Abstract
A variety of inflammatory cytokines are involved in spinal cord injury and influence the recovery of neuronal function. In the present study, we established a rat model of acute spinal cord injury by cerclage. The cerclage suture was released 8 or 72 hours later, to simulate decompression surgery. Neurological function was evaluated behaviorally for 3 weeks after surgery, and tumor necrosis factor α immunoreactivity and apoptosis were quantified in the region of injury. Rats that underwent decompression surgery had significantly weaker immunoreactivity of tumor necrosis factor α and significantly fewer apoptotic cells, and showed faster improvement of locomotor function than animals in which decompression surgery was not performed. Decompression at 8 hours resulted in significantly faster recovery than that at 72 hours. These data indicate that early decompression may improve neurological function after spinal cord injury by inhibiting the expression of tumor necrosis factor α.
Keywords: nerve regeneration, spinal cord injury, surgical decompression, tumor necrosis factor α, cell apoptosis, neurological function, neural regeneration
Introduction
Acute spinal cord injury (ASCI) can result in severe disability. At present, clinical treatment of ASCI relies mainly on surgery, but the optimal time at which to perform surgery remains debated. We therefore examined the effects of surgery at two time points in a rat model of ASCI.
Primary spinal cord injury is often irreversible, but secondary damage can be reduced with appropriate treatment. Thus, controlling secondary injury is an important goal of ASCI surgery in the restoration of motor function. Animal experiments have shown that surgical decompression soon after ASCI can promote the recovery of spinal nerve function (Kishan et al., 2005; Shields et al., 2005). A variety of inflammatory factors are released in the spinal cord after injury, including tumor necrosis factor α (TNF-α), whose expression correlates closely with the extent of recovery from nerve injury (Ding et al., 2010; Rouleau and Guertin, 2013; Wang et al., 2014). TNF-α is a cytokine involved in systemic inflammation and stimulation of the acute phase response. It is produced primarily by activated macrophages. TNF-α is a major mediator of the inflammatory response to a variety of biologically active factors. Traumatic ASCI causes a surge in the synthesis of TNF-α at the injury site (Bock et al., 2013; Cha et al., 2013), increasing local inflammation (Yune et al., 2003; Bachmeier et al., 2007). In the present study, we detected the expression of TNF-α in a cerclage-induced animal model of spinal cord injury, and proposed a theoretical basis for the treatment of ASCI.
Materials and Methods
Animals
One hundred clean-grade male Sprague-Dawley rats aged 13 weeks and weighing 255–376 g (323.6 ± 26.3 g) were provided by the Experimental Animal Center of Zhejiang Province in China (license No. SCXK (Zhe) 20080033). The study was approved by the Animal Ethics Committee of Yijishan Hospital Affiliated to Wannan Medical College, China. Rats were randomly and equally divided into four groups: control, model, 8-hour spinal decompression and 72-hour spinal decompression.
Preparation of ASCI models
Rat models of ASCI were established as described previously (Xu et al., 2011; Jones et al., 2012a). All rats were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (30–40 mg/kg). A median longitudinal incision (approximately 2.5 cm) was made above the T13 spinous process, using a portable X-ray machine (Siemens, Berlin, Germany) to confirm the correct position. Laminectomy was conducted at T13. A 5-0 silk suture (Covidien, Tyco Healthcare, USA) was passed through the gap between the anterior border of the dural sac and the posterior wall of the vertebral body, cut, and the needle was removed. One strand served as the measuring thread, and the other as the cerclage thread. Under a 10× objective (Olympus, Tokyo, Japan), the measuring thread was used to calculate the circumference (C1) of the dural sac. The length of the dural sac was measured with a waterproof digital vernier caliper (accuracy 0.01 mm). The mean spinal cord circumference for all rats was 7.82 mm (range, 6.62–8.97 mm). The target circumference after cerclage (C2) was calculated as C1 × 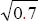 . The spinal cord was then ligated at T13, and the cross section of the dural sac was compressed to 70% of the original cross section.
. The spinal cord was then ligated at T13, and the cross section of the dural sac was compressed to 70% of the original cross section.
After model induction, the paraspinal muscles, fascia and skin incision were sutured under sterile conditions.
Rats in the control group underwent only laminectomy.
Spinal decompression
After establishment of the ASCI model, decompression surgery was performed 8 or 72 hours later by removing the cerclage suture. Animals in the model group did not undergo decompression surgery. All animals received postoperative care and manual bladder expression until the micturition reflex had returned (5–7 days).
Behavioral assessment
At 1, 3, 7, 14, and 21 days after modeling, five rats were selected from each group. Neurological recovery after SCI was evaluated in the morning under fasting conditions and after bladder evacuation, using the Basso Beattie and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995) and the inclined plane test score (Cheng et al., 1996; Rodicio and Barreiro-Iglesias, 2012). The maximum BBB score was 21 (no impairment) and lower scores indicated greater impairment in motor capacity. The inclined plane test assesses limb muscle strength, with lower scores indicating weakness. Each rat was evaluated three times, and the mean of the three measurements was taken.
Tissue collection
After behavioral assessment, rats were deeply anesthetized and perfused with 4% paraformaldehyde via the left ventricle. The full-length spinal cord was removed, and 1.5 cm tissue surrounding the damage site was obtained. The tissue was dehydrated conventionally, embedded in paraffin, and serially sectioned into 4 μm thick coronal slices. Eight sets of serial sections were obtained for each spinal cord.
Hematoxylin-eosin staining
Three sections of each group were used for hematoxylin and eosin staining (Pasyk and Hassett, 1989), and viewed under an optical microscope (Olympus). Slides were observed by two independent investigators, and morphological changes in nerve tissue were examined using Image-Pro Plus 6.0 image analysis software (Media Cybernetics, Bethesda, MD, USA).
Immunohistochemical staining
The remaining five sections from each group were used for immunohistochemical staining. The sections were dewaxed, hydrated, heated for antigen retrieval, treated with 3% H2O2 for inactivating endogenous enzymes, blocked in 5% bovine serum albumin, and incubated with rabbit anti-TNF-α polyclonal antibody (1:200; Wuhan Boster Biological Engineering Co., Ltd., Wuhan, Hubei Province, China) at 4°C overnight, according to the manufacturer's instructions. The following day, samples were incubated with the secondary antibody, biotinylated goat anti-rabbit IgG (1:100; Wuhan Boster Biological Engineering Co., Ltd.) in a wet box at 37°C for 30 minutes, and visualized using 3,3′-diaminobenzidine (Zhongshan Golden Bridge Biotechnology Co., Beijing, China) in a wet box at room temperature for 1 minute. The sections were washed three times with 0.01 mol/L PBS, for 5 minutes each time. Samples were then counterstained with hematoxylin, washed with running tap water, dehydrated through a graded alcohol series, permeabilized with xylene, and mounted with neutral resin. PBS was used in place of the primary antibody as a negative control. Cells with brown cytoplasm and/or nuclei were considered to have positive staining. Under a 400× objective, five fields of view per section were selected in the gray matter, and the number of positive cells in each field was quantified.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
Three sections were selected from each sample (48 sections in total). Staining was performed using a TUNEL apoptosis detection kit (Wuhan Boster Biological Engineering Co., Ltd.), according to the manufacturer's instructions. Apoptotic cells appeared brown under a microscope and were scattered among the gray matter. Apoptotic cells in the gray matter were counted in five fields of view per section, at 400× magnification.
Statistical analysis
Data were analyzed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA), and expressed as the mean ± SD. Group means were compared by one-way analysis of variance. Pairwise comparison was performed using independent-samples t-tests. P < 0.05 was considered statistically significant.
Results
Decompression promoted the recovery of neurological function in rat models of cerclage-induced spinal cord injury
The BBB and inclined plane test scores in the model group and both spinal decompression groups were lower than those in the control group (P < 0.05), and significantly higher in the decompression groups than in the model group (P < 0.05). Rats in the 8-hour spinal decompression group had higher BBB and inclined plane test scores than those in the 72-hour spinal decompression group (P < 0.05; Figure 1).
Figure 1.
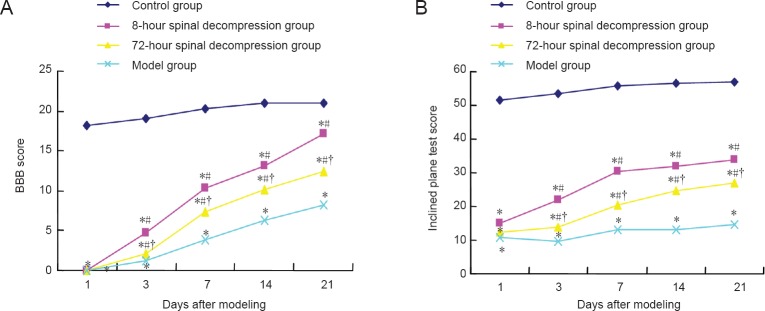
Recovery of neurological function in a rat model of cerclage-induced spinal cord injury, with or without spinal decompression at 8 or 72 hours after injury.
(A) Basso Beattie and Bresnahan (BBB) scores; (B) inclined plane test scores. n = 5 rats per group at each time point. *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. 8-hour spinal decompression group (one-way analysis of variance and independent sample t-test).
Decompression improved histopathological changes in rat models of cerclage-induced spinal cord injury
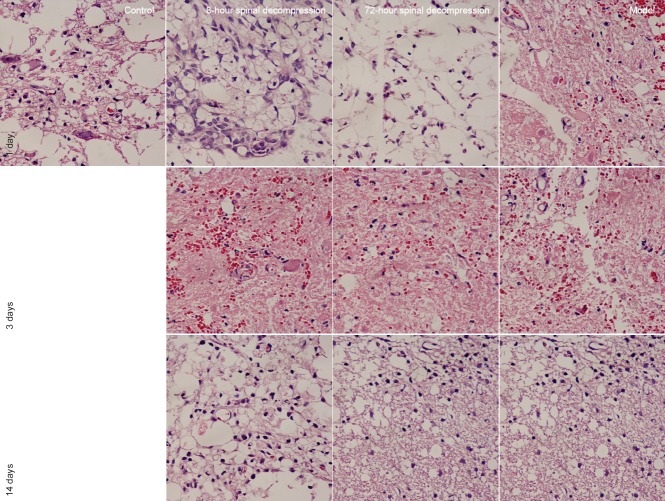
Hematoxylin-eosin staining showed that the morphology of spinal cord tissue in rats of the control group was well defined, without bleeding, edema or neuronal necrosis, whereas in the remaining groups the day after surgery, bleeding, edema, and neuronal death were evident. The damage was most obvious in the model group. In all groups, damage gradually reduced with time (Figure 2).
Figure 2.
Histopathological changes in the spinal cord of rats with cerclage-induced spinal cord injury, with or without spinal decompression at 8 or 72 hours after injury (hematoxylin-eosin staining, × 400).
Bleeding, edema and neuronal death are apparent in the model group on day 1. Decompression noticeably reversed these pathological characteristics, which also reduced with time.
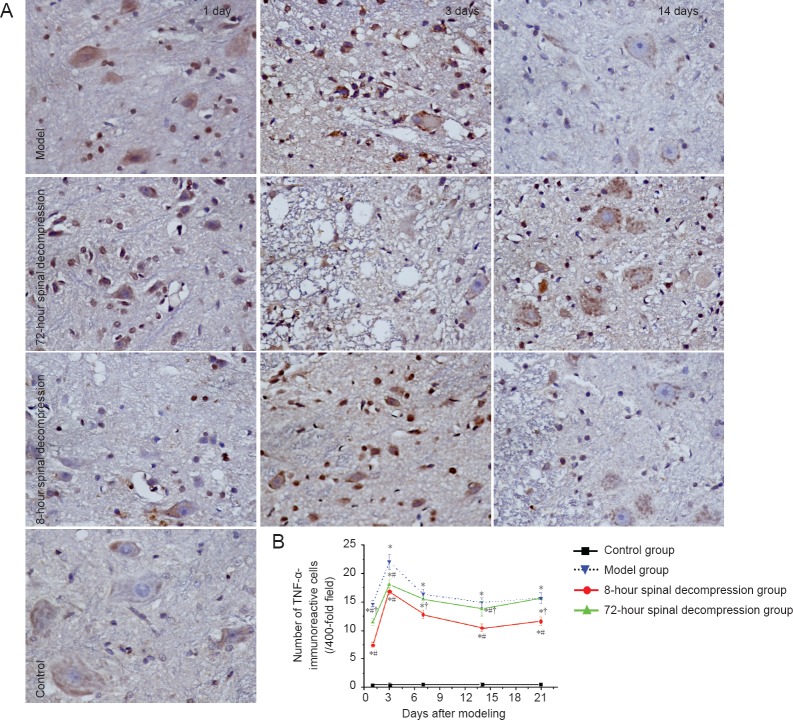
Decompression reduced TNF-α immunoreactivity in rat models of cerclage-induced spinal cord injury
Immunohistochemical staining revealed that TNF-α immunoreactivity was weak in the control group, but significantly higher in the spinal cord of rats from the model group (P < 0.05). Compared with the model group, TNF-α immunoreactivity was signifciantly weaker in both spinal decompression groups (P < 0.05). Moreover, TNF-α immunoreactivity was significantly weaker in the 8-hour spinal decompression group than in the 72-hour spinal decompression group (P < 0.05; Figure 3).
Figure 3.
Changes in TNF-α immunoreactivity in the spinal cord of rats with cerclage-induced spinal cord injury, with or without spinal decompression at 8 or 72 hours after injury.
(A) Brown staining showing TNF-α immunoreactivity (immunohistochemical staining, × 400). (B) Quantification of TNF-α-immunoreactive cells in the rat spinal cord. Data are expressed as the mean ± SD (n = 5 rats per group at each time point). *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. 8-hour spinal decompression group (one-way analysis of variance and independent-samples t-tests). TNF-α: Tumor necrosis factor alpha.
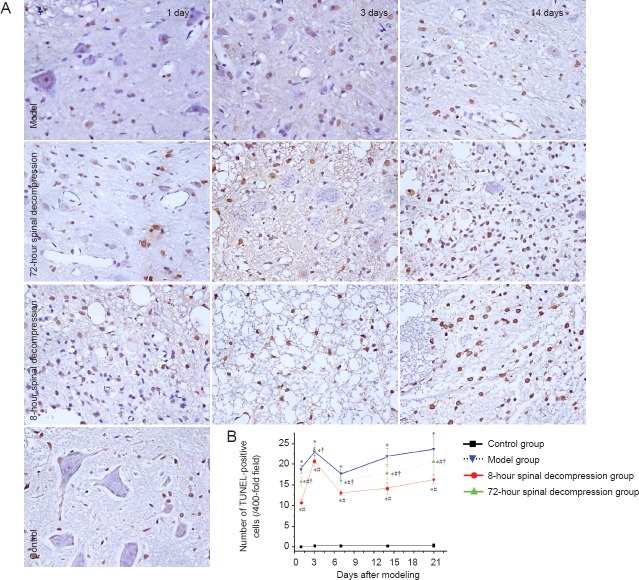
Decompression reduced the number of TUNEL-positive cells in the spinal cord of rats with cerclage-induced spinal cord injury
Few apoptotic cells were observable in the spinal cord of rats in the control group. There were significantly more TUNEL-positive cells in the model group than in the control group (P < 0.05) and fewer in both spinal decompression groups than in the model group (P < 0.05). The 8-hour spinal decompression group had fewer TUNEL-positive cells than the 72-hour spinal decompression group (P < 0.05; Figure 4).
Figure 4.
Alterations in apoptotic cells in the spinal cord of rats with cerclage-induced spinal cord injury, with or without spinal decompression at 8 or 72 hours after injury.
(A) TUNEL expression (brown stained cells) (× 400). (B) Quantification of TUNEL-positive cells in the rat spinal cord. Data are expressed as the mean ± SD (n = 5 rats per group at each time point). *P < 0.05, vs. control group; #P < 0.05, vs. model group; †P < 0.05, vs. 8-hour spinal decompression group (one-way analysis of variance and independent sample t-test).
Discussion
Preventing or ameliorating secondary injury remains a challenge in the treatment of ASCI. Damage caused by ASCI comprises primary mechanical damage and secondary injury. Secondary damage is mediated by multiple mechanisms including vascular factors, free radicals, excitatory amino acid toxicity, inflammation and apoptosis. Inflammation plays an important role in secondary pathological changes (Alexander and Popovich, 2009; Guimarães et al., 2009). The inflammatory response can further aggravate tissue damage, which is one of the main mechanisms leading to secondary damage after ASCI (Cheng et al., 2009).
TNF-α is produced by monocytes and macrophages. Its expression is first increased during the early stages after central nervous system injury. TNF-α upregulates the production of other cytokines (McCoy and Tansey, 2008), as well as inducing the release of arachidonic acid metabolites and lipid peroxides, increasing oxygen free radical generation, and damaging nerve cells, ultimately causing spinal cord tissue necrosis. All these factors have a damaging effect on cell membranes (Peng et al., 2006), and increases in TNF-α expression may be an important reason for the formation of edema after ASCI. Therefore, reducing nervous tissue inflammation is very important in the treatment of ASCI. TNF-α may also act as an exogenous signal to induce neuronal and glial cell apoptosis in the early stages after ASCI (Candolfi et al., 2004), by binding to its receptor, TNFR-1. The trimeric receptor binds to adaptor protein 1 and TNFR-associated death domain (TRADD). The death domain of TRADD binds to that of FADD, which transmits apoptotic signaling, activates caspase 8, and then activates downstream caspase 3, resulting in cell apoptosis (Tang et al., 2009). TNF-induced apoptosis may be mediated in part by nitric oxide (Genovese et al., 2008). TNF-α mRNA is rapidly upregulated within 3 hours of ASCI, peaking at 6 hours before decreasing after 7 days, and slowly returning to normal levels (McCoy and Tansey, 2008; Dong et al., 2010). Therefore, early detection of TNF can predict the extent of damage from ASCI. Miao et al. (2009) found that TNF-α expression was significantly elevated at 24 hours and increased further until at least 96 hours, as detected by enzyme linked immunosorbent assay. Similarly, Zhao (2010) demonstrated that TNF-α expression was elevated 1 day after ASCI, with high levels of TNF-α expression detected in spinal cord gray matter at 3 days, and remaining higher than normal levels at 7 days. Lv et al. (2008) also showed that TNF-α expression rapidly increased 6 hours after ASCI, returned to near basal levels at 24 hours, rose again at 3 days, and then diminished, but remained higher than normal levels 5 days later.
Results from a previous study on cerclage showed recovery of neurological function after ASCI (Jones et al., 2012b), with early decompression (8 hours) obtaining significantly better function than late decompression (72 hours) or no decompression. In the present study, we found fewer TNF-α- immunoreactive and TUNEL-positive cells in the spinal cord of the decompression groups, particularly in the 8-hour decompression group, than in the model group. When surgical decompression was delayed, neuronal damage increased and the number of apoptotic cells grew, correlating with poor functional recovery.
In summary, early spinal decompression surgery helps reduce neuronal injury and is conducive to functional recovery.
Footnotes
Funding: This study was supported by a grant from the Anhui Provincial Health Department-Funded Medical Research Project in 2009 in China, No. 09C33; a grant from the Key Scientific Research Project of Cultivating Fund of Wannan Medical College in China, No. WK2014ZF14.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, Boos N. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bock P, Spitzbarth I, Haist V, Stein VM, Tipold A, Puff C, Beineke A, Baumgärtner W. Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury. Brain Pathol. 2013;23:82–99. doi: 10.1111/j.1750-3639.2012.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Jaita G, Zaldivar V, Zárate S, Pisera D, Seilicovich A. Tumor necrosis factor-alpha-induced nitric oxide restrains the apoptotic response of anterior pituitary cells. Neuroendocrinology. 2004;80:83–91. doi: 10.1159/000081313. [DOI] [PubMed] [Google Scholar]
- Cha M, Ji Y, Masri R. Motor cortex stimulation activates the incertothalamic pathway in an animal model of spinal cord injury. J Pain. 2013;14:260–269. doi: 10.1016/j.jpain.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Wang KF, Li FT. Effect of pretreatment of aprotinin on nitric oxide and nitric oxide synthase contents after spinal cord ischemia-reperfusion injury in rabbits. Zhongguo Kangfu Lilun yu Shijian. 2009;15:109–111. [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Ding XJ, Wang HX, Wang T. Advances in sports training methods of spinal cord injury in rats. Zhongguo Kangfu Yixue Zazhi. 2010;25:589–591. [Google Scholar]
- Dong F, Lin JH, Wu ZY. Influence of intravenous administration of bone marrow mesenchymal stem cells on expression of TNF-α, IL-1β mRNA after spinal cord injury in rats. Zhongguo Gu yu Guanjie Sunshang Zazhi. 2010;25:697–699. [Google Scholar]
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E, Bramanti P, Cuzzocrea S. TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock. 2008;29:32–41. doi: 10.1097/shk.0b013e318059053a. [DOI] [PubMed] [Google Scholar]
- Guimarães JS, Freire MA, Lima RR, Souza-Rodrigues RD, Costa AM, dos Santos CD, Picanço-Diniz CW, Gomes-Leal W. Mechanisms of secondary degeneration in the central nervous system during acute neural disorders and white matter damage. Rev Neurol. 2009;48:304–310. [PubMed] [Google Scholar]
- Jones CF, Cripton PA, Kwon BK. Gross morphological changes of the spinal cord immediately after surgical decompression in a large animal model of traumatic spinal cord injury. Spine (Phila Pa 1976) 2012a;37:E890–899. doi: 10.1097/BRS.0b013e3182553d1d. [DOI] [PubMed] [Google Scholar]
- Jones CF, Newell RS, Lee JHT, Cripton PA, Kwon BK. The pressure distribution of cerebrospinal fluid responds to residual compression and decompression in an animal model of acute spinal cord injury. Spine (Phila Pa 1976) 2012b;37:E1422–1431. doi: 10.1097/BRS.0b013e31826ba7cd. [DOI] [PubMed] [Google Scholar]
- Kishan S, Vives MJ, Reiter MF. Timing of surgery following spinal cord injury. J Spinal Cord Med. 2005;28:11–19. doi: 10.1080/10790268.2005.11753793. [DOI] [PubMed] [Google Scholar]
- Lv ZG, Sun XL, Xu Y, Gong AH, Liang Y, Cui YH, Chen J, Zhang ZQ. A comparative study of the expression of inflammatory cytokines in cerebrospinal fluid and blood of rats with spinal cord injury. Linchuang Jianyan Zazhi. 2008;26:349–350. [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao WW, Yang YG, Cheng JP, Li CY. Tumor necrosis factor-α expression in rats with acute spinal cord injury. Zhongguo Laonian Xue Zazhi. 2009;29:535–537. [Google Scholar]
- Pasyk KA, Hassett CA. Modified hematoxylin and eosin staining method for epoxy-embedded tissue sections. Pathol Res Pract. 1989;184:635–638. doi: 10.1016/S0344-0338(89)80170-0. [DOI] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-α contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Rodicio MC, Barreiro-Iglesias A. Lampreys as an animal model in regeneration studies after spinal cord injury. Rev Neurol. 2012;55:157–166. [PubMed] [Google Scholar]
- Rouleau P, Guertin PA. A valuable animal model of spinal cord injury to study motor dysfunctions, comorbid conditions, and aging associated diseases. Curr Pharm Des. 2013;19:4437–4447. doi: 10.2174/1381612811319240010. [DOI] [PubMed] [Google Scholar]
- Shields CB, Zhang YP, Shields LBE, Han Y, Burke DA, Mayer NW. The therapeutic window for spinal cord decompression in a rat spinal cord injury model. J Neurosurg Spine. 2005;3:302–307. doi: 10.3171/spi.2005.3.4.0302. [DOI] [PubMed] [Google Scholar]
- Tang W, Wang W, Zhang Y, Liu S, Liu Y, Zheng D. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced chemokine release in both TRAIL-resistant and TRAIL-sensitive cells via nuclear factor kappa B. FEBS J. 2009;276:581–593. doi: 10.1111/j.1742-4658.2008.06809.x. [DOI] [PubMed] [Google Scholar]
- Wang YR, Mao HF, Chen JQ. Effects of huwentoxin on tumor necrosis factor apoptotic pathway in the hippocampus of a rat model of cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:5813–5818. [Google Scholar]
- Xu ZJ, Wang B, Yang M, Fang ZB. Evaluate the efficacy of early pressure at different times of experimental surgery after cerclage induced spinal cord injury. Zhonghua Shiyan Waike Zazhi. 2011;28:1191–1192. [Google Scholar]
- Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, Kim YC, Shin ML, Oh YJ, Han CT, Markelonis GJ, Oh TH. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma. 2003;20:207–219. doi: 10.1089/08977150360547116. [DOI] [PubMed] [Google Scholar]
- Zhao YP. The expression and distribution of endogenous NGF and TNF-α in acute phase of spinal cord injury. Shandong Yiyao. 2010;50:62–64. [Google Scholar]