour time point was significantly attenuated in ASMCs isolated from old versus young mice (Figure 5).
Figure 5.
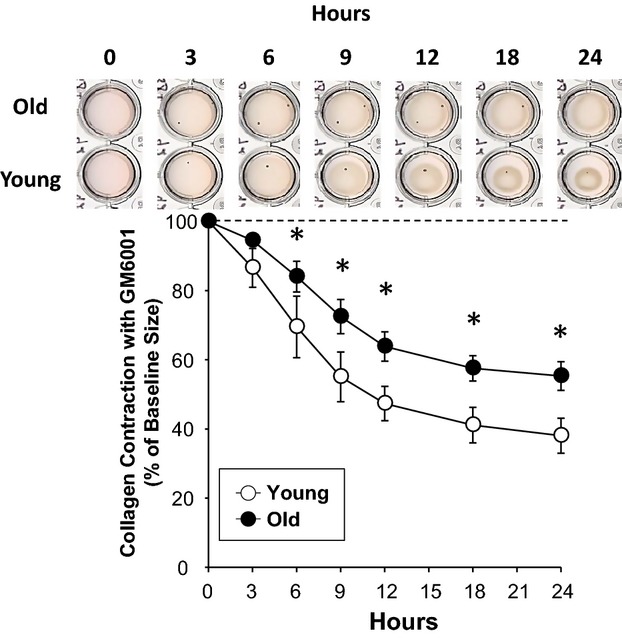
Collagen gel contraction by aortic smooth muscle cells decreases with age. Collagen gel contraction in the presence of the matrix metalloproteinase inhibitor GM6001 by aortic smooth muscle cells from old mice (n=6) is reduced over 24 hours relative to those from young mice (n=6). *P<0.05 vs young.
Discussion
Age‐related structural and cellular alterations within the aorta occur concomitantly with biomechanical dysfunction. Accordingly, this study tested the hypothesis that alterations within the ECM and cellular composition that occur with age directly affect the compliance and contractility of the thoracic aorta. The findings of the present study demonstrate that aortic diameter, thickness of the medial layer of the aortic wall, and collagen volume fraction were higher in the older mice. Moreover, there was an age‐related decrease in cell density, aortic compliance, and aortic contractility. Interestingly, changes in aortic compliance were correlated with collagen content but not with elastin content. This report demonstrates that the age‐dependent changes in the material properties of the thoracic aorta in a murine model contribute, at least in part, to reduced compliance and contractility of the thoracic aorta. Taken together, these age‐related changes in aortic material properties and mechanical characteristics may yield an aortic substrate that is more conducive to the development of aortopathies, such as aneurysm of the thoracic aorta.
Changes in the geometry of the thoracic aorta that occur with age include aortic dilation and increased aortic wall thickness.8,12 Mao et al reported that dilation of the thoracic aorta occurred in older but healthy humans.18 Similarly, Sonesson et al reported that there was dilation of the abdominal aorta with increasing age.19 Taken together, these previous studies suggest that age‐related dilation may occur throughout the aorta. The thickness of the aortic wall has also been reported to increase with age.20–22 Pearson et al reported that age was positively correlated with thoracic aortic wall thickness.23 Consistent with these past findings, the present study demonstrated that diameter, luminal perimeter, and wall thickness of the thoracic aorta were increased as a function of age. Because wall stress (Laplace's law) is directly proportional to vessel diameter, a physiological consequence of an increase in lumen diameter is increased wall stress; therefore, wall thickness, which is inversely related to wall stress, may have increased to normalize wall stress, as described previously in humans.24–25 Consequently, this murine aging model recapitulates the morphological hallmarks of human thoracic aortic aging and may serve as a valid model for human aortic disease.
The structure and composition of the aortic wall were examined to explore whether age‐related morphological changes occurred concomitantly with biochemical alterations to the aortic wall. In the present study, aortic wall collagen content increased in old mice, whereas elastin content remained the same between young and old mice. Moreover, both thin and thick collagen fibers were increased in the aortas from old animals. These findings are consistent with previous studies that reported increased collagen and collagen fiber size in the aortic walls of older rats and humans.12,26 Also similar, aortic elastin content has been reported to remain unchanged or to decrease with increasing age.27–28 The current study builds on these past reports by demonstrating that an age‐dependent change in aortic collagen content was correlated with a decrease in aortic stress relaxation. The stress relaxation of a vessel is related to its passive compliance, and an age‐dependent decline in stress relaxation suggests that the aorta becomes stiffer with age.29 Moreover, the negative correlation between aortic stress relaxation and collagen content suggests that collagen, but not elastin, is a key factor in determining the passive compliance of the thoracic aorta. Taken together, the findings from these past reports and the present study suggest that remodeling of the ECM within the thoracic aorta occurs as a function of age and that this remodeling appears to be driven by selective deposition of collagen. Importantly, increased aortic collagen in the setting of aortic dilation with age may initially compensate for increased wall stress; however, when these compensation mechanisms are exhausted, increases in wall stress in combination with a less compliant aorta may result in increased susceptibility to rupture.
In addition to changes in aortic structure and ECM composition, changes in cellular composition also occur. In the present study, the density of cells in the medial layer of the aortic wall was decreased in old mice; this finding is consistent with past studies.30–31 Given that SMCs make up most of the cellular volume of the aortic media, a functional consequence of a decrease in cell number may be reflected as a reduction in contractility of the aortic wall.31 Indeed, peak contractile force in aortic rings from old mice was reduced compared with that of young mice. A similar decline in contractility of aortic rings from old animals has been reported in rats.14 Nonetheless, these ex vivo contractility determinations do not directly delineate the contribution of SMCs to generate contractile force. To address this issue, the present study examined the effects of isolated SMCs on the contraction of collagen disks. In the collagen gel contraction assay, both cellular contraction and protease degradation can act together to reduce collagen disk area. To determine the contribution of only the contractile component, collagen gel compaction was measured in the presence of a broad‐spectrum matrix metalloproteinase inhibitor. Contraction of disks seeded with primary SMCs isolated from aortas of old mice was reduced compared with that of SMCs isolated from young mice. These results suggest an age‐dependent decline in contractile function of primary ASMCs.
Importantly, the mechanical findings of this study are aligned with previous work examining the effects of aging on the human aorta. O'Rourke et al found that aortic stiffness, when measured in vivo as pulse wave velocity, increased by ≈100% between the ages of 20 and 80 years.32 Using an intra‐aortic ultrasonic catheter, Stefanadis et al found a positive correlation between age and the slope of the aortic pressure–diameter loop (aortic elasticity).33 Increasing aortic stiffness with age has also been demonstrated by Sun et al with multiphase computed tomography imaging and through ex vivo biaxial mechanical testing of aortic specimens.34–35 Results presented in this report examining aortic aging in a murine model agree with these previous studies demonstrating a direct relationship between increased chronological age and increased aortic stiffness such that as age increased, so did aortic stiffness.
Increased aortic stiffness has a clinically significant impact on adjacent organ systems as well as on the aorta itself. Increased aortic stiffness, for example, is an independent risk factor for nonfatal cardiovascular events, ischemic stroke, and renal failure.36–38 Incorporation of aortic compliance along with other risk factors in selected patient populations has been suggested as a screening tool.39 Furthermore, reduced aortic compliance is a common feature in aortic dissection, aneurysm, and rupture that has been pathogenetically implicated because of its deleterious effects on wall integrity.35,40–41 Guidelines on addressing aortic mechanical function clinically have been established and include pharmacological approaches to reduce the effects of arterial stiffness, such as antihypertensive, hypolipidemic, and antidiabetic agents.42–45 Likewise, SMC dysfunction, which can result in reduced contractility, has been associated with aortic aneurysm and dissection.46 Novel approaches to ameliorate the mechanical effects of aging on the aorta, such as microRNA therapies to modulate the aortic ECM and smooth muscle function, have also been proposed.47–48 Long‐term therapeutic trials of these pharmacological approaches should be conducted to assess the value of treating these age‐related mechanical deficits in terms of cardiovascular risk reduction.
This study is not without limitations. First, other ECM components, including proteoglycans and matricellular proteins, may also change with age and affect aortic mechanical properties.27 In addition, although the correlation between elastin content and aortic stress relaxation was not statistically significant, a correlation of 0.28 could be meaningful in other settings and perhaps did not attain significance because of the sample size. Second, although a reduction in cell density with age was identified, the types of cells that were reduced were not identified. A shift in populations of noncontractile fibroblasts or contractile myofibroblasts relative to ASMCs may also affect aortic mechanical properties. Future studies could examine the relative abundance of cell types using cell type–specific markers. Finally, an index of SMC contractility was assessed using type 1 collagen disks. Care should be taken in extrapolating these results to more complex ECM environments like those found in vivo, which are difficult to replicate with in vitro assays. Nevertheless, the unique findings of the present study provide potential explanations for reduced compliance and contractility with aging. In conclusion, these results have identified age‐related changes in geometry, ECM collagen content, and medial cellular content within the thoracic aorta that contribute to alterations in aortic mechanical properties. Importantly, both aortic compliance and contractility were shown to decrease with age, and this decrease may have significant clinical implications for the risk of developing aortic disease with advanced age.
Acknowledgments
The authors would like to acknowledge Margaret Romano and the MUSC Histology Core for their assistance with histology specimens. This material is the result of work supported with resources and the use of facilities located in the Research Service of the Ralph H. Johnson VA Medical Center in Charleston, SC.
Sources of Funding
This study was supported by NIH National Institute on Aging Grant R01 AG036854 (J.S.I.), VA BLR&D Merit Award 2I01 BX000904‐04 (J.A.J.), and by T32 HL007260‐37 (J.B.W.).
Disclosures
None.
References
- Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009; 89:957-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopulos N, Westerhof N. Role of total arterial compliance and peripheral resistance in the determination of systolic and diastolic aortic pressure. Pathol Biol. 1999; 47:641-647. [PubMed] [Google Scholar]
- Silver FH, Christiansen DL, Buntin CM. Mechanical properties of the aorta: a review. Crit Rev Biomed Eng. 1989; 17:323-358. [PubMed] [Google Scholar]
- Sokolis DP. Passive mechanical properties and structure of the aorta: segmental analysis. Acta Physiol (Oxf). 2007; 190:277-289. [DOI] [PubMed] [Google Scholar]
- Tuna BG, Bakker EN, VanBavel E. Smooth muscle biomechanics and plasticity: relevance for vascular calibre and remodelling. Basic Clin Pharmacol Toxicol. 2012; 110:35-41. [DOI] [PubMed] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007; 211:157-172. [DOI] [PubMed] [Google Scholar]
- Hager A, Kaemmerer H, Rapp‐Bernhardt U, Blucher S, Rapp K, Bernhardt TM, Galanski M, Hess J. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002; 123:1060-1066. [DOI] [PubMed] [Google Scholar]
- Craiem D, Casciaro ME, Graf S, Chironi G, Simon A, Armentano RL. Effects of aging on thoracic aorta size and shape: a non‐contrast CT study. Conf Proc IEEE Eng Med Biol Soc. 2012; 2012:4986-4989. [DOI] [PubMed] [Google Scholar]
- Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO, Bluemke D, Lima JA. Age‐related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol. 2011; 58:1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanaki MG, Mora AL, Rojas M. Influence of age on wound healing and fibrosis. J Pathol. 2013; 229:310-322. [DOI] [PubMed] [Google Scholar]
- Selvin E, Najjar SS, Cornish TC, Halushka MK. A comprehensive histopathological evaluation of vascular medial fibrosis: insights into the pathophysiology of arterial stiffening. Atherosclerosis. 2010; 208:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013; 10:20121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Barbour JR, Lowry AS, Bouges S, Beck C, McClister DM, Jr, Mukherjee R, Ikonomidis JS. Spatiotemporal expression and localization of matrix metalloproteinas‐9 in a murine model of thoracic aortic aneurysm. J Vasc Surg. 2006; 44:1314-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough ER, Rice KM, Desai DH, Wehner P, Wright GL. Aging alters mechanical and contractile properties of the Fisher 344/Nnia X Norway/Binia rat aorta. Biogerontology. 2007; 8:303-313. [DOI] [PubMed] [Google Scholar]
- Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Differential effect of wall tension on matrix metalloproteinase promoter activation in the thoracic aorta. J Surg Res. 2010; 160:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond BR, Zellner JL, Dorman BH, Multani MM, Kratz JM, Crumbley AJ, III, Crawford FA, Jr, Spinale FG. Differential effects of calcium channel antagonists in the amelioration of radial artery vasospasm. Ann Thorac Surg. 2000; 69:1035-1040.‐ [DOI] [PubMed] [Google Scholar]
- Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red‐stained collagen determined only by the diameter of the fibers? Histochemistry. 1989; 93:27-29. [DOI] [PubMed] [Google Scholar]
- Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, Flores FR, Gao YL, Budoff MJ. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008; 15:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson B, Hansen F, Stale H, Lanne T. Compliance and diameter in the human abdominal aorta–the influence of age and sex. Eur J Vasc Surg. 1993; 7:690-697. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993; 73:413-467. [DOI] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of gender‐specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007; 116:669-676. [DOI] [PubMed] [Google Scholar]
- Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998; 32:437-443. [DOI] [PubMed] [Google Scholar]
- Pearson AC, Guo R, Orsinelli DA, Binkley PF, Pasierski TJ. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am Heart J. 1994; 128:344-351. [DOI] [PubMed] [Google Scholar]
- Astrand H, Sandgren T, Ahlgren AR, Lanne T. Noninvasive ultrasound measurements of aortic intima‐media thickness: implications for in vivo study of aortic wall stress. J Vasc Surg. 2003; 37:1270-1276. [DOI] [PubMed] [Google Scholar]
- Sundt TM. Indications for aortic aneurysmectomy: too many variables and not enough equations? J Thorac Cardiovasc Surg. 2013; 145:S126-S129. [DOI] [PubMed] [Google Scholar]
- Cox RH. Age‐related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev. 1983; 23:21-36. [DOI] [PubMed] [Google Scholar]
- Bruel A, Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996; 127:155-165. [DOI] [PubMed] [Google Scholar]
- Spina M, Garbisa S, Hinnie J, Hunter JC, Serafini‐Fracassini A. Age‐related changes in composition and mechanical properties of the tunica media of the upper thoracic human aorta. Arteriosclerosis. 1983; 3:64-76. [DOI] [PubMed] [Google Scholar]
- Klabunde RE. Cardiovascular Physiology Concepts. 2012Philadelphia, PA: Lippincott Williams & Wilkins/Wolters Kluwer; 2012 [Google Scholar]
- Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977; 39:13-20. [DOI] [PubMed] [Google Scholar]
- Collins JA, Munoz JV, Patel TR, Loukas M, Tubbs RS. The anatomy of the aging aorta. Clin Anat. 2014; 27:463-466. [DOI] [PubMed] [Google Scholar]
- Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983; 68:50-58. [DOI] [PubMed] [Google Scholar]
- Stefanadis C, Stratos C, Vlachopoulos C, Marakas S, Boudoulas H, Kallikazaros I, Tsiamis E, Toutouzas K, Sioros L, Toutouzas P. Pressure‐diameter relation of the human aorta. A new method of determination by the application of a special ultrasonic dimension catheter. Circulation. 1995; 92:2210-2219. [DOI] [PubMed] [Google Scholar]
- Martin C, Pham T, Sun W. Significant differences in the material properties between aged human and porcine aortic tissues. Eur J Cardiothorac Surg. 2011; 40:28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Sun W, Primiano C, McKay R, Elefteriades J. Age‐dependent ascending aorta mechanics assessed through multiphase CT. Ann Biomed Eng. 2013; 41:2565-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010; 55:1110-1115. [DOI] [PubMed] [Google Scholar]
- Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014; 16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Hozumi T, Sciacca RR, Miyake Y, Titova I, Gaspard G, Sacco RL, Homma S, Di Tullio MR. Impact of aortic stiffness on ischemic stroke in elderly patients. Stroke. 2002; 33:2077-2081. [DOI] [PubMed] [Google Scholar]
- Metafratzi ZM, Efremidis SC, Skopelitou AS, De Roos A. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J Cardiovasc Magn Reson. 2002; 4:481-491. [DOI] [PubMed] [Google Scholar]
- Wang X, LeMaire SA, Chen L, Shen YH, Gan Y, Bartsch H, Carter SA, Utama B, Ou H, Coselli JS, Wang XL. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation. 2006; 114:I200-I205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003; 75:1210-1214. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, Koide H. Effect of pioglitazone on carotid intima‐media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004; 53:1382-1386. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588-2605. [DOI] [PubMed] [Google Scholar]
- Kool MJ, Lustermans FA, Breed JG, Struyker Boudier HA, Hoeks AP, Reneman RS, Van Bortel LM. The influence of perindopril and the diuretic combination amiloride+hydrochlorothiazide on the vessel wall properties of large arteries in hypertensive patients. J Hypertens. 1995; 13:839-848. [DOI] [PubMed] [Google Scholar]
- Giannattasio C, Mangoni AA, Failla M, Stella ML, Carugo S, Bombelli M, Sega R, Mancia G. Combined effects of hypertension and hypercholesterolemia on radial artery function. Hypertension. 1997; 29:583-586. [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran‐Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha‐actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007; 39:1488-1493. [DOI] [PubMed] [Google Scholar]
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. Microrna‐29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011; 109:1115-1119. [DOI] [PubMed] [Google Scholar]
- Albinsson S, Sward K. Targeting smooth muscle micrornas for therapeutic benefit in vascular disease. Pharmacol Res. 2013; 75:28-36. [DOI] [PubMed] [Google Scholar]


