Abstract
Background:
De novo aneurysm formation after intracranial anastomotic surgery is a relatively rare complication with fewer than 20 reported cases, and the mechanism is still unclear.
Case Description:
A 63-year-old male treated for symptomatic internal carotid artery occlusion developed de novo aneurysms twice after anastomoses first of the superficial temporal artery-middle cerebral artery and second of the external carotid artery-radial artery-middle cerebral artery over a 10-year period. The first de novo aneurysm was successfully resected with pathological diagnosis of true aneurysm. The second de novo aneurysm thrombosed naturally after gradual growth. Genetic testing of the patient revealed the c.14576G>A (p.R4859K) variant in ring finger protein 213, which is a susceptibility gene for moyamoya disease.
Conclusions:
This genetic variant was probably involved in the repeated de novo aneurysm formation, and this case represents a rare phenotype of the genetic variant.
Keywords: de novo aneurysm, external carotid artery-internal carotid artery anastomosis, internal carotid artery occlusion, moyamoya disease, RNF213
INTRODUCTION
External carotid artery (ECA)-internal carotid artery (ICA) anastomosis is a well-established surgical procedure for the treatment of ischemic cerebrovascular disease such as moyamoya disease (MMD), and giant cerebral aneurysms combined with major artery ligation.[1,2,8] De novo aneurysm formation after intracranial anastomotic surgery is relatively rare but life-threatening, with fewer than 20 reported cases [Table 1].[1,3,4,7,9,10,11,12,13,20,21,22,23,25] The mechanism of de novo aneurysm formation after anastomotic surgery may involve mechanical arterial wall injuries caused by intraoperative manipulations,[13] disproportion between donor and recipient vessels,[12] and insufficiency of sutures.[24] In contrast, some cases with long intervals of more than 20 years from anastomotic surgery to aneurysm formation may involve hemodynamic stress due to the increased hemodynamic force from the donor artery.[7,11,21]
Table 1.
Series of de novo aneurysms after intracranial anastomotic surgery
We describe an extremely rare case of repeated de novo aneurysm formation after first and second ECA-ICA anastomoses for symptomatic ICA occlusion. Genetic analysis of the patient revealed the c.14576G>A (p.R4859K, rs112735431) variant in ring finger protein 213 (RNF213; a gene located in chromosome 17q; based on the National Center for Biotechnology Information Reference sequence NP_065965.4), which is known to be a susceptibility gene for MMD.[14] Recently, RNF213 c.14576G>A was reported to be associated with various phenotypes of intracranial major artery diseases.[18,19] We suggest that this genetic variant is associated with the repeated de novo aneurysm formation in the present case.
CASE REPORT
History and presentation
A 63-year-old male was admitted to Showa General Hospital with right hemiparesis. He had a history of hypertension, and no other risk factor of atherosclerosis. Magnetic resonance (MR) imaging revealed acute cerebral infarction in the left watershed area [Figure 1a], and MR angiography and digital subtraction angiography (DSA) demonstrated occlusion of the left ICA at the cervical portion [Figure 1b and c]. He was treated conservatively in the acute phase and scheduled for anastomotic surgery because technetium-99m-ethyl cysteinate dimer single photon emission computed tomography (99mTc-ECD SPECT) showed decreased cerebral blood flow.
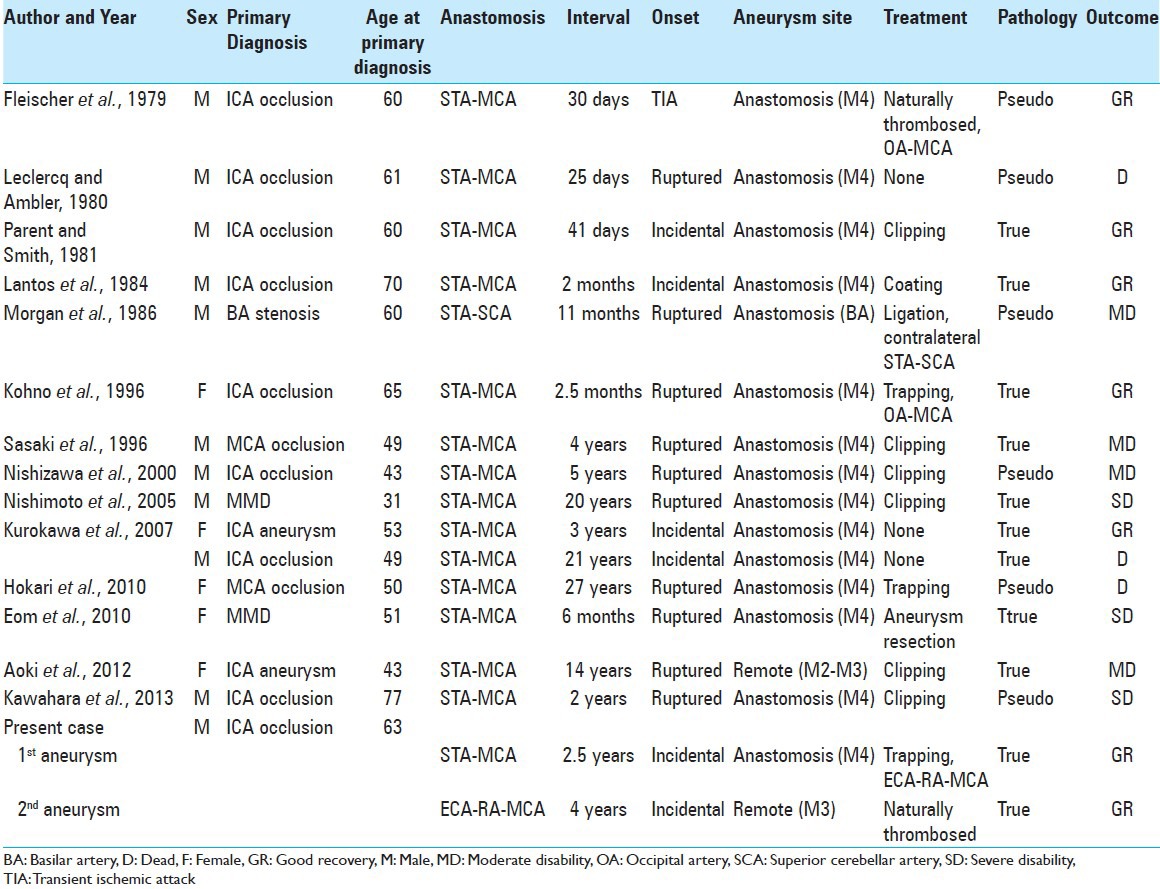
Figure 1.
(a) Diffusion-weighted MR image at onset indicating acute watershed infarction due to hemodynamic insufficiency. (b and c) MR angiogram (b) and lateral DSA of the left common carotid artery (c) at the onset showing occlusion of the left ICA at the cervical portion (arrow). (d) Lateral DSA of the left common carotid artery after the first anastomosis in which the blood flow from the STA perfuses both proximal and distal to the anastomotic site (arrow). (e and f) MR angiogram (e) and lateral DSA of the left common carotid artery (f) performed 2.5 years after the first anastomosis showing de novo aneurysm at the anastomotic site (arrow)
First anastomosis and postoperative course
The patient underwent a left superficial temporal artery (STA)-middle cerebral artery (MCA) single anastomosis at the M4 portion with 15 sutures of 10-0 monofilament nylon [Figure 2a]. Postoperative DSA showed good patency of the anastomosis [Figure 1d], and 99mTc-ECD SPECT demonstrated improvement of cerebral blood flow. Postoperatively, the right hemiparesis improved with rehabilitation, and he was discharged without neurological deficit. First follow-up MR angiography with an interval of 2 years (due to transient lost follow-up) showed de novo aneurysm [Figure 1e]. DSA performed 2.5 years after anastomosis (at the age of 66) revealed a 19.0-mm de novo saccular aneurysm at the anastomotic site [Figure 1f]. The left MCA territory was perfused mainly via the anastomosed STA, whereas cross flow via the anterior communicating artery, which had been detected immediately after anastomosis, had disappeared. He was scheduled for surgical intervention because the aneurysm was considered to be at high risk of rupture.
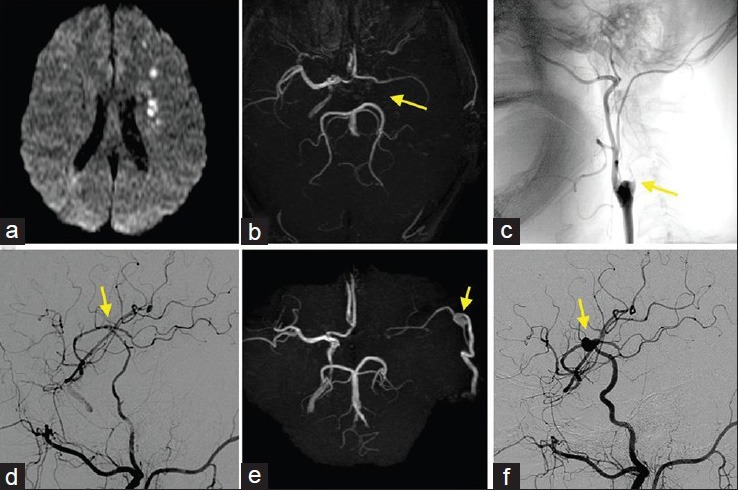
Figure 2.
(a) Intraoperative photograph of the first STA-MCA anastomosis (arrowhead). (b and c) Intraoperative photographs of the trapping of the aneurysm and second ECA-RA-M2 and STA-M4 anastomoses showing the de novo aneurysm (arrow) pretrapping view (b), and posttrapping and anastomoses (arrowhead) view (c). d: M4 portion of the MCA distal to the anastomosis; M2: M2 portion of the MCA; p: M4 portion of the MCA proximal to the anastomosis; RA: Radial artery; STA: Superficial temporal artery
Second anastomosis
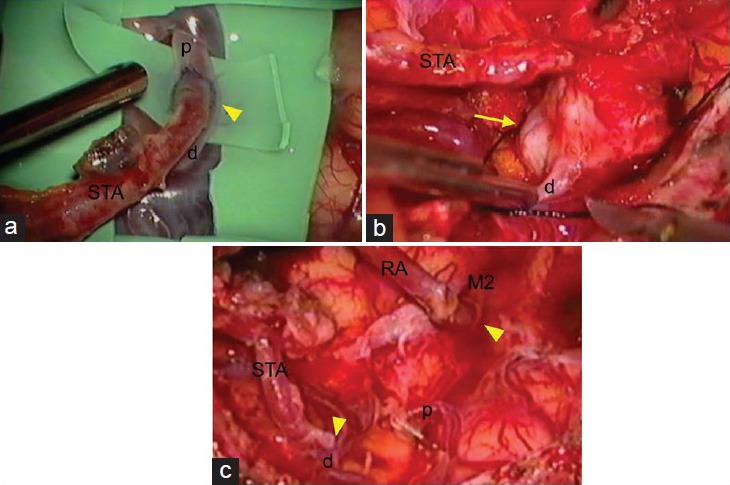
The patient underwent trapping of the aneurysm and left ECA-radial artery (RA)-MCA (M2 portion) and STA-MCA (M4 portion) anastomoses. Intraoperative findings showed that the de novo aneurysm at the anastomotic site had adhered to the superficial dura mater and the bottom brain surface with connective tissue. Clipping of the aneurysm was difficult, because the aneurysm was located exactly at the site of anastomosis [Figure 2b]. First, ECA-RA-MCA anastomosis was performed at the M2 portion to maintain perfusion of the MCA territory during the trapping procedure. Next, we resected the aneurysm, with ligation of the stump of the proximal M4. Finally, we performed anastomosis of the stumps of the STA and the distal M4 [Figure 2c]. Histological examination revealed that the aneurysm wall was thinned and consisted of collagen fibers, almost without media and internal elastic lamina, but immunohistochemistry revealed fragmented smooth muscle cells throughout the circumference, which established the diagnosis as true aneurysm [Figure 3]. Postoperative DSA showed good patency of the anastomoses [Figure 4a], and he was discharged without neurological deficit.
Figure 3.
Photomicrographs of resected de novo aneurysm. Left: Wall of the aneurysm dome (arrow) is thinned and consisted of collagen fibers. Asterisk = aneurysm lumen. Elastica van Gieson stain, original magnification ×20. Right: Fragmented smooth muscle cells (arrowhead) are detected throughout the circumference. Alpha-smooth muscle actin stain, original magnification ×20
Figure 4.
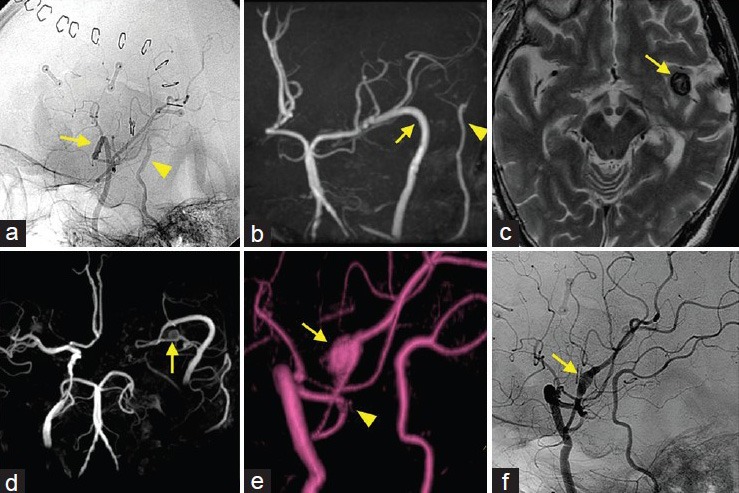
(a and b) Postoperative lateral DSA of the left common carotid artery (a) and follow-up MR angiogram (b) showing good patency of the ECA-RA-MCA (arrow) and STA-MCA (arrowhead) anastomoses. C–E: T2-weighted MR image (c) and MR angiograms (d and e) performed 6 years after the second anastomosis showing second de novo aneurysm located remote from the anastomotic site (arrow: Aneurysm, arrowhead: Anastomotic site) (f)Lateral DSA of the left common carotid artery exhibiting the dilated artery (M3 portion) without visualization of the aneurysm lumen (arrow)
Postoperative course
Follow-up MR angiography showed good patency of the anastomoses and no other abnormal findings over the next 3 years [Figure 4b]. However, MR angiography 4 years after the second anastomosis (at the age of 70) demonstrated a dilatation of the left M3 portion that was remote from the ECA-RA-MCA anastomotic site. Follow-up MR angiography for 3 years showed that the dilatation enlarged gradually and became a 12.0-mm saccular aneurysm at the nonbranching site [Figure 4c–e]. Therefore, he was scheduled for DSA. DSA revealed a slightly dilated vessel, but the aneurysm lumen was not detected [Figure 4f]. These findings suggested that the aneurysm lumen was completely thrombosed, and had little risk of rupture. The last follow-up MR imaging showed no enlargement of the thrombosed aneurysm. The patient remains under observation without ischemic event or neurological deficit.
Genetic analysis and ethical considerations
The patient's son had been incidentally diagnosed with asymptomatic right MCA occlusion, so these craniocervical major artery diseases were suspected to have some association with genetic factors. The patient was referred to The University of Tokyo Hospital and genetic analysis was performed there as described previously.[18,19] Genetic analysis revealed the patient had RNF213 c.14576G>A variant (heterozygote) [Figure 5]. The ethical reviews of the genetic analysis study in the two facilities (The University of Tokyo Hospital and Showa General Hospital) were both approved by the Human Genome, Gene Analysis Research Ethics Committee of the Faculty of Medicine, the University of Tokyo (approval number G10026-1). Written informed consent was obtained from the patient.
Figure 5.
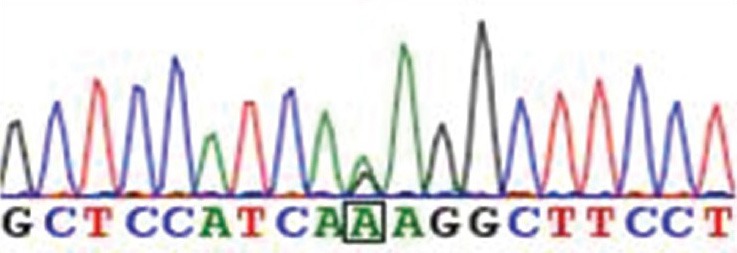
Sequencing chromatogram of the patient showing the heterozygote of the RNF213 c.14576G>A variant
DISCUSSION
In the present case, the first de novo aneurysm at the anastomotic site was detected at 2.5 years after the STA-MCA anastomosis surgery, and was successfully resected with a pathological diagnosis of true aneurysm. The second de novo aneurysm located remote from the anastomotic site was detected at 4 years after the ECA-RA-MCA anastomosis surgery, and was thrombosed naturally after gradual growth. This unique case represents repeated de novo aneurysm formation after ECA-ICA anastomosis. The extreme rarity of such repeated aneurysm formation and his family history suggested the involvement of genetic predisposing factors, leading us to identify the presence of the genetic variant RNF213 c.14576G>A.
The genetic variant c.14576G>A in RNF213 has significant associations with MMD and various phenotypes of non-MMD intracranial major artery stenosis and occlusion (ICASO).[14,18,19] This genetic variant is identified in about 80% of patients with MMD, and in 95% of patients with familial MMD.[17] In particular, this genetic variant is also found in about 20% of patients with non-MMD ICASO.[18,19] In contrast, the frequency of normal carriers is estimated at about 1–2% in East Asian population.[14,17] Due to such incomplete penetrance, RNF213 is regarded as a susceptibility gene, so the existence of other triggering factors should be considered,[14,16] and the clarification of the physiological and biochemical functions of RNF213 is important.
Vascular endothelial cells derived from induced pluripotent stem cells obtained from MMD patients carrying the RNF213 variant had reduced angiogenic activities, and overexpression of RNF213 variant in HeLa cells inhibited cell proliferation in vitro.[5,6] Accordingly, we speculate that the endothelial cells and smooth muscle cells were more vulnerable due to the genetic variant, so that increased hemodynamic stress due to the blood flow from the donor artery of the anastomosis resulted in de novo aneurysm formation in the present case.
We would refer to some limitations of this case report. First, the extreme rarity of the present case cannot be explained only by the presence of the genetic variant. STA-MCA anastomosis is a common surgical procedure for MMD and ICASO, and about 80% of patients with MMD and 20% of patients with ICASO had the genetic variant RNF213 c.14576G>A.[18,19] Consequently, most carriers of genetic variant RNF213 c.14576G>A who had undergone anastomosis did not develop de novo aneurysm. Second, the histological findings of the de novo aneurysm in the present case indicate atypical pathogenesis of occlusive arteries in MMD consisting of proliferative response of smooth muscle cells.[15] As triggering factors other than the genetic variant RNF213 c.14576G>A are considered to be present in MMD, such other factors in addition to the genetic variant RNF213 c.14576G>A were probably involved in the pathogenesis of the present case. Nevertheless, we believe that the present case represents an extremely rare phenotype of the genetic variant RNF213 c.14576G>A.
CONCLUSION
The present extremely rare case of repeated de novo aneurysm formation after the first STA-MCA and second ECA-RA-MCA anastomoses occurred in a patient with the genetic variant RNF213 c.14576G>A. We consider that the genetic variant was involved in the repeated de novo aneurysm formation, and this case represents a rare phenotype of the genetic variant RNF213 c.14576G>A.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research (B) (No. 25293304) to Dr. Saito and by grants from SENSHIN Medical Research Foundation to Dr. Miyawaki. The other authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/1/41/153709
Contributor Information
Yuta Fukushima, Email: fukushima-tky@umin.ac.jp.
Satoru Miyawaki, Email: miyawaki-tky@umin.ac.jp.
Tomohiro Inoue, Email: t.inoue-fujinsu@beige.plala.or.jp.
Seiichiro Shimizu, Email: sshimizubukuro@hotmail.com.
Gakushi Yoshikawa, Email: gyoshika-tky@umin.ac.jp.
Hideaki Imai, Email: hiimai-nsu@umin.ac.jp.
Nobuhito Saito, Email: nsaito-tky@umin.net.
Kazuo Tsutsumi, Email: k.tsutsumi-md@nifty.com.
REFERENCES
- 1.Aoki T, Yoshitomi M, Yamamoto M, Hirohata M, Morioka M. Ruptured de novo aneurysm arising at a site remote from the anastomosis 14 years after superficial temporal artery-middle cerebral artery bypass: A case report. Neurosurgery. 2012;71:E905–9. doi: 10.1227/NEU.0b013e318260ffcf. [DOI] [PubMed] [Google Scholar]
- 2.Barnett DW, Barrow DL, Joseph GJ. Combined extracranial-intracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92–8. doi: 10.1227/00006123-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Eom KS, Kim DW, Kang SD. Intracerebral hemorrhage caused by rupture of a giant aneurysm complicating superficial temporal artery-middle cerebral artery anastomosis for moyamoya disease. Acta Neurochir (Wien) 2010;152:1069–73. doi: 10.1007/s00701-009-0550-8. [DOI] [PubMed] [Google Scholar]
- 4.Fleischer AS, Faria MA, Jr, Hoffmann JC., Jr Pseudoaneurysm complicating superficial temporal artery-middle cerebral artery bypass. Surg Neurol. 1979;12:305–6. [PubMed] [Google Scholar]
- 5.Hitomi T, Habu T, Kobayashi H, Okuda H, Harada KH, Osafune K, et al. Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G>A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients. Biochem Biophys Res Commun. 2013;438:13–9. doi: 10.1016/j.bbrc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Hitomi T, Habu T, Kobayashi H, Okuda H, Harada KH, Osafune K, et al. The moyamoya disease susceptibility variant RNF213 R4810K (rs112735431) induces genomic instability by mitotic abnormality. Biochem Biophys Res Commun. 2013;439:419–26. doi: 10.1016/j.bbrc.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 7.Hokari M, Yasuda H, Iwasaki M, Kawabori M, Kuroda S, Abe S, et al. Intracerebral hemorrhage from a ruptured aneurysm at the site of anastomosis 27 years after superficial temporal artery-middle cerebral artery bypass. Neurol Med Chir (Tokyo) 2010;50:1012–4. doi: 10.2176/nmc.50.1012. [DOI] [PubMed] [Google Scholar]
- 8.Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H. Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg. 1992;77:84–9. doi: 10.3171/jns.1992.77.1.0084. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara I, Morofuji Y, Tsutsumi K, Takahata H, Ono T, Toda K, et al. De novo ruptured aneurysm at the site of anastomosis after superficial temporal artery-middle cerebral artery anastomosis--case report and literature review. Clin Neurol Neurosurg. 2013;115:457–60. doi: 10.1016/j.clineuro.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Kohno K, Ueda T, Kadota O, Sakaki S. Subdural hemorrhage caused by de novo aneurysm complicating extracranial-intracranial bypass surgery: Case report. Neurosurgery. 1996;38:1051–5. doi: 10.1097/00006123-199605000-00041. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa T, Harada K, Ishihara H, Fujisawa H, Kato S, Kajiwara K, et al. De novo aneurysm formation on middle cerebral artery branches adjacent to the anastomotic site of superficial temporal artery-middle cerebral artery bypass surgery in two patients: Technical case report. Neurosurgery. 2007;61:E297–8. doi: 10.1227/01.neu.0000303983.19375.a3. [DOI] [PubMed] [Google Scholar]
- 12.Lantos G, Fein JM, Knep S. Cortical artery aneurysm formation after extracranial to intracranial bypass surgery. Case report. J Neurosurg. 1984;60:636–9. doi: 10.3171/jns.1984.60.3.0636. [DOI] [PubMed] [Google Scholar]
- 13.Leclercq TA, Ambler MW. Fatal subdural bleeding following superficial temporal-middle cerebral artery anastomosis. Case report. J Neurosurg. 1980;52:392–4. doi: 10.3171/jns.1980.52.3.0392. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011;6:e22542. doi: 10.1371/journal.pone.0022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke. 1993;24:1960–7. doi: 10.1161/01.str.24.12.1960. [DOI] [PubMed] [Google Scholar]
- 16.Mineharu Y, Takagi Y, Takahashi JC, Hashikata H, Liu W, Hitomi T, et al. Rapid progression of unilateral moyamoya disease in a patient with a family history and an RNF213 risk variant. Cerebrovasc Dis. 2013;36:155–7. doi: 10.1159/000352065. [DOI] [PubMed] [Google Scholar]
- 17.Miyatake S, Miyake N, Touho H, Nishimura-Tadaki A, Kondo Y, Okada I, et al. Homozygous c. 14576G>A variant of RNF213 predicts early-onset and severe form of moyamoya disease. Neurology. 2012;78:803–10. doi: 10.1212/WNL.0b013e318249f71f. [DOI] [PubMed] [Google Scholar]
- 18.Miyawaki S, Imai H, Shimizu M, Yagi S, Ono H, Mukasa A, et al. Genetic variant RNF213 c. 14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke. 2013;44:2894–7. doi: 10.1161/STROKEAHA.113.002477. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N. Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke. 2012;43:3371–4. doi: 10.1161/STROKEAHA.112.663864. [DOI] [PubMed] [Google Scholar]
- 20.Morgan M, Besser M, Tuck R. Pseudoaneurysm complicating superficial temporal artery-superior cerebellar artery bypass. Surg Neurol. 1986;26:277–81. doi: 10.1016/0090-3019(86)90162-x. [DOI] [PubMed] [Google Scholar]
- 21.Nishimoto T, Yuki K, Sasaki T, Murakami T, Kodama Y, Kurisu K. A ruptured middle cerebral artery aneurysm originating from the site of anastomosis 20 years after extracranial-intracranial bypass for moyamoya disease: Case report. Surg Neurol. 2005;64:261–5. doi: 10.1016/j.surneu.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa S, Yokoyama T, Sugiyama K, Yokota N. Intracerebral hemorrhage from a ruptured pseudoaneurysm after STA-MCA anastomosis--case report. Neurol Med Chir (Tokyo) 2000;40:408–12. doi: 10.2176/nmc.40.408. [DOI] [PubMed] [Google Scholar]
- 23.Parent AD, Smith RR. Traumatic aneurysm complicating EC-IC bypass: Successful surgical clipping. Surg Neurol. 1981;15:229–31. doi: 10.1016/0090-3019(81)90151-8. [DOI] [PubMed] [Google Scholar]
- 24.Robertson JH, Robertson JT. The relationship between suture number and quality of anastomoses in microvascular procedures. Surg Neurol. 1978;10:241–5. [PubMed] [Google Scholar]
- 25.Sasaki T, Kodama N, Itokawa H. Aneurysm formation and rupture at the site of anastomosis following bypass surgery. Case report. J Neurosurg. 1996;85:500–2. doi: 10.3171/jns.1996.85.3.0500. [DOI] [PubMed] [Google Scholar]


