Abstract
Endocytic trafficking of signaling receptors is an important mechanism for limiting signal duration. Components of the Endosomal Sorting Complexes Required for Transport (ESCRT), which target ubiquitylated receptors to intra-lumenal vesicles (ILVs) of multivesicular bodies, are thought to terminate signaling by the epidermal growth factor receptor (EGFR) and direct it for lysosomal degradation. In a genetic screen for mutations that affect Drosophila eye development, we identified an allele of Vacuolar protein sorting 4 (Vps4), which encodes an AAA ATPase that interacts with the ESCRT-III complex to drive the final step of ILV formation. Photoreceptors are largely absent from Vps4 mutant clones in the eye disc, and even when cell death is genetically prevented, the mutant R8 photoreceptors that develop fail to recruit surrounding cells to differentiate as R1-R7 photoreceptors. This recruitment requires EGFR signaling, suggesting that loss of Vps4 disrupts the EGFR pathway. In imaginal disc cells mutant for Vps4, EGFR and other receptors accumulate in endosomes and EGFR target genes are not expressed; epistasis experiments place the function of Vps4 at the level of the receptor. Surprisingly, Vps4 is required for EGFR signaling even in the absence of Shibire, the Dynamin that internalizes EGFR from the plasma membrane. In ovarian follicle cells, in contrast, Vps4 does not affect EGFR signaling, although it is still essential for receptor degradation. Taken together, these findings indicate that Vps4 can promote EGFR activity through an endocytosis-independent mechanism.
Keywords: Vps4, Endocytosis, EGF receptor, Dynamin, Signaling
SUMMARY: The AAA ATPase Vps4 is required for and promotes EGFR activity during Drosophila eye development, acting via an endocytosis-independent mechanism.
INTRODUCTION
Endocytosis plays a dual role in signaling by many receptors; it is the route that leads to receptor degradation, but it can also alter the level of signaling activity by controlling receptor or ligand processing, recycling, localization or interaction with downstream components (Andersson, 2012; Callejo et al., 2011; Musse et al., 2012; Shilo and Schejter, 2011; Ueno et al., 2011). Activation of many receptors induces their ubiquitylation, internalization into early endosomes, sorting to multivesicular bodies (MVBs), where they are segregated from the cytoplasm, and ultimately lysosomal degradation (Piper and Lehner, 2011). The GTPase Dynamin catalyzes fission of Clathrin-coated endocytic vesicles from the plasma membrane (Schmid and Frolov, 2011). The subsequent sorting process is mediated by the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery (Hanson and Cashikar, 2012). ESCRT-0, which consists of the Hepatocyte growth factor regulated tyrosine kinase substrate (Hrs) and Signal transduction adaptor molecule (Stam) subunits, binds to and clusters ubiquitylated receptors. ESCRT-0 is recruited to endosomal membranes through interactions between Hrs and phosphatidylinositol 3-phosphate. It then recruits the four-subunit ESCRT-I complex, which brings in ESCRT-II, the substrate for assembly of ESCRT-III. The ESCRT-III subunit Vacuolar protein-sorting-associated protein 32 (Vps32) polymerizes into filaments that interact with Vps24 and Vps2 to deform the endosome membrane and promote budding of cargo-containing intra-lumenal vesicles (ILVs) (Henne et al., 2012; Wollert and Hurley, 2010). Vps2 also recruits the ATPase associated with a variety of cellular activities (AAA) protein Vps4, the only energy-utilizing ESCRT component (Hanson and Cashikar, 2012; Raiborg and Stenmark, 2009). Active Vps4 forms a hexameric complex that disassembles ESCRT-III, allowing recycling of its components, and also plays an active role in scission of the vesicle neck (Adell et al., 2014; Cashikar et al., 2014; Lata et al., 2008; Monroe et al., 2014; Mueller et al., 2012). In addition to their endocytic functions, ESCRT proteins, including Vps4, are required for cytokinesis, viral budding, protecting viral genomes from degradation, exosome secretion, receptor shedding on microvesicles, assembly of nuclear pore complexes, cholesterol transport and plasma membrane wound repair (Barajas et al., 2014; Choudhuri et al., 2014; Du et al., 2013; de Gassart et al., 2004; Jimenez et al., 2014; Morita, 2012; Nabhan et al., 2012; Tang, 2012; Webster et al., 2014).
The effect of endocytosis on signaling by the epidermal growth factor receptor (EGFR) is complex. Blocking EGFR internalization by removing Dynamin prevents its degradation, enhancing some downstream signaling events, but other aspects of EGFR signal transduction require an endosomal localization for the receptor (Jones and Rappoport, 2014; Legent et al., 2012; Miura et al., 2008; Teis et al., 2006; Vieira et al., 1996). Internalized EGFR can be either degraded or recycled to the plasma membrane, a choice that depends on the concentration and nature of the ligand, as ligands that remain bound in acidic late endosomes promote more extensive receptor ubiquitylation (Eden et al., 2012; French et al., 1995; Roepstorff et al., 2009; Sigismund et al., 2005). Recycling can alter the distribution of the receptor on the plasma membrane, controlling its exposure to ligands (Assaker et al., 2010; Jékely et al., 2005; Stetak et al., 2006; Vermeer et al., 2003). In Drosophila, EGFR targeted to the degradation pathway remains active on endosomes, as signaling is increased by ESCRT mutations that trap EGFR in the endocytic pathway (Vaccari et al., 2009). However, the transcriptional response in cultured mammalian cells depends primarily on EGFR activity at the plasma membrane (Brankatschk et al., 2012; Sousa et al., 2012). Observed effects on EGFR signaling could vary depending both on the stage at which endocytic sorting is blocked and the cellular context examined (Babst et al., 2000; Bache et al., 2006; Chanut-Delalande et al., 2010; Lloyd et al., 2002; Miura et al., 2008).
In the Drosophila retina, EGFR signaling is essential for the differentiation of all photoreceptors except for R8, the first to differentiate in each ommatidium (Freeman, 1996). Adult eye phenotypes thus provide a rapid screen for new regulators of the EGFR pathway (Legent et al., 2012; Miura et al., 2008; Roignant et al., 2006; Roignant and Treisman, 2010). Here, we report that a mutation in Vps4 disrupts EGFR signal transduction as well as the transduction of other signaling pathways. In Vps4 mutant cells in the eye disc, EGFR accumulates in endosomes but is unable to signal, resulting in the failure of non-R8 photoreceptors to differentiate. Interestingly, removing the Dynamin encoded by shibire does not restore EGFR signaling in the absence of Vps4, suggesting a new role for Vps4 upstream of EGFR internalization. Vps4 mutant ovarian follicle cells accumulate endocytosed EGFR, but show normal expression of EGFR target genes, indicating that defects in EGFR signaling in imaginal discs are not a consequence of its sequestration in the endocytic pathway. Taken together, these results suggest that Vps4 promotes EGFR activation independently of its effects on endocytosis.
RESULTS
Vps4 is required for R8 survival and R1-R7 differentiation
In a mosaic screen of the X chromosome (Legent et al., 2012), we recovered a mutation that prevents photoreceptor differentiation. In eye imaginal discs, clones of 3B1 mutant cells showed a cell-autonomous lack of expression of the pan-neuronal marker Elav (Fig. 1A), normally expressed by differentiating photoreceptors (Robinow and White, 1991). Genetic mapping and sequencing revealed that 3B1 was a missense mutation in the Vps4 gene. 3B1 transforms glutamate 209, which is adjacent to the first central pore motif of the AAA domain (Scott et al., 2005), into a lysine residue (Fig. 1B), a charge reversal that would probably disrupt protein folding. 3B1 failed to complement the lethality of Vps4Δ7b, a small deficiency that covers Vps4 and its flanking sequences (Rodahl et al., 2009a). Expression of an HA-tagged wild-type Vps4 cDNA in 3B1 clones fully rescued photoreceptor differentiation (Fig. 1C-E). Additionally, 98% (n=106) of hemizygous 3B1 males were rescued to viability by tubulin-GAL4-driven ubiquitous expression of UAS-HA::Vps4. These results confirm that the phenotypes observed in 3B1 mutants are due to loss of Vps4 function.
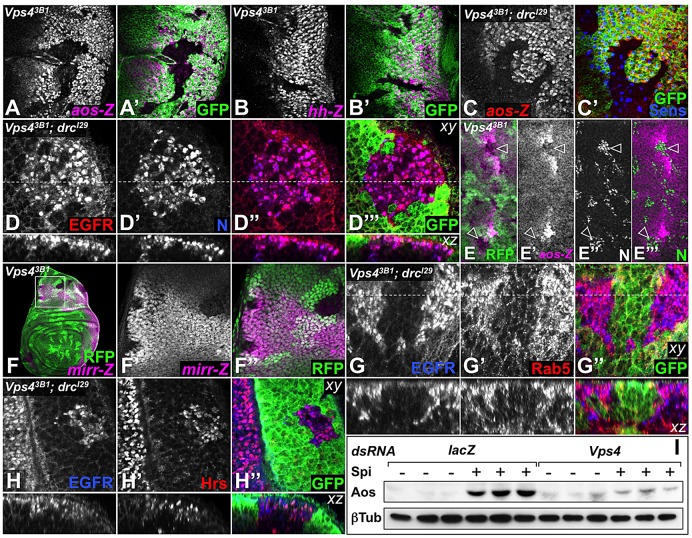
Fig. 1.
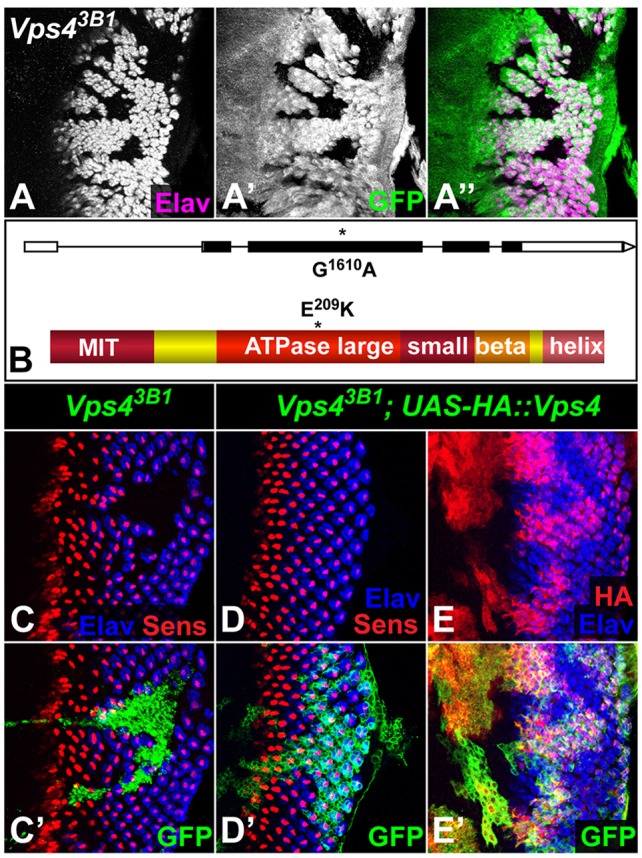
Vps4 is required for photoreceptor differentiation. (A) Vps43B1 mutant clones in third-instar eye discs, marked by the absence of GFP (A′, green in A″), lack photoreceptors stained with anti-Elav (A, magenta in A″). Anterior is to the left in this and all subsequent figures. (B) Diagram of the Vps4 gene and encoded protein indicating that the 3B1 mutation changes glutamic acid 209 in the large AAA ATPase domain into a lysine residue. MIT, microtubule interacting and trafficking domain. The ATPase domain is subdivided into large, small and beta domains. (C-E) Eye discs expressing GFP (green) alone (C) or together with HA-tagged wild-type Vps4 (D,E), within Vps43B1 mutant clones. HA::Vps4 (stained for HA, red in E) rescues the differentiation of photoreceptors labeled with Elav (blue) including R8, which is marked by Sens (red in C,D).
The absence of photoreceptors in Vps4 mutant clones could be due to either failure of differentiation or cell death. We observed that Vps43B1 mutant clones in third-instar eye discs underwent massive apoptosis, as indicated by their pyknotic nuclei and high levels of activated effector caspases (Fig. 2A). Both features were largely rescued in the absence of the initiator caspase Dronc (Steller, 2008) (Fig. 2B). Signaling pathways converging on c-Jun N-terminal Kinase (JNK) have been implicated in the regulation of programmed cell death in various contexts (Dhanasekaran and Reddy, 2008). As previously reported for Vps4 RNAi expression (Rodahl et al., 2009a), we found that Vps43B1 clones misexpressed the JNK transcriptional target puckered (puc)-lacZ (Martin-Blanco et al., 1998) (supplementary material Fig. S1A). To assess whether ectopic JNK signaling is instructive in photoreceptor cell death, we created clones mutant for both Vps4 and hemipterous (hep), which encodes the upstream activating kinase for JNK (Glise et al., 1995). However, removing hep did not restore photoreceptor differentiation or prevent caspase activation (supplementary material Fig. S1B-F). Similarly, inhibiting JNK signaling by overexpressing the JNK phosphatase Puc failed to rescue the Vps4 phenotypes (supplementary material Fig. S1G,H). JNK activity is thus not the primary driver of apoptosis in Vps4 mutant cells in the eye disc.
Fig. 2.
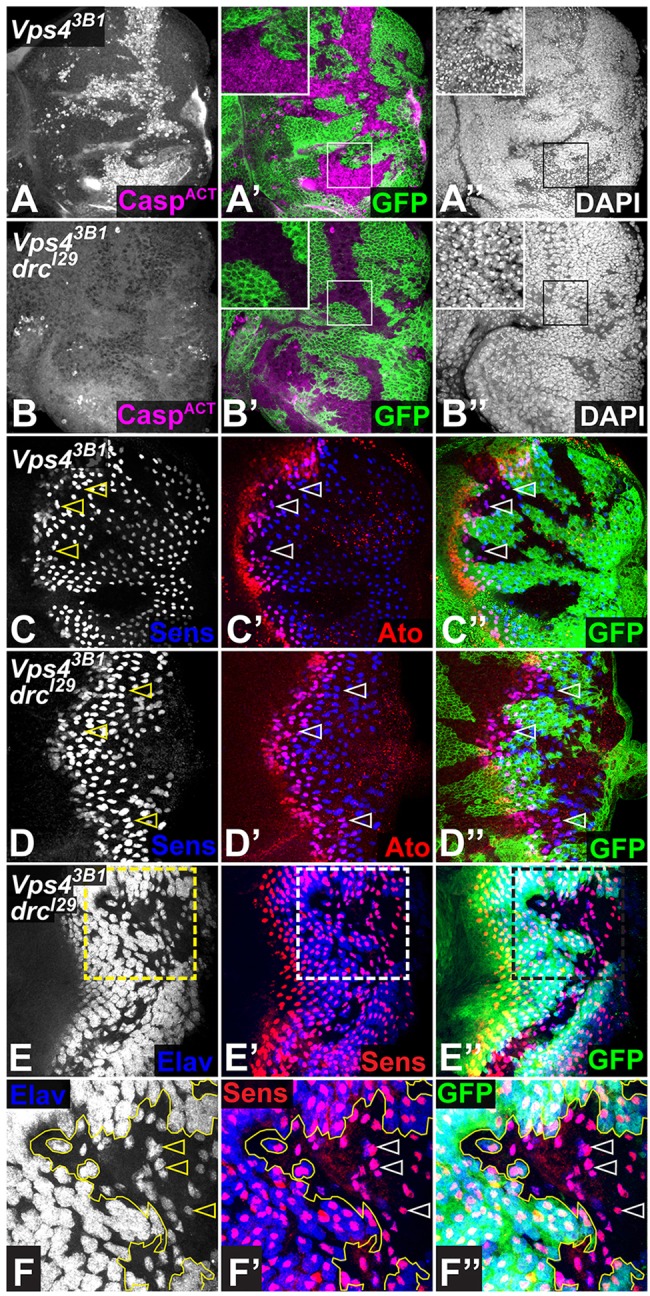
Vps4 is independently required for cell survival and R1-R7 differentiation. (A-F) Eye discs carrying Vps43B1 mutant clones marked by the absence of GFP (green), in a wild-type (A,C) or DroncI29 (drcI29) (B,D-F) background. Vps43B1 clones display elevated levels of activated Caspase 3 (A, magenta in A′) and pyknotic nuclei stained with DAPI (A″), which are greatly reduced in DroncI29 homozygotes (B). Very few Sens-positive R8 cells (C, blue in C′,C″) differentiate in Vps43B1 clones (arrowheads). Expression of Ato (red) in anterior proneural precursors is decreased but not abolished. (D) A substantial rescue of differentiated R8 photoreceptors stained with Sens (blue) and Ato (red) is observed in DroncI29 homozygotes (arrowheads). (E,F) Vps43B1 clones in DroncI29 discs stained for Elav (E, blue in E′,E″) and Sens (red). (F) An enlargement of the region boxed in E. Within the Vps43B1 clone (outlined), R8 cells are not surrounded by Elav-positive photoreceptors (arrowheads).
The rescue of cell death observed in the absence of Dronc allowed us to examine early markers of photoreceptor differentiation in Vps4 mutant cells. In each ommatidium, R8, marked by expression of Senseless (Sens) (Frankfort et al., 2001), is the first photoreceptor to differentiate. R8 cells are singled out of an anterior stripe of proneural precursors expressing Atonal (Ato) (Jarman et al., 1993). Only a few Sens-positive R8 cells were observed in anterior regions of Vps43B1 mutant clones (Fig. 2C). However, preventing cell death by removing Dronc rescued many R8 photoreceptors (Fig. 2D), indicating that Vps4 is required for R8 survival. R8 induces EGFR activation in surrounding cells to promote their differentiation into R1-R7 photoreceptors (Freeman, 1997; Tio et al., 1994). In Vps4 clones, most rescued R8 cells failed to recruit any Elav-positive neighboring cells (Fig. 2E,F). The absence of R1-R7 photoreceptors is thus not a secondary consequence of cell death, but results from a failure to transduce signals from R8.
Vps4 mutant cells accumulate inactive signaling receptors
Mutations in Tumor susceptibility gene 101 (TSG101) of the ESCRT-I complex and Vps25 of the ESCRT-II complex cause accumulation of the receptor Notch (N), which drives excessive signaling, leading to nonautonomous overproliferation (Moberg et al., 2005; Vaccari and Bilder, 2005). Similarly, we found that Vps4 mutant cells accumulated very high levels of both N and its ligand Delta (Dl) in punctate structures (Fig. 3A,B). However, unlike the upstream ESCRT mutants TSG101 and Vps25, N signaling was reduced in Vps4 mutant cells, as visualized by lower levels of the transcriptional reporter E(spl)mβ-CD2 (de Celis et al., 1998; Moberg et al., 2005) in the eye disc (Fig. 3C). We also investigated the requirement for Vps4 in N signaling during wing development. As Vps4 clones in the wing disc survived poorly (supplementary material Fig. S2), we induced them in a Dronc background, and again observed accumulation of N in large puncta (Fig. 3D). In the wing disc, N is activated in a stripe of cells along the dorsal-ventral (D-V) boundary, where it induces the transcription of cut (ct) (de Celis et al., 1996). Ct expression was lost in Vps4 clones (Fig. 3E), confirming that, in contrast to more-upstream ESCRT mutants, Vps4 promotes N signal transduction.
Fig. 3.
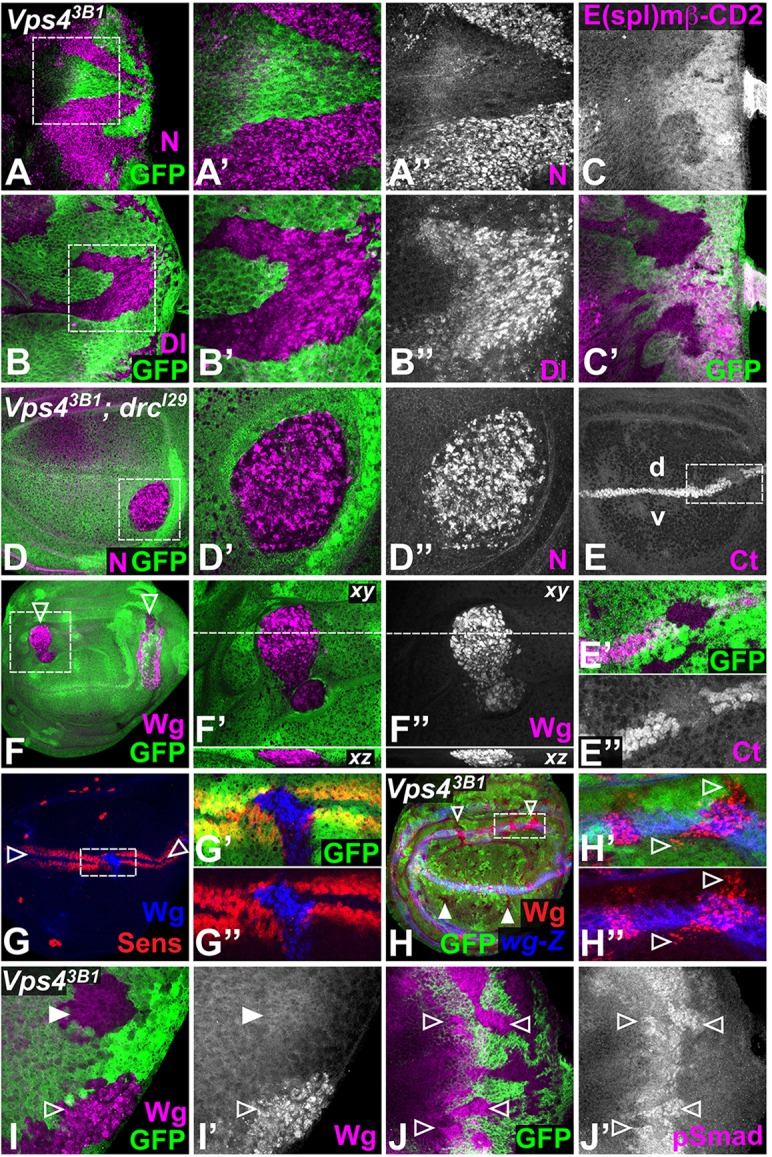
Loss of Vps4 affects multiple signaling pathways. Vps43B1 clones marked by the absence of GFP (green) in eye discs (A-C,I-J) or in DroncI29 (drcI29) homozygous wing discs (D-H). (A,B) Mutant cells accumulate high levels of N (A″, magenta in A,A′) and Dl (B″, magenta in B,B′) in large puncta (boxed regions are enlarged in A′,A″,B′,B″). (C) Expression of the Notch transcriptional reporter E(spl)-mβ-CD2 (C, magenta in C′) is decreased in Vps4 clones. (D) Mutant cells accumulate large N-positive puncta (D″, magenta in D,D′, the boxed region is enlarged in D′,D″). (E) The Notch transcriptional target Ct (E,E″, magenta in E′), normally expressed at the boundary between the dorsal (d) and ventral (v) compartments of the wing pouch, is lost in Vps4 clones (the boxed region is enlarged in E′,E″). (F) Wg (F″, magenta in F,F′) also accumulates in large puncta that span the depth of the cell in Vps4 clones (arrowheads; the boxed region is enlarged in F′,F″; xz sections are shown below). (G) The Wg target Sens (red) is expressed in two stripes (arrowheads) along the D-V boundary of the wing pouch, but is lost from Vps4 clones, despite accumulation of Wg (blue). The boxed region is enlarged in G′,G″. (H) Ectopic Wg (red) is observed in Vps4 clones in the vicinity of Wg-producing cells (open arrowheads), marked by wg-lacZ (blue), but not in more distant clones (filled arrowheads). The boxed region is enlarged in H′,H″. (I) In eye discs, Wg puncta (I′, magenta in I) are observed in clones along the lateral margins (open arrowhead) but not in more medial clones (filled arrowhead). (J) Vps4 clones display elevated levels of pSmad (J′, magenta in J) in the region of the morphogenetic furrow (arrowheads).
We next investigated whether other signaling pathways were similarly affected. In the wing disc, Wingless (Wg) secreted from both the D-V boundary and the hinge surrounding the wing pouch acts at a long range to organize wing growth and patterning (Neumann and Cohen, 1997). Like N and Dl, Wg accumulated in large punctate structures in Vps4 mutant cells (Fig. 3F). In both the wing and eye discs, Wg only accumulated in mutant cells within or close to its endogenous domain of expression, which is marked by the reporter wg-lacZ (Fig. 3H-I). wg-lacZ was unaffected by the loss of Vps4 (Fig. 3H), indicating that Wg protein accumulation results from internalization into receiving cells rather than increased transcription. Vps4 clones showed reduced expression of the Wg target genes sens in the wing disc (Fig. 3G) (Parker et al., 2002) and dachsous-lacZ in the eye disc (Yang et al., 2002) (data not shown), demonstrating that normal Wg signaling requires Vps4.
In contrast to the reduction in N and Wg signaling, we observed increased levels of the second messenger phosphorylated Smad (pSmad) (Tanimoto et al., 2000) in Vps4 mutant clones near the domain of expression of the Bone Morphogenetic Protein family member Decapentaplegic (Dpp) in the eye disc (Fig. 3J), suggesting that Vps4 mutant cells are able to transduce some signals and that, in contrast to its effects on other pathways, Vps4 negatively regulates signaling by Dpp. Taken together, our results indicate that unlike more-upstream ESCRT members, Vps4 is required for the transduction of several signaling pathways.
Vps4 is required for EGFR signaling
The effect of receptor internalization and endocytic processing on EGFR signaling remains controversial. Although some studies have demonstrated that it contributes to signal termination through lysosomal EGFR degradation (Bache et al., 2006; Razi and Futter, 2006), other results argue that it enables receptor signaling from intracellular organelles (Miaczynska et al., 2004; Shilo and Schejter, 2011). Vps4 clones in Dronc mutant eye discs resemble clones lacking positive intracellular effectors of EGFR transduction, in which R8 cells fail to recruit additional photoreceptors (Legent et al., 2012). We found that the expression of lacZ reporters for two direct transcriptional targets of the EGFR pathway in R1-R7, argos (aos) (Golembo et al., 1996) and hedgehog (hh) (Rogers et al., 2005), was downregulated in Vps4 mutant clones (Fig. 4A,B). Importantly, restoring R8 survival by removing Dronc did not rescue aos expression in surrounding cells (Fig. 4C). In the wing disc, EGFR signaling controls both the expression of aos in vein primordia (Sturtevant et al., 1993), and the expression of mirror (mirr) in the notum primordium (Zecca and Struhl, 2002). Consistent with a requirement for Vps4 in EGFR signaling, Vps4 mutant cells displayed a reduced expression of aos-lacZ and mirr-lacZ (Fig. 4E,F).
Fig. 4.
Vps4 is required for EGFR signaling. Eye discs (A-D,G-H) or wing discs (E,F) in which Vps43B1 mutant clones are marked by the absence of GFP (green, A-D,G-H) or RFP (green, E,F), in a wild-type (A-B,E-F) or DroncI29 (drcI29) homozygous (C-D,G-H) background. Vps43B1 clones lose expression of the EGFR transcriptional targets aos-lacZ (A, magenta in A′) and hh-lacZ (B, magenta in B′). (C) R8 cells marked by Sens (blue) in Vps4 clones in Dronc homozygotes fail to induce aos-lacZ (C, red in C′) in surrounding cells. (D) EGFR (D, red in D″,D‴) and N (D′, blue in D″,D‴) accumulate and colocalize in punctate structures in Vps4 mutant cells (xz sections are shown below). (E) aos-lacZ (E′, magenta in E,E‴) is normally expressed along presumptive veins but is lost in Vps43B1 clones (arrowheads), which accumulate high levels of N (E″, green in E‴). (F) In the notum, expression of the EGFR transcriptional target mirr-lacZ (F′, magenta in F,F″) is decreased in Vps4 mutant cells. The boxed region is enlarged in F′,F″. (G,H) In Vps4 mutant cells, punctate accumulations of EGFR (G,H, blue in G″,H″) colocalize extensively with Hrs (H′, red in H″) and partially with Rab5 (G′, red in G″). Both xy and xz sections are shown. (I) Western blots of Aos in the culture media and β-tubulin in the cell lysates of triplicate samples of D2F cells treated with lacZ or Vps4 dsRNA as indicated, and exposed (+) or not exposed (−) to purified sSpi for 16 h. Vps4 knockdown strongly reduces Aos production.
Despite the lack of EGFR target gene expression, EGFR protein levels were increased in Vps4 mutant cells (Fig. 4D,G,H). Intracellular EGFR colocalized with N in punctate structures, many of which also expressed the early endosomal markers Hrs, Rab5 and Syntaxin 7 (Syx7) (Lloyd et al., 2002; Lu and Bilder, 2005; Russell et al., 2012) (Fig. 4D,G,H; supplementary material Fig. S3A). Little colocalization was observed with markers for recycling endosomes (Rab11), the Golgi (Lava lamp; Lva) or secretory vesicles (Sec15) (Langevin et al., 2005; Sisson et al., 2000) (supplementary material Fig. S3B-D), arguing that the EGFR accumulation is in enlarged endosomes, known as class E compartments in yeast (Russell et al., 2012). Consistent with this view, loss of the ESCRT-I subunit TSG101 caused a similar accumulation of EGFR in punctate structures expressing Hrs (supplementary material Fig. S3F).
S2 cells stably expressing the EGFR (D2F cells) have been used to study EGFR signaling mechanisms (Schweitzer et al., 1995). Treatment of these cells with a purified secreted form of the EGFR ligand Spitz (Spi) (Miura et al., 2006) resulted in increased production and secretion of Aos protein (Fig. 4I). However, RNAi-mediated knockdown of Vps4 (supplementary material Fig. S4A) strongly reduced this response (Fig. 4I). Vps4 RNAi also reduced the phosphorylation of the downstream effector MAPK (also known as ERK and Rolled) in cells treated with Spi (supplementary material Fig. S4B). These results indicate that the effect of Vps4 on EGFR signaling is not dependent on the fate or epithelial organization of imaginal disc cells.
Vps4 acts at the level of EGFR activation
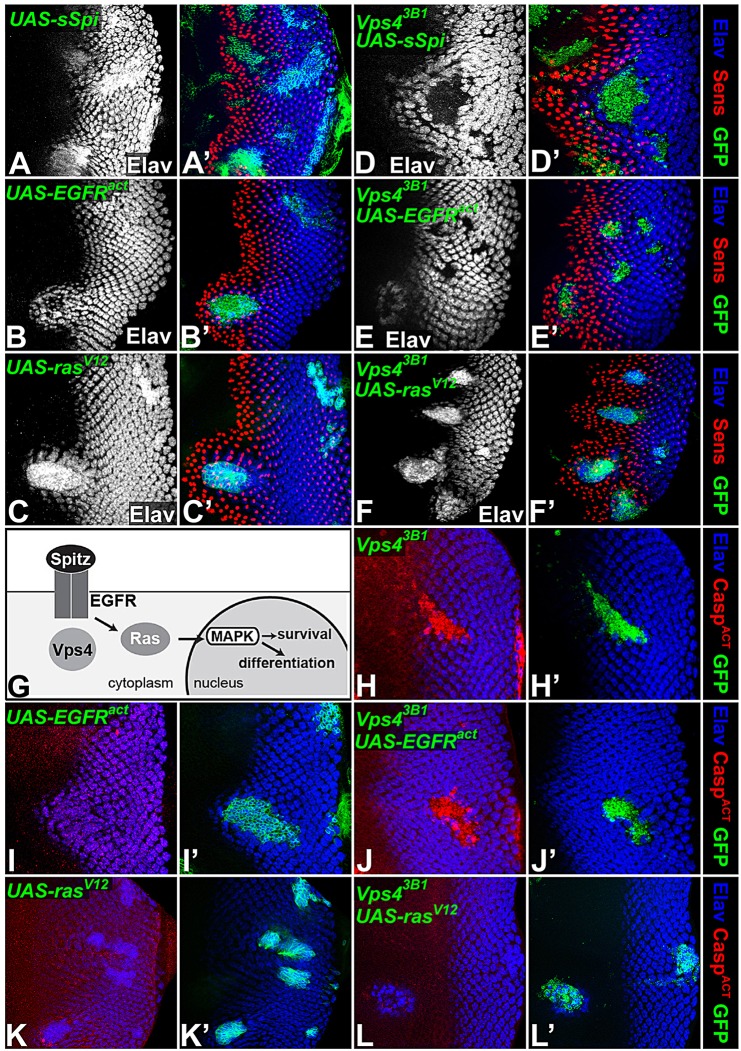
In order to determine at which step Vps4 influences EGFR signal transduction, we performed epistasis experiments. In the eye disc, Spi secreted by R8 is the primary ligand for EGFR in R1-R7 (Freeman, 1994; Tio et al., 1994). Its binding triggers receptor dimerization and activation by autophosphorylation, and subsequent recruitment of enzymes and adaptor proteins promotes conversion of Ras (also known as Ras85D) into its GTP-bound form. This small GTPase initiates a kinase cascade by activating Raf (also known as Pole hole), which phosphorylates MEK (also known as Dsor1), which in turn phosphorylates MAPK. Phosphorylated MAPK enters the nucleus and phosphorylates specific transcription factors to regulate target gene expression (Shilo, 2003). During eye development, expression of a secreted form of Spi (sSpi) (Schweitzer et al., 1995) or constitutively active forms of EGFR (Queenan et al., 1997) or Ras (Karim and Rubin, 1998) ectopically activates EGFR signaling and promotes the differentiation of extra photoreceptors (Fig. 5A-C). Expression of sSpi did not rescue photoreceptor differentiation in Vps4 mutant cells, although it triggered ectopic photoreceptor differentiation in wild-type cells surrounding the clone, indicating that Vps4 is not necessary for Spi secretion (Fig. 5D). The same result was obtained by overexpressing Rhomboid, a protease that activates endogenous Spi (Lee et al., 2001) (data not shown). Interestingly, a constitutively dimeric form of EGFR showed partial activity in Vps4 mutant cells; although photoreceptors still failed to differentiate within Vps4 mutant clones expressing activated EGFR, surrounding cells showed premature differentiation (Fig. 5E), probably due to hh expression induced within the clone (supplementary material Fig. S5). Expression of activated Ras was sufficient to bypass the requirement for Vps4 in EGFR transduction and promote the differentiation of extra photoreceptors both within and surrounding the clone (Fig. 5F). Similar epistasis experiments in the wing disc, using aos-lacZ to monitor pathway activation, showed that activated EGFR promoted strong ectopic aos expression in wild-type but not Vps4 mutant cells, whereas activated Ras could upregulate aos-lacZ even in the absence of Vps4 (supplementary material Fig. S6). The activity of Vps4 is thus required in the receiving cell, upstream of Ras activation but downstream or at the level of EGFR activation (Fig. 5G).
Fig. 5.
Vps4 acts at the level of the EGFR. (A-F,H-L) Eye discs with clones coexpressing GFP (green) with activated pathway components, stained for Elav (A-F and blue in A′-F′,H-L). Sens is shown in red in A′-F′. Expression of the constitutively active proteins encoded by sSpi (A), EGFRλtop (B) or RasV12 (C) in wild-type clones induces excessive photoreceptor differentiation. In Vps43B1 clones, expression of sSpi (D) induces extra photoreceptor differentiation in wild-type cells surrounding the clone, but has no effect on the mutant cells. EGFRλtop expression in Vps4 clones (E) does not rescue photoreceptor differentiation within the clones, but induces ectopic photoreceptors in surrounding wild-type tissue. In contrast, RasV12 can induce photoreceptor differentiation within Vps4 mutant clones (F). (G) A simplified diagram of the EGFR pathway showing that Vps4 acts upstream of Ras activation to promote photoreceptor differentiation and cell survival. (H-L) Activated Caspase 3 is stained in red. Control EGFRλtop (I) or RasV12 (K) expressing clones do not induce cell death. The caspase activation observed in Vps43B1 clones (H) is rescued by UAS-RasV12 (L) but not UAS-EGFRλtop (J).
Given that the EGFR pathway is required for cell survival in the eye disc (Halfar et al., 2001; Yang and Baker, 2003), we wondered whether restoring signaling downstream of the receptor could rescue the cell death observed in Vps4 mutant clones. Indeed, although activated EGFR did not prevent caspase activation in Vps4 cells (Fig. 5H-J), activated Ras abolished apoptosis (Fig. 5K,L). These results demonstrate that, rather than ectopic JNK signaling (supplementary material Fig. S1) (Rodahl et al., 2009a), impaired EGFR signal transduction is the primary reason for the death of Vps4 mutant cells.
Vps4 functions upstream of EGFR endocytosis
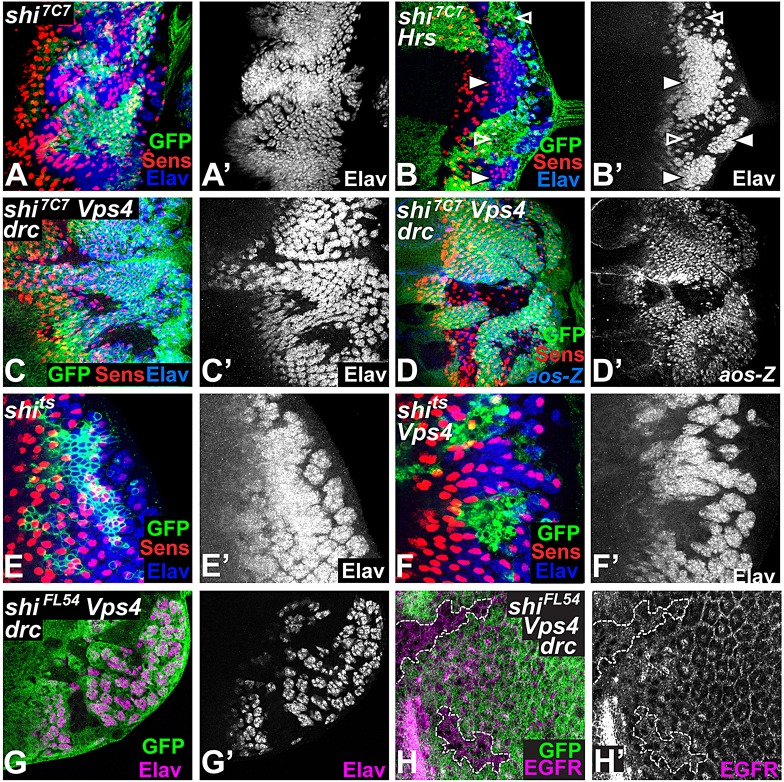
Because the effect of Vps4 on EGFR signaling was opposite to the negative effect of other ESCRT components (Vaccari et al., 2009) (see supplementary material Fig. S3E), we wanted to test whether Vps4 acted during endocytosis to affect EGFR signaling. We therefore generated double mutant clones for Vps4 and shibire (shi), which encodes Dynamin, a GTPase required to internalize EGFR and other receptors from the plasma membrane (Henriksen et al., 2013; Sousa et al., 2012; Windler and Bilder, 2010). shi7C7 mutant cells have enhanced EGFR signaling, leading to increased photoreceptor differentiation (Legent et al., 2012) (Fig. 6A). The ESCRT-0 component Hrs has a positive role in EGFR signaling and photoreceptor differentiation in the eye disc (Miura et al., 2008). As expected, given that Dynamin acts prior to Hrs in endocytosis, shi mutant cells still showed increased photoreceptor differentiation in Hrs mutant eye discs, although loss of Hrs reduced photoreceptor differentiation in regions wild-type for shi (Miura et al., 2008) (Fig. 6B). Surprisingly, however, shi7C7 Vps43B1 double mutants showed the Vps4 phenotype of missing photoreceptors and loss of aos-lacZ expression, even in a Dronc background in which cell death was prevented (Fig. 6C,D). Vps4 was similarly epistatic to shi in clones expressing a dominant-negative thermo-sensitive allele of shi (Kitamoto, 2001) (Fig. 6E,F). As the molecular nature of the shi7C7 allele is unknown, we repeated the epistasis analysis with the previously described null allele shiFL54 (Windler and Bilder, 2010). shiFL54 Vps43B1 double mutant cells also failed to differentiate as photoreceptors, although removing shi prevented the endosomal accumulation of EGFR in these cells (Fig. 6G,H). These results are not consistent with an effect of Vps4 only on internalized EGFR, and suggest that Vps4 can influence EGFR function prior to its endocytosis.
Fig. 6.
Vps4 blocks EGFR signaling upstream of Dynamin-mediated internalization. All panels show eye discs with clones marked by the absence of GFP (green) in A-D,G-H, or the presence of GFP (E,F). R8 is stained with Sens (red in A-F). Photoreceptors are stained with Elav (A′-C′,E′,F′, blue in A-C,E,F) or aos-lacZ is stained with anti-β-galactosidase (D′, blue in D). (A) shi7C7 clones differentiate excessive photoreceptors. (B) In an HrsD28/Df(Hrs) mutant disc in which photoreceptor differentiation is reduced (open arrowheads), shi7C7 clones still overproduce photoreceptors (filled arrowheads). (C,D) shi7C7 Vps43B1 double mutant clones in a DroncI29 (drcI29) mutant background show loss of photoreceptors other than R8 and loss of aos-lacZ expression. (E,F) Clones expressing shits in otherwise wild-type cells (E) or in Vps43B1 mutant cells (F). Vps4 is still required for photoreceptor differentiation in the presence of this dominant-negative form of Shi. (G,H) shiFL54 Vps43B1 clones (outlined in H) in a DroncI29 mutant background are stained with Elav (G′, magenta in G) or EGFR (H′, magenta in H). Clones mutant for both Vps4 and a shi-null allele lack photoreceptors but do not show punctate accumulation of EGFR.
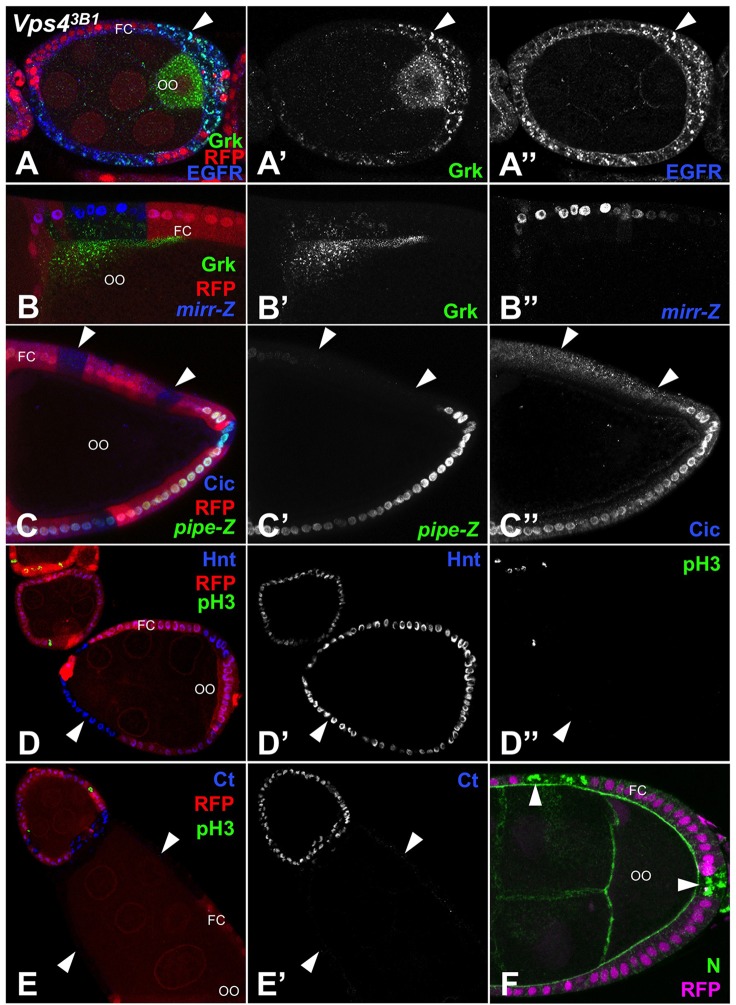
One possibility is that Vps4 might promote EGFR trafficking to the plasma membrane. To investigate this, we made use of the ovarian model system, in which the ligand-producing and responding cells can be distinguished. During egg chamber development, the EGFR ligand Gurken (Grk) is secreted from the oocyte and internalized into first posterior and later dorsal anterior follicle cells to specify their fates (Chang et al., 2008; Nilson and Schüpbach, 1998). This internalization requires both shi and Egfr (Chang et al., 2008), indicating that it is mediated by binding to surface EGFR and subsequent endocytosis. We found that clones of follicle cells mutant for Vps4 were still able to internalize Grk, and in fact accumulated abnormally high levels of Grk that colocalized with internalized EGFR in punctate structures (Fig. 7A). EGFR must therefore be present on the surface of Vps4 mutant follicle cells, allowing Grk reception. Surprisingly, Vps4 was not required for EGFR signaling in these cells. In this system, EGFR signaling acts by preventing nuclear localization of the transcriptional repressor Capicua (Cic) (Astigarraga et al., 2007). Cic represses the expression of mirr (Atkey et al., 2006), which encodes a transcription factor that represses the ventral determinant pipe (Andreu et al., 2012). In dorsal follicle cells mutant for Vps4, a mirr-lacZ reporter was expressed normally, and no ectopic pipe-lacZ or nuclear localization of Cic was observed (Fig. 7B,C). The large clone size also indicated normal survival of these mutant cells. Notch signaling was likewise unaffected in Vps4 mutant follicle cells. We saw no loss of its target gene hindsight (hnt; FlyBase – peb) or misexpression of ct, a gene repressed by Hnt (Sun and Deng, 2007), and mitotic cells marked by phosphorylated histone H3 were not observed in egg chambers older than stage 6 (Fig. 7D,E), despite strong intracellular accumulation of Notch protein (Fig. 7F). These results show that trapping of the EGFR with its ligand in the endocytic pathway is not sufficient to block EGFR signaling. Vps4 thus appears to promote EGFR signaling by a new non-endocytic and cell type-dependent mechanism, which could also be applicable to Notch and other receptors.
Fig. 7.
Vps4 is not required for EGFR or Notch signaling in follicle cells. All panels show egg chambers with Vps43B1 mutant clones in the follicle cells (FC) marked by the absence of RFP (red in A-E, magenta in F). OO, oocyte. Posterior is to the right. Arrowheads indicate representative clones. (A) Staining for Grk (A′, green in A) and EGFR (A″, blue in A) reveals that internalized Grk and EGFR accumulate and colocalize in mutant follicle cells. (B-C) Dorsal is up. (B) A mutant clone in follicle cells dorsal to the oocyte shows normal mirr-lacZ expression (B″, blue in B) and Grk internalization (B′, green in B). (C) Dorsal mutant follicle cell clones do not misexpress pipe-lacZ (C′, green in C) and Cic remains cytoplasmic, rather than localizing to the nucleus as seen in the ventral region (C″, blue in C). (D,E) Hnt (D′, blue in D) is expressed normally and there is no ectopic phospho-histone H3 (pH3) staining (D″, green in D,E) in Vps4 clones. Ct (E′, blue in E) is not misexpressed in Vps4 clones. Loss of Notch signaling would cause loss of Hnt and ectopic Ct and pH3 in egg chambers older than stage 6. (F) Notch (green) accumulates in puncta in Vps4 clones.
DISCUSSION
Vps4 affects multiple signaling pathways
We describe here a point mutation that specifically disrupts the function of Drosophila Vps4. A previous study used a deletion allele that unlike our mutation, could not be rescued by a wild-type Vps4 construct, and thus might also disrupt one or both of the neighboring genes (Rodahl et al., 2009a). We confirmed previous findings that loss of Vps4 results in JNK activation and apoptosis (Rodahl et al., 2009a). However, we found that blocking JNK activity did not prevent cell death or restore photoreceptor differentiation, indicating that JNK is not the primary driver of these effects of Vps4 mutations. In contrast, activating EGFR signaling downstream of the receptor was sufficient to restore survival of R8 cells and differentiation of R1-R7, highlighting this pathway as central to the function of Vps4 in eye development.
As expected, given the known role for Vps4 and other ESCRT proteins in targeting receptors for lysosomal degradation (Hanson and Cashikar, 2012), EGFR and other receptors accumulate in enlarged endosomes in Vps4 mutant cells. However, this accumulation has distinct effects on their activity; loss of Vps4 reduces EGFR, Notch and Wg signaling, but increases Dpp signaling. The effect on Wg target genes is consistent with previous findings that endocytosis and MVBs promote Wnt signaling by sequestering Glycogen synthase kinase 3β, which would otherwise inhibit β-catenin (Dobrowolski et al., 2012; Seto and Bellen, 2006; Taelman et al., 2010). Dpp signaling is also thought to require endocytosis, because Smads are recruited to activated TGFβ family receptors by the endosomal protein Smad anchor for receptor activation (Sara) (Bennett and Alphey, 2002; Panopoulou et al., 2002; Tsukazaki et al., 1998). As Sara is present on early endosomes, signaling can be prolonged when progression of the receptor to late endosomes is blocked by loss of the ESCRT-II subunit Vps25 (Thompson et al., 2005) and perhaps also Vps4.
Vps4 has effects distinct from other ESCRT complex subunits
Vps4 mutants differ from previously described ESCRT mutations in their effects on Notch and EGFR signaling. Internalization of these receptors into the ILVs of MVBs segregates their intracellular domains from cytoplasmic effectors and should thus terminate signaling (Piper and Lehner, 2011). This model is consistent with the excessive Notch and EGFR signaling observed in eye and wing discs in the absence of many ESCRT proteins (Aoyama et al., 2013; Moberg et al., 2005; Rodahl et al., 2009b; Thompson et al., 2005; Vaccari and Bilder, 2005; Vaccari et al., 2009). This increased signaling has been attributed to the lack of lysosomal degradation of these receptors and their continued activity on the endosomal membrane.
Vps4 mutant cells instead show reduced expression of EGFR and Notch target genes, despite endosomal accumulation of the receptors. Vps43B1 cells also fail to induce the non-autonomous overgrowth that results from misexpression of the Notch target gene unpaired (FlyBase – outstretched) in cells mutant for other ESCRT subunits (Thompson et al., 2005; Vaccari and Bilder, 2005). Notch cleavage and signaling is thought to occur in partially acidified endosomes and thus to require progression through the endocytic pathway (Schneider et al., 2013; Vaccari et al., 2010, 2008; Yan et al., 2009). Although EGFR signaling can occur at the plasma membrane (Brankatschk et al., 2012; Legent et al., 2012; Sousa et al., 2012), some studies suggest that receptor progression from early to late endosomes has a positive effect on signaling (Kim et al., 2007; Miura et al., 2008; Teis et al., 2006). Loss of Vps4 might block endocytosis so late in the process of ILV formation that the intracellular domains of receptors are trapped in nascent ILV buds and no longer have access to the cytoplasm. Alternatively, an early block in receptor progression through endocytosis due to failure to recycle other ESCRT subunits (Babst et al., 1998) could reduce signaling.
Vps4 might contribute to EGFR activation
Another possible explanation for the effect of Vps4 on EGFR signaling is suggested by our finding that loss of shi function does not restore EGFR signaling to Vps4 mutant cells, even though it blocks receptor accumulation in the endocytic pathway. Dynamin is required to internalize mammalian EGFR by both clathrin-dependent and -independent mechanisms following its activation with all the ligands tested (Henriksen et al., 2013). In the absence of Dynamin, prolonged signaling of EGFR on the plasma membrane should be unaffected by defects in the late stages of endocytosis. The disruption of EGFR signaling by Vps4 in shi mutant cells thus suggests that Vps4 has a role upstream of cell surface EGFR activation that is distinct from its function in endocytic MVBs. This model is supported by our observation that a constitutively active form of EGFR can partially rescue Vps4 mutant cells (Fig. 5E). In addition, although Vps4 affects the progression of EGFR and its ligand Grk, as well as Notch, through the endocytic pathway in follicle cells, signaling by both receptors remains intact in these cells, indicating that the two functions of Vps4 are separable.
The mechanism of this new effect of Vps4 is still unknown. As Vps4 mutant clones accumulate high levels of intracellular EGFR, Vps4 is not required for EGFR transcription or translation. Although some studies have implicated ESCRT-III and Vps4 in recycling Ras and associated EGFR to the plasma membrane (Baldys and Raymond, 2009; Tu et al., 2011; Zheng et al., 2012), Drosophila Ras need not be associated with the membrane to function in photoreceptor recruitment (Sung et al., 2010), and a requirement for Vps4 in EGFR recycling would not explain its effect in the absence of Dynamin-mediated internalization. Vps4 has been reported to transport some newly synthesized proteins to the plasma membrane via endosomes (Futter et al., 1995; Yoshimori et al., 2000). However, the ability of Vps4 mutant follicle cells to internalize Grk indicates that EGFR is present on the plasma membrane of these cells. It is possible that EGFR is trafficked by distinct routes in imaginal discs and in follicle cells. Grk is internalized through the apical domain of follicle cells (Tanentzapf et al., 2000), whereas active forms of Spi are localized basolaterally in discs (Steinhauer et al., 2013). A role for Vps4 in targeting the EGFR to the appropriate membrane domain would, however, not explain the requirement for Vps4 in cultured S2 cells, which lack apical-basal polarity. An alternative possibility is that Vps4 affects EGFR activation independently of its trafficking. Vps4 is involved in transporting cholesterol to the endoplasmic reticulum (Du et al., 2013), and could indirectly affect EGFR activation by altering the composition of membrane rafts (Balbis and Posner, 2010). Loss of Vps4 in yeast was recently shown to result in the accumulation of misassembled nuclear pore complexes (Webster et al., 2014); although it seems unlikely that this would influence EGFR activation, it supports the existence of previously undescribed functions for Vps4. Further investigation of the effect of Vps4 on EGFR activity might define a new molecular mechanism of action for this versatile protein.
MATERIALS AND METHODS
Drosophila genetics
3B1 is an EMS-induced lethal allele of Vps4 isolated previously (Legent et al., 2012). Using rescue by X chromosomal duplications followed by recombination with P-element markers (Zhai et al., 2003), we mapped it to a 0.35 cM interval in 16F1-5. The coding region of Vps4 amplified from homozygous mutant larvae contained a missense mutation, E209K, that was absent from the y,w, FRT19A isogenic strain used for the mutagenesis. The rescuing duplication Dp(1;3)JC153 (R1) was obtained from Alberto Ferrus (Cajal Institute, Madrid, Spain). hepr75 (Glise et al., 1995) is a deletion of nucleotides 486 to 1346 that removes the start codon. Other stocks used were wg-lacZP, puc-lacZE69, UAS-puc2A, HrsD28, Df(Hrs) [Exel6277], aos-lacZW11, hh-lacZP30, mirr-lacZcre2 (Bloomington Drosophila Stock Center), E(spl)mβ-CD2 (de Celis et al., 1998), pipe-lacZ (Andreu et al., 2012), vps4Δ7b (Rodahl et al., 2009a), DroncI29 (Xu et al., 2005), shi7C7 (Legent et al., 2012), shiFL54 (Windler and Bilder, 2010), UAS-shits (Kitamoto, 2001), TSG1012 (Moberg et al., 2005), UAS-rhomboid (Lee et al., 2001), UAS-sSpi (Schweitzer et al., 1995), UAS-EGFRλtop (Queenan et al., 1997) and UAS-RasV12 (Karim and Rubin, 1998).
Stocks used to generate clones were: (1) w, hsFLP122, P[w+, ubi-GFP], FRT19A, (2) y,w, hsFLP122, P[w+, ubi-RFP], FRT19A, (3) w, eyFLP1, tub-GAL80, FRT19A;; tub-GAL4, UAS-CD8::GFP/SM6-TM6B, (4) w, hsFLP122, tub-GAL80, FRT19A;; tub-GAL4, UAS-CD8::GFP/SM6-TM6B, (5) w, eyFLP1, P[w+, ubi-GFP], FRT19A; DroncI29/TM6B, (6) w, hsFLP122, P[w+, ubi-GFP], FRT19A; DroncI29/TM6B and (7) w, hsFLP122, P[w+, ubi-RFP], FRT19A; DroncI29/TM6B.
Molecular biology
The full-length Vps4 (CG6842) coding region was amplified by PCR from the GH02678 EST clone (Drosophila Genomics Resource Center) using Pfu Turbo and cloned into pUAST-HA as an EcoRI-XhoI fragment to generate pUAS-HA::Vps4. Transgenic flies were generated by Genetic Services, Inc.
Immunohistochemistry
Third-instar eye and wing discs were dissected and stained as described previously (Legent and Treisman, 2008). Ovaries were stained as described previously (Miura et al., 2006). Antibodies used were: rabbit anti-β-galactosidase (1:5000, Cappel), chicken anti-GFP (1:500, Aves), mouse anti-HA (1:100, Covance), mouse anti-CD2 (1:50, Serotec), rabbit anti-active Caspase 3 (1:500, CM1, BD Pharmingen), rabbit anti-Ato (1:5000; Jarman et al., 1993), guinea pig anti-Sens (1:1000; Nolo et al., 2000), mouse anti-NECD (1:10), mouse anti-DlECD (1:10), mouse anti-Wg (1:10), mouse anti-Ct (1:10), mouse anti-Grk (1:10), rat anti-Elav (1:100) (all Developmental Studies Hybridoma Bank, University of Iowa), rabbit anti-EGFRCTER (1:500; Rodrigues et al., 2005), mouse anti-EGFREXT (1:200, Sigma E2906), rabbit anti-phospho-Smad (1:50, Cell Signaling 9516), guinea pig anti-Hrs (1:200; Lloyd et al., 2002), rabbit anti-Rab5 (1:50; Wucherpfennig et al., 2003), rabbit anti-Rab11 (1:1000; Satoh et al., 2005), chicken anti-Syx7 (1:500; Lu and Bilder, 2005), rabbit anti-Lva (1:5000; Sisson et al., 2000), guinea pig anti-Sec15 (1:500; Mehta et al., 2005), and rabbit anti-Cic (1:5000; Astigarraga et al., 2007). Secondary antibodies used were from Jackson Immunoresearch (1:200) or Molecular Probes (1:1000). Images were taken on a Leica SP5 confocal microscope. For shits stainings, larvae were maintained at 31°C for 48 h prior to dissection.
Antibodies used for western blotting were mouse anti-diphosphorylated ERK (dpERK, 1:2500, Sigma M8159), rabbit anti-ERK (1:20,000, Cell Signaling 4695), mouse anti-Aos (1:100, Developmental Studies Hybridoma Bank), and mouse anti-β-Tubulin (1:3000, Covance MMS-410P) antibodies.
Cell culture
EGFR-expressing S2 (D2F) cells (Schweitzer et al., 1995) were maintained in Schneider's medium supplemented with 10% fetal calf serum and 150 µg/ml G418. Double-stranded RNAs (dsRNAs) were generated using the MEGAscript T7 and T3 Kits (Ambion) as described previously (Roignant et al., 2006) and 15 µg dsRNA were used to treat 106 cells per well for 5 days. EGFR expression was induced for 3 h with 60 µM Cu2SO4. For dpERK (pMAPK) western blots, the cells were treated with purified His-sSpiCS (Miura et al., 2006) for 10 min, and lysed as described previously (Miura et al., 2008). For Aos western blots, cells were serum-starved in medium containing dsRNA, and treated with purified His-sSpiCS for 16 h. Media were then harvested and the cells were lysed in ice-cold 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100 and protease inhibitors (Roche). Western blotting was carried out as described previously (Miura et al., 2006). Total RNA was extracted from D2F cells using Trizol (Invitrogen). RT-PCR was performed on 1 µg of total RNA using the Invitrogen SuperScript First-Strand Kit. Primer sequences are available on request.
Supplementary Material
Acknowledgements
We thank Minrong Ai, Hugo Bellen, David Bilder, Alberto Ferrus, Marcos Gonzalez-Gaitan, Andrew Jarman, Gerardo Jimenez, Daniel Marenda, Don Ready, Aloma Rodrigues, Hyung Don Ryoo, Benny Shilo, Harald Stenmark, the Drosophila Genomics Resource Center, the Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We are grateful to Antoine Guichet for his support. The manuscript was improved by the critical comments of Sergio Astigarraga, Justine Oyallon, Josefa Steinhauer and Annabelle Suisse.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health [EY013777 to J.E.T.]. Deposited in PMC for release after 12 months.
Author contributions
K.L. and J.E.T. designed experiments; K.L., H.H.L. and J.E.T. performed experiments; K.L. and J.E.T. wrote the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.117960/-/DC1
References
- Adell M. A. Y., Vogel G. F., Pakdel M., Muller M., Lindner H., Hess M. W. and Teis D. (2014). Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 205, 33-49 10.1083/jcb.201310114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson E. R. (2012). The role of endocytosis in activating and regulating signal transduction. Cell. Mol. Life Sci. 69, 1755-1771 10.1007/s00018-011-0877-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu M. J., Gonzalez-Perez E., Ajuria L., Samper N., Gonzalez-Crespo S., Campuzano S. and Jimenez G. (2012). Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila. Development 139, 1110-1114 10.1242/dev.076562 [DOI] [PubMed] [Google Scholar]
- Aoyama N., Yamakawa T., Sasamura T., Yoshida Y., Ohori M., Okubo H., Iida E., Sasaki N., Ueda R. and Matsuno K. (2013). Loss- and gain-of-function analyses of vacuolar protein sorting 2 in Notch signaling of Drosophila melanogaster. Genes Genet. Syst. 88, 45-57. [DOI] [PubMed] [Google Scholar]
- Assaker G., Ramel D., Wculek S. K., Gonzalez-Gaitan M. and Emery G. (2010). Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc. Natl. Acad. Sci. USA 107, 22558-22563 10.1073/pnas.1010795108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astigarraga S., Grossman R., Díaz-Delfín J., Caelles C., Paroush Z. and Jiménez G. (2007). A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 26, 668-677 10.1038/sj.emboj.7601532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkey M. R., Lachance J.-F. B., Walczak M., Rebello T. and Nilson L. A. (2006). Capicua regulates follicle cell fate in the Drosophila ovary through repression of mirror. Development 133, 2115-2123 10.1242/dev.02369 [DOI] [PubMed] [Google Scholar]
- Babst M., Wendland B., Estepa E. J. and Emr S. D. (1998). The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982-2993 10.1093/emboj/17.11.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Odorizzi G., Estepa E. J. and Emr S. D. (2000). Mammalian Tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1, 248-258 10.1034/j.1600-0854.2000.010307.x [DOI] [PubMed] [Google Scholar]
- Bache K. G., Stuffers S., Malerød L., Slagsvold T., Raiborg C., Lechardeur D., Wälchli S., Lukacs G. L., Brech A. and Stenmark H. (2006). The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell 17, 2513-2523 10.1091/mbc.E05-10-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbis A. and Posner B. I. (2010). Compartmentalization of EGFR in cellular membranes: role of membrane rafts. J. Cell. Biochem. 109, 1103-1108 10.1002/jcb.22505 [DOI] [PubMed] [Google Scholar]
- Baldys A. and Raymond J. R. (2009). Critical role of ESCRT machinery in EGFR recycling. Biochemistry 48, 9321-9323 10.1021/bi900865u [DOI] [PubMed] [Google Scholar]
- Barajas D., de Castro Martin I. F., Pogany J., Risco C. and Nagy P. D. (2014). Noncanonical role for the host Vps4 AAA+ ATPase ESCRT protein in the formation of Tomato bushy stunt virus replicase. PLoS Pathog. 10, e1004087 10.1371/journal.ppat.1004087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. and Alphey L. (2002). PP1 binds Sara and negatively regulates Dpp signaling in Drosophila melanogaster. Nat. Genet. 31, 419-423. [DOI] [PubMed] [Google Scholar]
- Brankatschk B., Wichert S. P., Johnson S. D., Schaad O., Rossner M. J. and Gruenberg J. (2012). Regulation of the EGF transcriptional response by endocytic sorting. Sci. Signal. 5, ra21 10.1126/scisignal.2002351 [DOI] [PubMed] [Google Scholar]
- Callejo A., Bilioni A., Mollica E., Gorfinkiel N., Andres G., Ibanez C., Torroja C., Doglio L., Sierra J. and Guerrero I. (2011). Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disc epithelium. Proc. Natl. Acad. Sci. USA 108, 12591-12598 10.1073/pnas.1106881108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashikar A. G., Shim S., Roth R., Maldazys M. R., Heuser J. E. and Hanson P. I. (2014). Structure of cellular ESCRT-III spirals and their relationship to HIV budding. eLife e02184 10.7554/eLife.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.-L., Liou W., Pen H.-C., Chou H.-Y., Chang Y.-W., Li W.-H., Chiang W. and Pai L.-M. (2008). The gradient of Gurken, a long-range morphogen, is directly regulated by Cbl-mediated endocytosis. Development 135, 1923-1933 10.1242/dev.017103 [DOI] [PubMed] [Google Scholar]
- Chanut-Delalande H., Jung A. C., Baer M. M., Lin L., Payre F. and Affolter M. (2010). The Hrs/Stam complex acts as a positive and negative regulator of RTK signaling during Drosophila development. PLoS ONE 5, e10245 10.1371/journal.pone.0010245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri K., Llodrá J., Roth E. W., Tsai J., Gordo S., Wucherpfennig K. W., Kam L. C., Stokes D. L. and Dustin M. L. (2014). Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 507, 118-123 10.1038/nature12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis J. F., Garcia-Bellido A. and Bray S. J. (1996). Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359-369. [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Tyler D. M., de Celis J. and Bray S. J. (1998). Notch signalling mediates segmentation of the Drosophila leg. Development 125, 4617-4626. [DOI] [PubMed] [Google Scholar]
- de Gassart A., Géminard C., Hoekstra D. and Vidal M. (2004). Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic 5, 896-903 10.1111/j.1600-0854.2004.00223.x [DOI] [PubMed] [Google Scholar]
- Dhanasekaran D. N. and Reddy E. P. (2008). JNK signaling in apoptosis. Oncogene 27, 6245-6251 10.1038/onc.2008.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R., Vick P., Ploper D., Gumper I., Snitkin H., Sabatini D. D. and De Robertis E. M. (2012). Presenilin deficiency or lysosomal inhibition enhances Wnt signaling through relocalization of GSK3 to the late-endosomal compartment. Cell Rep. 2, 1316-1328 10.1016/j.celrep.2012.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Kazim A. S., Dawes I. W., Brown A. J. and Yang H. (2013). The AAA ATPase VPS4/SKD1 regulates endosomal cholesterol trafficking independently of ESCRT-III. Traffic 14, 107-119 10.1111/tra.12015 [DOI] [PubMed] [Google Scholar]
- Eden E. R., Huang F., Sorkin A. and Futter C. E. (2012). The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic 13, 329-337 10.1111/j.1600-0854.2011.01305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort B. J., Nolo R., Zhang Z., Bellen H. and Mardon G. (2001). Senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron 32, 403-414 10.1016/S0896-6273(01)00480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. (1994). The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech. Dev. 48, 25-33 10.1016/0925-4773(94)90003-5 [DOI] [PubMed] [Google Scholar]
- Freeman M. (1996). Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87, 651-660 10.1016/S0092-8674(00)81385-9 [DOI] [PubMed] [Google Scholar]
- Freeman M. (1997). Cell determination strategies in the Drosophila eye. Development 124, 261-270. [DOI] [PubMed] [Google Scholar]
- French A. R., Tadaki D. K., Niyogi S. K. and Lauffenburger D. A. (1995). Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J. Biol. Chem. 270, 4334-4340 10.1074/jbc.270.9.4334 [DOI] [PubMed] [Google Scholar]
- Futter C. E., Connolly C. N., Cutler D. F. and Hopkins C. R. (1995). Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J. Biol. Chem. 270, 10999-11003 10.1074/jbc.270.18.10999 [DOI] [PubMed] [Google Scholar]
- Glise B., Bourbon H. and Noselli S. (1995). hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83, 451-461 10.1016/0092-8674(95)90123-X [DOI] [PubMed] [Google Scholar]
- Golembo M., Schweitzer R., Freeman M. and Shilo B. Z. (1996). argos transcription is induced by the Drosophila EGF receptor pathway to form an inhibitory feedback loop. Development 122, 223-230. [DOI] [PubMed] [Google Scholar]
- Halfar K., Rommel C., Stocker H. and Hafen E. (2001). Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development 128, 1687-1696. [DOI] [PubMed] [Google Scholar]
- Hanson P. I. and Cashikar A. (2012). Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 28, 337-362 10.1146/annurev-cellbio-092910-154152 [DOI] [PubMed] [Google Scholar]
- Henne W. M., Buchkovich N. J., Zhao Y. and Emr S. D. (2012). The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 151, 356-371 10.1016/j.cell.2012.08.039 [DOI] [PubMed] [Google Scholar]
- Henriksen L., Grandal M. V., Knudsen S. L. J., van Deurs B. and Grøvdal L. M. (2013). Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS ONE 8, e58148 10.1371/journal.pone.0058148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A. P., Grau Y., Jan L. Y. and Jan Y. N. (1993). atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell 73, 1307-1321 10.1016/0092-8674(93)90358-W [DOI] [PubMed] [Google Scholar]
- Jékely G., Sung H.-H., Luque C. M. and Rørth P. (2005). Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev. Cell 9, 197-207 10.1016/j.devcel.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Jimenez A. J., Maiuri P., Lafaurie-Janvore J., Divoux S., Piel M. and Perez F. (2014). ESCRT machinery is required for plasma membrane repair. Science 343, 1247136 10.1126/science.1247136 [DOI] [PubMed] [Google Scholar]
- Jones S. and Rappoport J. Z. (2014). Interdependent Epidermal growth factor receptor signalling and trafficking. Int. J. Biochem. Cell Biol. 51, 23-28 10.1016/j.biocel.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Karim F. D. and Rubin G. M. (1998). Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125, 1-9. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Taylor L. J. and Bar-Sagi D. (2007). Spatial regulation of EGFR signaling by Sprouty2. Curr. Biol. 17, 455-461 10.1016/j.cub.2007.01.059 [DOI] [PubMed] [Google Scholar]
- Kitamoto T. (2001). Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81-92 10.1002/neu.1018 [DOI] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Sibarita J.-B., Aresta S., Murthy M., Schwarz T., Camonis J. and Bellaïche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365-376 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Lata S., Schoehn G., Jain A., Pires R., Piehler J., Gottlinger H. G. and Weissenhorn W. (2008). Helical structures of ESCRT-III are disassembled by VPS4. Science 321, 1354-1357 10.1126/science.1161070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. R., Urban S., Garvey C. F. and Freeman M. (2001). Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107, 161-171 10.1016/S0092-8674(01)00526-8 [DOI] [PubMed] [Google Scholar]
- Legent K. and Treisman J. E. (2008). Wingless signaling in Drosophila eye development. Methods Mol. Biol. 469, 141-161 10.1007/978-1-60327-469-2_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legent K., Steinhauer J., Richard M. and Treisman J. E. (2012). A screen for X-linked mutations affecting Drosophila photoreceptor differentiation identifies Casein kinase 1alpha as an essential negative regulator of Wingless signaling. Genetics 190, 601-616 10.1534/genetics.111.133827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G. and Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261-269 10.1016/S0092-8674(02)00611-6 [DOI] [PubMed] [Google Scholar]
- Lu H. and Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232-1239 10.1038/ncb1324 [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., Gampel A., Ring J., Virdee K., Kirov N., Tolkovsky A. M. and Martinez-Arias A. (1998). puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557-570 10.1101/gad.12.4.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. Q., Hiesinger P. R., Beronja S., Zhai R. G., Schulze K. L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M. C. et al. (2005). Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron 46, 219-232 10.1016/j.neuron.2005.02.029 [DOI] [PubMed] [Google Scholar]
- Miaczynska M., Pelkmans L. and Zerial M. (2004). Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400-406 10.1016/j.ceb.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Miura G. I., Buglino J., Alvarado D., Lemmon M. A., Resh M. D. and Treisman J. E. (2006). Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10, 167-176 10.1016/j.devcel.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Miura G. I., Roignant J.-Y., Wassef M. and Treisman J. E. (2008). Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development 135, 1913-1922 10.1242/dev.017202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg K. H., Schelble S., Burdick S. K. and Hariharan I. K. (2005). Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell 9, 699-710 10.1016/j.devcel.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Monroe N., Han H., Gonciarz M. D., Eckert D. M., Karren M. A., Whitby F. G., Sundquist W. I. and Hill C. P. (2014). The oligomeric state of the active Vps4 AAA ATPase. J. Mol. Biol. 426, 510-525 10.1016/j.jmb.2013.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E. (2012). Differential requirements of mammalian ESCRTs in multivesicular body formation, virus budding and cell division. FEBS J. 279, 1399-1406 10.1111/j.1742-4658.2012.08534.x [DOI] [PubMed] [Google Scholar]
- Mueller M., Adell M. A. Y. and Teis D. (2012). Membrane abscission: first glimpse at dynamic ESCRTs. Curr. Biol. 22, R603-R605 10.1016/j.cub.2012.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musse A. A., Meloty-Kapella L. and Weinmaster G. (2012). Notch ligand endocytosis: mechanistic basis of signaling activity. Semin. Cell Dev. Biol. 23, 429-436 10.1016/j.semcdb.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan J. F., Hu R., Oh R. S., Cohen S. N. and Lu Q. (2012). Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 109, 4146-4151 10.1073/pnas.1200448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C. J. and Cohen S. M. (1997). Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development 124, 871-880. [DOI] [PubMed] [Google Scholar]
- Nilson L. A. and Schüpbach T. (1998). EGF receptor signaling in Drosophila oogenesis. Curr. Top. Dev. Biol. 44, 203-243 10.1016/S0070-2153(08)60471-8 [DOI] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A. and Bellen H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349-362 10.1016/S0092-8674(00)00040-4 [DOI] [PubMed] [Google Scholar]
- Panopoulou E., Gillooly D. J., Wrana J. L., Zerial M., Stenmark H., Murphy C. and Fotsis T. (2002). Early endosomal regulation of Smad-dependent signaling in endothelial cells. J. Biol. Chem. 277, 18046-18052 10.1074/jbc.M107983200 [DOI] [PubMed] [Google Scholar]
- Parker D. S., Jemison J. and Cadigan K. M. (2002). Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565-2576. [DOI] [PubMed] [Google Scholar]
- Piper R. C. and Lehner P. J. (2011). Endosomal transport via ubiquitination. Trends Cell Biol. 21, 647-655 10.1016/j.tcb.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan A. M., Ghabrial A. and Schupbach T. (1997). Ectopic activation of Torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development 124, 3871-3880. [DOI] [PubMed] [Google Scholar]
- Raiborg C. and Stenmark H. (2009). The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445-452 10.1038/nature07961 [DOI] [PubMed] [Google Scholar]
- Razi M. and Futter C. E. (2006). Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 17, 3469-3483 10.1091/mbc.E05-11-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S. and White K. (1991). Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J. Neurobiol. 22, 443-461 10.1002/neu.480220503 [DOI] [PubMed] [Google Scholar]
- Rodahl L. M., Haglund K., Sem-Jacobsen C., Wendler F., Vincent J.-P., Lindmo K., Rusten T. E. and Stenmark H. (2009a). Disruption of Vps4 and JNK function in Drosophila causes tumour growth. PLoS ONE 4, e4354 10.1371/journal.pone.0004354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodahl L. M., Stuffers S., Lobert V. H. and Stenmark H. (2009b). The role of ESCRT proteins in attenuation of cell signalling. Biochem. Soc. Trans. 37, 137-142 10.1042/BST0370137 [DOI] [PubMed] [Google Scholar]
- Rodrigues A. B., Werner E. and Moses K. (2005). Genetic and biochemical analysis of the role of Egfr in the morphogenetic furrow of the developing Drosophila eye. Development 132, 4697-4707 10.1242/dev.02058 [DOI] [PubMed] [Google Scholar]
- Roepstorff K., Grandal M. V., Henriksen L., Knudsen S. L. J., Lerdrup M., Grøvdal L., Willumsen B. M. and van Deurs B. (2009). Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 10, 1115-1127 10.1111/j.1600-0854.2009.00943.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E. M., Brennan C. A., Mortimer N. T., Cook S., Morris A. R. and Moses K. (2005). Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development 132, 4833-4843 10.1242/dev.02061 [DOI] [PubMed] [Google Scholar]
- Roignant J.-Y. and Treisman J. E. (2010). Exon junction complex subunits are required to splice Drosophila MAP kinase, a large heterochromatic gene. Cell 143, 238-250 10.1016/j.cell.2010.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roignant J.-Y., Hamel S., Janody F. and Treisman J. E. (2006). The novel SAM domain protein Aveugle is required for Raf activation in the Drosophila EGF receptor signaling pathway. Genes Dev. 20, 795-806 10.1101/gad.1390506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. R. G., Shideler T., Nickerson D. P., West M. and Odorizzi G. (2012). Class E compartments form in response to ESCRT dysfunction in yeast due to hyperactivity of the Vps21 Rab GTPase. J. Cell Sci. 125, 5208-5220 10.1242/jcs.111310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A. K., O'Tousa J. E., Ozaki K. and Ready D. F. (2005). Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132, 1487-1497 10.1242/dev.01704 [DOI] [PubMed] [Google Scholar]
- Schmid S. L. and Frolov V. A. (2011). Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell Dev. Biol. 27, 79-105 10.1146/annurev-cellbio-100109-104016 [DOI] [PubMed] [Google Scholar]
- Schneider M., Troost T., Grawe F., Martinez-Arias A. and Klein T. (2013). Activation of Notch in lgd mutant cells requires the fusion of late endosomes with the lysosome. J. Cell Sci. 126, 645-656 10.1242/jcs.116590 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Shaharabany M., Seger R. and Shilo B. Z. (1995). Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518-1529 10.1101/gad.9.12.1518 [DOI] [PubMed] [Google Scholar]
- Scott A., Chung H.-Y., Gonciarz-Swiatek M., Hill G. C., Whitby F. G., Gaspar J., Holton J. M., Viswanathan R., Ghaffarian S., Hill C. P. et al. (2005). Structural and mechanistic studies of VPS4 proteins. EMBO J. 24, 3658-3669 10.1038/sj.emboj.7600818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E. S. and Bellen H. J. (2006). Internalization is required for proper Wingless signaling in Drosophila melanogaster. J. Cell Biol. 173, 95-106 10.1083/jcb.200510123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B.-Z. (2003). Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp. Cell Res. 284, 140-149 10.1016/S0014-4827(02)00094-0 [DOI] [PubMed] [Google Scholar]
- Shilo B.-Z. and Schejter E. D. (2011). Regulation of developmental intercellular signalling by intracellular trafficking. EMBO J. 30, 3516-3526 10.1038/emboj.2011.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P. and Polo S. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760-2765 10.1073/pnas.0409817102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson J. C., Field C., Ventura R., Royou A. and Sullivan W. (2000). Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J. Cell Biol. 151, 905-918 10.1083/jcb.151.4.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa L. P., Lax I., Shen H., Ferguson S. M., De Camilli P. and Schlessinger J. (2012). Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc. Natl. Acad. Sci. USA 109, 4419-4424 10.1073/pnas.1200164109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer J., Liu H. H., Miller E. and Treisman J. E. (2013). Trafficking of the EGFR ligand Spitz regulates its signaling activity in polarized tissues. J. Cell Sci. 126, 4469-4478 10.1242/jcs.131169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H. (2008). Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132-1138 10.1038/cdd.2008.50 [DOI] [PubMed] [Google Scholar]
- Stetak A., Hoier E. F., Croce A., Cassata G., Di Fiore P. P. and Hajnal A. (2006). Cell fate-specific regulation of EGF receptor trafficking during Caenorhabditis elegans vulval development. EMBO J. 25, 2347-2357 10.1038/sj.emboj.7601137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant M. A., Roark M. and Bier E. (1993). The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 7, 961-973 10.1101/gad.7.6.961 [DOI] [PubMed] [Google Scholar]
- Sun J. and Deng W.-M. (2007). Hindsight mediates the role of Notch in suppressing Hedgehog signaling and cell proliferation. Dev. Cell 12, 431-442 10.1016/j.devcel.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P. J., Rodrigues A. B., Kleinberger A., Quatela S., Bach E. A. and Philips M. R. (2010). Cytosolic Ras supports eye development in Drosophila. Mol. Cell. Biol. 30, 5649-5657 10.1128/MCB.00635-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taelman V. F., Dobrowolski R., Plouhinec J.-L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D. and De Robertis E. M. (2010). Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136-1148 10.1016/j.cell.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Smith C., McGlade J. and Tepass U. (2000). Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151, 891-904 10.1083/jcb.151.4.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L. (2012). Membrane trafficking components in cytokinesis. Cell Physiol. Biochem. 30, 1097-1108 10.1159/000343301 [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Itoh S., ten Dijke P. and Tabata T. (2000). Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell 5, 59-71 10.1016/S1097-2765(00)80403-7 [DOI] [PubMed] [Google Scholar]
- Teis D., Taub N., Kurzbauer R., Hilber D., de Araujo M. E., Erlacher M., Offterdinger M., Villunger A., Geley S., Bohn G. et al. (2006). p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J. Cell Biol. 175, 861-868 10.1083/jcb.200607025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Mathieu J., Sung H.-H., Loeser E., Rørth P. and Cohen S. M. (2005). Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 9, 711-720 10.1016/j.devcel.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Tio M., Ma C. and Moses K. (1994). Spitz, a Drosophila homolog of Transforming growth factor-alpha, is required in the founding photoreceptor cells of the compound eye facets. Mech. Dev. 48, 13-23 10.1016/0925-4773(94)90002-7 [DOI] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang T. A., Davison A. F., Attisano L. and Wrana J. L. (1998). SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95, 779-791 10.1016/S0092-8674(00)81701-8 [DOI] [PubMed] [Google Scholar]
- Tu C., Ahmad G., Mohapatra B., Bhattacharyya S., Ortega-Cava C., Chung B. M., Wagner K.-U., Raja S. M., Naramura M., Band V. et al. (2011). ESCRT proteins: double-edged regulators of cellular signaling. Bioarchitecture 1, 45-48 10.4161/bioa.1.1.15173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Huang X., Tanaka Y. and Hirokawa N. (2011). KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev. Cell 20, 60-71 10.1016/j.devcel.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Vaccari T. and Bilder D. (2005). The Drosophila tumor suppressor Vps25 prevents nonautonomous overproliferation by regulating Notch trafficking. Dev. Cell 9, 687-698 10.1016/j.devcel.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M. E. and Bilder D. (2008). Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755-762 10.1083/jcb.200708127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Rusten T. E., Menut L., Nezis I. P., Brech A., Stenmark H. and Bilder D. (2009). Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 122, 2413-2423 10.1242/jcs.046391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Duchi S., Cortese K., Tacchetti C. and Bilder D. (2010). The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 137, 1825-1832 10.1242/dev.045484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer P. D., Einwalter L. A., Moninger T. O., Rokhlina T., Kern J. A., Zabner J. and Welsh M. J. (2003). Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422, 322-326 10.1038/nature01440 [DOI] [PubMed] [Google Scholar]
- Vieira A. V., Lamaze C. and Schmid S. L. (1996). Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086-2089 10.1126/science.274.5295.2086 [DOI] [PubMed] [Google Scholar]
- Webster B. M., Colombi P., Jäger J. and Lusk C. P. (2014). Surveillance of nuclear pore complex assembly by ESCRT-III/Vps4. Cell 159, 388-401 10.1016/j.cell.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler S. L. and Bilder D. (2010). Endocytic internalization routes required for Delta/Notch signaling. Curr. Biol. 20, 538-543 10.1016/j.cub.2010.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T. and Hurley J. H. (2010). Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864-869 10.1038/nature08849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Brauninger M. and Gonzalez-Gaitan M. (2003). Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609-624 10.1083/jcb.200211087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Li Y., Arcaro M., Lackey M. and Bergmann A. (2005). The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development 132, 2125-2134 10.1242/dev.01790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Denef N. and Schüpbach T. (2009). The vacuolar proton pump, V-ATPase, is required for Notch signaling and endosomal trafficking in Drosophila. Dev. Cell 17, 387-402 10.1016/j.devcel.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. and Baker N. E. (2003). Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev. Cell 4, 359-369 10.1016/S1534-5807(03)00059-5 [DOI] [PubMed] [Google Scholar]
- Yang C.-H., Axelrod J. D. and Simon M. A. (2002). Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108, 675-688 10.1016/S0092-8674(02)00658-X [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Yamagata F., Yamamoto A., Mizushima N., Kabeya Y., Nara A., Miwako I., Ohashi M., Ohsumi M. and Ohsumi Y. (2000). The mouse SKD1, a homologue of yeast Vps4p, is required for normal endosomal trafficking and morphology in mammalian cells. Mol. Biol. Cell 11, 747-763 10.1091/mbc.11.2.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M. and Struhl G. (2002). Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129, 1357-1368. [DOI] [PubMed] [Google Scholar]
- Zhai R. G., Hiesinger P. R., Koh T.-W., Verstreken P., Schulze K. L., Cao Y., Jafar-Nejad H., Norga K. K., Pan H., Bayat V. et al. (2003). Mapping Drosophila mutations with molecularly defined P element insertions. Proc. Natl. Acad. Sci. USA 100, 10860-10865 10.1073/pnas.1832753100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.-Y., Cheng C.-M., Fu X.-R., Chen L.-Y., Xu L., Terrillon S., Wong S. T., Bar-Sagi D., Songyang Z. and Chang E. C. (2012). CHMP6 and VPS4A mediate the recycling of Ras to the plasma membrane to promote growth factor signaling. Oncogene 31, 4630-4638 10.1038/onc.2011.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.