Abstract
Background
Catheter ablation of persistent atrial fibrillation yields an unsatisfactorily high number of failures. The hybrid approach has recently emerged as a technique that overcomes the limitations of both surgical and catheter procedures alone.
Methods and Results
We investigated the sequential (staged) hybrid method, which consists of a surgical thoracoscopic radiofrequency ablation procedure followed by radiofrequency catheter ablation 6 to 8 weeks later using the CARTO 3 mapping system. Fifty consecutive patients (mean age 62±7 years, 32 males) with long‐standing persistent atrial fibrillation (41±34 months) and a dilated left atrium (>45 mm) were included and prospectively followed in an unblinded registry. During the electrophysiological part of the study, all 4 pulmonary veins were found to be isolated in 36 (72%) patients and a complete box‐lesion was confirmed in 14 (28%) patients. All gaps were successfully re‐ablated. Twelve months after the completed hybrid ablation, 47 patients (94%) were in normal sinus rhythm (4 patients with paroxysmal atrial fibrillation required propafenone and 1 patient underwent a redo catheter procedure). The majority of arrhythmias recurred during the first 3 months. Beyond 12 months, there were no arrhythmia recurrences detected. The surgical part of the procedure was complicated by 7 (13.7%) major complications, while no serious adverse events were recorded during the radiofrequency catheter part of the procedure.
Conclusions
The staged hybrid epicardial–endocardial treatment of long‐standing persistent atrial fibrillation seems to be extremely effective in maintenance of normal sinus rhythm compared to radiofrequency catheter or surgical ablation alone. Epicardial ablation alone cannot guarantee durable transmural lesions.
Clinical Trial Registration
URL: www.ablace.cz Unique identifier: cz‐060520121617
Keywords: hybrid approach, persistent atrial fibrillation, radiofrequency ablation, sequential, surgical treatment
Introduction
Atrial fibrillation (AF) represents the most common sustained supraventricular arrhythmia, which is also noteworthy for its growing incidence in the elderly population.1–2 It is associated with increased mortality, risk of stroke, and exacerbation of heart failure. Since pharmacological treatment of arrhythmias have been found to be unsatisfactory (in terms of rhythm control), there have been a number of nonpharmacological treatment modalities introduced into routine clinical practice over the past 10 years.3 However, catheter‐based therapies have been shown to have variable results. This is particularly true with regard to persistent forms of arrhythmia, which usually require repeated procedures to establish a stable sinus rhythm (SR).4–6 Long‐standing persistent AF represents a significant therapeutic challenge due to left atrial (LA) dilatation and fibrotic structural remodeling. Ablation strategies include not only pulmonary vein (PV) isolation but also creation of transmural linear lines connecting anatomical barriers. Various epicardial structures, such as the ligament of Marshall, are often impossible to ablate from the endocardial surface.
Surgical treatment of AF has shifted from open heart surgery and cut‐and‐sew techniques toward minimally invasive procedures using endoscopic instruments to isolate the PVs, create linear lesions, and occlude the left atrial appendage (LAA)—all on the beating heart.7 Although this approach achieves higher arrhythmia‐free success rates after a single procedure than catheter ablations,8 contiguous and transmural lesions cannot always be guaranteed.9 The mitral isthmus and the tricuspid isthmus lines are particularly difficult to ablate from the epicardial surface of the heart.10
The recently introduced hybrid approach seems to be a very attractive alternative for overcoming the limitations of the epi‐ or endocardial ablation approach alone. However, there is limited data regarding the staged approach on a well‐defined cohort of patients with long‐standing persistent AF. Published studies offer only a mix of different AF types (paroxysmal, persistent, and long‐standing persistent, with or without previous catheter ablation) and even employ heterogeneous ablation techniques. When patients with long‐standing persistent AF are included, they often represent only a small percentage of the sample. Therefore, this study was designed to provide 1‐year results from a staged hybrid procedure in patients with long‐standing persistent AF using a combined endoscopic surgical radiofrequency (RF) ablation of the LA with occlusion of LAA, followed by an electrophysiological diagnostic study and a RF catheter ablation of the left and right atrium (RA) (primary endpoint). The catheter ablation took place 6 to 8 weeks after the surgical phase to allow healing and maturation of the surgical ablation sites. Secondary end points included overall arrhythmia‐free survival during the entire follow‐up period, prevalence of transmural linear and circumferential lines after the surgical part of the hybrid procedure, and overall safety of both surgical and endocardial ablation procedures.
Methods
Patients with symptomatic drug‐refractory, long‐standing persistent AF were included in an unblinded registry and followed prospectively. The enrollment of patients started in July 2012 and was finished in November 2013. The definitions of paroxysmal, persistent, and long‐standing persistent AF were based on recent guidelines.3 All patients underwent the surgical part of the hybrid arrhythmia treatment, which followed a standardized protocol. Six to 8 weeks later, patients were admitted for the transvenous catheter part of the procedure. To be recruited to the registry, patients had to have LA dilation >45 mm. Exclusion criteria were the presence of severe coronary artery disease (>60% stenosis of the main coronary arteries), moderate or severe heart valve disease, a history of stroke, previous catheter ablation, pulmonary surgery or cardiac surgery, inability to use anticoagulant drugs, or the presence of a thrombus in the LAA.
All patients underwent transesophageal echocardiography, cardiac computed tomography with contrast, and pulmonary function tests. Patients older than 50 years or with symptoms of angina pectoris also underwent coronary angiography. Transesophageal echocardiography and computed tomography scans were both repeated in all patients prior to RF catheter ablation; of particular interest was any PV narrowing (defined as 20% to 50% reduction in the diameter of the vein with detected maximum pulsed‐wave Doppler velocity at the orifice of the vein <100 cm/s) or stenosis (defined as ≥50% reduction in the diameter with pulsed‐wave Doppler velocity ≥100 cm/s) after the surgical ablation. Oral anticoagulation therapy was discontinued 7 days before surgery/catheter ablation, and low‐molecular‐weight heparin was administered twice a day until the evening before the procedure. The study was approved by the local institutional ethics committee, all patients signed informed consents, and the data were recorded prospectively in a web‐based database.
Surgical Technique
The surgical phase was performed with patients under general anesthesia with a double‐lumen endotracheal tube for selective lung ventilation. The procedure started on the right side with placement of endoscopic tools: a 10‐mm camera port in the right fifth intercostal space on the midaxillary line, a 5‐mm working port in the third intercostal space on the anterior axillary line, and a 10‐mm working port in the seventh intercostal space on the midaxillary line. After right lung deflation, the pericardium was opened anterior to the phrenic nerve from the superior vena cava to the diaphragm and gently separated and secured with 3 stitches to ensure optimal visibility. The interatrial groove was dissected with endoscopic scissors to gain access to the oblique sinus between the inferior vena cava and the right lower PV. An articulating and illuminating dissector (LumiTip Dissector; AtriCure, OH) was used to surround the right PVs with a rubber tourniquet, which was subsequently used as a guide for insertion of an ablation clamp (Isolator Synergy; AtriCure). A series of ablations (minimally 5) around the right PVs was performed followed by verification of an entry block. If any electrical activity was recorded inside the veins, further RF energy applications were administered until entry block was confirmed. A roof line (connecting the superior right‐side and left‐side PVs) and an inferior line (connecting the inferior right‐side and left‐side PVs) were created using a linear pen device (Bipolar Linear Pen; AtriCure). According to the protocol, a “trigone” line on the roof of the LA extending from the right superior PV to the left fibrous trigonum was made. No verification of completeness of this line was performed during surgery. After that, a ganglionated plexi ablation, guided by high‐frequency stimulation and the vagal response, was performed. The procedure on the right side was finished by putting 1 approximating stich in the pericardium and placing a drain into the right pleural space.
The left‐side approach was similar to the right side, but the ports were placed more posteriorly and the pericardium was opened posteriorly to the phrenic nerve. The ligament of Marshall was dissected using scissors and both ends were burned using a linear pen. A LumiTip with a rubber tourniquet was used to place the bipolar clamp, and a series of ablations were made until entry block was documented. At this time, if the patient was in AF, an electric cardioversion was performed and the epicardial bidirectional block was confirmed for the PVs and also for the box‐lesion. The LAA was excluded, whenever deemed safe and feasible by the surgeon; this was done using an AtriClip device (Atricure). A drain was introduced into the left pleural space and the patient was transferred to the intensive care unit. Low‐molecular‐weight heparin was administered 6 hours after surgery and warfarin was commenced after removal of both pleural drains.
Catheter‐Based Technique
All electrophysiology studies were performed 6 to 8 weeks after surgery to allow time for tissue healing, edema resorption, and lesion consolidation. If SR was present at the beginning of the procedure, RF ablation of the cavotricuspid isthmus was performed first before moving to the LA. RF energy was applied using a 3.5‐mm irrigated‐tip ThermoCool Smart Touch catheter (Biosense Webster Inc, Diamond Bar, CA) with the output of 30 to 35 W (cool‐flow 20 mL/min). A bidirectional block of conduction across the isthmus was confirmed using standard criteria. In patients who were not in SR and did not have an isthmus‐dependent atrial flutter at the beginning of the study, a cavotricuspid isthmus ablation was performed at the end of the procedure after SR has been achieved. After a double transseptal puncture, 2 steerable transseptal sheaths (8F, Channel; BARD Electrophysiology, Lowell, MA) were introduced into the LA. Next, using the CARTO 3 mapping system, a virtual three‐dimensional reconstruction of the anatomy and a bipolar voltage map were constructed by acquiring at least 300 points. Low‐voltage areas were considered to reflect previous epicardial lines created by surgeons. A circular mapping catheter (Lasso; Biosense Webster Inc) was positioned in all PVs to confirm isolation or electrical reconnection by demonstrating the presence of both entry and exit blocks.
RF energy was applied using a 3.5‐mm irrigated‐tip ThermoCool Smart Touch catheter (Biosense Webster Inc) that used a software module, which enabled contact force sensing with a temperature limitation of 44°C and RF energy ranging from 25 W up to 35 W (cool‐flow 20 mL/min). If an electrical reconnection between the PVs and LA was suspected, a detailed mapping of the PV antra was performed to find the earliest PV potential within the presumed epicardial ablation line. Such regions (from now on referred to as a “gaps”) were targeted for ablation first. This was repeated until all gaps had been closed and electrical silence inside the circumferential ablation line was observed.
After achieving isolation of all PVs, the posterior LA wall was mapped in detail. For such purposes, a Lasso catheter was placed so that it touched the posterior wall perpendicularly. If no potentials were recorded, a complete box‐lesion was deemed present. If any potentials were recorded, both the superior and inferior connecting lines were meticulously mapped to look for gaps, which were subsequently ablated (Figure 1).
Figure 1.
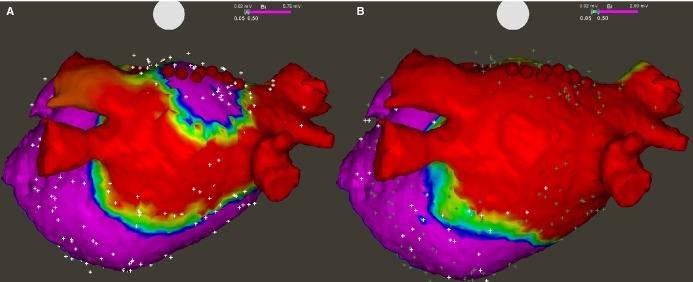
Bipolar voltage map of the left atrium after epicardial radiofrequency thoracoscopic ablation, posterior view. A, All 4 pulmonary veins were found isolated, while a gap was found in the middle of the roof line allowing electrical activation of the posterior wall. Red dots show sites that were re‐ablated with endocardial touch‐up. B, Remapping after ablation revealed no potentials in the posterior wall, which confirmed a complete box‐lesion.
Following this step, the anterior mitral isthmus line was completed. The line started close to the mitral annulus and extended toward the right superior PV to connect with the epicardial line that had been made during the surgical phase. Completeness of the anterior mitral isthmus line (trigone lesion) was confirmed using standard criteria demonstrating a clockwise activation pattern around the mitral annulus when pacing lateral to the ablation line and counterclockwise activation pattern when pacing medial to the ablation line.
Additionally, incremental atrial pacing, up to 300 bpm, was performed at the end of the procedure. If atrial tachycardia (AT) was induced, a detailed activation map of the LA or RA was performed; the mechanism of the arrhythmia was elucidated and the tachycardia source was eliminated with a RF ablation. Mapping and ablation of ATs were performed initially if sustained arrhythmias were recorded at the very beginning of the procedure. If AF was present at the beginning of the procedure, patients were cardioverted to SR first.
Postoperative Care and Follow‐up
After completion of the catheter phase of the procedure, all patients underwent continuous telemetric monitoring until discharge from the hospital. No anti‐arrhythmic drugs were prescribed at the time of discharge. Patients had followed‐up visits at 3, 6, 9, and 12 months after the completed hybrid procedure and thereafter every 6 months. A 7‐day ECG Holter monitor was performed before each follow‐up visit. In addition, if patients complained of palpitations and no arrhythmia was detected during Holter monitoring, they were equipped with an ECG event recorder and were instructed to record their ECG whenever they felt palpitations. Success was defined as the absence of AF and/or any other supraventricular arrhythmias lasting more than 30 s on any of the 7‐day Holter monitors (or casual 12‐lead ECG or event recorder tracings) during the entire follow‐up.
Statistics
Continuous variables are expressed as mean±SD. Categorical variables are presented as absolute numbers and percentages. Comparisons between categorical variables were made using the Fisher's exact test. A P<0.05 was considered statistically significant. Arrhythmia‐free survival during the entire follow‐up period was visualized using Kaplan–Meier curves.
Results
Clinical characteristics of patients are listed in Table 1. Fifty‐one patients with long‐standing persistent AF and a dilated LA (median LA diameter=48 mm) underwent the surgical phase of the procedure, but 1 patient failed to return for the catheter phase of the hybrid procedure and was lost to follow‐up.
Table 1.
Clinical and Demographical Data
| N | 50 |
| Age, y | 62.2±7.3 |
| Males/females | 32 (64%)/18 (36%) |
| AF duration, mo | 41.7±34.8 |
| BMI, kg/m2 | 30.5±4.8 |
| Number of failed AA drugs | 1.7±0.6 |
| Previous failed ECV | 41 (82%) |
| Hypertension | 32 (64%) |
| COPD | 10 (20%) |
| LA diameter in PLAX, mm | 48.1±4.4 |
| LA volume, mL | 141±31 |
| LV ejection fraction, % | 63±8 |
AA indicates antiarrhythmic; AF, atrial fibrillation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECV, electrical cardioversion; LA, left atrium; LV, left ventricle; PLAX, parasternal long axis view.
Surgical procedure characteristics are listed in Table 2. All PVs were shown to be isolated at the end of surgical ablation with the exception of 1 patient. In this particular patient, a diaphragmatic hernia that was not diagnosed prior to the operation prevented right‐side lesions. Complete isolation of the posterior LA wall (box‐lesion) was accomplished in 48 (96%) patients. Dissection of the ligament of Marshall was performed in 49 (98%) patients and occlusion of the LAA was carried out in 42 (84%) patients.
Table 2.
Surgical and Catheter Procedure Variables
| Surgical Ablation | Catheter Ablation | |
|---|---|---|
| N | 51 | 50 |
| Procedure time, min | 190±30 | 137±41 |
| X‐ray time, min | NA | 8±4 |
| Total radiofrequency energy duration, min | NA | 28.1±12.4 |
| In‐hospital stay, days | 4.1±3 | 3.1±1.1 |
| Major complications | 7 (13.7%) | 0 (0%) |
| Conversion to sternotomy | 2 (3.9%) | |
| Permanent phrenic nerve injury | 4 (7.8%) | |
| Tamponade | 1 (2%) | |
| Minor complications | 10 (20%) | 0 (0%) |
| Nonsignificant pulmonary vein narrowing* | 7 (13.7%) | |
| Temporary phrenic nerve injury | 1 (2%) | |
| Postoperative infection | 2 (3.9%) |
NA indicates not applicable.
Narrowing <50%.
Two patients had to undergo an urgent median sternotomy due to intraoperative bleeding from the left pulmonary artery. Unilateral wound infections were noted during the postoperative course in 2 obese female patients (body mass index 43.2 and 34.4); however, both were successfully treated with intravenous antibiotics.
One patient was readmitted to the hospital with signs of cardiac tamponade and with an international normalized ratio of 7.1 on day 24 after surgery, but was successfully treated with a pericardial drain.
There were no operative or postoperative deaths in the group. Additionally, there were no in‐hospital strokes and none of the patients required implantation of a pacemaker.
Additional surgical complications were discovered at the time of the electrophysiology study. Five phrenic nerve palsies with immobility of the right side of the diaphragm were noted during skiascopy. One of these patients underwent a surgical plication of the diaphragm 6 months later. Two patients underwent special rehabilitation training alleviating the symptoms of dyspnea and 1 remained completely asymptomatic. In the fifth patient, there was a spontaneous full recovery of phrenic nerve function 8 months after surgery. Insignificant PV narrowing was found in 7 patients (13.7%) and was confirmed with a computed tomography scan. PV narrowings occurred in 7 of the first 15 patients to enter the thoracoscopic ablation program. A slight change in the surgical technique, with clamps positioned more proximally, eliminated this complication.
All but 1 patient (previously mentioned) underwent the catheter phase of the hybrid procedure, which was performed on average 82±14 days following the surgical procedure. Thus, only 50 patients completed a minimum 12‐month follow‐up after the hybrid procedure and were analyzed as part of this study.
Thirty‐nine patients (78%) were in normal SR at the time of admission for the catheter ablation procedure. Eleven patients (22%) had an ongoing arrhythmia: 3 patients were in AF, 3 patients had typical isthmus‐dependent RA flutter, and 5 patients had other left‐ or right‐sided ATs (Figure 2).
Figure 2.
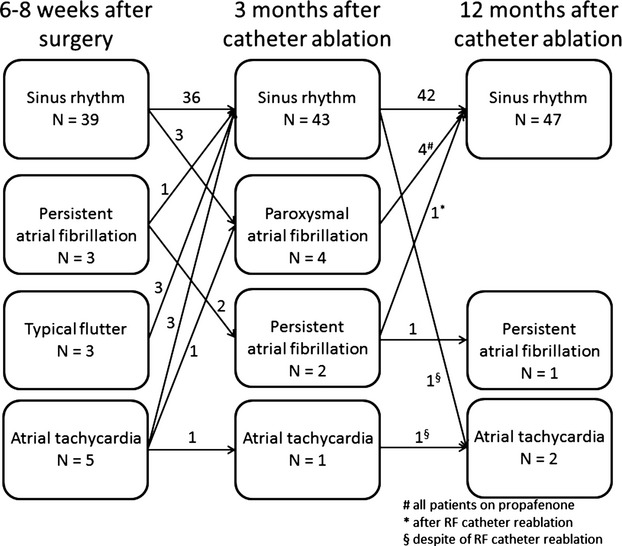
Cardiac rhythm 6 to 8 weeks after the surgical thoracoscopic epicardial radiofrequency (RF) ablation and 3 and 12 months after the transvenous catheter ablation.
During the electrophysiological evaluation, complete left‐ and right‐sided PV isolation was found in 40 (80%) and 47 (94%) patients, respectively (Figure 3). All 4 PVs were isolated in 37 (74%) patients, which was a significantly lower proportion than observed during surgery, when isolation of all 4 PVs was demonstrated in 98% of patients (P<0.001). The roof line was found complete in 17 (34%) patients, while the connecting line between the inferior veins was found complete in 30 (60%) patients, resulting in an overall box‐lesion success rate of 30% (15 patients), again a significantly lower proportion than found during surgery (96%, P<0.001). A permanent box‐lesion was achieved in a smaller proportion of patients compared to PV isolation (P<0.001). The trigone line was found complete in 5 (10%) patients. The percentage of patients with complete PV isolation and linear lines did not differ between the group of patients presenting with SR and ongoing arrhythmias (Table 3).
Figure 3.
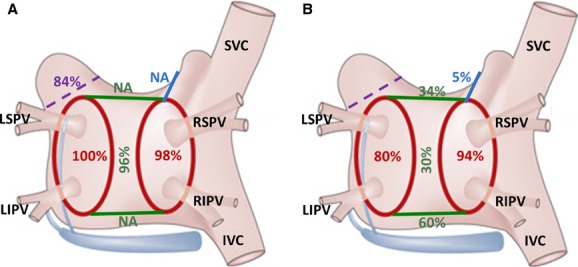
Success rate of surgical thoracoscopic epicardial radiofrequency isolation of pulmonary veins, linear ablation lines connecting both superior and inferior pulmonary veins, and the trigone line connecting the right superior pulmonary vein across the left atrial roof toward the noncoronary aortic cusp as they were assessed (A) immediately after the ablation during surgery and (B) during the electrophysiological examination 6 to 8 weeks following the index procedure. Percentage of deployed left atrial appendage clips is also depicted (violet color). IVC indicates inferior vena cava; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; NA, not applicable; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava.
Table 3.
Electrophysiological Findings in Relation to Arrhythmia Presentation During Catheter Ablation Phase of the Hybrid Procedure
| N | Left PVs Isolated | Right PVs Isolated | All PVs Isolated | Posterior LA Exclusion | |
|---|---|---|---|---|---|
| SR | 39 | 32 (82.1%) | 36 (92.3%) | 29 (74.4%) | 11 (28.2%) |
| SVA | 11 | 8 (72.7%) | 11 (100%) | 8 (72.7%) | 4 (36.4%) |
| P Value | NS | NS | NS | NS |
LA indicates left atrium; N, number of patients; NS, not significant; PVs, pulmonary veins; SR, sinus rhythm at the beginning of the electrophysiological study; SVA, any ongoing supraventricular arrhythmia at the beginning of the electrophysiological study (namely, atrial flutter in 3 patients, atrial fibrillation in another 3 patients, and atrial tachycardia in 5 patients).
Incomplete linear lines in the LA and PV reconnections were successfully re‐ablated in all patients. The cavotricuspid isthmus line was also successfully ablated in all patients. In addition, superior vena cava isolation was performed in 4 patients (8%), since frequent ectopy arising from this vein was observed during the electrophysiological study. Ablations of right‐ and left‐side ATs, occurring either spontaneously or during testing, were performed in 8 and 10 patients, respectively. No serious adverse events occurred during the RF catheter ablation phase of the hybrid procedure or during the following 30 days.
During the 12‐month follow‐up completed by all patients in the study, SR was documented, using 7‐day ECG Holters, in 47 patients (94%). One patient (2%) remained in AF and 2 (4%) suffered from ATs (Figure 2). However, 4 patients required class Ic antiarrhythmic (AA) drugs for paroxysmal AF diagnosed at the 3‐month follow‐up (lasting <2 minutes in 2 patients and 2 to 6 hours in the other 2 patients) to achieve stable SR; therefore, without any AA drugs, the success rate dropped to 84%. One patient with persistent AF and 1 with AT, diagnosed post–hybrid ablation, underwent a second catheter ablation, but in both cases the procedure was unsuccessful and arrhythmias remained unresolved. Since there were no recurrences of AF after 12 months, the overall success rate during the extended follow‐up period (513±138 days) remained 94% (Figure 4). Interestingly, most recurrences actually occurred during the first 3 months after the completion of the hybrid procedure.
Figure 4.
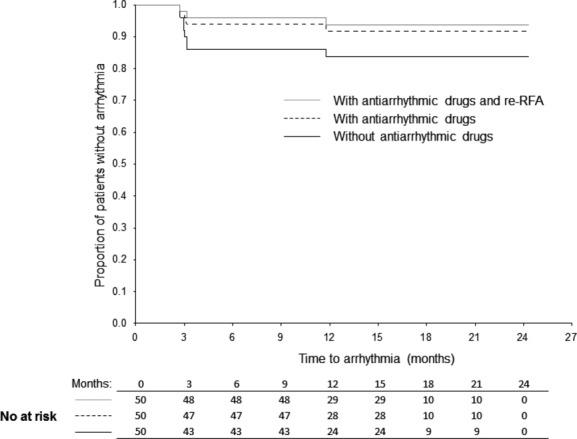
Kaplan–Meier arrhythmia‐free survival during the mean follow‐up of 513±138 days. RFA indicates radiofrequency ablation.
Discussion
Our study highlights for the first time several important findings: (1) the extremely high midterm effectiveness of the hybrid approach in a well‐defined homogeneous cohort of patients with long‐standing persistent AF and a dilated LA, (2) the importance of delaying the endocardial phase of the procedure, and (3) the surprising ineffectiveness of epicardial RF ablations in creating transmural linear lesions. Additionally, and for the first time, we provide systematic three‐dimensional endocardial mapping data on surgically created epicardial linear and circular lesions after a healing period both in patients with arrhythmia recurrence and in patients with SR, presumably successfully treated for AF, by surgical ablation.
PV isolation has been established as the cornerstone of treatment for paroxysmal AF. However, the effectiveness of simple PV isolation in patients with persistent and especially long‐standing persistent AF is as low as 21%.11 Repeated procedures and arrhythmia substrate ablations are usually needed to increase the success rate above 50%. Indeed, patients with long‐standing persistent AF and a dilated LA represent the most difficult group to treat. In both controlled trials and daily clinical practice, such patients are usually excluded from ablative treatments since the results of AF ablation are likely to be very poor.
Effectiveness of Hybrid Approach
Hybrid procedures have been recently proposed as a method to overcome the shortcomings of both surgical and catheter‐based ablation procedures alone. Currently, thoracoscopic minimally invasive surgery uses predominately bipolar RF energy. In a recent meta‐analysis by Gelsomino et al,12 bipolar RF energy was found to yield better results in terms of freedom from AF. Published results range from 85.7% to 92% in studies employing bipolar RF and from 36.8% to 88.9% in those utilizing monopolar RF. However, studies published to date, on the hybrid approach using bipolar RF energy, have included rather diverse patient groups, small samples, and extremely heterogeneous methodologies.12
A study by Mahapatra et al13 included only 15 patients with persistent AF, who were unsuccessfully treated, despite having a complex history, that is, after at least 1 catheter ablation and treatment with 1 AA drug. The authors performed an epicardial PV isolation, roof line, trigone line, superior vena cava isolation, LAA amputation, and ablation of the ganglionated plexis. After 3 to 5 days, all patients were brought to the electrophysiological lab for cavotricuspid isthmus and coronary sinus ablation. In contrast to our study, which included all patients, be they in the SR or in any supraventricular arrhythmia, Mahapatra et al checked PV isolation and completeness of the LA linear lines only in patients with inducible arrhythmias, in whom they also tried to ablate regular ATs or complex fractionated ECGs. The authors reported a success rate of 86.7% and 93.3%, off and on AA drugs, respectively, during a mean follow‐up of 20.7±4.5 months.
Krul et al14 investigated 31 patients: 16 with paroxysmal, 13 with persistent, and 2 with long‐standing persistent AF. Using a variation of the hybrid methodology, they performed epi‐ and endocardial ablations, which were carried out simultaneously. PV isolation and ganglionated plexi ablation were performed in all patients, while roof and inferior lines were ablated in 13 and 8 patients, respectively. LAA was excluded in 29 patients. At the 12‐month follow‐up, the authors reported 86% success without arrhythmia recurrence and without need of AA drugs.
Pison et al15 studied a cohort of 78 consecutive patients, again with a number of disease variations (29 with paroxysmal, 34 with persistent, and 15 with long‐standing persistent AF), who underwent a simultaneous epicardial and endocardial ablation. Surgeons created lines around the PVs and a box‐lesion on the posterior LA, while electrophysiologists ascertained entry and exit blocks in the isolated regions and, if necessary, closed conduction gaps using cool‐tip RF ablation. In cases where AF transitioned to LA flutter, a mitral isthmus line (extending from left inferior PV toward the mitral annulus) was added from both the endo‐ and epicardial sides. A cavotricuspid isthmus ablation was performed in patients with a history of typical RA flutter or whenever it was observed during the procedure. A superior vena cava ablation and a superior vena cava to inferior vena cava line were created, but only in patients with persistent and long‐standing persistent AF with a dilated RA. The single‐procedure success rate was 74% for the total, rather diverse, group. After an additional catheter ablation procedure in 10 patients, the success rate increased to 87% and 92% of patients, off and on AA drugs, respectively.
In contrast to the abovementioned studies, our study followed a well‐defined surgical protocol in a homogeneous group of patients with long‐standing persistent AF, who were naive to previous catheter ablations or surgery. The success rate of a staged hybrid approach using our protocol was astonishingly high. Interestingly, the majority of recurrences occurred during the first 3 months after the procedure and midterm results seem to be promising and very stable, with no late recurrences (Figure 4). Finally, only 3 patients in our cohort were unable to maintain a stable SR: One patient remained in permanent AF and repeated electrical cardioversions failed despite successful completion of the hybrid procedure and therapy with amiodarone. An extremely dilated LA (LA volume=210 mL!) with extensive fibrotic remodeling was considered to be the reason for failure. The remaining 2 patients had sustained regular ATs. One AT was confined to the LAA; however, ablating the arrhythmia would have probably led to complete electrical isolation of the unclipped LAA and a consequent prothrombogenic state. Therefore, an RF ablation was considered ill‐advised. The third patient, despite repeated ablations, suffered from multi‐re‐entry ATs, which were all confined to the RA. In 1 of our patients with successful catheter re‐ablation, AF was also maintained by a rotor in the LAA, which was not excluded during the surgical part of the procedure. These data confirm the observation by Di Biase et al16 that, in up to 27% of redo cases, AF is triggered or maintained by the LAA and therefore excision or clipping of the LAA (which causes its electrical isolation) may potentially eliminate this mechanism of arrhythmia recurrence. If the LAA had been excluded in all patients, 2 of the failures would have most likely never occurred. We can only speculate that systematic exclusion of all LAAs would have also eliminated the paroxysms of AF in 2 other patients in our study group, since 2 of 4 patients with a recurrence of paroxysmal AF had no LAA clip deployed.
Despite the fact that LAA exclusion was an integral part of our surgical protocol wherever feasible and safe, ultimately only 84% of patients had LAA exclusion. The reason was that in the first 21 patients, we used first‐generation AtriClip devices, which did not include a distal device articulation as part of deployment, which led to implantation failures in a significant proportion of patients. In the remaining 30 patients, we utilized second‐generation AtriClips (AtriClip Pro), which raised our LAA exclusion rate to >90%.
Transmurality of Epicardial Lines and Value of the Staged Approach
Transmurality of ablation lines is considered to be of utmost importance for successful treatment of AF according to the Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) consensus.11 For the first time we were able to analyze data on the recurrence of electrical reconduction of previously epicardially ablated lines. Despite direct visualization of the epicardial RF lesions, and despite the use of multiple burns during surgery (usually six to eight burns on each pair of PVs), acutely observed electrical isolation turned out to be only temporary in a significant proportion of patients, which was most likely due to tissue stunning and edema. This confirms concerns by some authors that simultaneous epicardial–endocardial procedures may be associated with a high number of false‐negative results (demonstration of entry or exit blocks that are only transient and do not endure long‐term) and also false‐positive results in terms of early inducible arrhythmias, which usually fade away after “maturation” of the ablative lesions.17 Furthermore, maturation of epicardial “burns” allows easier identification of boundaries of nonisolated substrate and target points during subsequent endocardial mapping.
Not surprisingly, linear ablation lines especially represented the weakest point of the epicardial approach. One possible explanation is the large amount of epicardial fat tissue in the areas of the ablation lines, which can dissipate RF energy. The use of different instruments for the creation of lines (linear pen for lines versus ablation clamps used for PVs) could also have contributed to the problems. There are no comparable data published in the literature on systematic delayed endocardial mapping in patients both with and without arrhythmia recurrence, with regard to completeness of linear lesions, after surgery. During the acute period, Pison et al15 described the need for an endocardial touch‐up, to complete box‐lesions during a simultaneous hybrid approach, in 36% of patients.
Completeness of lines and/or PV isolation is not directly related to the appearance of arrhythmias over the course of time. There is a lack of relevant data in the literature, especially postsurgery, and one can only speculate about the relationship between completeness of linear lesions and actual incidence of recurring arrhythmias. Presence or absence of complete lines after surgery does not seem to affect acute arrhythmia recurrence in our study, since a similar proportion of patients with or without arrhythmia showed gaps in the surgical ablation lines (Table 3). However, based on the knowledge gained from catheter ablation studies, any incomplete lines or PV reconduction could potentially hamper the long‐term results of the procedure. It is also important to stress that the completion of both PV isolation and connecting linear lines was extremely easy in all patients thanks to the epicardial burns, which could be readily identified using bipolar voltage maps.
Apart from assessing chronic conduction properties of epicardial lesions, other potential advantages of a 2‐stage procedure may include (1) lower overall procedural time and consequently lower general anesthesia time, (2) fewer bleeding and infection complications, (3) the possibility of using a dedicated operating theater and an advanced three‐dimensional mapping system for targeting induced or ongoing ATs, and (4) advantageous reimbursement in some countries, as well as other logistic benefits. Although the benefits of a 2‐stage hybrid approach, as a treatment modality, are easy to imagine, its ultimate value and utility should be tested in randomized multicenter trials.
Complications of Hybrid Surgery
Serious complications from catheter ablations of AF, in a high‐volume center like ours, are extremely rare.18 In our series, we did not record any major or minor complications related to the endocardial procedure. However, there were 5 cases of phrenic nerve injury associated with the surgical part of the hybrid procedure. This is a very serious complication, with 2 likely causes. The first is a proximity‐related mechanical injury to the phrenic nerve during opening of the pericardium. After placing pericardial stitches, to prevent the pericardium from obstructing the surgeon's view, manipulation with endoscopic tools while creating the inferior line can easily stretch or tear the pericardium, resulting in damage to the phrenic nerve. The second potential cause is heat injury during the ablation process. There is evidence that if the phrenic nerve is only partly damaged, the potential for healing is around 70% after 1 year and 90% after 2 years.19 In our series, only 1 patient fully recovered phrenic nerve function after 8 months. There is an unfortunate lack of literature discussing this complication. One‐sided injury of the phrenic nerve with limited/no movement of the diaphragm does not necessarily lead to the sensation of dyspnea. Therefore, such a complication is most likely underestimated in surgical literature since consistent follow‐up examinations aimed at accessing diaphragm movement, using skiascopy, are usually missing.
Similarly, there is no data in the surgical literature that examines PV narrowing or stenosis after epicardial PV isolation. Nonsignificant PV narrowings found in our study were most likely caused by overly distal clamping of the PVs during RF ablation. Two conversions to open‐heart surgery, due to bleeding from the pulmonary artery, occurred during the early stages of our hybrid program, and 2 infectious complications were most likely related to extreme obesity and concomitant diabetes. One recorded pericardial bleed was directly caused by an overdose of anticoagulant drugs, and the complication was not deemed directly related to the surgery itself.
Study Limitation
The main limitation of the trial was that we did not include a control group of patients, in whom catheter ablation was not carried out after surgery. It might have happened that if catheter ablation was performed only in patients with arrhythmia recurrence, the overall clinical results would be similar and patients would not be exposed to an additional, albeit very low, risk of invasive procedure. Therefore, it still remains to be elucidated whether endocardial ablation aimed at completing epicardial surgical ablation lines should be performed routinely in all patients.
Conclusions
Hybrid thoracoscopic and transvenous catheter ablation of long‐standing persistent AF shows extremely encouraging midterm results, which may represent an important and possibly more effective alternative to repeated RF catheter ablation therapy. Complications during the surgical part of the procedure are mostly related to the learning curve and probably should be expected on introduction of the surgical component during development of, and transition to, a hybrid AF treatment protocol.
Single thoracoscopic RF ablations fail to ensure permanent and durable transmural lesions in the LA. Of the two hybrid approaches, the staged hybrid approach offers several advantages over the simultaneous hybrid procedure. Randomized multicenter clinical trials with long‐term follow‐up are needed to confirm the superiority of staged hybrid procedures over simultaneous procedures and over repeated catheter ablations using currently available novel technologies.
Acknowledgment
We thank Tom Secrest for the English revision of the manuscript.
Sources of Funding
This study was partially funded by the Faculty of Health and Social Studies, University of South Bohemia in Ceske Budejovice (BOV 2012_001).
Disclosures
None.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006; 27:949-953. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De CR, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al‐Attar N, Alfieri O, Angelini A, Blomstrom‐Lundqvist C, Colonna P, De SJ, Ernst S, Goette A, Gorenek B, Hatala R, Heidbuchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FW. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. *Developed with the special contribution of the European Heart Rhythm Association. Europace. 2012; 14:1385-1413. [DOI] [PubMed] [Google Scholar]
- Bertaglia E, Tondo C, De Simone A, Zoppo F, Mantica M, Turco P, Iuliano A, Forleo G, La Rocca V, Stabile G. Does catheter ablation cure atrial fibrillation? Single‐procedure outcome of drug‐refractory atrial fibrillation ablation: a 6‐year multicentre experience. Europace. 2010; 12:181-187. [DOI] [PubMed] [Google Scholar]
- Fiala M, Chovancik J, Wojnarova D, Bulkova V, Pindor J, Szymeczek H, Labrova R, Toman O, Januska J, Spinar J. Characterization of residual coronary sinus‐related tachycardia during ablation of longstanding persistent atrial fibrillation. Vnitr Lek. 2011; 57:33-42. [PubMed] [Google Scholar]
- Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow‐up? J Am Coll Cardiol. 2011; 57:160-166. [DOI] [PubMed] [Google Scholar]
- La MM. New technologies and hybrid surgery for atrial fibrillation. Rambam Maimonides Med J. 2013; 4:e0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, Sandoval E, Calvo N, Brugada J, Kelder J, Wijffels M, Mont L. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2‐center randomized clinical trial. Circulation. 2012; 125:23-30. [DOI] [PubMed] [Google Scholar]
- Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012; 60:54-61. [DOI] [PubMed] [Google Scholar]
- Kurfirst V, Mokracek A, Bulava A, Canadyova J, Hanis J, Pesl L. Two‐staged hybrid treatment of persistent atrial fibrillation: short‐term single‐centre results. Interact Cardiovasc Thorac Surg. 2014; 18:451-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012; 14:528-606. [DOI] [PubMed] [Google Scholar]
- Gelsomino S, Van Breugel HN, Pison L, Parise O, Crijns HJ, Wellens F, Maessen JG, La Meir M. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg. 2014; 45:401-407. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, LaPar DJ, Kamath S, Payne J, Bilchick KC, Mangrum JM, Ailawadi G. Initial experience of sequential surgical epicardial‐catheter endocardial ablation for persistent and long‐standing persistent atrial fibrillation with long‐term follow‐up. Ann Thorac Surg. 2011; 91:1890-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul SP, Driessen AH, van Boven WJ, Linnenbank AC, Geuzebroek GS, Jackman WM, Wilde AA, de Bakker JM, de Groot JR. Thoracoscopic video‐assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical‐electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011; 4:262-270. [DOI] [PubMed] [Google Scholar]
- Pison L, Gelsomino S, Luca F, Parise O, Maessen JG, Crijns HJ, La Meir M. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg. 2014; 3:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase BL, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM, Zagrodzky JD, Santangeli P, Hao S, Hongo R, Beheiry S, Themistoclakis S, Bonso A, Rossillo A, Corrado A, Raviele A, Al‐Ahmad A, Wang P, Cummings JE, Schweikert RA, Pelargonio G, Dello RA, Casella M, Santarelli P, Lewis WR, Natale A. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010; 122:109-118. [DOI] [PubMed] [Google Scholar]
- Magnano AR, Argenziano M, Dizon JM, Vigilance D, Williams M, Yegen H, Rueter K, Oz M, Garan H. Mechanisms of atrial tachyarrhythmias following surgical atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2006; 17:366-373. [DOI] [PubMed] [Google Scholar]
- Aldhoon B, Wichterle D, Peichl P, Cihak R, Kautzner J. Complications of catheter ablation for atrial fibrillation in a high‐volume centre with the use of intracardiac echocardiography. Europace. 2013; 15:24-32. [DOI] [PubMed] [Google Scholar]
- Kolář P. Rehabilitation in Clinical Practise. 20101st edPrague, Czech Republic: Galén; 2010 [Google Scholar]


