Abstract
Edaravone has been shown to delay neuronal apoptosis, thereby improving nerve function and the microenvironment after spinal cord injury. Edaravone can provide a favorable environment for the treatment of spinal cord injury using Schwann cell transplantation. This study used rat models of complete spinal cord transection at T9. Six hours later, Schwann cells were transplanted in the head and tail ends of the injury site. Simultaneously, edaravone was injected through the caudal vein. Eight weeks later, the PKH-26-labeled Schwann cells had survived and migrated to the center of the spinal cord injury region in rats after combined treatment with edaravone and Schwann cells. Moreover, the number of PKH-26-labeled Schwann cells in the rat spinal cord was more than that in rats undergoing Schwann cell transplantation alone or rats without any treatment. Horseradish peroxidase retrograde tracing revealed that the number of horseradish peroxidase-positive nerve fibers was greater in rats treated with edaravone combined withSchwann cells than in rats with Schwann cell transplantation alone. The results demonstrated that lower extremity motor function and neurophysiological function were better in rats treated with edaravone and Schwann cells than in rats with Schwann cell transplantation only. These data confirmed that Schwann cell transplantation combined with edaravone injection promoted the regeneration of nerve fibers of rats with spinal cord injury and improved neurological function.
Keywords: nerve regeneration, spinal cord injury, Schwann cells, cell transplantation, edaravone, motor function, electrophysiological function, neural regeneration
Introduction
Schwann cells are important support cells surrounding the nerve cells that play a key role in neuronal regeneration, can secrete various neurotrophic factors, contribute to neuronal survival and differentiation and support and guide nervous processes (Zong et al., 2013; Liu et al., 2014; Sun et al., 2014). Schwann cell transplantation promotes functional recovery after spinal cord injury, which provided a new direction in the treatment of spinal cord injury (Nout et al., 2011).
Free radicals exert a crucial secondary effect following spinal cord injury. After spinal cord injury, ischemia and hypoxia lead to energy dysmetabolism, excitatory amino acid accumulation and free radical increase (Zhang et al., 2013). These cause lipid peroxidation, which injures the structure of cell membranes by increasing their fluidity and permeability, which then induces Na+/K+ ATP activity decrease, cellular energy metabolism disorders and intracellular calcium overload, finally resulting in tissue necrosis and loss of function (Chen et al., 2011). Edaravone is a free radical scavenger that suppresses vascular endothelial cell damage, delays neuronal apoptosis, improves neurological function and lessens nerve ischemia. Edaravone effectively inhibits free radical-induced secondary injury of spinal cord injury (Uji et al., 2008; Hisano et al., 2009).
This study was designed to test whether edaravone could improve the microenvironment in the spinal cord injury region and encourage the actions of Schwann cell transplantation on the recovery of neurological function in rats with spinal cord injury.
Materials and Methods
In vitro culture, purification and identification of Schwann cells
A total of 10 healthy inbred-line female Sprague-Dawley rats aged 2 months and weighing 200–250 g were purchased from the Animal Laboratory of Chinese Academy of Medical Sciences in China (license No. SCXK (Jin) 20050076). The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee from Tianjin Fourth Central Hospital in China. Rats were intraperitoneally anesthetized with 2.5% ketamine (20 mg/kg) and decapitated. In accordance with previous methods (Hou et al., 2013; Zaminy et al., 2013), bilateral sciatic nerve was aseptically stripped under a microscope, digested with 0.25% trypsin (Gibco BRL, Gaithersburg, MD, USA)/0.2% collagenase for 40 minutes, centrifuged at 1,000 r/min for 5 minutes, and incubated with Dulbecco's modified Eagle's Medium (DMEM)/F12 (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum in a 5% CO2 incubator at 37°C for 30 minutes. Fibroblasts were removed by differential adherence. Twenty-four hours later, 100 μL cytarabine (1 × 10–5 mM) was added for 48 hours to further kill fibroblasts. The medium was replaced by a fresh one. From then on, half the medium was replaced by a fresh one, twice a week. One week later, passage was conducted at the proportion of 1:3. Fourth passage Schwann cells were incubated on a coverslip for 48 hours, washed three times with PBS, fixed with 4% paraformaldehyde (pH 7.4) at room temperature for 20 minutes, and washed three times with PBS. These cells were then treated with rabbit anti-myelin basic protein polyclonal antibodies (1:1,000; Hyclone) in a wet box at 4°C overnight, washed three times with PBS, incubated with fluorescein isothiocyanate (FITC) fluorescence-labeled goat anti-rabbit IgG (1:1,000; Hyclone) in an incubator at 37°C for 2 hours, and then treated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 minutes, followed by three washes in PBS. The samples were mounted and observed under a light microscope (Olympus, Tokyo, Japan).
PKH-26-labeled Schwann cells
Schwann cells were digested in trypsin and single-cell suspensions were obtained. Schwann cells (2 × 107) were placed in conical centrifuge tubes, washed with serum-free low-glucose DMEM (Gibco BRL), and centrifuged at 800 r/min for 5 minutes. After removal of the supernatant, 1 mL diluent was added. Cells were resuspended to be fully dispersed. Before staining, 4 × 106 M PKH-26 staining solution (Sigma, St. Louis, MO, USA) was placed in a centrifuge tube. One mL 2 × cells (2 × 109/mL) was added in 1 mL 2 × dye, and rapidly stirred with a pipette, for incubation for 2–5 minutes at 25°C. The centrifuge tube was gently inverted every 6 minutes to ensure an entire mix at 25°C. Serum was added to terminate the staining reaction. The above reaction solution was diluted with low-glucose DMEM containing an equal volume of serum, centrifuged at 400 × g and 25°C for 10 minutes. The supernatant was discarded. Cell masses were moved to an additional tube, washed three times, incubated with 10 mL low-glucose DMEM, centrifuged, and then resuspended.
Establishment of animal models of spinal cord injury
A total of 60 healthy inbred-line female Sprague-Dawley rats weighing 200–250 g were purchased from the Animal Laboratory of Chinese Academy of Medical Sciences in China. After the rats were acclimatized in the Laboratory for 2 weeks, they were intraperitoneally anesthetized with 2.5% ketamine 20 mg/kg and fixed on the bench in the prone position. The lower back was shaved. A median incision was made on the back taking T8–9 spinous process as a center to expose T7–10 spinous processes and the lamina. The T8–9 spinous process and part of the lamina tissue were removed, without reserving the spinal dura mater. This exposed spinal cord tissue was the lesion area. In accordance with a modified Allen's method (Young, 2002), a 10-g object was dropped vertically from a 2.5-cm height and directly impacted the rat spinal cord. The wound was washed with penicillin saline, and the tissue was sutured layer by layer. After establishment of the model, rats were injected intraperitoneally with penicillin 30,000 U/kg × 2 daily, for 7 days. Abdominal massage and extrusion were performed in the morning and afternoon daily to assist urination until the micturition reflex was restored (about 2 weeks). Paralysis of the lower limbs was observed after the rat's tail suffered from swing and spasm, indicating successful establishment of the model.
Schwann cell transplantation combined with edaravone
Rat models were equally and randomly assigned to the model, Schwann cell and combination groups. Rats in the model group were injected with 1 mL of low-glucose DMEM in the head and tail ends of the lesion area at 6 hours after model induction. Rats in the Schwann cell group were slowly injected with 10 μL (1 × 1010/L) Schwann cell suspension in the head and tail ends of the lesion area with a microsyringe at 6 hours after model induction. The injection was completed within 3 minutes and the needle was maintained in place for 5 minutes. The needle hole was blocked with medical biological glue to avoid cell suspension effluence, and then the wound was sutured layer by layer. Rats in the combination group were subjected to Schwann cell transplantation and simultaneously injected with 3 mg/kg edaravone (Kunming Jida Pharmaceutical Co., Ltd., China; Approval No. GYZZ H20080495) through the caudal vein, at 6 hours after model establishment.
Evaluation of motor function in the rat lower limb
Before model establishment and at 1, 2, 4, 6 and 8 weeks after model establishment, 10 rats were obtained from each group. Motor function in the lower limb was assessed with the inclined plane test and on the Basso, Beattie and Bresnahan locomotor scale. In the inclined plane test, rats were placed horizontally on a modified Rivilin tiltboard with their heads to the right. From the horizontal position (0°), the angle was increased gradually. The maximum angle at which the rats stayed on the board for 5 seconds was recorded. The experiments were conducted in triplicate, and the average value was calculated. The range of Basso, Beattie and Bresnahan locomotor scale scores was between 0 and 21. Twenty-one points refer to normal rats (Leech et al., 2014; Zhang and He, 2014). The test was done six times in each rat, and the average value was calculated.
Electrophysiological testing in rats
At 8 weeks after model establishment, six rats were collected from each group. In accordance with a previous method (Pallini et al., 2005), a Keypoint 4 induced potential instrument (Dantec Dynamics, Copenhagen, Denmark) was used to measure somatosensory-evoked potentials and motor-evoked potentials. The rats were intraperitoneally anesthetized with 10% chloral hydrate, and then laid on the horizontal plane. Hind limbs were stimulated with stimulating electrode. A recording electrode was placed under the scalp at the intersection of the coronal suture and sagittal suture healing line (i.e., hindlimb cortical sensory area). A reference electrode was placed at 0.5 cm posterior to the hindlimb cortical sensory area. Direct current, square wave, electrical pulses were given until the hind limb affected a slight tic. The conditions were as follows: current intensity 5–15 mA, pulse width 0.2 ms, frequency 3 Hz, and superimposed 50–60 times. Neurophysiological recovery was monitored by recording alterations in somatosensory-evoked potential latency and amplitude, and detection of motor-evoked potential by their latencies and amplitudes. These were obtained after anesthesia by placing the stimulating electrodes under the scalp at 2 mm anterior to the coronal suture and 2 mm lateral to the sagittal suture (i.e., motor cortex) (Paxinos and Watson, 2005), at the stimulus intensity of 40 mA, pulse width of 0.1 ms, frequency of 1 Hz, superimposed 300–500 times, at a scanning speed of 5 ms/div and sensitivity of 5 μV/div.
Pathological observation of spinal cord tissue of rats with spinal cord injury
At 8 weeks after model establishment, four rats were randomly obtained from each group and decapitated. Spinal cord tissue was sliced into 5-μm-thick sections. Sections were stained with hematoxylin for 5 minutes, and then immersed in acid followed by ammonia, each for 1 minute. The sections were washed with running water for 1 hour, immersed in distilled water for a moment, dehydrated through a graded alcohol series, stained with eosin for 2–3 minutes, and then observed under a light microscope.
Schwann cell distribution in the injured spinal cord of rats
The injured spinal cord tissue of rats was fixed in 4% paraformaldehyde, and sliced into 5-μm-thick frozen sections. Sections were observed with a fluorescence microscope (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Ten fields were obtained from each section under a high power lens (× 200). The number of PKH26-positive cells was calculated in each field, and an average value was obtained.
Horseradish peroxidase retrograde tracing
At 8 weeks after model establishment, horseradish peroxidase was dissolved using physiological saline (5 mg horseradish peroxidase in 1 mL physiological saline; Santa Cruz Biotechnology). Four rats were randomly selected from each group, and anesthetized. The spinal cord was exposed. Then 50% horseradish peroxidase (1 μL) was injected over 10 minutes into the region 1 mm left and right of the T12 spinal dorsal median vein using a microsyringe. The needling depth was 1.5 mm. The needle was maintained in place for 15 minutes. After 3 days, the rats were anesthetized with chloral hydrate. Their hearts were perfused with 4% paraformaldehyde. T3–11 segments of spinal cord were immersed in 30% sucrose solution (4°C) for 20 hours, and then sliced into 5-μm-thick frozen sections. The sections were visualized with 3,3′-diaminobenzidine. Horseradish peroxidase-positive nerve fiber bundles on cross-sections of the spinal cord were observed using a light microscope. Ten sections were randomly collected from each group. The number of positive nerve fiber bundles was calculated under 200× magnification using Image-Pro Plus 7.0 image processing software (Beijing Yicheng Hengda Technology Co., Ltd., Beijing, China), and the average value was obtained.
Statistical analysis
Statistical analysis was performed by the second author using SPSS 16.0 software (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SD and analyzed with repeated measures analysis of variance and the Student-Newman-Keuls test. A value of P < 0.05 was considered statistically significant between groups.
Results
Morphology of cultured Schwann cells
Inverted phase contrast microscopy demonstrated that cells were confluent after 5 or 6 days of culture, and most of them were Schwann cells; a few were fibroblasts. The cells were spindle-shaped, narrow, with small nuclei, and surrounded by abundant secretion (Figure 1A). Immunohistochemical staining revealed that the myelin basic protein-positive cells were spindle-shaped and narrow, with small nuclei, which indicated that these were Schwann cells, accounting for more than 98% of the total (Figure 1B).
Figure 1.
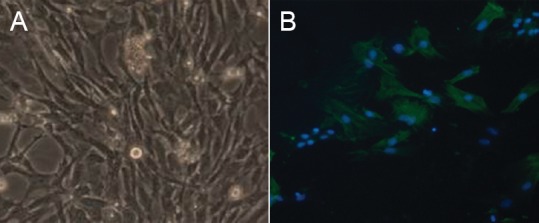
Morphological identification of cultured Schwann cells (× 40).
(A) Phase contrast microscopy showed that Schwann cells were tightly arranged, spindle-shaped, narrow, with small nuclei. (B) Immuno-histochemical staining shows the myelin basic protein, expressed in Schwann cell bodies and processes, as green fluorescence (fluorescein isothiocyanate, FITC). Nuclei exhibited blue fluorescence (4′,6-diamidino-2-phenylindole, DAPI). The cytoplasm of fibroblasts was not stained, but their nuclei were stained blue.
Combination of edaravone and Schwann cell transplantation improved motor function in the rat lower limb after spinal cord injury
Scores on the inclined plane test and modified Basso, Beattie and Bresnahan locomotor scale were 21 in rats from each group before model establishment. Compared with the control model group, scores on the inclined plane test and the modified Basso, Beattie and Bresnahan locomotor scale were significantly higher in the Schwann cell and combination groups (P < 0.05 or P < 0.01). Moreover, the scores on the inclined plane test and the modified Basso, Beattie and Bresnahan locomotor scale were higher in the combination group than in the Schwann cell group (P < 0.05; Figure 2).
Figure 2.
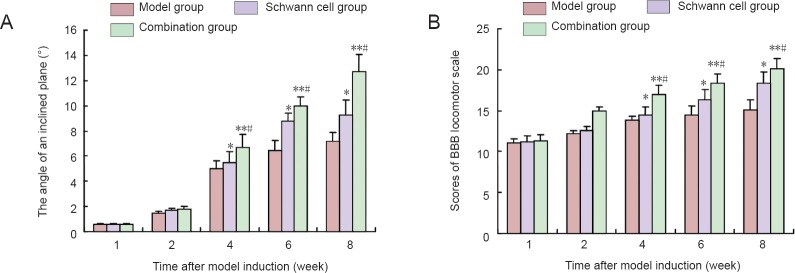
Evaluation results of motor function in rats from inclined plane test (A) and modified Basso, Beattie and Bresnahan (BBB) locomotor scale (B).
Data are expressed as the mean ± SD, with 10 rats in each group at each time point. Data were analyzed with repeated measures analysis of variance and Student-Newman-Keuls test. *P < 0.05, **P < 0.01, vs. model group; #P < 0.05, vs. Schwann cell group.
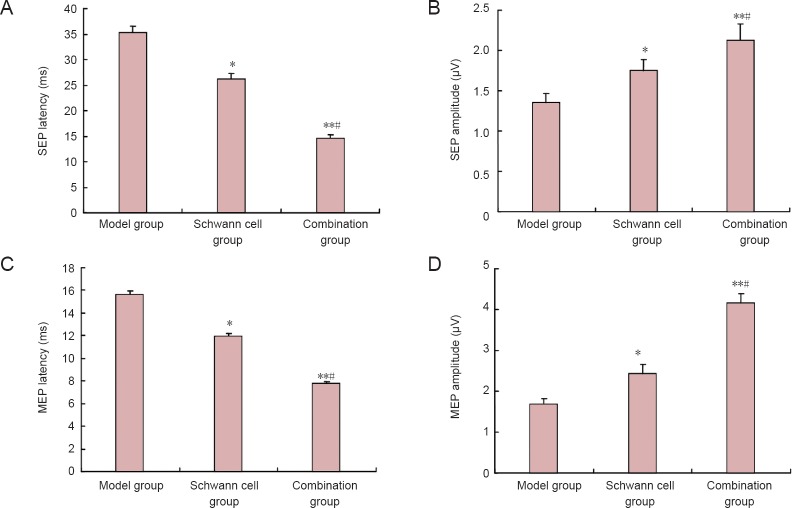
Combination of edaravone and Schwann cell transplantation improved nerve conduction in rats with spinal cord injury
After establishing rat models of spinal cord injury, detection of somatosensory-evoked potentials and motor-evoked potentials showed that the evoked potential waveforms completely disappeared in rats from each group. At 8 weeks after model establishment, somatosensory-evoked potentials and motor-evoked potentials returned to normal in the control model group. Compared with the control model group, somatosensory-evoked potential latencies were significantly shortened, and amplitude significantly increased in both the Schwann cell and combination groups (P < 0.05 or P < 0.01, respectively). Compared with the Schwann cell group, somatosensory-evoked potential latencies in the combination group were significantly shortened, and amplitude significantly increased (P < 0.05; Figure 3A and B). Similar results were obtained for motor-evoked potentials (Figure 3C and D).
Figure 3.
Effects of edaravone and Schwann cell transplantation on nerve conduction in rats with spinal cord injury at 8 weeks after surgery.
(A, B) Somatosensory-evoked potential (SEP) latencies and amplitudes; (C, D) motor-evoked potential (MEP) latencies and amplitudes. Data are expressed as the mean ± SD, with 12 rats in each group. Data were analyzed with repeated measures analysis of variance and Student-Newman-Keuls test. *P < 0.05, **P < 0.01, vs. model group; #P < 0.05, vs. Schwann cell group.
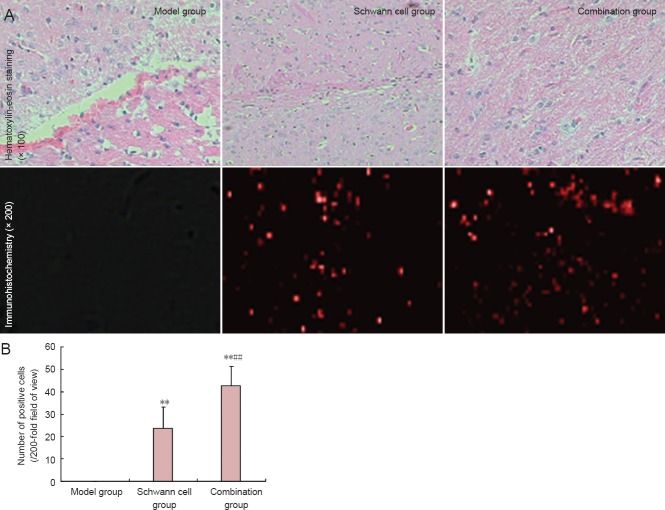
Edaravone combined with Schwann cell transplantation improved pathological morphology of injured spinal cord in rats
Eight weeks after model establishment, hematoxylin-eosin-stained sections demonstrated that spinal cord tissue was disrupted at the injury site; a clear cavity, scarring and structural disorder were visible in rats of the model group. These typical morphological changes to nerve cells following the spinal cord contusion were observed in the transplanted region of rats in the Schwann cell group. However, the cavity was smaller in the Schwann cell group than in the model group. Typical nerve cell-like morphological changes in the lesioned area of rats from the combination group were detected, but the cavity and scarring were not seen (Figure 4A).
Figure 4.
Effects of edaravone and Schwann cell transplantation on pathological morphology of injured spinal cord in rats at 8 weeks after surgeny.
(A) Pathological morphology of rat spinal cord tissue. (B) Number of PKH26-positive cells in rat spinal cord. Data are expressed as the mean ± SD, with 12 rats in each group. Data were analyzed with repeated measures analysis of variance and Student-Newman-Keuls test. **P < 0.01, vs. model group; ##P < 0.01, vs. Schwann cell group.
PKH26 expression could not be detected by immunofluorescence staining in the injured spinal cord of rats in the model group. Scattered PKH26 expression (red fluorescence) was visible both in the Schwann cell and combination groups (P < 0.01, vs. model group), especially in the combination group (P < 0.01, vs. Schwann cell group) (Figure 4B).
Edaravone combined with Schwann cell transplantation promoted extension of spinal cord nerve fiber bundles
Color reaction of 3,3′-diaminobenzidine demonstrated a region with a darkly stained center surrounded by a lightly stained area at the injection site. In the model group, 2 days after horseradish peroxidase was injected through the intumescentia lumbalis and horseradish peroxidase was transported retrogradely, a few nerve fibers labeled by horseradish peroxidase were observed in T8 or above segments. Many horseradish peroxidase-positive nerve fibers were detected in the injured spinal cord of rats in the Schwann cell and combination groups (Figure 5A). Moreover, the number of horseradish peroxidase-positive nerve fibers was greater in the combination group than that in the Schwann cell group (P < 0.01; Figure 5B).
Figure 5.
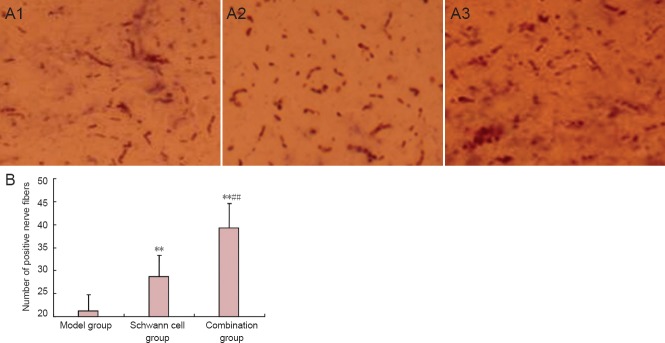
Effects of edaravone and Schwann cell transplantation on nerve fiber bundles in rats at 8 weeks after spinal cord injury.
(A) Morphology of nerve fiber bundles of rat spinal cord (× 200); (A1) model group; (A2) Schwann cell group; (A3) combination group. (B) Number of horseradish peroxidase (HRP)-positive nerve fibers in rat spinal cord. Data are expressed as the mean ± SD, with 12 rats in each group. Data were analyzed with repeated measures analysis of variance and Student-Newman-Keuls test. **P < 0.01, vs. model group; ##P < 0.01, vs. Schwann cell group.
Discussion
Treatment of spinal cord injury has been one of the most challenging problems of neuroscience. Spinal cord injury is a severe injury with a high disability rate, and serious sequelae such as paralysis. The traditional view was that the central nervous system did not have any regenerative capacity. However, some studies found that after spinal cord injury, injured axons could have regenerative capacity if the local environment was changed (Saberi et al., 2008). Recent studies have probed more deeply into the changes in the microenvironment after spinal cord injury, cytoskeleton transplantation in the repair of spinal cord injury and nerve cell regeneration (Li et al., 2013; Zhao et al., 2013). Numerous studies have found changes in the microenvironment, the nerve cells, and their expression of gene, protein, mitochondrion and nerve cell factors after spinal cord injury, and confirmed that injured axons could have regenerative capacity if the local environment changed (Chi et al., 2010; Deng et al., 2011; Wang et al., 2011; Bunge and Wood, 2012; Enomoto et al., 2013; Yazdani et al., 2013). Schwann cells are the main structural and functional support cells in the peripheral nerve, and play important roles in peripheral nerve injury, regeneration and repair.
Neurological damage of spinal cord injury is induced by two mechanisms: primary and secondary injuries. Of them, secondary injury is the major pathological damage of spinal cord injury (Moore et al., 2006; Lu et al., 2007; Koda et al., 2008; Kocsis et al., 2009; King et al., 2010; Zhang et al., 2011; Ohnishi et al., 2013). Previous studies suggested that neuronal apoptosis played an important role in secondary spinal cord injury, and apoptotic cells were mainly oligodendrocytes, but that neurons in the gray matter did not present characteristics of apoptosis (Crowe et al., 1997; Liu et al., 1997; Lai et al., 2013). Inflammatory reaction exerts a crucial effect on secondary injury after spinal cord injury (Ma et al., 2014; Zong et al., 2014). A moderate inflammatory reaction is helpful to damage repair. Excessive inflammatory reaction causes oligodendrocyte apoptosis, aggravates spinal cord injury, and impacts functional recovery after spinal cord injury. Edaravone is a radical scavenging agent and inhibits lipid peroxidation, suppressing oxidative damage of nerve cells and vascular endothelial cells (Zhao et al., 2013). This lessens the edema of nervous tissue and improves the microenvironment in the lesioned area. Edaravone has been shown to reduce the malondialdehyde content and water content by scavenging free radicals, to suppress cell apoptosis by up-regulating Bcl-2 gene expression and down-regulating caspase-3 gene expression. It also relieved secondary injury, producing anti-apoptotic effects and neuroprotective effects against spinal cord injury (Banno et al., 2005; Ohta et al., 2005).
In summary, we have found that edaravone combined with Schwann cell transplantation can elevate the survival rate of the transplanted cells. The number of PKH-26-labeled Schwann cells was more than that in rats undergoing Schwann cell transplantation alone, or without any treatment, suggesting that edaravone substantially improves the effect of transplantation. The therapeutic effects of edaravone combined with Schwann cell transplantation were shown to be better histologically and functionally than Schwann cell transplantation alone in rats with spinal cord injury.
Footnotes
Conflicts of interest: None declared.
Copyedited by Dawes E, Raye W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Banno M, Mizuno T, Kato H, Zhang G, Kawanokuchi J, Wang J, Kuno R, Jin S, Takeuchi H, Suzumura A. The radical scavenger edaravone prevents oxidative neurotoxicity induced by peroxynitrite and activated microglia. Neuropharmacology. 2005;48:283–290. doi: 10.1016/j.neuropharm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Wood PM. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handbk Clin Neurol. 2012;109:523–540. doi: 10.1016/B978-0-444-52137-8.00032-2. [DOI] [PubMed] [Google Scholar]
- Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Watanabe S, Guerrero AR, Kobayashi S, Ma WY, Liu SY, Baba H. Tumor necrosis factor-α antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine (Phila Pa 1976) 2011;36:1350–1358. doi: 10.1097/BRS.0b013e3181f014ec. [DOI] [PubMed] [Google Scholar]
- Chi GF, Kim MR, Kim DW, Jiang MH, Son Y. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinated axons in the spinal cord injury. Exp Neurol. 2010;222:304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J Neurochem. 2008;106:1483–1492. doi: 10.1111/j.1471-4159.2008.05485.x. [DOI] [PubMed] [Google Scholar]
- Deng LX, Hu J, Liu N, Wang X, Smith GM, Wen X, Xu XM. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011;229:238–250. doi: 10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchossoy Y, David S, Baulieu EE, Robel P. Treatment of experimental spinal cord injury with 3β-methoxy-pregnenolone. Brain Res. 2011;1403:57–66. doi: 10.1016/j.brainres.2011.05.065. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Bunge MB, Tsoulfas P. A multifunctional neurotrophin with reduced affinity to p75 NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury. Exp Neurol. 2013;248:170–182. doi: 10.1016/j.expneurol.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Hisano K, Watanabe M, Morimoto Y. Protective effects of the free radical scavenger edaravone against glutamate neurotoxicity in nearly pure neuronal culture. J Anesth. 2009;23:363–369. doi: 10.1007/s00540-009-0766-z. [DOI] [PubMed] [Google Scholar]
- Hou X, Liang Q, Wu Y. Transplantation of Schwann cells co-cultured with brain-derived neurotrophic factor for the treatment of experimental autoimmune neuritis. J Neuroimmunol. 2013;263:83–90. doi: 10.1016/j.jneuroim.2013.08.004. [DOI] [PubMed] [Google Scholar]
- King VR, Alovskaya A, Wei DY, Brown RA, Priestley JV. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials. 2010;31:4447–4456. doi: 10.1016/j.biomaterials.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Lankford KL, Sasaki M, Radtke C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neurosci Lett. 2009;456:137–142. doi: 10.1016/j.neulet.2008.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda M, Someya Y, Nishio Y, Kadota R, Mannoji C, Miyashita T, Okawa A, Murata A, Yamazaki M. Brain-derived neurotrophic factor suppresses anoikis-induced death of Schwann cells. Neurosci Lett. 2008;444:143–147. doi: 10.1016/j.neulet.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Lai BQ, Wang JM, Duan JJ, Chen YF, Gu HY, Ling EA, Wu JL, Zeng YS. The integration of NSC-derived and host neural networks after rat spinal cord transection. Biomaterials. 2013;34:2888–2901. doi: 10.1016/j.biomaterials.2012.12.046. [DOI] [PubMed] [Google Scholar]
- Leech KA, Kinnaird CR, Hornby TG. Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J Neurotrauma. 2014;31:1334–1342. doi: 10.1089/neu.2013.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Shen LF, Wang GS, Guo GH. Neural stem cell transplantation for the treatment of rat spinal cord injury in recovery period. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:3508–3514. [Google Scholar]
- Liu L, Wang L, Tong YL, Mo YL, Lv L, Chen YP, Yang WX, Lv LF, Zhan Q, Zhu FJ, Xin HM, Gong ZY. Effect of different concentrations of human amniotic homogenate supernatant on the proliferation of rat Schwann cells. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:3218–3222. [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203:8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Ma C, Xu Z, Wang ZQ, Deng SY. Interleukin-17 expression in the injured site of a rat model of complete spinal cord transection. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2824–2829. [Google Scholar]
- Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ, Yaszemski MJ. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–429. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Morizane K, Ogata T, Morino T, Horiuchi H, Yamaoka G, Hino M, Miura H. A novel thermoelectric cooling device using Peltier modules for inducing local hypothermia of the spinal cord: the effect of local electrically controlled cooling for the treatment of spinal cord injuries in conscious rats. Neurosci Res. 2012;72:279–282. doi: 10.1016/j.neures.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Nout YS, Culp E, Schmidt MH, Tovar CA, Pröschel C, Mayer-Pröschel M, Noble MD, Beattie MS, Bresnahan JC. Glial restricted precursor cell transplant with cyclic adenosine monophosphate improved some autonomic functions but resulted in a reduced graft size after spinal cord contusion injury in rats. Exp Neurol. 2011;227:159–171. doi: 10.1016/j.expneurol.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y-i, Iwatsuki K, Shinzawa K, Ishihara M, Moriwaki T, Umegaki M, Kishima H, Yoshimine T. Adult olfactory sphere cells are a source of oligodendrocyte and Schwann cell progenitors. Stem Cell Res. 2013;11:1178–1190. doi: 10.1016/j.scr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Ohta S, Iwashita Y, Takada H, Kuno S, Nakamura T. Neuroprotection and enhanced recovery with edaravone after acute spinal cord injury in rats. Spine (Phila Pa 1976) 2005;30:1154–1158. doi: 10.1097/01.brs.0000162402.79482.fd. [DOI] [PubMed] [Google Scholar]
- Pallini R, Vitiani LR, Bez A, Casalbore P, Facchiano F, Di Giorgi Gerevini V, Falchetti ML, Fernandez E, Maira G, Peschle C, Parati E. Homologous transplantation of neural stem cells to the injured spinal cord of mice. Neurosurgery. 2005;57:1014–1025. doi: 10.1227/01.neu.0000180058.58372.4c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Sun HW, Zhang TH, You XY, Ren YF, Zhong S. Polylactic-co-glycolic acid complex with different concentrations of Schwann cells for peripheral nerve regeneration. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:7579–7584. [Google Scholar]
- Uji Y, Yamamoto H, Mori T, Akabori H, Tsuchihashi H, Shimizu T, Endo Y, Tani T. Edaravone improves the survival of rats subjected to hemorrhagic shock without resuscitation. Surg Today. 2008;38:476–477. doi: 10.1007/s00595-007-3666-6. [DOI] [PubMed] [Google Scholar]
- Wang JM, Zeng YS, Wu JL, Li Y, Teng YD. Cograft of neural stem cells and schwann cells overexpressing TrkC and neurotrophin-3 respectively after rat spinal cord transection. Biomaterials. 2011;32:7454–7468. doi: 10.1016/j.biomaterials.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, Soleimani M. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15:782–791. doi: 10.1016/j.jcyt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]
- Zaminy A, Shokrgozar MA, Sadeghi Y, Norouzian M, Heidari MH, Piryaei A. Transplantation of schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch Iran Med. 2013;16:533–541. [PubMed] [Google Scholar]
- Zhang D, He X. A meta-analysis of the motion function through the therapy of spinal cord injury with intravenous transplantation of bone marrow mesenchymal stem cells in rats. PLoS One. 2014;9:e93487. doi: 10.1371/journal.pone.0093487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Huang F, Gates M, Holmberg EG. Scar ablation combined with LP/OEC transplantation promotes anatomical recovery and P0-positive myelination in chronically contused spinal cord of rats. Brain Res. 2011;1399:1–14. doi: 10.1016/j.brainres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Li Y, Cao Y, Dong MY, Lü G. Effect of hyperbaric oxygen preconditioning on expression of Bcl-2 and Bax after spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:8018–8023. [Google Scholar]
- Zhao ZJ, Sun ZM, Zhang CY, Han JG, Shi RC, Wang WZ. Edaravone combined with neural stem cell transplantation for treatment of spinal cord injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:1862–1867. [Google Scholar]
- Zong HB, Jia JL, Dong YZ, Zhou HC. Transfection of Schwann cells with neurotrophic factor-3 nanoparticles. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:1362–1366. [Google Scholar]
- Zong SH, Fang Y, Peng JZ, Gao TH. The expression of inflammatory factors in different periods after acute spinal cord injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:2806–2811. [Google Scholar]