Abstract
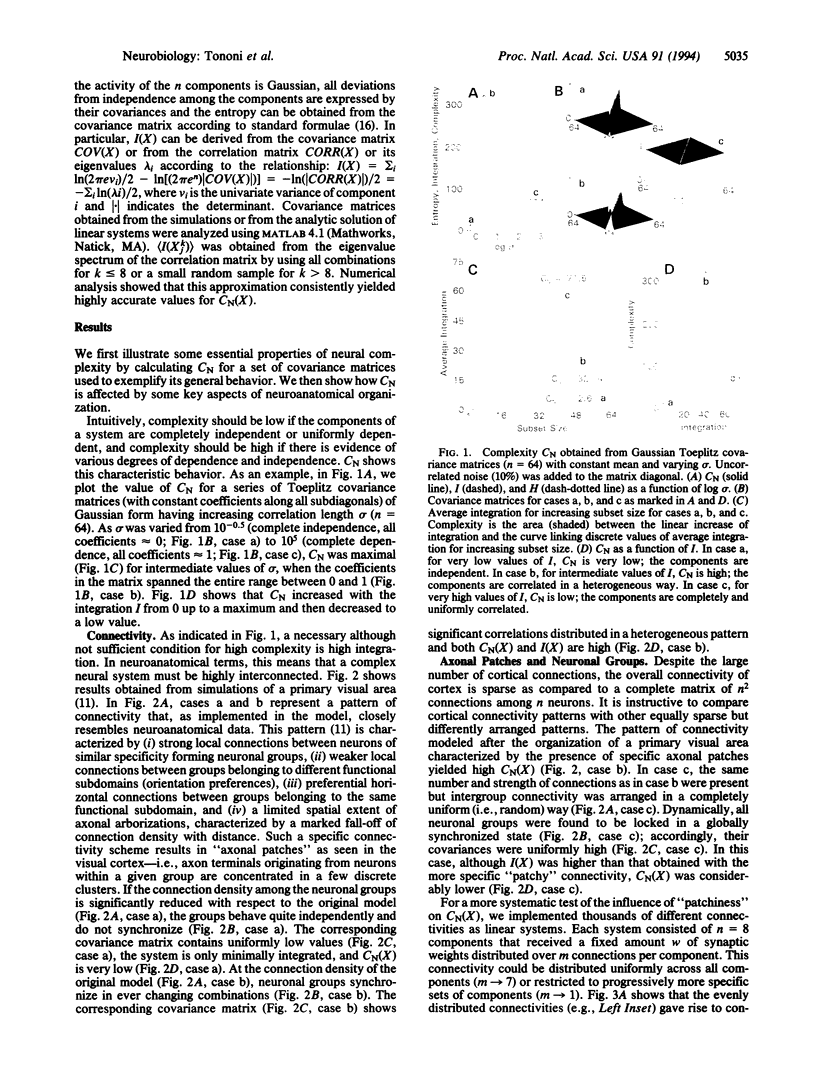
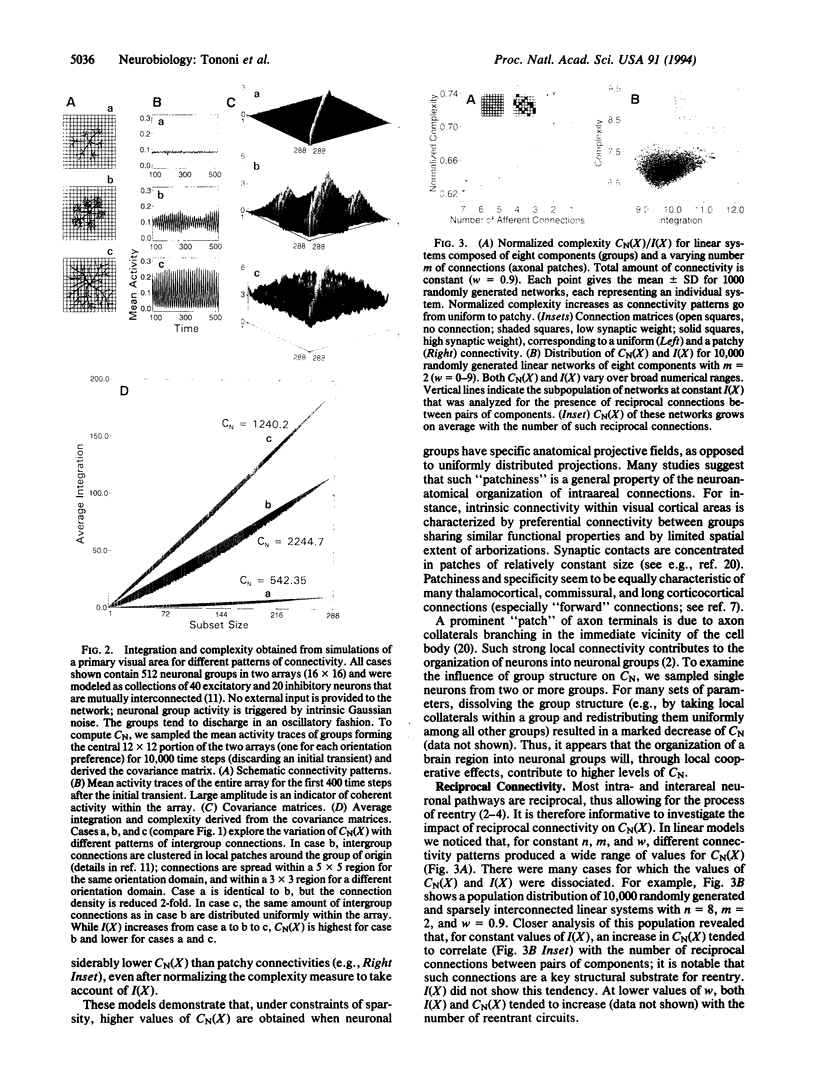
In brains of higher vertebrates, the functional segregation of local areas that differ in their anatomy and physiology contrasts sharply with their global integration during perception and behavior. In this paper, we introduce a measure, called neural complexity (CN), that captures the interplay between these two fundamental aspects of brain organization. We express functional segregation within a neural system in terms of the relative statistical independence of small subsets of the system and functional integration in terms of significant deviations from independence of large subsets. CN is then obtained from estimates of the average deviation from statistical independence for subsets of increasing size. CN is shown to be high when functional segregation coexists with integration and to be low when the components of a system are either completely independent (segregated) or completely dependent (integrated). We apply this complexity measure in computer simulations of cortical areas to examine how some basic principles of neuroanatomical organization constrain brain dynamics. We show that the connectivity patterns of the cerebral cortex, such as a high density of connections, strong local connectivity organizing cells into neuronal groups, patchiness in the connectivity among neuronal groups, and prevalent reciprocal connections, are associated with high values of CN. The approach outlined here may prove useful in analyzing complexity in other biological domains such as gene regulation and embryogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir Y., Harel M., Malach R. Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortex. J Comp Neurol. 1993 Aug 1;334(1):19–46. doi: 10.1002/cne.903340103. [DOI] [PubMed] [Google Scholar]
- Bressler S. L., Coppola R., Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993 Nov 11;366(6451):153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- Damasio A. R. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989 Nov;33(1-2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron. 1993 Feb;10(2):115–125. doi: 10.1016/0896-6273(93)90304-a. [DOI] [PubMed] [Google Scholar]
- Engel A. K., König P., Kreiter A. K., Schillen T. B., Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci. 1992 Jun;15(6):218–226. doi: 10.1016/0166-2236(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Felleman D. J., Van Essen D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991 Jan-Feb;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Finkel L. H., Edelman G. M. Integration of distributed cortical systems by reentry: a computer simulation of interactive functionally segregated visual areas. J Neurosci. 1989 Sep;9(9):3188–3208. doi: 10.1523/JNEUROSCI.09-09-03188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Nelson J. I., Salin P. A., Munk M. H., Arzi M., Bullier J. Spatial and temporal coherence in cortico-cortical connections: a cross-correlation study in areas 17 and 18 in the cat. Vis Neurosci. 1992 Jul;9(1):21–37. doi: 10.1017/s0952523800006349. [DOI] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Edelman G. M. Modeling perceptual grouping and figure-ground segregation by means of active reentrant connections. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):129–133. doi: 10.1073/pnas.88.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G. M. Reentry and the problem of integrating multiple cortical areas: simulation of dynamic integration in the visual system. Cereb Cortex. 1992 Jul-Aug;2(4):310–335. doi: 10.1093/cercor/2.4.310. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978 Aug 3;274(5670):423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]