Abstract
Background/Aims:
Success of colorectal cancer (CRC) screening is dependent in part on the proportion of uptake by the targeted population. We aimed in this study to identify factors that were associated with willingness to undergo CRC screening based on the health belief model (HBM).
Patients and Methods:
This was a cross-sectional study among citizens of Riyadh, Saudi Arabia. Demographic data collected included gender, age, education, marital status, employment status, a history of CRC in the family or knowing a friend with CRC, as well as income. A questionnaire was developed in Arabic based on the HBM and included enquiries on knowledge about CRC symptoms and risk factors, types of CRC screening tests, perceived risk of CRC, previously undergoing CRC screening, intent to undergo CRC screening, perceived barriers to CRC screening, perceived severity of CRC, as well as attitudes toward CRC and its screening.
Results:
Five hundred participants were included. The mean age was 41.0 years (SD 10.7). Males were 50% and only 6.7% of those between 50 and 55 years of age had undergone CRC screening. Of those surveyed, 70.7% were willing to undergo CRC screening. Also, 70.5% thought that CRC is curable, 73.3% believed it was preventable, whereas 56.7% thought it was a fatal disease. Neither gender, level of education, occupation, income, marital status, nor general knowledge about CRC was found to be associated with the willingness to undergo CRC screening. Recognizing that colonoscopy was a screening test (OR 1.55, 95% CI; 1.04–2.29) was associated with a strong desire to undergo CRC screening while choosing a stool-based test was associated with not willing to undergo CRC screening (OR 0.59, 95%CI; 0.38–0.91).
Conclusion:
We found that the majority of those interviewed were willing to undergo CRC screening and identified a number of barriers as well as potential areas that could be targeted in the promotion of CRC screening uptake if such a national program were to be implemented.
Keywords: Colon cancer, colonoscopy, early detection, screening, endoscopy, epidemiology, health belief model, Saudi Arabia
Screening programs have been proven to decrease both the incidence and the mortality[1] associated with colorectal cancer (CRC). CRC ranks third in cancer-related mortality for males and females alike with an estimated 96,830 new cases and 50,310 deaths in the United States in 2014.[2] In Saudi Arabia, CRC ranks second in incidence among all cancers with a median age of diagnosis of 60 years for males and 58 years for females.[3] In 2008, the age-standardized rate for the incidence for CRC in Saudi Arabia was 12.1, associated mortality was 8.6, whereas the 5-year prevalence was 21.5 per 100,000 population-years.[4,5]
Screening programs for CRC require considerable financial as well as logistic allocation in an era of finite resources, thus prior to starting such projects, factors associated with its success should be optimized. There is a large body of evidence that indicates that public awareness for CRC in Asia is generally low,[6] but there is a belief that educating the general population, improving access to health care resources as well as removing barriers to screening can improve outcomes.[6,7] One of the barriers to participation in CRC screening is lack of knowledge about the impact of CRC, its risk factors, and the benefits that could be gained through screening.[8,9,10] We aimed in this study to identify knowledge, attitude, and behavioral factors among the public that are associated with willingness to undergo CRC screening based on the health belief model (HBM).
PATIENTS AND METHODS
Data collection
This was an observational, cross-sectional study among selected citizens of Riyadh, Saudi Arabia. The study was conducted between March and April 2013.
Sample size calculation was based on a priori baseline knowledge about CRC in the population of 20%, which was based on the opinion of experienced gastroenterologists, as there are no data from the region, to the best of our knowledge, prior to this study. Using the rule of 10 outcome events per predictor variable,[11] and our wish to include up to 10 variables in the multivariable model, we estimated that 500 individuals would be needed to provide sufficient accuracy.
To have a better representation of the various residential and socioeconomic groups of the city, it was divided into four areas and a list of all the malls was generated. Eight malls were selected using a simple number generator. Individuals of 18–75 years of age who were willing to be surveyed were included and only residents of Riyadh city were included. The questionnaires were distributed by six of the investigators and a convenience sampling method was used. The institutional review board at King Saud University Medical city approved the study.
Survey Instrument
The questionnaire was specially designed for the purposes of this study, after a thorough literature review, based on the health belief model (HBM).[12,13] It was converted into an Arabic version and was reviewed by two bilingual epidemiologists and a gastroenterologist. The questionnaire we used in our study was derived based on the HBM, which is a sociopsychological model that explains health-seeking behavior of individuals by focusing on the attitudes and behaviors that are influenced by perceived susceptibility, severity, benefit, barriers, and cues to actions.[13] This model was chosen as it was mostly adopted to assess the construct of health-seeking behavior with regard to uptake of cancer screening in general as well as CRC screening in particular and was the most valid tool to measure such an association.[14] Collected data included demographics, gender, age, education, marital status, employment status, history of CRC in the family or having a friend with CRC, as well as monthly income. The knowledge section included enquiries about: CRC symptoms and risk factors, types of CRC screening tests, perceived risk of CRC, previous screening for CRC, intent to undergo CRC screening, perceived barriers to CRC screening, and perceived severity of CRC. We also asked the participants about the age at which CRC screening should begin; this was categorized into five age ranges starting at the age of 20 till the age of 70 years.
We included five CRC screening tests as possible options: Fecal occult blood tests (FOBT), colonoscopy, flexible sigmoidoscopy, or computed tomographic colonography (CTC).
Attitudes toward CRC and its screening were assessed using a 5-point Likert scale starting with responses of “strongly agree” and ranging to “strongly disagree.” The survey also included a question about which screening test the person surveyed would accept, if given an option. The participants were requested not to answer the last question until one of the investigators used a standardized layman description of each test to ensure proper understanding of the CRC screening method, time intervals between each test, and the benefits as well as limitations of each of the listed screening test. The data presented to the participants was based on the joint guideline from the American Cancer Society, the US Multi-Society Task Force on CRC, and the American College of Radiology.[15]
For the purpose of analysis we formulated a knowledge score according to correct responses in the questionnaire, where each correct response was awarded a point. This knowledge score was used as a continuous variable when analyzing the data. Although the American College of Gastroenterology[16] as well as the U.S. Preventive Services Task Force[17] recommend starting screening at the age of 50 years for average risk individuals, there is no formal CRC screening program in Saudi Arabia and hence no nationally recommended age to initiate CRC screening. Thus we considered the age groups (40–49 years) and (50–59 years) as correct responses and included both in the knowledge score we formulated. Each correct response was appointed a single point and the maximum score that could be achieved was 26.
A pilot study was performed on a convenient sample of 22 individuals that assessed the face and content validity of the questionnaire; those were not included in the study. On average, each participant required about 7 min to complete the questionnaire.
Statistical analysis
Data analysis included descriptive statistics computed for continuous variables, including means, standard deviations (SDs), and minimum and maximum values. Frequency distributions were used for categorical variables. When hypothesis testing was conducted, the t-test with unequal variances, as well as Fisher's exact test was used where appropriate. When comparing more than one group; a one-way analysis of variance (ANOVA) was used to test for differences among these groups. Univariable and multivariable logistic regressions were used to examine the possible association between independent variables and the willingness to undergo a screening test for CRC, when appropriate. Odds ratio (OR) and their corresponding 95% confidence intervals (CIs) were calculated. Results are shown with a Bonferroni adjustment for multiple comparisons.
We used STATA 11.2 (Stata Corp., College Station, TX, USA) software for our analysis. A statistical significance threshold of P = 0.05 was adopted. No attempt at imputation was made for missing data.
RESULTS
Demographics and historical data
A total of 500 participants were included in the study. The mean age of participants was 41.0 years (SD 10.7 years). Saudi nationals comprised 80% of the study sample. Of the surveyed population 2.0% were illiterate, 3.2% had primary education or less, 26.6% had intermediate or high school education, and 68.1% had completed university or postgraduate studies. The majority of participants worked in government paid jobs (37.9%), followed by those employed by the private sector (24.3%), whereas 19.6% were housewives. The majority of the respondents were married (83.6%), whereas 13.0% were single [Table 1]. The majority of the respondents had heard about CRC (81.6%). Only 3.6% reported a history of CRC in a first-degree relative, whereas 14.5% mentioned having a friend who had CRC. Only 6.7% of the surveyed sample between the ages of 50 and 55 years and 6.5% of those aged between 55 and 60 years reported having undergone a screening test for CRC with the majority of those being screened with a colonoscopy.
Table 1.
Basic demographic variables
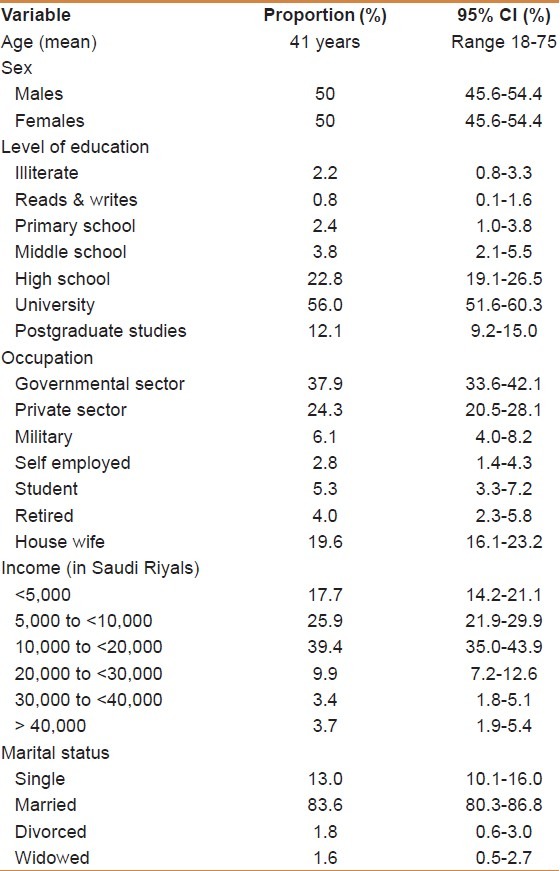
Neither gender (P = 0.51), level of education (P = 0.72), occupation (P = 0.34), income (P = 0.22), nor marital status (P = 0.88) were found to be statistically associated with willingness to undergo CRC screening.
Knowledge score
Of those included in the study, 35.6% thought that CRC was common, whereas 35.6% thought it was not. The mean knowledge score was 10.50 (SD 4.4, range 2–23). We found that the knowledge score increased if the participant had heard about CRC (OR 17.2, 95%CI; 6.50–45.58), when the surveyed individual was married (OR, 2.63, 95%CI; 1.13–6.17) and with age (OR 1.08, 95%CI; 1.04–1.12, per year). On the other hand, there was no statistical association between the knowledge score and the gender, occupation, nationality, education, income, or if the individual had a relative or friend who was affected by CRC. There was no difference in the knowledge score between those who were generally willing to undergo CRC screening and those who were not (10.7 vs. 10.0, P = 0.13).
Knowledge about CRC risk factors
The proportion of respondents who correctly identified the following as risk factors for CRC were: 62.2% for alcohol consumption, 54.2% for diet, 50.8% for inflammatory bowel disease, 37.6% for family history of CRC, 35.3% for smoking, 22.1% for obesity, 19.3% for age, 7.8% for diabetes mellitus, 7.4 for hypertension, and 6.8% for sex. However, 18.3% thought wrongly that having hemorrhoids was a risk factor for CRC and 13.9% admitted not knowing risk factors for CRC. Participants who thought that age was a risk factor were more likely to be willing to undergo CRC screening (80.6% vs. 68.2, P = 0.02; OR 1.95, 95%CI; 1.11-3.40), as well as those who thought male gender was a risk factor (90.6% vs. 69.2%, P = 0.01; OR 4.31, 95%CI; 1.29-14.38).
There was no difference in the willingness to undergo CRC screening based on having a relative with CRC (P = 0.31), having a friend with CRC (P = 0.95), having heard of CRC (P = 0.81), or whether the person thought CRC was common or not (P = 0.60). Also there was no difference in the willingness to undergo CRC screening between those who thought that the following were risk factors for CRC: diet (P = 0.88), family history of CRC (P = 0.93), smoking (P = 0.19), alcohol consumption (P = 0.36), diabetes mellitus (P = 0.15), hypertension (P = 0.99), irritable bowel disease (P = 0.19), obesity (P = 0.39), hemorrhoids (P = 0.36), as well as self-perception about knowing risk factors for CRC (P = 0.76).
Knowledge about CRC symptoms
The proportion of individuals who correctly identified the following possible symptoms for CRC was 41.2% for bleeding per rectum, 38.2% for abdominal pain, 38.2% for change in bowel habits, 32.6% for fatigue, 32.2% for weight loss, and 27.8% for melena. Difficulty in swallowing was correctly identified as not being a symptom by 93.0% of the respondents; whereas 28.8% admitted not knowing the symptoms of CRC, only 31.4% of the participants correctly thought that CRC could present without symptoms.
Those who thought that abdominal pain was a symptom for CRC were more likely to want screening for CRC (81.1% vs. 66.6%, P < 0.01). While knowledge about the following possible symptoms was not associated with increased willingness to undergo CRC screening; melena (P = 0.92), bleeding per rectum (P = 0.59), change in bowel habits (P = 0.08), weight loss (P = 0.25), fatigue (P = 0.65), difficulty in swallowing (P = 0.24), self-perception about knowing symptoms of CRC (P = 0.49), or that CRC can present without symptoms (P = 0.18).
Knowledge about CRC screening tests
The most commonly recognized tool for screening for CRC was colonoscopy (50.56%), followed by computed tomography colonography (CTC) (32.7%), stool-based screening (24.7%), whereas the least appreciated method was flexible sigmoidoscopy (14.7%). Of the participants, 21.9% thought that a complete blood count was a screening method for CRC, whereas 19.9% did not know of any screening tests for CRC.
On univariable analysis, those who recognized that colonoscopy was a screening test for CRC were more likely to strongly want screening for CRC (OR 1.55, 95% CI; 1.04–2.29), whereas those who chose a stool-based test as a screening method were less likely to strongly want to undergo CRC screening (OR 0.59, 95%CI; 0.38–0.91).
Appropriate age to initiate CRC screening
Most of those surveyed thought that screening for CRC should start at the age of 40–49 years (35.1%) followed by the age range of 30–39 (24.1%), whereas the minority thought that it should start between 60 and 69 years of age (2.2%).
Those who thought that screening should start at the age of 70–79 years of age were less likely to strongly want to undergo CRC screening (OR 0.53, 95% CI; 0.30–0.93).
Attitudes toward CRC and its screening
Of those surveyed, 70.5% thought that CRC is curable, 73.3% believed it was preventable, whereas 56.7% thought it was a fatal disease. Although most of those surveyed either strongly disagreed or disagreed about the statement “If I had cancer, I would rather not know about it” (63.3%), yet 27.6% either agreed or strongly agreed with that statement. Furthermore, 60.7% of those surveyed either agreed or strongly agreed with the statement “The thought of CRC scares me.” In addition, 56.6% of those surveyed did not think that colonoscopy was embarrassing or harmful (65.6%), whereas 38.4% thought that a colonoscopy was painful [Figure 1].
Figure 1.
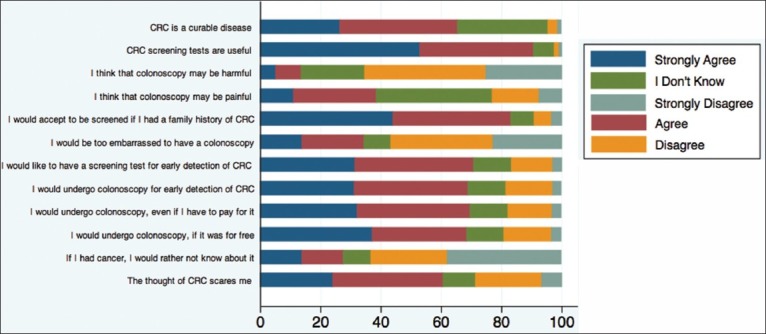
Answers to knowledge questions based on a 5-point Likert scale
Those who strongly disagreed with the statement that colonoscopy may be harmful were more likely to be willing to undergo CRC screening compared with those who did not (P < 0.01). Also those who chose a colonoscopy as a screening test were generally willing to undergo CRC screening (OR 3.01, 95%CI; 1.85–4.90).
All respondents who did not want to know that they had cancer were less likely to be willing to undergo CRC screening (P < 0.01).
Willingness to undergo CRC screening
The majority of the surveyed population was willing to undergo a screening test for CRC (70.7%) and the proportion increased to 83% if there was a family history of CRC. Colonoscopy was accepted as a screening test in the majority of individuals (68.9%) with no difference between its acceptance as a screening test whether it was for free (68.5%) or if the individual had to pay for such a service (69.7%).
DISCUSSION
Numerous CRC screening efforts are being conducted worldwide, either as population-based programs or as part of research protocols[18] and these programs vary with regard to the instrument used as a screening test ranging from guaiac-based or immunochemical-based fecal occult blood tests to sigmoidoscopy or complete colonoscopy.[18]
Although CRC is one of the leading tumors in incidence in Saudi Arabia it appears that its age-adjusted rate is much less than that reported in North America and Western Europe,[19] and there is data that suggests that adenomas might be less prevalent in Saudi Arabia compared with those populations.[20,21] Nonetheless, CRC in Saudi Arabia tends to present at an earlier age and at advanced stages[22] and with a worse 5-year survival.[3] Whether a national CRC screening program would be able to improve outcomes remains undetermined.
A major determinant of the success of CRC screening programs is the rate of uptake by the targeted population. Knowledge is only one factor that affects participation in CRC screening programs. Other identified barriers include not having access to physicians, the setting and organization of the screening intervention, access to the health care delivery system, lack of time for those intended to be screened, transportation, financial barriers as well as fear from receiving unwanted result as well as embarrassment or shame.[7,10] Furthermore, these perceived barriers varied in their effect on different ethnic groups within communities[7] as well as between countries,[12] signifying a more complex nature for uptake of CRC screening. A multinational study involving 14 Asia pacific countries, including 7915 participants demonstrated that the uptake of screening for CRC in those older than 50 years of age was 27%.[12] Furthermore, this was different between countries with it being highest in the Philippines (69%), Australia (48%), and Japan (38%), and lowest in India (1.5%), Malaysia (3%), Indonesia (3%), Pakistan (7.5%), and Brunei (13.7%).[12]
Although our study included a wider age range than usually targeted in such surveys, it had a similar age representation for those older than 50 years of age (22.8%) as those reported from China and India.[12] Additionally, Keighley et al.[23] studied 20,710 participants from 21 European countries and included those who were older than 16 years in age. This wider inclusion criterion that we used aimed to provide at having a better appreciation of the general knowledge of the public, what barriers exist and to have an idea of which intervention to choose and at what age.
In our study, the majority of respondents were willing to undergo CRC screening (>70%) and was even higher among those who had a family history of CRC, this is similar to what was reported from a Palestinian study.[24] Moreover, there were no differences seen between males and females with regard to the willingness to undergo CRC screening, nor was there a difference between both genders in the chosen screening method, which was similar to the study by Qumseya et al.[24] These findings are informative if a CRC program were to be implemented but may not result in optimum utilization of such a program. This dissociation between willingness and the actual undergoing of a CRC screening test is demonstrated in a study from Spain where it was reported that 78.8% of individuals older than 50 years were willing to participate in CRC screening[9] but only 12% had ever undergone a screening test[9] demonstrating that there are other barriers to undergoing CRC screening. In a study by Gimeno-Garcia et al.[25] neither the gender, marital status, employment, or smoking status of those targeted with CRC screening were associated with undergoing CRC screening, even among those who reported having a family history of CRC,[25] which resembles our findings. Nevertheless, a study from Singapore reported that there were different barriers for CRC screening between males and females, where females perceived colonoscopy as painful and embarrassing compared with males.[26]
One of the commonly reported barriers to CRC screening programs is financial burden.[27,28,29] This does not appear to be the case in our study where health care is publically funded in government hospitals, in addition to a proportion of population being health insured. Nonetheless, access to health care facilities can be difficult due to long waiting times in public hospitals. Also considering potential differences in the socioeconomic situation between Saudis and Palestinians, cost for CRC screening tests did not seem to influence the willingness to undergo a screening test, as reported by Qumseya et al.[24] This should be viewed with caution, as the sample we chose for our survey, being in shopping malls, may not represent the broader public.
Consistent HBM constructs that were found to influence acceptance to undergo CRC screening included perceived barriers such as fear and embarrassment.[29] In our study, 27.6% did not want to know that they had CRC in the event they did, whereas more than half (60.8%) stated that the thought of CRC scared them, a fact which has been addressed in a number of qualitative studies where interviews were conducted to address populations where the uptake of CRC was low.[27,28] Such fears are not limited to the public but the perception of colonoscopy being a painful procedure was found to be high in even 4th year Greek medical students, where 57% thought it was painful and 85% preferred an alternative method to colonoscopy for CRC screening.[30] These fears could be well addressed by awareness campaigns as well as discussions with health care providers who are in a good position to advocate undergoing CRC screening. It was consistently found that recommendations by physicians generally predicted acceptance to undergo CRC screening.[12,31] This puts emphasis on physicians' knowledge and attitudes toward CRC screening.
We found that educational level did not impact willingness to undergo CRC screening in our sample, although lower educational level was reported to be associated with a decrease in CRC screening in two systematic reviews.[29,32] Our finding might be partially explained by the high rate of willingness across all strata of education in our study and the fact that at least 90% of the participants had attained a minimum of high school education, thus we may have been underpowered to detect such an association.
Our study population preferentially chose CTC as a screening test for CRC followed by a stool-based test, then colonoscopy, and the least preferred was a flexible sigmoidoscopy [Figure 2]. Despite the general notion that females would not choose a colonoscopy as a screening method, in our population there was no difference between males and females in the choice of colonoscopy as a screening tool: 38.3% versus 28.3%, respectively [Figure 2].
Figure 2.
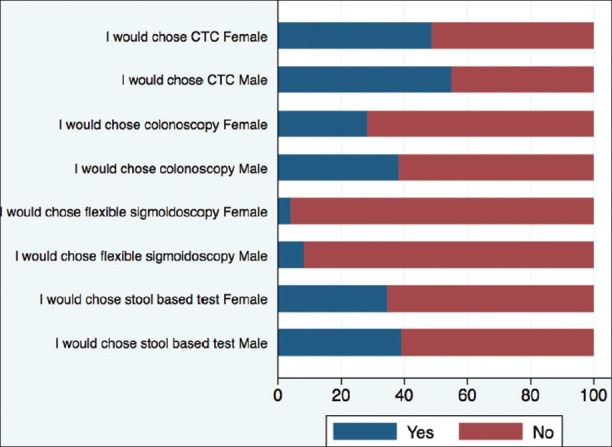
The choice of colorectal screening test based on gender
Some limitations of the study are that all of the study population were residents of an urban area, as a nonurban background has been reported to be associated with a less probability of willingness to undergo CRC screening.[24] Also it was conducted in a single city in Saudi Arabia. Furthermore, we could not obtain a random sample from the population due to the difficulty in acquiring a well-defined sampling frame, which may limit the generalizability of its outcomes. Also the respondents were from shopping centers, and those could have characteristics that do not represent the general population at large. Nonetheless, we did attempt to sample different areas of the city with the aim of having a more representative sample of Riyadh residents. Of note, this study does address the willingness to undergo a screening test for CRC, which is a one-time event as apposed to adherence where an individual is asked to undergo periodic screening tests, as characteristics of the former may differ from the latter.[29] Also, the majority of studies addressing the issue of knowledge and behaviors with CRC uptake do not look at the temporal relationship between these factors and actual uptake or adherence with CRC screening.[29] Nonetheless, this study was a community-based one, which may be perceived as being more representative than those conducted in health care facilities (such as clinics or hospitals).[12] This may in fact be an important step when considering the initiation of a national CRC screening program in Saudi Arabia, including description of factors, which could be targeted to increase CRC screening uptake, once initiated.
Footnotes
Source of Support: The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project number RGP-VPP-279
Conflict of Interest: None declared.
REFERENCES
- 1.Garborg K, Holme O, Loberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24:1963–72. doi: 10.1093/annonc/mdt157. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ahwal MS, Shafik YH, Al-Ahwal HM. First national survival data for colorectal cancer among Saudis between 1994 and 2004: What's next? BMC Public Health. 2013;13:73. doi: 10.1186/1471-2458-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ren JS, Masuyer E, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J SH, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10 [Ferlay J SH, #19] Lyon, France: International Agency for Research on Cancer. 2010. [Last accessed on 2013 Oct 25]. Available from: http://globocan.iarc.fr .
- 6.Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull. 2013;105:29–42. doi: 10.1093/bmb/lds040. [DOI] [PubMed] [Google Scholar]
- 7.Ma GX, Wang MQ, Toubbeh J, Tan Y, Shive S, Wu D. Factors Associated with Colorectal Cancer Screening Among Cambodians, Vietnamese, Koreans and Chinese Living in the United States. N Am J Med Sci (Boston) 2012;5:1–8. doi: 10.7156/v5i1p001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroy PC, 3rd, Glick JT, Robinson PA, Lydotes MA, Evans SR, Emmons KM. Has the surge in media attention increased public awareness about colorectal cancer and screening? J Community Health. 2008;33:1–9. doi: 10.1007/s10900-007-9065-5. [DOI] [PubMed] [Google Scholar]
- 9.Gimeno-Garcia AZ, Quintero E, Nicolas-Perez D, Jimenez-Sosa A. Public awareness of colorectal cancer and screening in a Spanish population. Public Health. 2011;125:609–15. doi: 10.1016/j.puhe.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Senore C, Malila N, Minozzi S, Armaroli P. How to enhance physician and public acceptance and utilisation of colon cancer screening recommendations. Best Pract Res Clin Gastroenterol. 2010;24:509–20. doi: 10.1016/j.bpg.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 12.Koo JH, Leong RW, Ching J, Yeoh KG, Wu DC, Murdani A, et al. Knowledge of, attitudes toward, and barriers to participation of colorectal cancer screening tests in the Asia-Pacific region: A multicenter study. Gastrointest Endosc. 2012;76:126–35. doi: 10.1016/j.gie.2012.03.168. [DOI] [PubMed] [Google Scholar]
- 13.Sung JJ, Choi SY, Chan FK, Ching JY, Lau JT, Griffiths S. Obstacles to colorectal cancer screening in Chinese: A study based on the health belief model. Am J Gastroenterol. 2008;103:974–81. doi: 10.1111/j.1572-0241.2007.01649.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: A review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psychooncology. 2011;20:1023–33. doi: 10.1002/pon.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Dominic OG, McGarrity T, Dignan M, Lengerich EJ. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104:2626–7. doi: 10.1038/ajg.2009.419. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 18.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. International Colorectal Cancer Screening Network. Colorectal cancer screening: A comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–67. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 19.Ferlay J SH BF, Forman D, Mathers C, Parkin DM. GLOBOCAN, 2008 v2.0 CIaMWIC, No. 10 [Ferlay J SH, #19] Lyon FIAfR, on Cancer. 2010. [Last accessed on 2014 Oct 13]. Available from: http://globocan.iarc.frao .
- 20.Almadi MA, Alharbi O, Azzam N, Wadera J, Sadaf N, Aljebreen AM. Prevalence and characteristics of colonic polyps and adenomas in 2654 colonoscopies in Saudi Arabia. Saudi J Gastroenterol. 2014;20:154–61. doi: 10.4103/1319-3767.132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsanea N. The dilemma of the threshold age to start screening for colorectal cancer in Saudi Arabia. Saudi J Gastroenterol. 2014;20:141–2. doi: 10.4103/1319-3767.132976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aljebreen AM. Clinico-pathological patterns of colorectal cancer in Saudi Arabia: Younger with an advanced stage presentation. Saudi J Gastroenterol. 2007;13:84–7. doi: 10.4103/1319-3767.32183. [DOI] [PubMed] [Google Scholar]
- 23.Keighley MR, O'Morain C, Giacosa A, Ashorn M, Burroughs A, Crespi M, et al. Public awareness of risk factors and screening for colorectal cancer in Europe. Eur J Cancer Prev. 2004;13:257–62. doi: 10.1097/01.cej.0000136575.01493.9b. [DOI] [PubMed] [Google Scholar]
- 24.Qumseya BJ, Tayem YI, Dasa OY, Nahhal KW, Abu-Limon IM, Hmidat AM, et al. Barriers to colorectal cancer screening in Palestine: A national study in a medically underserved population. Clin Gastroenterol Hepatol. 2014;12:463–9. doi: 10.1016/j.cgh.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno Garcia AZ, Quintero E, Nicolas Perez D, Hernandez M, JimenezSosa A. Colorectal cancer screening in first-degree relatives of colorectal cancer: Participation, knowledge, and barriers against screening. Eur J Gastroenterol Hepatol. 2011;23:1165–71. doi: 10.1097/MEG.0b013e32834a289e. [DOI] [PubMed] [Google Scholar]
- 26.Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: A national cross sectional study based on the health belief model. BMC Public Health. 2013;13:677. doi: 10.1186/1471-2458-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James AS, Daley CM, Greiner KA. Knowledge and attitudes about colon cancer screening among African Americans. Am J Health Behav. 2011;35:393–401. doi: 10.5993/ajhb.35.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients' understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30:67–74. doi: 10.1016/j.cdp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–59. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 30.Papanikolaou IS, Sioulas AD, Kalimeris S, Papatheodosiou P, Karabinis I, Agelopoulou O, et al. Awareness and attitudes of Greek medical students on colorectal cancer screening. World J Gastrointest Endosc. 2012;4:513–7. doi: 10.4253/wjge.v4.i11.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gili M, Roca M, Ferrer V, Obrador A, Cabeza E. Psychosocial factors associated with the adherence to a colorectal cancer screening program. Cancer Detect Prev. 2006;30:354–60. doi: 10.1016/j.cdp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN. National Colorectal Cancer Roundtable Screening Among the 65 Plus Task Group. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]


