Abstract
Background:
Intraventricular craniopharyngiomas are difficult to remove. We combined an interhemispheric transcallosal approach with a flexible endoscope (videoscope) for successful tumor removal.
Case Description:
A 52-year-old male complained of general fatigue and memory disturbance. Magnetic resonance imaging revealed a well-enhanced third ventricle mass with dilatation of lateral ventricles. During removal with the interhemispheric transcallosal approach, a videoscope that was inserted into the left lateral ventricle revealed the interface of the tumor and the ventricular wall. The tumor was pushed to the right using forceps and removed totally through the right foramen of Monro without any fornix injury.
Conclusion:
This procedure is a safe option for removing third ventricular tumors especially in the case with hydrocephalus.
Keywords: Craniopharyngioma, flexible endoscope, third ventricle, transcallosal approach, videoscope
INTRODUCTION
Purely intraventricular craniopharyngiomas are rare.[3] These tumors show an intact third ventricle floor, a suprasellar cistern, a normal pituitary stalk, and an absence of sellar abnormalities.[12] Surgeries for these lesions pose significant technical challenges because of important surrounding structures.
The two main surgical approaches for these lesions are the translamina terminalis and the transventricular approaches. The translamina terminalis approach using subfrontal, pterional, or basal interhemispheric approaches has been used to treat suprasellar or intraventricular craniopharyngiomas.[9,11,17] Although this approach is easy for accessing tumors in the inferior part of the third ventricle, it is difficult to remove large lesions.[24] The transventricular approach through a transcortical or transcallosal approach allows access to the third ventricle with transforaminal, subchoroidal, or transfornician approaches. The usefulness of these approaches have been reported for intraventricular tumor removal,[5,6,13,26] but there is a risk of injuries to the surrounding neuronal or vascular structures, such as the body of the fornix. We experienced a case of a large craniopharyngioma that occupied the third ventricle, which was successfully treated by the combination of a microscopic transcallosal approach and a flexible endoscope. We describe the operative technique and the usefulness of this procedure.
CASE REPORT
History and presentation. A 52-year-old male truck driver experienced headache, fatigue, and lethargy for 3 months. His family described memory disturbances, and he was referred to our hospital. On admission, he had mild disorientation, disturbance of short-term memory, and a left temporal visual field defect. His motor and sensory functions were intact. Brain magnetic resonance imaging (MRI) revealed a large, well-enhanced mass in the third ventricle that was adherent to the pituitary stalk and enlarged lateral ventricles [Figure 1a and b]. The optic chiasm was compressed with an anterior-downward shift. A small 3-mm aneurysm was observed in the left anterior communicating artery. Endocrinological examinations revealed hypopituitarism with growth hormone deficiency and polyuria.
Figure 1.
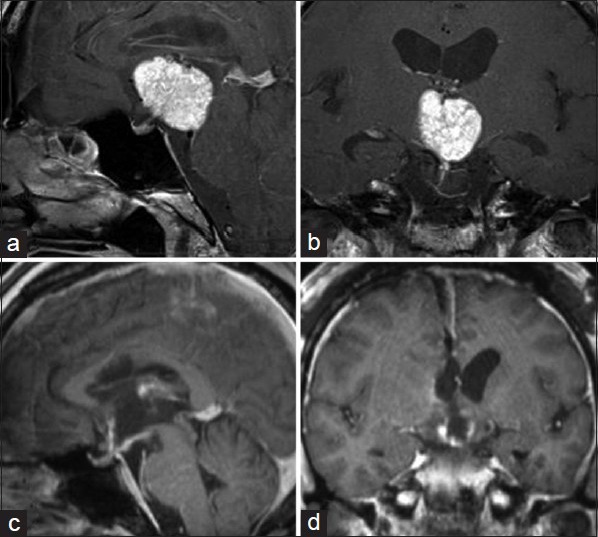
Gadolinium (Gd)-enhanced magnetic resonance (MR) images. In the preoperative images, the tumor was homogeneously enhanced by Gd, which extended to the dorsal part in the third ventricle (a and b). The MR images obtained 5 days after the operation. The tumor was totally removed with mild enhancement of the ventricular wall reflecting postoperative changes (c and d)
Operation and postoperative course. Due to the aneurysm in the anterior communicating artery and hypoplasia of the right A1 segment, an interhemispheric transcallosal approach was selected, instead of a translamina terminalis approach. A craniotomy was made in the right frontal region over the coronal suture, and one burr hole was made in the left frontal bone [Figure 2a]. A clear 6-mm sheath (Neurosheath™, Medikit Co., Ltd., Tokyo, Japan) was set into the left anterior bone, and a flexible videoscope (VEF-V, Olympus Corporation, Tokyo, Japan) was inserted. An enlarged left foramen of Monro and tumor in the third ventricle were observed. The right dura was incised in an arc, and the interhemispheric space was enlarged. A 2.0-cm incision was applied to the corpus callosum. The right lateral ventricle was opened, and the enlarged foramen of Monro with a yellowish tumor was observed [Figure 2a]. The choroidal fissure was incised to obtain working space. The soft tumor without visible hemorrhage was aspirated with an ultrasonic aspirator. Although the posterior tumor in the third ventricle was easy to remove, it was difficult to confirm the intact contralateral wall and the distance to the anterior third ventricle wall. The videoscope in the left lateral ventricle revealed the interaction of the tumor with third ventricle wall. By pressing the tumor with forceps [Figure 2b and c], the tumor was debulked easily. Thus, tumor dissection from the ventricular wall was conducted safely with videoscope visualization [Figure 2d and e] without fornix retraction. The tumor was detached from the infundibular recess and removed totally. Videoscopy confirmed no residual mass, hemostasis, third ventricular wall integrity, and no cerebrospinal fluid obstruction.
Figure 2.
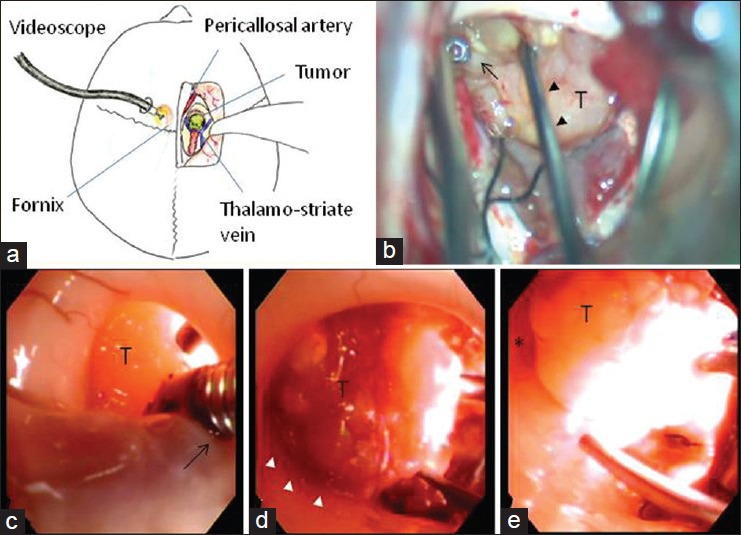
Intraoperative images and photographs. (a): Schematic sketch of the combined surgical approach with a videoscope for the third ventricular craniopharyngioma. (b): Microscopic view of the tumor removal. The tumor (T) was dissected by a dissector (arrow heads) with the assistance of forceps through the contralateral videoscope (arrow). (c-e) Videoscopic view of the tumor removal through the contralateral ventricle. The tumor was pushed by the forceps (arrow) through the videoscope (c). The interface between the tumor and the left wall of the third ventricle was well observed (white arrowheads) (d). The tumor was almost removed. The anterior wall of the third ventricle (asterisk) was observed through the videoscope (e)
His postoperative course was uneventful. His preoperative disorientation and memory disturbances were diminished. His general condition and activity improved with appropriate hormonal replacement of cortisol, thyroxine, and vasopressin. Postoperative MRI revealed total tumor removal without ventricular wall injury [Figure 1c and d] and a normal pituitary gland. The histological diagnosis was papillary craniopharyngioma. He was discharged 10 days after the surgery.
DISCUSSION
Intraventricular craniopharyngiomas account for 0.5–11% of all craniopharyngiomas.[3,25] Third ventricle tumors are surgical challenges because of the complex surrounding structures, including the hypothalamus, infundibulum, optic pathways, limbic system, and nearby vasculature.[26] Tumor dissection within the third ventricle can cause various injuries, such as hemiparesis, memory loss, increased endocrinopathy, or visual loss.[6]
Various surgical approaches have been used. The translamina terminalis approach after pterional or subfrontal approach enables easy access to the inferior third ventricle, where the tumor is attached at the tuber cinereum side.[7,14] The basal interhemispheric and translamina terminalis approaches are advocated because the posterior third ventricle lesions can be resected under direct visualization.[17,23] Hori et al.[11] presented a modified anterior interhemispheric approach with the translamina terminalis approach, which can access the tumor growing anteriorly from the line joining the anterior ridge of the foramen of Monro and the cerebral aqueduct. However, complete resection though the lamina terminalis alone may not permit sufficient removal for large lesions invading the dorsal part of the third ventricle.
The interhemispheric transcallosal approach allows access to the third ventricle through the foramen of Monro with minimal brain retraction.[12,13,26] Three different transforaminal, transchoroidal, and interforniceal approaches provide third ventricle access through its roof.[27] Transcallosal approaches allow symmetrical access to both lateral ventricles and both third ventricle walls. Danaila et al.[6] performed the transcallosal–transventricular approach on the third ventricle region in 58.3% of 120 patients and described this approach as the best method for third ventricle tumors. Chamoun and Couldwell[5] presented a video of the frontal transventricular approach through the usually dilated foramen of Monro for optimal tumor visualization while minimizing injury risks to the hypothalamus and pituitary stalk. However, anterior tumors invading the optic chiasm and adhering to the anterior cerebral artery complex are difficult to remove[24] and can damage the fornices and venous systems.
Thus, for tumors invading the dorsal anterior third ventricle, anterior callosal sectioning and an anterior interhemispheric approach have been recently described.[24] Alternatively, the transventricular preforniceal approach[30] may be adopted for anterior third ventricle tumors, although this approach is a two-stage surgery with an interhemispheric translamina terminalis approach to remove the anterior tumor in the chiasmatic regions.
To remove the intraventricular tumor, surgeons should maintain the plane between the tumor and the ependymal surface during the removal. However, the main difficulty in third ventricular tumor removal is their resection through a small opening and a very deep corridor. Tomassello et al.[26] advocated that multiple points of access to the lesion are key for success. Roth et al.[21] reported the benefits of endoscopes in narrow working spaces in their combined microsurgical and endoscopic resection of a hypothalamic hamartoma. Chamoun et al.[5] reported that endoscopes can explore blind angles that are hidden from microscopic view.
There have been many case series and reports of endoscopic resections of intraventricular tumors.[1,8,16,18,22,28] A rigid endoscope was considered useful through a single trajectory because of its superior solid rod lens visualization, but rigid endoscopes are inflexible and may damage brain tissue. To avoid surrounding structure injuries and change the direction during tumor removal, a flexible endoscope is more suitable. A videoscope (VEF-V) places a miniature charge-coupled device chip at the distal endoscope end so that the digital image quality is as high as that of images obtained using from rigid endoscopes and microscopes.[19] Recently, a high-definition flexible endoscope was described that provided clear and wide vision and contributed significantly to safer surgical procedures.[8,15]
However, problem when we use the flexible endoscope is sterilization. Because flexible endoscope cannot be autoclaved and cannot withstand aggressive chemical disinfection, the risk of transmission of Creutzfeldt–Jakob disease (CJD) and its variants is inevitable.[10] In our institute, according to the guideline of the infection prophylaxis against prion disease 2008 edition (in Japanese) that the Ministry of Health, Labor and Welfare has created in 2008, videoscope and other flexible endoscopes were sterilized by low-temperature hydrogen peroxide gas plasma (STERRAD® 100S, Johnson and Johnson company, USA), which was proved inactivation of prion protein in vivo.[29] Recently Rogez-Kreuz et al.[20] summarized the efficacy and sustainability of this method in the inactivation of prions on the surfaces of medical devices. Although we should destroy the flexible endoscopy after surgery according to the guidelines of the European Society of Gastrointestinal Endoscopy (ESGE)[2] if the patient has been diagnosed with CJD at surgery, fortunately the patient in this study was not CJD.
In our case, we expected a difficult removal through the foramen of Monro because the tumor was over 3 cm. As mentioned earlier, the anterior tumor may remain without fornix retraction, even if the subchoroidal approach is selected. To avoid fornix retraction, we adopted endoscopic assistance through the contralateral foramen of Monro. Initially, we applied the rigid endoscope,[4] which has a working channel system and is suitable in both dry and wet fields. Although the rigid scope provided clear vision, the working area was restricted though the burr hole, and we needed to enlarge the cortical incision to obtain suitable handling. Then, we changed to a videoscope that went through a burr hole. We initially used irrigation, but it was also clear in the dry field. During tumor removal under the microscope, we could see the interface between the tumor and the ventricular wall by the endoscope. The endoscopic surgeon assisted the tumor dissection with forceps by pushing or retracting through the contralateral foramen of Monro. In addition, information about the remaining depth to the anterior wall of the third ventricle and confirmation of the opening of all of the ventricles after tumor removal were useful. Finally, the tumor was removed totally without injury to the fornix or other surrounding structures.
With just one burr hole, the videoscope could explore the microscope blind spots in the third ventricle and assist removal through the contralateral ventricle. With a trained endoscopic surgeon, this procedure may be a good choice for the removal of third ventricular tumors.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/3/113/153653
Contributor Information
Shigetoshi Yano, Email: yanos@kumamoto-u.ac.jp.
Takuichiro Hide, Email: thide@fc.kuh.kumamoto-u.ac.jp.
Naoki Shinojima, Email: nshinojima@hotmail.com.
Yutaka Ueda, Email: ytkedy@gmail.com.
Jun-Ichi Kuratsu, Email: jkuratsu@kumamoto-u.ac.jp.
REFERENCES
- 1.Ahmad F, Sandberg DI. Endoscopic management of intraventricular brain tumors in pediatric patients: A review of indications, techniques, and outcomes. J Child Neurol. 2010;25:359–67. doi: 10.1177/0883073809340318. [DOI] [PubMed] [Google Scholar]
- 2.Axon AT, Beilenhoff U, Bramble MG, Ghosh S, Kruse A, McDonnell GE, et al. Variant Creutzfeldt-Jakob disease (vCJD) and gastrointestinal endoscopy. Endoscopy. 2001;33:1070–80. doi: 10.1055/s-2001-18937. [DOI] [PubMed] [Google Scholar]
- 3.Behari S, Banerji D, Mishra A, Sharma S, Chhabra DK, Jain VK. Intrinsic third ventricular craniopharyngiomas: Report on six cases and a review of the literature. Surg Neurol. 2003;60:245–52. doi: 10.1016/s0090-3019(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 4.Cai R, Di X. Combined intra- and extra-endoscopic techniques for aggressive resection of subependymal giant cell astrocytomas. World Neurosurg. 2010;73:713–8. doi: 10.1016/j.wneu.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 5.Chamoun R, Couldwell WT. Transcortical-transforaminal microscopic approach for purely intraventricular craniopharyngioma. Neurosurg Focus. 2013;34(1 Suppl) doi: 10.3171/2013.V1.FOCUS12347. Video 4. [DOI] [PubMed] [Google Scholar]
- 6.Danaila L, Radoi M. Surgery of tumors of the third ventricle region. Chirurgia (Bucar) 2013;108:456–62. [PubMed] [Google Scholar]
- 7.Davies MJ, King TT, Metcalfe KA, Monson JP. Intraventricular craniopharyngioma: A long-term follow-up of six cases. Br J Neurosurg. 1997;11:533–41. doi: 10.1080/02688699745691. [DOI] [PubMed] [Google Scholar]
- 8.Endo H, Fujimura M, Kumabe T, Kanamori M, Watanabe M, Tominaga T. Application of high-definition flexible neuroendoscopic system to the treatment of primary pineal malignant B-cell lymphoma. Surg Neurol. 2009;71:344–8. doi: 10.1016/j.surneu.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas: Experience with 168 patients. J Neurosurg. 1999;90:237–50. doi: 10.3171/jns.1999.90.2.0237. [DOI] [PubMed] [Google Scholar]
- 10.Gaab MR. Instrumentation: Endoscopes and equipment. World Neurosurg. 2013;79(2 Suppl):S14.e11–1. doi: 10.1016/j.wneu.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Hori T, Kawamata T, Amano K, Aihara Y, Ono M, Miki N. Anterior interhemispheric approach for 100 tumors in and around the anterior third ventricle. Neurosurgery. 2010;66(3 Suppl Operative):S65–74. doi: 10.1227/01.NEU.0000365550.84124.BB. [DOI] [PubMed] [Google Scholar]
- 12.Jung TY, Jung S, Jang WY, Moon KS, Kim IY, Kang SS. Operative outcomes and adjuvant treatment of purely third ventricle craniopharyngioma after a transcallosal approach. Br J Neurosurg. 2012;26:355–60. doi: 10.3109/02688697.2011.631615. [DOI] [PubMed] [Google Scholar]
- 13.Liu JK. Interhemispheric transcallosal approach for resection of intraventricular central neurocytoma. Neurosurg Focus. 2013;34(1 Suppl) doi: 10.3171/2013.V1.FOCUS12353. Video 3. [DOI] [PubMed] [Google Scholar]
- 14.Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: Trans-lamina terminalis approach. Neurosurgery. 2000;47:857–63. doi: 10.1097/00006123-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Nishijima Y, Fujimura M, Nagamatsu K, Kohama M, Tominaga T. Neuroendoscopic management of symptomatic septum pellucidum cavum vergae cyst using a high-definition flexible endoscopic system. Neurol Med Chir (Tokyo) 2009;49:549–52. doi: 10.2176/nmc.49.549. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien DF, Hayhurst C, Pizer B, Mallucci CL. Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg. 2006;105(3 Suppl):219–26. doi: 10.3171/ped.2006.105.3.219. [DOI] [PubMed] [Google Scholar]
- 17.Ohata K, Hakuba A, Nagai K, Morino M, Iwa Y. A biorbitofrontobasal interhemispheric approach for suprasellar lesions. Mt Sinai J Med. 1997;64:217–21. [PubMed] [Google Scholar]
- 18.Oi S, Shibata M, Tominaga J, Honda Y, Shinoda M, Takei F, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: A prospective study. J Neurosurg. 2000;93:245–53. doi: 10.3171/jns.2000.93.2.0245. [DOI] [PubMed] [Google Scholar]
- 19.Oka K. Introduction of the videoscope in neurosurgery. Neurosurgery. 2008;62(5 Suppl 2):ONS337–40. doi: 10.1227/01.neu.0000326016.84315.56. [DOI] [PubMed] [Google Scholar]
- 20.Rogez-Kreuz C, Yousfi R, Soufflet C, Quadrio I, Yan ZX, Huyot V, et al. Inactivation of animal and human prions by hydrogen peroxide gas plasma sterilization. Infect Control Hosp Epidemiol. 2009;30:769–77. doi: 10.1086/598342. [DOI] [PubMed] [Google Scholar]
- 21.Roth J, Bercu MM, Constantini S. Combined open microsurgical and endoscopic resection of hypothalamic hamartomas. J Neurosurg Pediatr. 2013;11:491–4. doi: 10.3171/2013.2.PEDS12275. [DOI] [PubMed] [Google Scholar]
- 22.Selvanathan SK, Kumar R, Goodden J, Tyagi A, Chumas P. Evolving instrumentation for endoscopic tumour removal of CNS tumours. Acta Neurochir. 2013;155:135–8. doi: 10.1007/s00701-012-1561-4. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya M, Takayasu M, Suzuki Y, Saito K, Sugita K. Bifrontal basal interhemispheric approach to craniopharyngioma resection with or without division of the anterior communicating artery. J Neurosurg. 1996;84:951–6. doi: 10.3171/jns.1996.84.6.0951. [DOI] [PubMed] [Google Scholar]
- 24.Shiramizu H, Hori T, Matsuo S, Niimura K, Yoshimoto H, Ishida A, et al. Anterior callosal section is useful for the removal of large tumors invading the dorsal part of the anterior third ventricle: Operative technique and results. Neurosurg Rev. 2013;36:467–75. doi: 10.1007/s10143-013-0455-0. [DOI] [PubMed] [Google Scholar]
- 25.Sipos L, Vajda J. Craniopharyngioma of the third ventricle. Acta Neurochir. 1997;139:92–3. doi: 10.1007/BF01850877. [DOI] [PubMed] [Google Scholar]
- 26.Tomasello F, Cardali S, Angileri FF, Conti A. Transcallosal approach to third ventricle tumors: How I do it. Acta Neurochir. 2013;155:1031–4. doi: 10.1007/s00701-013-1714-0. [DOI] [PubMed] [Google Scholar]
- 27.Ulm AJ, Russo A, Albanese E, Tanriover N, Martins C, Mericle RM, et al. Limitations of the transcallosal transchoroidal approach to the third ventricle. J Neurosurg. 2009;111:600–9. doi: 10.3171/2008.7.JNS08124. [DOI] [PubMed] [Google Scholar]
- 28.Yamini B, Refai D, Rubin CM, Frim DM. Initial endoscopic management of pineal region tumors and associated hydrocephalus: Clinical series and literature review. J Neurosurg. 2004;100(5 Suppl Pediatrics):S437–41. doi: 10.3171/ped.2004.100.5.0437. [DOI] [PubMed] [Google Scholar]
- 29.Yan ZX, Stitz L, Heeg P, Pfaff E, Roth K. Infectivity of prion protein bound to stainless steel wires: A model for testing decontamination procedures for transmissible spongiform encephalopathies. Infect Control Hosp Epidemiol. 2004;25:280–3. doi: 10.1086/502392. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto K, Shono T, Matsukado K, Sasaki T. The transventricular preforniceal approach for exophytic chiasmatic/hypothalamic astrocytomas extending into the anterior third ventricle. Acta Neurochir. 2013;155:727–32. doi: 10.1007/s00701-013-1642-z. [DOI] [PubMed] [Google Scholar]


