Summary
Inducible PGE2 production by mPGES-1 is critical for the colonic mucosal homeostasis. This function is exploited in the presence of the neoplastic transformation in ApcΔ14/+ mice as PGE2 contributes to the growth and expansion of the early cryptal structures.
Abstract
Microsomal PGE2 synthase-1 (mPGES-1), the terminal enzyme in the formation of inducible PGE2, represents a potential target for cancer chemoprevention. We have previously shown that genetic abrogation of mPGES-1 significantly suppresses tumorigenesis in two preclinical models of intestinal cancer. In this study, we examined the role of mPGES-1 during colon tumorigenesis in the presence of dextran sulfate sodium (DSS)-induced inflammatory microenvironment. Using Apc Δ14/+ in which the mPGES-1 gene is either wild-type (D14:WT) or deleted (D14:KO), we report that mPGES-1 deficiency enhances sensitivity to acute mucosal injury. As a result of the increased epithelial damage, protection against adenoma formation is unexpectedly compromised in the D14:KO mice. Examining the DSS-induced acute injury, cryptal structures are formed within inflamed areas of colonic mucosa of both genotypes that display the hallmarks of early neoplasia. When acute epithelial injury is balanced by titration of DSS exposures, however, these small cryptal lesions progress rapidly to adenomas in the D14:WT mice. Given that mPGES-1 is highly expressed within the intestinal stroma under the inflammatory conditions of DSS-induced ulceration, we propose a complex and dual role for inducible PGE2 synthesis within the colonic mucosa. Our data suggest that inducible PGE2 is critical for the maintenance of an intact colonic epithelial barrier, while promoting epithelial regeneration. This function is exploited during neoplastic transformation in Apc Δ14/+ mice as PGE2 contributes to the growth and expansion of the early initiated cryptal structures. Taken together, inducible PGE2 plays a complex role in inflammation-associated cancers that requires further analysis.
Inducible PGE2 production by mPGES-1 is critical for the colonic mucosal homeostasis. This function is exploited in the presence of the neoplastic transformation in Apc Δ14/+ mice as PGE2 contributes to the growth and expansion of the early cryptal structures.
Introduction
Prostaglandin E2 (PGE2) is a bioactive lipid derived from arachidonic acid by the sequential enzymatic actions of cyclooxygenases (COXs) and terminal prostaglandin E synthases (PGES) (1). PGE2 is the most abundant of the COX-derived prostanoids, where it plays an important role in the maintenance of tissue homeostasis (2). Under certain circumstances, however, PGE2 levels can be greatly increased, thereby contributing to a range of pathologies including inflammation and cancer (3). Coordinated activation of inducible COX-2 and microsomal PGES-1 (mPGES-1) are found in many types of cancers, including colorectal cancer (reviewed in (4)). The association of PGE2 and increased cancer risk is based upon a substantial body of evidence obtained from rodent studies, as well as several decades of clinical research on the chemopreventive efficacy of COX inhibitors (5). Although non-steroidal anti-inflammatory agents (NSAIDs), most notably aspirin (6) and sulindac (7), have proven beneficial in cancer prevention, the widespread application of selective COX-2 inhibitors (Coxibs) for long-term preventive use has been limited. This is due in part to their dose-dependent toxicities, including cardiovascular and gastrointestinal effects, which are caused by the non-specific reduction in the levels of other essential prostanoid metabolites of arachidonic acid (8). Given these impediments to the widespread application of COX-2 inhibitors, significant efforts have been made to develop alternative strategies for the selective targeting of PGE2 synthesis. Of these various approaches, mPGES-1 has emerged as an attractive target due to its direct role in the synthesis of inducible PGE2 (3).
Several recent preclinical studies have shown that mPGES-1 deficient mice are significantly protected against tumor formation, including those occurring in the breast (9) and colon (2,10,11), as well as prostate cancer metastasis to the lung (12). Howe et al. (9). has shown that mPGES-1 deficiency causes a substantial reduction in tumor multiplicity in a mouse mammary tumor model driven by HER2/neu over-expression. We have previously demonstrated in two intestinal cancer models that global genetic inactivation of mPGES-1 reduces the levels of PGE2, while markedly suppressing tumor formation (2,11). In the first study of its kind, we showed that introducing a mPGES-1 deletion onto Apc Δ14/+ mice resulted in tumor blockade of up to 65 and 50% in the small intestine and colon, respectively (11). In this model, mPGES-1 appears to play a critical role in tumor promotion. Although the formation of smaller adenomas (~1mm) was not affected by mPGES-1 status, knockout (KO) mice developed significantly fewer large tumors (11). In a subsequent study, the direct impact of mPGES-1 deletion was examined using the azoxymethane carcinogen model in highly sensitive strain A mice (A/J) (2,13). Suppression of inducible PGE2 formation significantly reduced colon adenoma formation by up to 95% (2).
During the course of the azoxymethane study, we observed the presence of synchronous, localized colonic ulcerations, in some cases affecting up to 15% of the colonic epithelium in A/J mice harboring the mPGES-1 gene deletion (2). These ulcerated lesions were found to develop independently of carcinogen exposure and were characterized histologically by the presence of regenerative atypia. The mucosal ulcerations resembled the active phase of ulcerative colitis and the mice developed accompanying splenomegaly and macroscopically inflamed mesenteric lymph nodes (2). Taken together, these findings suggest that mPGES-1 exerts a complex and multifaceted role within the colonic epithelium, whereby its activity contributes both to mucosal homeostasis as well as tumor promotion (2). PGE2 is a key driver of acute inflammation, but also elicits powerful immunosuppressive effects that contribute to disease resolution and mucsosal repair (3). The restorative properties of PGE2 within the gut mucosa are underscored by clinical observations that strongly contraindicate the use of NSAIDs in inflammatory bowel disease (IBD) patients undergoing active disease (14).
The increased risk of colorectal cancer development associated with long-standing IBD is well-documented (15). Given the dramatic protection against colon carcinogenesis we observed by genetic inactivation of mPGES-1 (2), we sought to assess the potential benefit of targeting inducible mPGES-1 in an inflammation-associated tumor model by using dextran sodium sulfate (DSS). DSS is a commonly used experimental tool for recapitulating many of the clinical and histopathological features found in human IBD, and it has been shown to cause a redistribution of adenomas to the colon in Apc Min/+ mice (16). In this study, Apc Δ14/+ mice harboring a wild-type (D14:WT) or inactivated mPGES-1 gene (D14:KO) were treated with DSS to induce acute colitis, and the formation of colon adenomas was evaluated for up to 4 weeks after cessation of DSS treatment (17). We report here that mPGES-1 plays a complex role within the colonic epithelium. While mPGES-1 protects the colonic mucosa against acute DSS-induced injury, its absence impairs the expansion and growth of Apc initiated cryptal lesions. These studies provide important insights into how selective mPGES-1 inhibition can impact intestinal tissue homeostasis and repair while contributing to cancer development in the colon.
Materials and methods
Animals
Apc Δ14/+ mice were provided by Dr. Christine Perret at Universite Paris (18). Apc Δ14/+: mPGES-1 wild type (D14:WT) and Apc Δ14/+: mPGES-1 knockout (D14:KO) mice were generated as described previously (11), and maintained in the animal facility of University of Connecticut Health Center (UCHC). All protocols were approved by the UCHC Center for Comparative Medicine (CCM). Both males and female mice were randomly placed on study with the access to maintenance diet (Teklad Global 19% Protein Extruded Rodent Diet) and drinking water ad libitum.
DSS treatment
At 5 weeks of age, mice were given 0, 0.1, 0.5, 1 or 2% DSS (molecular weight: 36 000–50 000; MP Biomedicals) in drinking water for 5 days, and switched to regular water thereafter until killing. Body weight was recorded by the percent of body weight change, calculated by dividing the weight at the time of killing by the initial weight at the start of the study. Spleen weight was normalized by the body weight of each mouse. Colons were harvested, flushed immediately with ice-cold phosphate-buffered saline and excised longitudinally. Specimens were fixed-flat in 10% neutral buffered formalin for 4h and stored in 70% ethanol for further analysis.
Quantification of lesions
For adenoma count, whole-mount small intestines and colons were stained with 0.2% methylene blue and the number and size of adenomas were scored under a dissecting microscope. Colon tumor load per mouse was determined using tumor diameter to calculate the spherical tumor volume (V = 4/3πr 3). The extent of inflamed mucosal area in the colon was determined as the ratio of ulcerated tissue within the entire length of the colon, assessed by evaluation of H&E-stained sections.
Immunohistochemistry and immunofluorescence
Colons were Swiss-rolled, paraffin-embedded and sectioned at 5-μm thickness. Sections were treated with 1–3% hydrogen peroxide, blocked and incubated with anti-β-catenin (1:2000; Sigma-Aldrich), anti-mPGES-1 (1:1000; Abnova, Taipei City, Taiwan) and anti-Cyclin D1 (1:50; Cell Signaling). Sections were incubated with biotinylated-secondary antibody, followed by ABC reagent (Vector Laboratories Inc, Burlingame, CA). Signal was detected using diaminobenzidene solution (Vector Laboratories). Tissues were counterstained with hematoxylin. For immunofluorescence, sections were incubated with anti-mPGES-1 (1:1000) and anti-vimentin (1:400; Cell Signaling), followed by detection with Alexa Fluor® 488 and Alexa Fluor® 568-conjugated secondary antibodies (1:200; Invitrogen). Images were captured using QCapture PRO software (QImaging, Surrey, BC, Canada).
Statistical analyses
Statistical analyses were performed using GraphPad Prism V software (GraphPad Software, Inc, La Jolla, CA). Data are shown as mean ± SEM. P values were calculated by the Student’s t-test or one-way analysis of variance with Bonferroni’s multiple comparison tests where appropriate as indicated in the Figure legends. A P value less than 0.05 was considered statistically significant.
Results
Formation of intestinal adenomas in Apc Δ14/+ mice treated with DSS
Based on our previously published findings (11), we sought to determine whether tumor protection by mPGES-1 blockade is extended to an inflammation-associated intestinal cancer model. To induce colitis, D14:WT and D14:KO mice were treated with DSS and analyzed 4 weeks later (Figure 1A). As shown in Figure 1B, DSS treatment caused a modest, but significant increase (1.5-fold, P < 0.05) in the number of small intestinal tumors in the D14:WT mice, consistent with earlier findings (16). Genetic inactivation of mPGES-1 resulted in a significant reduction in the number of adenomas present in the small intestine, in both the plain drinking water (3.4-fold, P < 0.0001) and 1% DSS (6.5-fold, P < 0.0001) groups at 4 weeks after treatment. These findings are consistent with the well-established tumor-promoting role of mPGES-1.
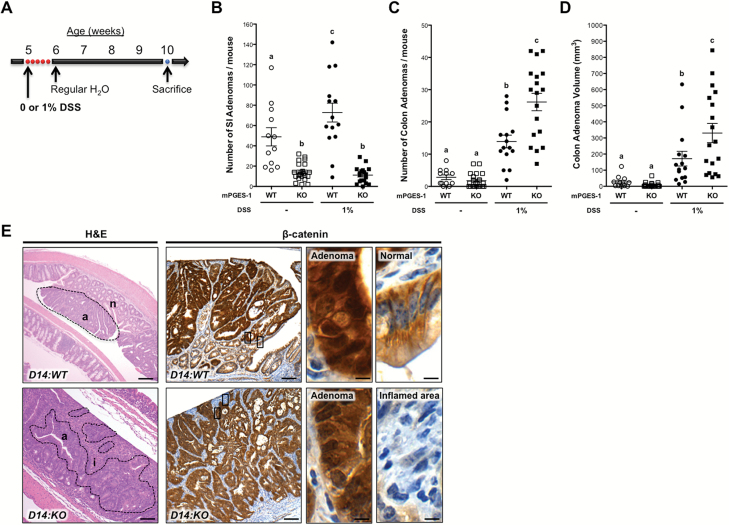
Figure 1.
Formation of intestinal adenomas in Apc Δ14/+ mice treated with 1% DSS. (A) Experimental scheme for D14:WT and D14:KO mice treated with 0% (WT, n = 12 and KO, n = 22) or 1% DSS (WT, n = 15 and KO, n = 18) and analyzed 4 weeks after DSS treatment. Number of small intestinal adenomas (B), number of colon adenomas (C) and volume of colon adenomas (D) in the D14:WT and D14:KO mice. (E) Representative sections of colon adenomas formed in Apc Δ14/+ mice, showing tumor morphology (left) and β-catenin immunostaining (right). While the majority of adenomas in the D14:WT mice were found within areas of normal colonic mucosa, adenomas in the D14:KO mice were more often identified within areas of inflamed epithelium. (a), (n) and (i) identifies adenomas, normal and inflamed lesions, respectively. Intense nuclear β-catenin staining is found in the adenomas of both mPGES-1 genotypes, while adjacent normal mucosa only shows membrane staining. Enlarged areas are identified by the boxes. Bars indicate means ± SEM. Letters within the graphs indicate significance measured by one-way analysis of variance with Bonferroni’s multiple comparison tests. Scale bars, 100 and 10μm (insets).
The effects of mPGES-1 inactivation in the colon, however, were more complex. As shown in Figure 1C, genetic deletion of mPGES-1 caused only a moderate, but non-significant decrease (1.5-fold, P = 0.211) in the number of colon adenomas in mice receiving plain drinking water. In the D14:WT mice, DSS treatment caused a significant increase in the number of adenomas when compared to mice receiving plain drinking water (4.9-fold, P < 0.0001; Figure 1C). Under the same inflammatory conditions associated with DSS exposure, the absence of mPGES-1 (D14:KO) resulted in a further increase in the number of colon adenomas (1.9-fold, P < 0.0001; Figure 1C). A similar trend in tumor volume was also observed in the D14:KO mice (1.9-fold, P < 0.0001; Figure 1D).
As shown in Figure 1E, a range of pathologies are present within the colons of Apc Δ14/+ mice at 4 weeks after DSS treatment. In the D14:WT mice, the majority of adenomas are found within and above areas of normal-appearing colonic mucosa (Figure 1E: D14:WT H&E). Furthermore, these adenomas stain positively for nuclear β-catenin (Figure 1E: D14:WT β-catenin), indicating that they have undergone neoplastic transformation associated with Apc loss of heterozygosity (LOH). In striking contrast, most of the adenomas in the D14:KO mice occurred within areas of inflammation or ulcerated mucosa and were associated with fibroblast and lymphocyte infiltration, all hallmarks of DSS-induced tissue injury (Figure 1E: D14:KO H&E). Epithelial cells within the D14:KO lesions were also strongly positive for nuclear β-catenin, indicating that loss of full-length Apc also occurred in these mice (Figure 1E: D14:KO β-catenin).
The disparate findings between the small intestine and colon are most probably result from the fact that DSS-induced epithelial injury is largely absent in the small bowel (Supplementary Figure 1 is available at Carcinogenesis Online). In the colon, however, the impact of mPGES-1 genotype is clearly dependent upon DSS-induced mucosal injury. Our observations suggest that more extensive acute injury occurs in the D14:KO mice, thereby abrogating the cancer protection afforded by mPGES-1 deletion.
Dose-dependent effects of DSS on acute mucosal injury
During the acute phase of DSS-induced colitis, the colonic mucosa exhibits histological abnormalities, including mucin depletion, epithelial degeneration and necrosis, leading to the disappearance of a normal epithelial structure (19). This acute phase of epithelial injury is followed by neutrophil infiltration, the formation of crypt abscesses and epithelial erosion (19). Based upon the results of a previous study (reviewed in (20)), we postulated that mPGES-1 status might directly affect the mucosal response to acute intestinal injury. To test this possibility, D14:WT and D14:KO mice were treated with increasing concentrations of DSS (0, 0.1, 0.5, 1 and 2%) and analyzed 2 days later (Figure 2A). As shown in Figure 2B, there were no genotypic differences in neither body weight nor spleen size observed until a concentration of 1% DSS was reached. At that dose level, D14:KO mice showed a significant reduction in body weight (1.3-fold, P < 0.0006) and increase in spleen size (1.7-fold, P < 0.0001) in comparison to the D14:WT mice. Comparable changes to the D14:WT mice were not observed until a concentration of 2% DSS was administered. However, D14:KO mice could not tolerate 2% DSS, and 100% mortality was observed within 7 days of DSS treatment.
Figure 2.
mPGES-1 deletion sensitizes the colonic mucosa to DSS-induced injury. (A) Experimental scheme for D14:WT (n = 7–10) and D14:KO (n = 3–16) mice treated with 0, 0.1, 0.5, 1 or 2% DSS and analyzed 2 days after DSS treatment. (B) Percent body weight change and spleen weights normalized to body weights were measured for each mouse. (C) Representative colon sections with ulcerated areas (demarcated by the brackets) in both genotypes with increasing concentrations of DSS. Sections were stained with H&E. Scale bars, 100 μm. (D) Quantification of affected areas within the entire length of colon is represented as a % ulceration. Bars indicate means ± SEM. Letters in the graph indicate significance measured by one-way analysis of variance with Bonferroni’s multiple comparison tests.
Histological evaluation of the colons was consistent with the clinical findings. D14:WT mice were not affected by DSS treatment at concentrations of up to 0.5%, whereas the D14:KO mice developed small lesions with evidence of crypt abscesses at this dose level (Figure 2C: 0.1% DSS and 0.5% DSS). D14:KO mice treated with 1% DSS demonstrated a comparable level of acute mucosal injury present in the D14:WT mice exposed to 2% DSS (Figure 2C: 1% DSS and 2% DSS). Regardless of mPGES-1 genotype, however, acute mucosal injury was accompanied by inflammatory cell infiltrates and crypt abscesses extending across approximately 40–70% of the entire length of the colons (Figure 2D). These results confirmed a previous report from Hara et al. (20), underscoring the critical contribution of the mPGES-1/PGE2 axis to the maintenance of mucosal integrity and intact epithelial barrier function.
Preneoplastic lesions develop within severely ulcerated areas in Apc Δ14/+ mice
Recovery from DSS-induced mucosal injury is initiated by epithelial restitution and regeneration contributed by normal crypts residing at the edges of the ulcerated areas (21). As described above, D14:WT mice treated with 1% DSS showed moderate levels of ulceration and the presence of regenerative crypts at 2 days after DSS withdrawal (Figures 2C, 3 A: H&E). These mice showed a normal repair process with evidence of epithelial restitution along the surface of the mucosa, indicated by the arrows (Figure 3A: H&E). When the levels of acute colonic injury were balanced by giving 2% DSS to D14:WT and 1% DSS to D14:KO mice, epithelial lesions consisting of clusters of colonic crypts confined within the ulcerated areas were evident 2 days after DSS withdrawal (Figure 3B: H&E). These lesions did not appear to arise from neighboring normal-appearing crypts and are probably to have undergone early neoplastic transformation, indicated by intense nuclear β-catenin staining (Figure 3B: β-catenin). Activation of β-catenin is confirmed by the positive staining for cyclin D1, a major downstream target of Wnt signaling pathway (22) (Supplementary Figure 2 is available at Carcinogenesis Online).
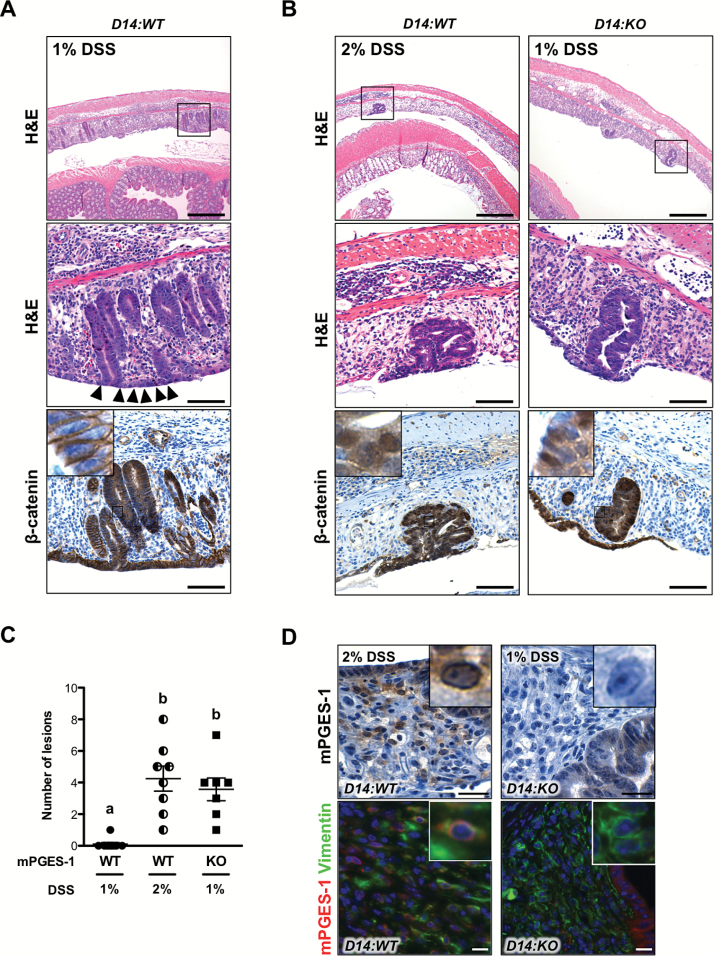
Figure 3.
Development of preneoplastic colon lesions within severely ulcerated areas in Apc Δ14/+ mice. Representative colon sections showing the presence of regenerative crypts in D14:WT mice treated with 1% DSS (A), and preneoplastic lesions in D14:WT treated with 2% DSS and D14:KO treated with 1% DSS (B). Enlarged areas are indicated by the boxes. Serial sections showing membrane or nuclear localization of β-catenin within the lesions. (C) The number of nuclear β-catenin-positive preneoplastic lesions in D14:WT and D14:KO treated with 1 or 2% DSS. Bars indicate means ± SEM. Letters indicate significance measured by one-way analysis of variance with Bonferroni’s multiple comparison tests. Scale bars 100 and 50 μm (insets). (D) Representative sections of colons immunostained for mPGES-1, showing a perinuclear localization within the infiltrating stromal cells in the D14:WT mice. Representative sections of colons analyzed by double immunofluorescence microscopy, showing co-localization of mPGES-1 with vimentin within the ulcerated areas. Scale bars, 50 μm.
As shown in Figure 3C, further quantification of these lesions show that they occur infrequently in the D14:WT mice treated with 1% DSS. However, when the extent of initial mucosal injury is balanced, these lesions are equally distributed and develop independently of mPGES-1 genotype (Figure 3C). To gain further insight into the potential role of mPGES-1 in the formation of these inflammation-associated pre-neoplastic lesions, serial sections of the colon were subjected to mPGES-1 immunostaining. As shown in Figure 3D, mPGES-1 was abundantly expressed within the perinuclear regions of cells infiltrating the ulcerated areas. Some of the mPGES-1-expressing cells within the colonic ulcer were co-localized with vimentin, suggesting that these cells are mesenchymal/fibroblast cells (Figure 3D: mPGES-1 Vimentin).
Severe DSS-induced injury increases adenoma formation
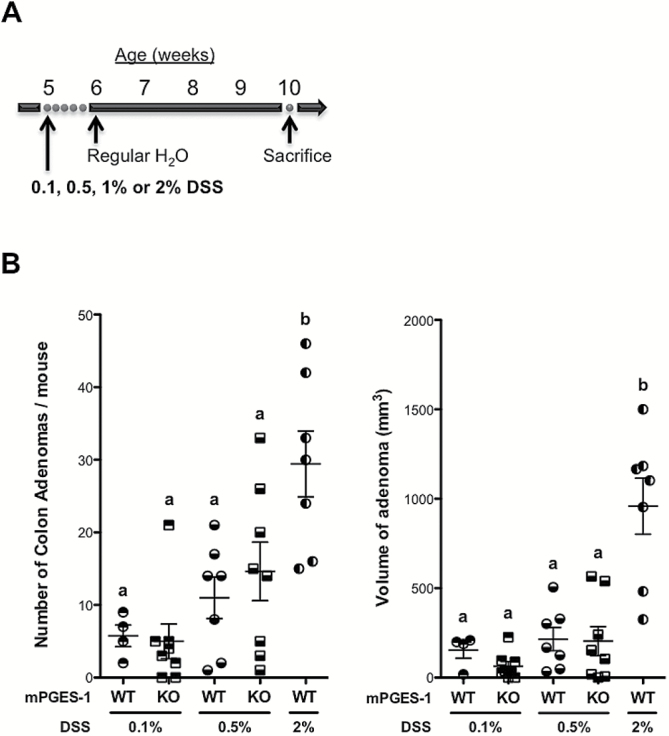
Based on these results, we developed the hypothesis that the initial severity of mucosal injury induced by DSS may ultimately influence the extent of tumorigenesis in the Apc Δ14/+ mice. To test this possibility, D14:WT and D14:KO mice were treated with increasing concentrations of DSS, ranging from 0.1 up to 2% and analyzed 4 weeks after treatment (Figure 4A). As shown in Figure 4B, there was a trend towards tumor suppression in the D14:KO mice at 0.1% DSS. However, this suppressive effect was mitigated as the concentration of DSS was increased to 0.5%, a level associated with acute mucosal injury in the D14:KO mice (Figure 4B). We then compared colon tumor multiplicity and size, in which the extent of initial mucosal injury was balanced by giving 2 versus 1% DSS, respectively, to D14:WT and D14:KO mice. As summarized in Table 1, when the extent of acute ulceration was equivalent, the total number of adenomas was comparable in both genotypes. However, the size of the adenomas was significantly reduced (2.9-fold, P < 0.0001) in the DSS-treated D14:KO mice.
Figure 4.
Suppression of inducible PGE2 production protects against inflammation-associated tumorigenesis. (A) Experimental scheme for D14:WT (n = 4–7) and D14:KO (n = 8) mice treated with 0.1, 0.5, 1 or 2% DSS. (B) Number and volume of colon adenomas in D14:WT and D14:KO mice 4 weeks after DSS treatment. Bars indicate means ± SEM. Letters in the graph indicate significance measured by one-way analysis of variance with Bonferroni’s multiple comparison tests.
Table 1.
Number and volume of colon adenomas in Apc Δ14/+ mice treated with DSS
| DSS (%) | n | % Ulceration (± SEM) | Adenomas | |||
|---|---|---|---|---|---|---|
| n | Number (± SEM) | Volume (± SEM) | ||||
| D14:WT | 2 | 8 | 73.11 (4.035) | 7 | 29.43 (4.535) | 958.9* (157.0) |
| D14:KO | 1 | 8 | 59.46 (2.720) | 18 | 26.17 (2.696) | 330.6 (59.49) |
*P < 0.0001.
Overall, these results indicate that genetic blockade of mPGES-1 affords significant protection to the colon in this inflammation-associated tumor model under conditions in which the extent of initial injury is comparable.
Discussion
The accumulation of bioactive lipids, most notably PGE2, is often found in colorectal tumors, underscoring the important role of this bioactive lipid in the carcinogenic process (23). Accordingly, considerable efforts have been made to target this metabolic pathway as a strategy for colon cancer prevention. To this end, NSAIDs have been widely applied for their chemopreventive efficacy, taking advantage of their ability to inhibit COX activities (7,24–26). Despite their considerable chemopreventive efficacy, however, the prolonged use of selective COX-2 inhibitors, such as Rofecoxib (Vioxx), have been associated with increased risk of cardiovascular disease, thus limiting their widespread application to high-risk populations (27). The adverse effects of the Coxibs are due in large measure to their global suppression of a panel of critical prostanoids required for normal homeostasis, including the pro-thrombotic TxA2 and antithrombotic PGI2 (28).
The off-target toxicities attributed to COX-2 inhibition have stimulated the development of mPGES-1 inhibitors as an alternative enzymatic target within this metabolic pathway that may afford cancer protection without the accompanying adverse effects associated with the Coxibs (1). Recent preclinical studies from our laboratory and others have established the potential benefit of targeting mPGES-1 for colon cancer prevention (2,10,11). Importantly, earlier studies have shown that targeting mPGES-1 can achieve highly selective inhibition of inducible PGE2 synthesis without affecting the levels of other important prostanoid metabolites (11,29–32).
In addition to its well-established role in tumor promotion, PGE2 also exerts pleiotropic effects with respect to inflammation. As described by Hanahan and Weinberg (33), inflammation is one of the fundamental hallmarks of cancer, and chronic inflammation accounts for up to 20% of cancer incidence in human populations (34). For example, patients with IBD, including ulcerative colitis and Crohn’s disease, have an increased risk of developing colorectal cancer, ranging from 2 to 18% depending on the severity, extent and duration of the disease (35–37). Chemopreventive options for treating colitis-associated cancer remain limited; most importantly, the use of NSAIDs in IBD patients represents a significant risk factor for exacerbation of the disease (38). Emerging evidence indicates that the adverse effects are probably due to the inhibitory effects of this drug class on PGE2 synthesis, leading in some cases to impaired mucosal barrier function (14). The important role of PGE2 with respect to epithelial homeostasis has been studied extensively (39–43). For example, exogenous administration of PGE2 has been shown to protect the gastric mucosa against ulcerogenic stimuli, including stress, necrotizing agents and NSAIDs (39,43). In addition, studies with DSS in PGE2 receptor (EP)-deficient mice have shown that PGE2 signaling via the EP4 receptor contributes to the maintenance of colonic mucosal integrity (40–42). In fact, the susceptibility of mPGES-1 deficient mice to DSS-induced acute colonic injury has been reported by Hara et al. (20). Moreover, we have observed that mPGES-1-deficient C57BL/6 mice failed to fully recover from DSS-induced tissue injury, even after withdrawal from DSS, and KO mice develop a phenotype that resembles chronic inflammation (44). These earlier studies and our present results confirm the fact that the mPGES-1/PGE2 axis is an important component of acute intestinal injury and clearly facilitates recovery from DSS-induced epithelial damage.
Intestinal wound healing is a complex process, involving an intricate array of cellular factors that regulate the interconnected mechanisms of epithelial restitution, proliferation and maturation (21). During this process, intestinal epithelial cells migrate from adjacent normal crypts to cover the wound bed (restitution), followed by restoration of the intact crypt architecture (45). Although DSS administration to WT C57BL/6 mice results in severe acute injury, the colonic mucosa is entirely repaired within only a few days after withdrawal of DSS (46), a process that is dependent upon epithelial restitution. Similarly, we have shown that in D14:WT mice that have sustained only mild ulcerative damage, wound healing is robust, indicated by the presence of normal-appearing regenerative crypts repopulating the epithelium (Figure 3A). This repair process is delayed in the D14:KO colons, probably accounting for incomplete reconstitution of the mucosal epithelium and their greater sensitivity to acute injury. However, as the extent of mucosal injury is increased, Apc Δ14/+ mice of either mPGES-1 genotype develop sporadic cryptal structures that form within the most severely ulcerated areas. Furthermore, these lesions do not appear to be ‘seeded’ from the adjacent unaffected normal epithelium, indicating that their formation is independent of epithelial restitution (Figure 3B). Importantly, these sporadic cryptal lesions share morphological features with early microadenomas, such as depletion of goblet cells, cellular dysplasia and the accumulation of nuclear β-catenin (Figure 3B). Overall, these observations suggest that they represent an early stage of neoplastic transformation occurring within a field of damaged colonic mucosa.
The formation of these sporadic cryptal lesions occurs in an mPGES-1-independent manner, indicating that their formation does not require inducible PGE2 synthesis. This is consistent with our previous observations that genetic inactivation of mPGES-1 does not affect early stages of tumor initiation in Apc Δ14/+ mice (11). The expansion and histological fate of these early neoplastic lesions found within ulcerated areas of the colon, however, may be highly dependent upon mPGES-1 status. In fact, our results support the contention that these isolated cryptal lesions are more effectively promoted to advanced adenomas within a tissue microenvironment that is enriched in PGE2 (Table I). Supporting these observations, Manieri et al. (47) have shown that colonic mesenchymal stem cells (cMSCs) express high levels of COX-2 following mucosal injury and elevated levels of PGE2 and PGI2 are found within a surgically induced wound bed. In fact, the accumulation of these COX-2 metabolites within the stroma was essential to the proliferative phase of intestinal wound repair (47). In a related study, the addition of PGE2 to intestinal subepithelial myofibroblasts induced the production of growth factors that promote intestinal epithelial proliferation, transformation and angiogenesis (48). These observations suggest that PGE2 contributes to intestinal mucosal homeostasis in part by orchestrating an epithelial-stromal cell communication (49).
Although the identity of the progenitor cells that give rise to the isolated cryptal lesions described in this study is unclear, it is possible that they are derived from crypt stem cells (50). Support for this mechanism comes from the observations of Barker et al. (50)., in which intestinal stem cells (Lgr5+ cells) undergoing loss of heterozygosity (LOH) of the Apc gene have enhanced proliferative properties, giving rise to a more advanced neoplasia. Based on these observations, it is possible that surviving intestinal stem cells residing within the widespread denuded areas of the DSS-treated colons may spontaneously acquire LOH of Apc prior to completion of the restitution process. If this were the case, elevated levels of PGE2 in the D14:WT mice would favor the growth and expansion of these early neoplastic lesions. This notion is supported by the results of several studies demonstrating that exogenous addition of PGE2 has been shown to increase LGR5 protein expression in colon cancer cell lines (51). Similarly, Fan et al. (52). recently demonstrated that arachidonic acid-derived PGE2 stimulates the growth of organoids isolated from mouse colonic crypts and significantly increases the number of Lgr5-GFP-positive stem cells in vitro. Furthermore, short-term treatment of Apc Min/+ mice with sulindac suppresses adenoma formation and induces apoptosis in Lgr5+ stem cells, suggesting that oncogenically transformed stem cells, including those without functional Apc, may be sensitive to NSAID treatment (53). These observations suggest that PGE2 is an essential component for the maintenance of stem cells by virtue of its growth stimulating properties. Moreover, the growth-promoting effects of inducible PGE2 may be context-dependent within an epithelial field harboring oncogenically transformed cells (Figure 5).
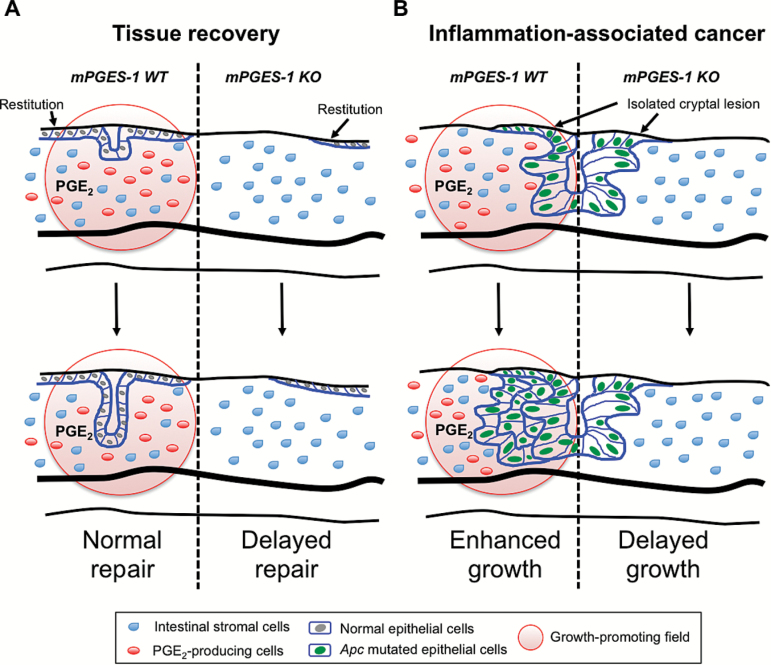
Fig.ure 5.
A schematic model depicting the functional roles of mPGES-1 within the colonic mucosa. Abundant expression of mPGES-1 generates a PGE2-enriched microenvironment following DSS-induced injury. (A) PGE2 plays a critical role in epithelial repair in the D14:WT mice. In the D14:KO mice, wound healing is delayed, resulting in incomplete tissue repair. (B) Apc-mutated epithelial cells form aberrant cryptal lesions, the growth of which is promoted by the PGE2-enriched microenvironment present in the D14:WT. In the D14:KO mice, lesion progression is severely impaired.
In summary, we have extended the potential chemopreventive benefit of targeting mPGES-1 to an inflammation-associated colon cancer model. By introducing the mPGES-1 null mutation onto Apc Δ14/+ mice, we have uncovered a dual role for inducible PGE2 synthesis within the intestinal mucosa; on the one hand, mPGES-1 activity is critical for maintaining epithelial homeostasis and mucosal integrity under injurious conditions. However, in colon tissue that harbors early neoplastic transformation, as evidenced by the presence of sporadic cryptal lesions found within areas of mucosal ulceration, mPGES-1 activity may actually contribute to colon cancer pathogenesis. Further studies to refine the chemoprotective benefit of targeting mPGES-1, while limiting the potential for mucosal injury are presently underway.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institute of Health (CA-13870205).
Supplementary Material
Acknowledgements
We thank Nicole Horelik and Yuichi Igarashi for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- COX
cyclooxygenase
- DSS
dextran sulfate sodium
- KO
knockout
- mPGES-1
microsomal PGE2 synthase-1
- NSAID
non-steroidal anti-inflammatory agents
- PGE2
prostaglandin E2
- PGES
prostaglandin E synthase
References
- 1. Jakobsson P.J., et al. (1999). Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA, 96, 7220–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakanishi M., et al. (2011). Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev. Res. (Phila)., 4, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakanishi M., et al. (2013). Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol., 35, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakanishi M., et al. (2010). mPGES-1 as a target for cancer suppression: A comprehensive invited review “Phospholipase A2 and lipid mediators”. Biochimie, 92, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer S.M., et al. (2011). Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev. Res. (Phila)., 4, 1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thun M.J., et al. (2012). The role of aspirin in cancer prevention. Nat. Rev. Clin. Oncol., 9, 259–267. [DOI] [PubMed] [Google Scholar]
- 7. Arber N., et al. (2008). Chemoprevention of colorectal neoplasia: the potential for personalized medicine. Gastroenterology, 134, 1224–1237. [DOI] [PubMed] [Google Scholar]
- 8. Wang D., et al. (2010). The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene, 29, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howe L.R., et al. (2013). Genetic deletion of microsomal prostaglandin E synthase-1 suppresses mouse mammary tumor growth and angiogenesis. Prostaglandins Other Lipid Mediat., 106, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasaki Y., et al. (2012). Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene, 31, 2943–2952. [DOI] [PubMed] [Google Scholar]
- 11. Nakanishi M., et al. (2008). Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res., 68, 3251–3259. [DOI] [PubMed] [Google Scholar]
- 12. Takahashi R., et al. (2014). Roles of microsomal prostaglandin E synthase-1 in lung metastasis formation in prostate cancer RM9 cells. Biomed. Pharmacother., 68, 71–77. [DOI] [PubMed] [Google Scholar]
- 13. Rosenberg D.W., et al. (2009). Mouse models for the study of colon carcinogenesis. Carcinogenesis, 30, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feagins L.A., et al. (2010). Do non-steroidal anti-inflammatory drugs cause exacerbations of inflammatory bowel disease? Dig. Dis. Sci., 55, 226–232. [DOI] [PubMed] [Google Scholar]
- 15. Lewis J.D., et al. (1999). Cancer risk in patients with inflammatory bowel disease. Gastroenterol. Clin. North Am., 28, 459–477. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T., et al. (2006). Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int. J. Cancer, 118, 25–34. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimi K., et al. (2009). Enhanced colitis-associated colon carcinogenesis in a novel Apc mutant rat. Cancer Sci., 100, 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colnot S., et al. (2004). Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab. Invest., 84, 1619–1630. [DOI] [PubMed] [Google Scholar]
- 19. Perše M., et al. (2012). Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol., 2012, 718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hara S., et al. (2010). Prostaglandin E synthases: Understanding their pathophysiological roles through mouse genetic models. Biochimie, 92, 651–659. [DOI] [PubMed] [Google Scholar]
- 21. Iizuka M., et al. (2011). Wound healing of intestinal epithelial cells. World J. Gastroenterol., 17, 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shtutman M., et al. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA, 96, 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rigas B., et al. (1993). Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med., 122, 518–523. [PubMed] [Google Scholar]
- 24. Kune G.A., et al. (1988). Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res., 48, 4399–4404. [PubMed] [Google Scholar]
- 25. Flossmann E., et al. (2007). Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet, 369, 1603–1613. [DOI] [PubMed] [Google Scholar]
- 26. Leshno M., et al. (2008). Point/Counterpoint: Aspirin is clinically effective in chemoprevention of colorectal neoplasia: point. Cancer Epidemiol. Biomarkers Prev., 17, 1558–1561. [DOI] [PubMed] [Google Scholar]
- 27. FitzGerald G.A., et al. (2001). COX-2 inhibitors and the cardiovascular system. Clin. Exp. Rheumatol., 19(6 suppl. 25), S31–S36. [PubMed] [Google Scholar]
- 28. Bresalier R.S., et al. (2005). Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med., 352, 1092–1102. [DOI] [PubMed] [Google Scholar]
- 29. Degousee N., et al. (2008). Microsomal prostaglandin E2 synthase-1 deletion leads to adverse left ventricular remodeling after myocardial infarction. Circulation, 117, 1701–1710. [DOI] [PubMed] [Google Scholar]
- 30. Wang M., et al. (2006). Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc. Natl. Acad. Sci. USA, 103, 14507–14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu D., et al. (2009). Comparison of microsomal prostaglandin E synthase-1 deletion and COX-2 inhibition in acute cardiac ischemia in mice. Prostaglandins Other Lipid Mediat., 90, 21–25. [DOI] [PubMed] [Google Scholar]
- 32. Cheng Y., et al. (2006). Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J. Clin. Invest., 116, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanahan D., et al. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 34. Aggarwal B.B., et al. (2006). Inflammation and cancer: how hot is the link? Biochem. Pharmacol., 72, 1605–1621. [DOI] [PubMed] [Google Scholar]
- 35. Canavan C., et al. (2006). Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment. Pharmacol. Ther., 23, 1097–1104. [DOI] [PubMed] [Google Scholar]
- 36. Rutter M., et al. (2004). Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology, 126, 451–459. [DOI] [PubMed] [Google Scholar]
- 37. Eaden J.A., et al. (2001). The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut, 48, 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Triantafillidis J.K., et al. (2011). Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Devel. Ther., 5, 185–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takeuchi K., et al. (2010). Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J. Pharmacol. Sci., 114, 248–261. [DOI] [PubMed] [Google Scholar]
- 40. Nitta M., et al. (2002). Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand. J. Immunol., 56, 66–75. [DOI] [PubMed] [Google Scholar]
- 41. Kabashima K., et al. (2002). The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest., 109, 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirata I., et al. (2001). Estimation of mucosal inflammatory mediators in rat DSS-induced colitis. Possible role of PGE(2) in protection against mucosal damage. Digestion, 63 (suppl. 1), 73–80. [DOI] [PubMed] [Google Scholar]
- 43. Robert A., et al. (1979). Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology, 77, 433–443. [PubMed] [Google Scholar]
- 44. Montrose D.C., et al. (2014). The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat., 116-117C, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taupin D., et al. (2003). Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol., 4, 721–732. [DOI] [PubMed] [Google Scholar]
- 46. Yan Y., et al. (2009). Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One, 4, e6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manieri N.A., et al. (2012). Igf2bp1 is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice. Gastroenterology, 143, 110–21.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shao J., et al. (2006). Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res., 66, 846–855. [DOI] [PubMed] [Google Scholar]
- 49. Powell D.W., et al. (2012). Mesenchymal stem cells and prostaglandins may be critical for intestinal wound repair. Gastroenterology, 143, 19–22. [DOI] [PubMed] [Google Scholar]
- 50. Barker N., et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457, 608–611. [DOI] [PubMed] [Google Scholar]
- 51. Al-Kharusi M.R., et al. (2013). LGR5 promotes survival in human colorectal adenoma cells and is upregulated by PGE2: implications for targeting adenoma stem cells with NSAIDs. Carcinogenesis, 34, 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fan Y.Y., et al. (2014). Differential effects of 2- and 3-series E-prostaglandins on in vitro expansion of Lgr5+ colonic stem cells. Carcinogenesis, 35, 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qiu W., et al. (2010). Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc. Natl. Acad. Sci. USA, 107, 20027–20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.