Abstract
The mechanism involved in neural regeneration after spinal cord injury is unclear. The myelin-derived protein Nogo-A, which is specific to the central nervous system, has been identified to negatively affect the cytoskeleton and growth program of axotomized neurons. Studies have shown that Nogo-A exerts immediate and chronic inhibitory effects on neurite outgrowth. In vivo, inhibitors of Nogo-A have been shown to lead to a marked enhancement of regenerative axon extension. We established a spinal cord injury model in rats using a free-falling weight drop device to subsequently investigate Nogo-A expression. Nogo-A mRNA and protein expression and immunoreactivity were detected in spinal cord tissue using real-time quantitative PCR, immunohistochemistry and western blot analysis. At 24 hours after spinal cord injury, Nogo-A protein and mRNA expression was low in the injured group compared with control and sham-operated groups. The levels then continued to drop further and were at their lowest at 3 days, rapidly rose to a peak after 7 days, and then gradually declined again after 14 days. These changes were observed at both the mRNA and protein level. The transient decrease observed early after injury followed by high levels for a few days indicates Nogo-A expression is time dependent. This may contribute to the lack of regeneration in the central nervous system after spinal cord injury. The dynamic variation of Nogo-A should be taken into account in the treatment of spinal cord injury.
Keywords: nerve regeneration, spinal cord injury, contusion, Nogo-A, axon growth, immunohistochemistry, fluorescent quantitative PCR, neural regeneration
Introduction
The discovery of the Nogo family of proteins provides an opportunity to develop interventions to promote axonal regeneration in the central nervous system after injury. Basic research and clinical study of Nogo has increased our understanding of the mechanisms underlying spinal cord injury, multiple sclerosis, and neurodegenerative diseases. There are three major isoforms of Nogo: Nogo-A, Nogo-B and Nogo-C. The inhibitory actions of Nogo-A are dependent on two domains, Nogo-66 which is present in all three isoforms, and a sequence referred to as Nogo-delta 20, that is unique to the long N-terminal domain of Nogo-A. Nogo-A exerts its effects by binding to Nogo Receptor 1, via the Nogo-66 domain (Wu et al., 2014). Nogo-A specifically negatively regulates axonal regeneration in the central nervous system because Nogo receptor is only expressed by limited classes of neurons (Hunt et al., 2002b; Josephson et al., 2002). However, Nogo-A has additional inhibitory domains in its unique sequence (Prinjha et al., 2002) that do not require Nogo receptor for activity (Niederost et al., 2002). Growing evidence shows that lack of nerve regeneration after spinal cord injury is associated with a high expression of myelin-associated inhibitory molecules. Nogo-A is considered to be the major growth inhibitor, but its expression after spinal cord injury is not entirely clear (Schweigreiter et al., 2006; Cafferty et al., 2010; Lee et al., 2010; Pernet et al., 2012). Understanding its expression pattern after injury will allow the development of methods to effectively manipulate its levels and will be beneficial for the treatment of axonal regeneration after spinal cord injury (Walmsley et al., 2007; Müllner et al., 2008; Montani et al., 2009; Kern et al., 2013). In this study, we used weight-drop-caused spinal cord injury rats to investigate the variation of Nogo-A expression in spinal cord tissue at different time points using immunohistochemistry, real-time quantitative PCR and western blot analysis. A comparative study using normal and sham-operated rat spinal cord tissue was carried out to clarify the role of Nogo-A protein in the nerve regeneration process.
Materials and Methods
Animals
A total of 108 adult female Sprague-Dawley rats weighing 180–220 g (Experimental Animal Center of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China) were maintained separately and were given free access to food and water at the Animal Experimental Center of Nanjing University of Traditional Chinese Medicine in China (license No. SCXK (Zhe) 2008-0033). The protocol was performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals made by the Ministry of Science and Technology of China.
Animal grouping and model establishment
The rats were randomly and equally assigned to three groups: normal group, sham-operated group and model group (n = 36). The rats in the normal group were not operated. In the sham-operated and model groups, the vertebral column was exposed and a laminectomy was performed. The skin, subcutaneous tissue and deep fascia of rats in the sham-operated group were sutured and they received no spinal cord injury.
A contusion injury was performed on rats in the model group according to Allen's (1911) advanced method. Under anesthesia of chloral hydrate (0.4 mL/100 g, intraperitoneal injection, Aoxin, Jiangsu Province, China), the vertebral column of the rats was exposed and a laminectomy was performed at the T9–11 level. A contusion injury was inflicted using an advanced self-made weight-drop device in rats. A weight of 10 g was dropped from a height of 25 mm on the exposed spinal cord. Spastic convulsions could be seen in the rats’ hindlimb several times which was followed by flaccid paralysis. Following contusion, the incision was closed with wound clips. Penicillin (80,000 units/day, intraperitoneal injection, Yakang, China) was used to protect from postoperative infection. During recovery, urine was expressed manually, twice daily, to assist in urination untilper intrinsic function returned to normal.
Spinal cord extraction
Rats were anesthetized and decapitated at 24 hours, 3 days, 7 days or 14 days postoperatively according to the studies of Josephson et al. (2001), Hunt et al. (2003) and Wang et al. (2008). At each time point, three rats in each group were randomly selected for immunohistochemistry, western blot analysis and real-time quantitative PCR test. Using aseptic procedures, rats were flushed with saline and fixed with 4% paraformaldehyde/PBS for 2 hours. The injured region was removed (about 1 cm) from the spinal cords and immediately frozen at −70°C. These were used for the detection of Nogo mRNA and protein expression. Samples for immunohistochemistry were fixed in 4% paraformaldehyde/PBS overnight and then frozen at 4°C.
Immunohistochemistry
Tissue samples of spinal cord were dehydrated and embedded in paraffin following routine methods. Ten serial sections were cut (4–5 μm thick) coronally through the lesion site. Five sections were randomly selected to detect Nogo-A using the EnVision Kit (Dako, Produktionsvej, Denmark) according to the manufacturer's protocol (Primary antibody: rabbit anti-mouse polyclonal antibody, 1:400, Abcam, HK, China. Secondary antibody: goat anti-rabbit IgG-Horseradish Peroxidase, 1:2,000, Bioworld, MN, USA). 3,3′-Diaminobenzidine coloration was used with hematoxylin as a counterstain. Sections were observed using an inverted biological microscope, and images taken on a digital camera were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). Five fields of view were randomly chosen per section within each slice at 200× magnification. Nogo-A immunoreactivity, indicated by pale brown staining, was quantified using the mean value of optical density in five fields of view.
Western blot analysis
Spinal cords were homogenized in RIPA buffer for 30 minutes on ice, and the homogenate was centrifuged at 15,000 × g for 30 minutes at 4°C. Protein was extracted from the supernatant and concentrations were then determined using the Bradford method. Loading buffer was used for protein denaturation. Equal amounts of protein were then resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% for β-actin, 6% for Nogo-A) and transferred onto polyvinylidene fluoride membrane with Trans-blot®SD Semi-dry Electroblotting Apparatus (25 V, 30 minutes for Nogo-A, 25 V, 22 minutes for β-actin). The membrane was blocked for 2 hours at room temperature with PBS containing 5% skimmed milk and 0.1% Tween-20, washed with 1 × TBST for five times and then incubated for 12 hours at 4°C with primary antibodies specific for β-actin (rabbit anti-mouse monoclonal antibody, 1:1,000, CST, Danvers, MA, USA) and Nogo-A (rabbit anti-mouse polyclonal antibody, 1:1,000, Abcam, HK, China) in the blocking buffer. The membrane was subsequently incubated with goat anti-rabbit IgG-horseradish peroxidase (for Nogo: 1:1,500, for β-actin: 1:3,000, Bioworld, MN, USA), then visualized using a chemiluminescence system (Image Quant LAS 4000 mini, GE, NY, USA). Fuji X-ray film (Fuji, Kanagawa, Japan) with cassette closure times was used to obtain adequate exposure and to visualize bands. Bands were analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, MD, USA). β-Actin was taken as an internal control. The relative protein expression of Nogo-A was demonstrated by the ratio of gray scale between Nogo-A and β-actin.
Real-time quantitative PCR
RNA from tissue specimens in each group were purified using Trizol RNA isolation reagent according to the manufacturer's protocol (Biyuntian, Nanjing, Jiangsu Province, China). A reverse transcription kit (Biyuntian) was used to make cDNA, and PCR amplification was performed. The upstream primer sequence of Nogo-A was 5′-GAC AGA AAT GGG CAG CAT AGT-3′; the downstream primer sequence was 5′-CAG AGA CAG CAG CAG GAA TAA-3′. The PCR program began with an initial 30 second denaturation step at 95°C. This was followed by 40 cycles of 5 seconds at 95°C for denaturation and 34 seconds at 60°C for annealing and extension. The relative expression of Nogo-A mRNA was estimated using the CT comparative method (2−ΔΔCt) by Liebscher (2005). GAPDH was taken as the internal reference gene. The upstream primer sequence of GAPDH was 5′-CTG AGC ACT CTC CCT CAC AAT TC-3′; the downstream primer sequence was 5′-GTG CAG CGA ATC TTA TTG ATG GT-3′.
Statistical analysis
All normally distributed values are presented as the mean ± SD. Statistical analysis was performed using SPSS version 19.0 software (SPSS, Chicago, IL, USA). The significance of any differences between three groups was evaluated using one-way analysis of variance and the least significant difference test. A probability of 95% was taken to indicate significant difference.
Results
Immunoreactivity of Nogo-A in rat spinal cord
In the sham-operated group, weak Nogo-A immunoreactivity was observed (compared with the normal group) at each time point (24 hours, 3 days, 7 days, 14 days post-injury) compared with the normal group and there was no difference compared with that in the normal group (P > 0.05). The immunoreactivity of Nogo-A slightly increased at 24 hours after spinal cord injury in the model group, compared with the sham-operated group, however, there was no significant difference between the two groups (P > 0.05). In the model group, the immunoreactivity, as measured by mean optical density of Nogo-A staining, markedly decreased at 3 days post-injury (P < 0.01), significantly increased to a peak at 7 days post-injury (P < 0.01), and then declined at 14 days post-injury (P < 0.01; Figure 1).
Figure 1.
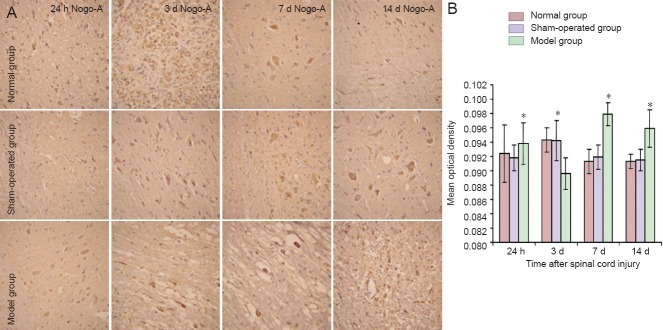
Sequential immunohistochemical expression of Nogo-A in the rat spinal cord after injury.
(A) Nogo-A immunoreactivity in injured spinal cord (T9–11). Nogo-A immunoreactivity is indicated by pale brown staining. Images were taken at 200 × magnification. (B) Immunohistochemical positive cells were found in the model group at 3 days post-injury, reaching a peak at 7 days and then slowly decreasing at 14 days. *P < 0.01, vs. sham-operated group. Data are expressed as the mean ± SD of three rats in each group, one-way analysis of variance and the least significant difference test were used. h: Hours; d: days.
Protein expression of Nogo-A in rat spinal cord
Western blot analysis showed that Nogo-A protein expression was stable and similar in the normal group and sham-operated group and there was no difference at all time points (P > 0.05). However, in the model group, the expression of Nogo-A dynamically varied after spinal cord injury. Nogo-A expression was initially low at 24 hours after spinal cord injury, and then dropped to its lowest level at 3 days before gradually rising to a peak at 7 days, and then declining again after 14 days. Although lower, the expression at 14 days remained at a high level and was significantly different compared with the sham-operated group at all time points (P < 0.05; Figure 2).
Figure 2.

Protein expression of Nogo-A in injured spinal cord at different time points after injury.
Nogo-A expression was stable and similar between the normal group (normal) and sham-operated group (sham), without significant difference (P > 0.05). The expression of Nogo-A after spinal cord injury in the model group (model) varied dynamically. The expression of Nogo-A in the model group at 24 hours after spinal cord injury was low, and dropped to the lowest level at 3 days, then gradually rose to a peak at 7 days, and gradually declined after 14 days. However, the expression in the model group remained at a high level, which was significantly different from that in the sham group at all time points (*P < 0.05, vs. sham-operated group). Data were expressed as the mean ± SD of three rats in each group. One-way analysis of variance and the least significant difference test were used. h: Hours; d: days.
The mRNA expression of Nogo-A in rat spinal cord
At each time point, Nogo-A mRNA expression in the sham-operated group was not significantly different compared with that in the normal group (P > 0.05). However, in the model group, the expression of Nogo-A mRNA was significantly lower at 24 hours post-injury compared with the sham-operated group. It dropped to its lowest level at 3 days, then rapidly rose to a peak at 7 days before declining slightly at 14 days post-injury. At both 7 and 14 days post-injury, the expression of Nogo-A mRNA in the model group was still significantly higher than that in the sham-operated group. The differences in expression were significantly different at every time point between sham-operated group and model group (P < 0.01; Figure 3).
Figure 3.
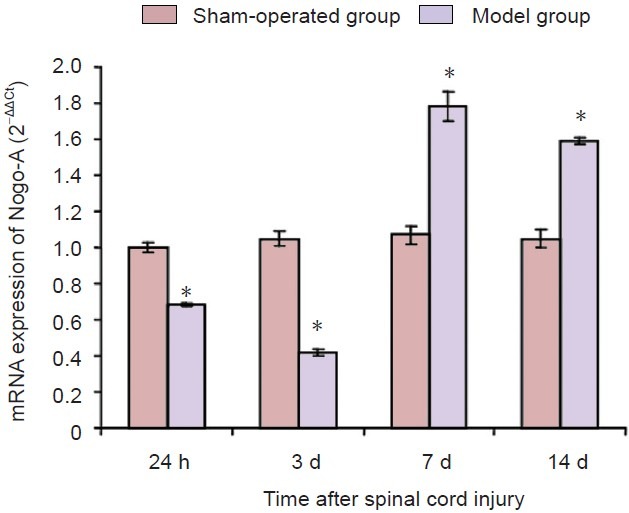
Expression of Nogo-A mRNA in injured spinal cord at different time points after spinal cord injury (real-time quantitative PCR).
The expression of Nogo-A mRNA in the model group was low at 24 hours after spinal cord injury, and dropped to its lowest level at 3 days, then rapidly rose to a peak at 7 days, and gradually declined after 14 days. The expression of Nogo-A mRNA in the model group was still higher than that in the sham-operated group at all time points (*P < 0.05, vs. sham-operated group). Data were expressed as the mean ± SD of three rats in each group. One-way analysis of variance and the least significant difference test were used. h: Hours; d: days.
Discussion
The microenvironment of the central nervous system is crucial to the survival and regeneration of damaged nerve tissue. Nogo protein is one of the myelin-associated growth inhibitory proteins and is thought to play an important role in spinal cord regeneration (Hunt et al., 2003; Baumann et al., 2009; Hånell et al., 2010; Hou et al., 2010; Raiker et al., 2010; Anja et al., 2012). Nogo-A is a myelin-related axonal growth inhibitory factor secreted by oligodendrocytes and stored in the white matter of the central nervous system, which is rich in myelinated membranes (Vitellaro-Zuccarello et al., 2007; Chong et al., 2012; Saha et al., 2014). Intracellular Nogo-A is released by oligodendrocytes and myelin into the extracellular matrix after spinal cord injury, and inhibits axonal regeneration. As an important nerve growth inhibitory factor, Nogo-A is currently at the forefront of biomedical research (Liebscher et al., 2005; Weinmann et al., 2006).
In this study, we found that a spinal cord injury model had low protein and mRNA expression at 24 hours after spinal cord injury, and dropped to the lowest level at 3 days, rapidly rose to a peak at 7 days, and gradually declined at 14 days. Compared with sham-operated group, the expression of Nogo-A protein and mRNA was significantly increased at 7 and 14 days post-injury. This confirms that spinal cord injury was responsible for the high expression of Nogo-A.
It is widely believed that Nogo-A launches a signaling cascade after binding with its receptors and induces transformation of actin-cytoskeleton in growth cones and contraction of both filopodia and lamellipodia. All of these change the shape and stability of growth cones and then cause degeneration, which inhibits axon regeneration (Broggini et al., 2010; Joset et al., 2010; Wu et al., 2010; Hui et al., 2013). As a result, the high expression of Nogo-A after spinal cord injury may be one of the main reasons why nerves do not regenerate in the central nervous system.
The dynamic variation of Nogo-A in the rat spinal cord has been previously reported (Wang et al., 2013). Huber's team (2002) found expression levels of Nogo-A of postnatal Lewis rats were not changed significantly after traumatic lesions to the spinal cord. Hunt et al. (2003) showed that following injury to the spinal cord, Nogo-A mRNA was upregulated around the lesion and Nogo-A protein was strongly expressed in injured dorsal column fibres and their sprouts which entered the lesion site. Guo et al. (2005) showed high expression of Nogo-A appeared immediately after spinal cord injury in rats. The differences observed in these studies may be explained by the use of different breeds of animals, type of spinal cord injury surgery and the time points observed in the experiment. However, assessment of Nogo-A immunohistochemistry, real-time quantitative PCR and western blot analysis and observation for 14 days had not previously been studied. Our study showed that after spinal cord injury, Nogo-A was first expressed at a lower level, dropped even further, and then rapidly rose to a peak, which is similar to the study by Wang et al. (2008). In contrast to Wang's study, we used three different methods of Nogo-A measurement and found a slow decline in process in the last 7 days. We speculate that the initial low expression is relevant to acute injury and necrosis of spinal cord. The linearized expression of Nogo-A indicates 7 days post spinal cord injury as a time window for intervention.
Acknowledgments
We would like to thank Yalan Pan and Hao Chen from Nanjing University of Traditional Chinese Medicine, China for their participation in this research.
Footnotes
Funding: This study was financially supported by a grant from the Natural Science Foundation of Jiangsu Province, No. BK2011180; Ordinary University Graduate Student Scientific Research Innovation Projects of Jiangsu Province, No. CXZZ13-0614, CXZZ12-0609.
Conflicts of interest: None declared.
Copyedited by Paul P, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J Am Med Assoc. 1911;LVII:878–880. [Google Scholar]
- Baumann MD, Austin JW, Fehlings MG, Shoichet MS. A quantitative ELISA for bioactive anti-Nogo-A, a promising regenerative molecule for spinal cord injury repair. Methods. 2009;47:104–108. doi: 10.1016/j.ymeth.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Broggini T, Nitsch R, Savaskan NE. Plasticity-related gene 5 (PRG5) induces filopodia and neurite growth and impedes lysophosphatidic acid- and nogo-A-mediated axonal retraction. Mol Biol Cell. 2010;21:521–537. doi: 10.1091/mbc.E09-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJ, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. doi: 10.1073/pnas.1113540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Li SR, Su BY. Nogo-A immunoreactivity in injuried spinal cord of adul trats. Neurosci Bull. 2005;21:28–290. [Google Scholar]
- Hånell A, Clausen F, Björk M, Jansson K, Philipson O, Nilsson LN, Hillered L, Weinreb PH, Lee D, McIntosh TK, Gimbel DA, Strittmatter SM, Marklund N. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27:1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Shi Y, Cheng S, Yang X, Li L, Xiao C. Nogo-A expresses on neural stem cell surface. Int J Neurosci. 2010;120:201–205. doi: 10.3109/00207450903506502. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SP, Monaghan JR, Voss SR, Ghosh S. Expression pattern of Nogo-A, MAG, and NgR in regenerating urodele spinal cord. Dev Dyn. 2013;242:847–860. doi: 10.1002/dvdy.23976. [DOI] [PubMed] [Google Scholar]
- Hunt D, Coffin RS, Prinjha RK, Campbell G, Anderson PN. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol. 2001;169:319–328. doi: 10.1006/exnr.2001.7659. [DOI] [PubMed] [Google Scholar]
- Joset A, Dodd DA, Halegoua S, Schwab ME. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 2010;188:271–285. doi: 10.1083/jcb.200906089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern F, Stanika RI, Sarg B, Offterdinger M, Hess D, Obermair GJ, Lindner H, Bandtlow CE, Hengst L, Schweigreiter R. Nogo-A couples with Apg-1 through interaction and co-ordinate expression under hypoxic and oxidative stress. Biochem J. 2013;455:217–227. doi: 10.1042/BJ20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injure drats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Montani L, Gerrits B, Gehrig P, Kempf A, Dimou L, Wollscheid B, Schwab ME. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/Cofilin in the unlesioned adult nervous system. J Biol Chem. 2009;284:10793–10807. doi: 10.1074/jbc.M808297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME. Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci. 2008;27:326–333. doi: 10.1111/j.1460-9568.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Schwab ME. The role of Nogo-A in axonal plasticity regrowth and repair. Cell Tissue Res. 2012;349:97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- Saha N, Kolev M, Nikolov DB. Structural features of the Nogo receptor signaling complexes at the neuron/myelin interface. Neurosci Res. 2014 doi: 10.1016/j.neures.2014.06.003. doi:10.1016/j.neures.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Scholze AR, Barres BA. A Nogo signal coordinates the perfect match between myelin and axons. Proc Natl Acad Sci USA. 2012;109:1003–1004. doi: 10.1073/pnas.1120301109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigreiter R, Bandtlow CE. Nogo in the injured spinal cord. J Neurotrauma. 2006;23:384–396. doi: 10.1089/neu.2006.23.384. [DOI] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Gorio A, De Biasi S. Erythropoietin-mediated preservation of the white matter in rat spinal cord injury. Neuroscience. 2007;144:865–877. doi: 10.1016/j.neuroscience.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Wang CF, Wu CL, Ma YC. The expression of nerve growth inhibiting factor Nogo-A after spinal cord injury in rats. Linchuang Guke Zazhi. 2008;11:572–575. [Google Scholar]
- Wang D, Fan YH, Zhang JJ. Transplantation of Nogo-66 receptor gene-silenced cells in a poly(D, L-lactic-co-glycolic acid) scaffold for the treatment of spinal cord injury. Neural Regen Res. 2013;8:677–685. doi: 10.3969/j.issn.1673-5374.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhao H, Fan YP, Gong HY, Li M, Qi F, Liu Y. Expression of nerve growth inhibitory factor of Nogo-A after spinal cord injury in rats. Chin J Integr Med. 2008;16:167–172. [Google Scholar]
- Weinmann O, Schnell L, Ghosh A, Montani L, Wiessner C, Wannier T, Rouiller E, Mir A, Schwab ME. Intrathecally infused antibodies against Nogo-A penetrate the CNS and downregulate the endogenous neurite growth inhibitor Nogo-A. Mol Cell Neurosci. 2006;32:161–173. doi: 10.1016/j.mcn.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang H, Qiu Z, Zhang Q, Ding T, Geng D. Effect of combined treatment with methylprednisolone and Nogo-A monoclonal antibody after rat spinal cord injury. J Int Med Res. 2010;38:570–582. doi: 10.1177/147323001003800219. [DOI] [PubMed] [Google Scholar]
- Wu SX, Yu ZH, Liu F, Lin HY, Zhang ZY, Zhang CS. Role and mechanism of retinoic acid in axonal regeneration. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2450–2454. [Google Scholar]


