Neurodevelopmental disorders are characterized by an abnormal development of the central nervous system, leading to a myriad of symptoms and diseases, including intellectual disability, attention deficits, impairments in learning and memory, speech disorders and repetitive behavior (Telias and Ben-Yosef, 2014). Common major neurodevelopmental disorders include autism and autism spectrum disorders (ASDs), fragile X syndrome (FXS), Down syndrome (DS), and Rett syndrome (RTT). They can be collectively described as disorders in which the plasticity of the brain has been severely impaired. The concept of plasticity refers to the brain's ability to adapt to and process new information and react accordingly, and it can be classified into three categories: a) molecular plasticity, whenever specific receptors, ion channels, enzymes, neurotransmitters or other molecules that participate in neuronal function undergo up- or down-regulation in response to electrochemical inputs or outputs; b) cellular plasticity, when dendrites and axons grow or retract new spines and terminals to develop or eliminate connections within the neuronal network; c) tissue plasticity, when resident neural stem cells (NSCs) in the adult brain differentiate asymmetrically and produce new neurons and glia, which migrate and replenish brain areas in which neurons had died.
Neurodevelopmental disorders are apparently caused by mechanisms that affect neuronal plasticity in all three categories. For example, the expression of specific synaptic proteins in ASDs was found to be mis-regulated, and neurons affected by FXS displayed an excess of synaptic spines in their dendrites (Telias and Ben-Yosef, 2014). Notably, a common motif among neurodevelopmental disorders is a reduced capacity of affected NSCs to proliferate, differentiate and migrate. For example, one study that used a knock-out animal model for FXS demonstrated that FMRP, the protein missing in FXS, regulates the proliferation and differentiation of NSCs in the mouse brain via CDK4 and GSK3β (Luo et al., 2010). Different isoforms of MeCP2 (the genetic cause of RTT) were also shown to affect the differentiation potential of NSCs in vitro (Liyanage et al., 2013). Furthermore, a dosage increase in the chromosome 21 genes, DYRK1A and DSCR1, which simulates trisomy 21 in DS, was recently reported to cause a delay in NSCs differentiation and in a cell-fate shift through suppression of the transcription factor NFATc (Kurabayashi and Sanada, 2013). Based on these findings, it is reasonable to speculate that potential treatments for neurodevelopmental disorders could be aimed at rescuing impaired molecular, cellular or tissue plasticity of the brain. In this perspective article, we will discuss the potential therapeutic strategy of NSCs replacement in neurodevelopmental disorders and its foreseeable challenges (Figure 1).
Figure 1.
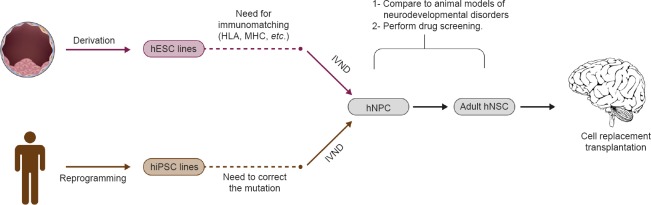
Flowchart illustrating different approaches for neural stem cell replacement in the brain as a therapy for neurodevelopmental disorders.
hESC: Human embryonic stem cell; HLA: human leukocyte antigen; MHC: major histocompatibility complex; hiPSC: human induced pluripotent stem cell; IVND: in vitro neural differentiation; hNPC: human neural precursor cell; hNSC: human neural stem cell.
Generation of NSCs from pluripotent SCs: NSCs in the postnatal and adult brain are found in both the subventricular zone (SVZ) and the subgranular zone (SGZ). They undergo asymmetric mitotic division that results in one identical daughter cell (self-renewal) and one cell that undergoes the initial steps of differentiation and commitment towards a final neuronal cell fate (Jessberger and Gage, 2014). The origin of adult NSCs is uncertain, but it is reasonable to hypothesize that they are formed during embryonic neurogenesis from a specific subset of embryonic neural precursor/progenitor cells (NPCs), which mature into adult NSCs and migrate and settle in the SVZ and SGZ (Grabel, 2012; Li et al., 2013). Unlike adult NSCs, embryonic NPCs do not self-renew in vivo due to their transient state during the process of embryonic development. However, embryonic NPCs can self-renew in vitro through advanced cell-culture techniques. Most importantly, self-renewing NPCs can be generated in vitro from human pluripotent SCs (hPSCs), including embryonic SCs (hESCs) and induced pluripotent SCs (hiPSCs). Several studies have reported in vitro neural differentiation of hPSCs into NPCs that are able to self-renew without further differentiation. However, whenever final neuronal differentiation is induced, division is symmetric and the resulting daughter cells will be composed of two neuron/glia cells. Therefore, the first and foremost challenge in NSC therapy is to establish reliable protocols for the generation of in vitro self-renewing multipotent NSCs from hPSCs and NPCs. Successful differentiation of hPSCs into NPCs that can then be further differentiated into NSCs must be validated not only at the functional level in bioassays that demonstrate asymmetric division of the putative NSCs, but also through the establishment of reliable biomarkers that can be easily detected. Moreover, it is important to find NPCs-NSCs biomarkers (preferably membrane proteins) that will enable sorting of the desired population of cells in order to achieve homogenous cell populations. While many such markers have been established for identifying NSCs within the SVZ and the SGZ, there is no detailed list of markers that are capable of showing the differentiation of hPSCs into NSCs (Gage and Temple, 2013).
Pluripotent SCs as a source for NSCs in modeling neurodevelopmental disorders: Before hPSCs were introduced into the field of disease modeling, the most prominent studies that explored the pathology of neurodevelopmental disorders were performed on transgenic animal models, such as drosophila, zebrafish and mice. When reliable protocols for differentiation of hPSCs into NSCs will be established, they could be used in basic research in order to confirm or reject the findings obtained in animal models by demonstrating that neurodevelopmental disorders are associated with reduced numbers of NSCs in the SVZ and/or reduced capability for these NSCs to differentiate into neurons (Luo et al., 2010; Kurabayashi and Sanada, 2013; Liyanage et al., 2013). Additionally, hPSCs can then be utilized to discover new molecular and cellular phenotypes for the disease in question. Therefore, generating NSCs from hPSCs will not only be beneficial for possible future therapies, but it will also be invaluable in disease modeling.
NSCs as a human in vitro platform for drug screening: NSCs that have been differentiated in vitro from hPSCs and NPCs can serve as a powerful tool in drug screening for the development of new therapeutic molecules for neurodevelopmental disorders. Once a human cellular phenotype has been established for any given neurodevelopmental disorder under investigation, bioassays can be devised which will be used to measure the response of these cells to various potential drugs. At the molecular level, gene expression in these cells can be tested directly by whole transcriptome analysis or analyzed specifically for a known target gene of interest by reverse-transcriptase PCR. Similarly, cellular and morphological tests, including differentiation, migration and survival assays, can be used to explore the differences between control and diseased NSCs, and examine whether certain drugs and compounds can rescue the diseased phenotype. Furthermore, in vitro NSCs that had been differentiated from control and diseased hPSCs can be used to test the toxicity of prospective drugs under examination. As such, NSCs offer the possibility of testing a potential therapeutic molecule that has been shown as being beneficial in animal models, and validate its effectiveness (or lack of) in a human-based model. Currently, most clinical trials for drug treatment of neurodevelopmental disorders move from the preclinical phase of animal studies directly to testing in human patients, without being evaluated or validated in any human in vitro model beforehand. Therefore, we consider that NSCs and NPCs that had been differentiated from hPSCs can be used as a reproducible and reliable human in vitro platform for drug screening and discovery.
Cell replacement in the brain: The idea of replacing impaired NSCs with healthy ones in the brains of individuals with neurodevelopmental disorders is certainly a very appealing prospect. Following engraftment, healthy NSCs would start re-populating the hippocampus and other brain areas with normal neurons that can generate correct synaptic connections. Furthermore, if proven successful, such a treatment will potentially require only one surgical intervention since NSCs’ asymmetric division will ensure the generation of new neurons together with a sustainable pool of self-renewing NSCs. This is in contrast to the approach of cell therapy in which post-mitotic neurons are transplanted but are limited by a relatively short lifespan, and thus may require repeated surgical interventions for additional cell transplantation. Furthermore, whereas in degenerative disorders like Alzheimer's or Parkinson's disease the main pathological hallmark is reduced number of neurons due to increased cell death, in neurodevelopmental disorders such as autism and FXS the main concern is reduced synaptic plasticity and the subsequent generation of incorrect synaptic connections between neurons. Therefore, while adding exogenous neurons to the brain would probably fail to improve neuronal function in neurodevelopmental disorders, slow replacement of resident cells by the activity of healthy NSCs would probably succeed.
Supposing that the differentiation protocol indeed generates bona-fide NSCs that express the expected markers and show asymmetric division, the next challenge will be to choose the correct type of hPSCs to use as a source for in vitro neural differentiation. hiPSCs offer the possibility of autologous transplantation, having been generated from the patient's own cells and therefore altogether precluding immune rejection. For neurodevelopmental disorders in which the specific mutation is unknown (e.g., ASDs), however, differentiation of hiPSCs will result in diseased NSCs that will not be useful for cell therapy. For neurodevelopmental disorders with known mutations (e.g., FXS, RTT, DS), hiPSCs established from the patient's own cells will have to undergo genetic manipulation (gene therapy) to rescue the mutation, such as the stable introduction of FMR1 (in FXS) or MeCP2 (in RTT) or the elimination of the extra chromosome 21 (in DS). It is possible that such manipulations will also result in the introduction of undesirable mutations and epigenetic abnormalities that cannot be predicted. The use of hESCs will certainly circumvent this limitation, although it will generate the need for the establishment of a large collection of hESC lines that will comprise a vast variety of HLA types in order to increase the chances for achieving an HLA-matched hESC line. However, even if an HLA-matched hESC line is found, patients will probably have to be treated with immunosuppression drugs to eliminate any immune rejection.
Two more obstacles will need to be overcome when dealing with both hiPSCs and hESCs. First, differentiation of NSCs for transplants will require growing large quantities of hPSCs for long culture periods. This can result in the accumulation of mutations related to adaptation to culture conditions (Ben-Yosef et al., 2013). Second, the presence of undifferentiated cells among the differentiated NSCs can lead to the development of tumors upon transplantation. Finally, timing of transplantation can be crucial. Current views in neuroscience hold that the brain's plasticity is reduced as it ages. Therefore, it should follow that there is a specific “window of opportunity” in which the introduction of healthy NSCs into the brains of patients with neurodevelopmental disorders will succeed in healing the brain by correcting impaired synaptic connections. The determination of such a window of opportunity continues to be elusive. However, if strategies are devised to solve these problems, it is possible to envision a future routine medical practice in which patients with neurodevelopmental disorders can be helped by replacing the NSCs in their brains with healthy ones. It is indeed a vision of the distant future, but the first steps towards achieving it are already being taken today.
References
- Ben-Yosef D, Boscolo FS, Amir H, Malcov M, Amit A, Laurent LC. Genomic analysis of hESC pedigrees identifies de novo mutations and enables determination of the timing and origin of mutational events. Cell Rep. 2013;4:1288–1302. doi: 10.1016/j.celrep.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Grabel L. Developmental origin of neural stem cells: the glial cell that could. Stem Cell Rev. 2012;8:577–585. doi: 10.1007/s12015-012-9349-8. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol. 2014;24:558–563. doi: 10.1016/j.tcb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Kurabayashi N, Sanada K. Increased dosage of DYRK1A and DSCR1 delays neuronal differentiation in neocortical progenitor cells. Genes Dev. 2013;27:2708–2721. doi: 10.1101/gad.226381.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fang L, Fernandez G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage VR, Zachariah RM, Rastegar M. Decitabine alters the expression of Mecp2 isoforms via dynamic DNA methylation at the Mecp2 regulatory elements in neural stem cells. Mol Autism. 2013;4:46. doi: 10.1186/2040-2392-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, Li W, Liu C, Jin P, Zhao X. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telias M, Ben-Yosef D. Modeling nNeurodevelopmental Disorders using human pluripotent stem cells. Stem Cell Rev. 2014;10:494–511. doi: 10.1007/s12015-014-9507-2. [DOI] [PubMed] [Google Scholar]


