Abstract
Most studies on spinal cord neuronal injury have focused on spinal cord tissue histology and the expression of nerve cell damage and repair-related genes. The importance of the microcirculation is often ignored in spinal cord injury and repair research. Therefore, in this study, we established a rat model of intervertebral disc extrusion by inserting a silica gel pad into the left ventral surface of T13. Electroacupuncture was used to stimulate the bilateral Zusanli point (ST36) and Neiting point (ST44) for 14 days. Compared with control animals, blood flow in the first lumbar vertebra (L1) was noticeably increased in rats given electroacupuncture. Microvessel density in the T13 segment of the spinal cord was increased significantly as well. The number of normal neurons was higher in the ventral horn of the spinal cord. In addition, vacuolation in the white matter was lessened. No obvious glial cell proliferation was visible. Furthermore, hindlimb motor function was improved significantly. Collectively, our results suggest that electroacupuncture can improve neuronal morphology and microcirculation, and promote the recovery of neurological functions in a rat model of intervertebral disc extrusion.
Keywords: nerve regeneration, electroacupuncture, intervertebral disc, blood circulation, inflammation, neuroprotection, motor function, neurons, NSFC grants, neural regeneration
Introduction
Intervertebral disc protrusion is a pathological condition where the nucleus pulposus and anulus fibrosus of the intervertebral disc protrude into the surrounding tissue and compress the spinal cord or nerve root. Intervertebral disc disease belongs to the category of “backleg pain”, “arthromyodynia” and “paralysis syndrome” according to traditional Chinese medical theory (Jiang, 2005). As such, acupuncture can adjust the body's inherent function, and play a therapeutic role mainly through “dredging meridians, promoting blood circulation and removing blood stasis, and regulating qi and blood”. Using dogs with thoracolumbar intervertebral disc disease, we established an electroacupuncture treatment method, mainly consisting of stimulation of Yangming stomach meridian (such as Zusanli point (ST36) and Neiting point (ST44)), Taiyang bladder meridian (such as Shenshu point (BL23) and Pangguangshu point (BL28)). Over more than 10 years of clinical practice, we observed a good outcome with electroacupuncture, with greater therapeutic efficacy than that of conservative treatments based on Western medicine and surgery. In the rat intervertebral disc extrusion model developed in our laboratory, only the Zusanli point and Neiting point of the hindlimb Yangming stomach meridian were stimulated, due to limitations associated with the back operation procedure. An account of “taking the acupoints of Yangming meridian alone to treat paralysis syndrome” was described as early as in “Huangdi's Canon of Medicine”, and modern research has shown that acupuncture of Zusanli of the Yangming stomach meridian can improve spinal cord neuronal function (Song et al., 2006; Han et al., 2010; Zhu and Zhang, 2012). In addition, Tsuchiya et al. (2007) reported that needling the palm and forearm can promote nitric oxide production in the ipsilateral axillary vein, with an ensuing significant increase in the ipsilateral subcutaneous blood flow of the palm. Because nitric oxide expands blood vessels, we speculated that an elevation in spinal cord blood flow would be observed in the rat intervertebral disc extrusion model when needling Zusanli and Neiting points.
Dogs suffering from athletic performance dysfunction have been shown to respond favorably to electroacupuncture therapy (Yang et al., 2003; Hayashi et al., 2007).
The majority of studies on electroacupuncture have been limited to examination of changes in spinal tissue morphology and the expression of genes associated with neuronal damage and repair. However, the important role of the microcirculation (e.g., spinal cord blood flow and microvessel density) in spinal cord injury and repair processes has been neglected (Benton et al., 2008). Therefore, in this study, we examined local changes in spinal cord blood flow, microvessel density and neuronal morphology induced by electroacupuncture treatment for intervertebral disc extrusion. This study was performed to clarify the pathological changes produced by intervertebral disc extrusion and to elucidate the mechanisms of action of electroacupuncture treatment.
Materials and Methods
Animals
Fifty-two clean male Sprague-Dawley rats weighing 200–250 g and aged 60 days were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences of the People's Liberation Army (License No. SCXK (Army) 2012-0004), Beijing, China. Rats were acclimatized to the laboratory for at least 3 days before testing. All rats were housed in a temperature controlled room (20–23°C) with controlled lighting (lights on from 7:00 a.m. to 6:00 p.m.). The rats were allowed free access to food pellets and tap water. All animal experiments were approved by the Animal Care and Use Committee of the Beijing University of Agriculture and were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Rats were randomly divided into control (n = 14), model (n = 19) and electroacupuncture (n = 19) groups. The rats in the model and electroacupuncture groups were both used to create the intervertebral disc extrusion model, and rats in the electroacupuncture group also received electroacupuncture treatment.
Establishment of the rat models of intervertebral disc extrusion
Rats were anesthetized intraperitoneally with sodium pentobarbital (40 mg/kg) and placed face down, and the skin above the thoracolumbar spinal cord was shaved and sterilized. The operation was carried out under strict aseptic conditions, with an approximately 2-cm-long incision made into the skin along the dorsal midline of the first lumbar vertebra (L1). The L1 spinal process, vertebral plate and left articular process were exposed using conventional methods. The dorsal part of L1 and the left articular process were ground using a Surgic XT swing saw (NSK, Tochigi, Japan), and the entire vertebral plate and left articular process were carefully removed with forceps, allowing the back of the L1 spinal cord to be fully exposed. The self-made silica gel pad was inserted into the left ventral position of T13, from the left of L1 (Figure 1), and the incision was sutured after spinal cord blood flow was tested. All rats received penicillin and streptomycin sulfate (30,000 U/kg and 15 mg/kg, respectively, twice a day for 3 days) by intramuscular injection after the operation, and were kept in separate cages with free access to drinking water and food. Rats in the control group had their spinal cords exposed only.
Figure 1.
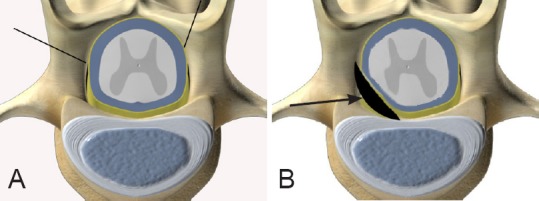
Schematic diagram of the laminectomy site.
The first lumbar vertebra (A, indicated by black lines) and the self-made silica gel pad compressing the thirteenth thoracic ventral horn (B, arrow).
Electroacupuncture treatment
An electroacupuncture apparatus (Beijing Xindonghua Electronic Instrument Co., Ltd., Beijing, China) was used. Zusanli point (situated posterolateral in the knee, about 5 mm under the fibular head (Li, 2007)) and Neiting point (posterior to the 1st and 2nd metatarsophalangeal joints (Li, 2007)) of both hindlimbs Yangming Stomach Meridian were selected. After the steel needles (0.20 × 25 mm, Suzhou Acupuncture & Moxibustion Appliance Co., Ltd., Suzhou, Jiangsu Province, China) were vertically inserted into the points, they were connected to the electroacupuncture apparatus (frequency, 2 Hz; the strength of the stimulation was adjusted until the rat hindlimb displayed rhythmic contraction and relaxation). The treatment was administered daily in the morning for approximately 20 minutes, which was identical to the length of treatment used in the canine electroacupuncture clinic. The depth of the electroacupuncture needles was 0.5 cm, and the intensity of stimulation was considered sufficient when the region between the electrodes vibrated lightly. The duration of therapy was 14 days.
Detection of blood flow in the L1 segment of the spinal cord
After exposing the L1 segment of the spinal cord of rats in both model and electroacupuncture groups, dorsal blood flow was observed and monitored for 10 minutes with the PeriCam PSI System (Perimed, Stockholm, Sweden) before and after applying pressure onto the spinal cord with the silica gel pad. The PeriCam PSI System parameters were as follows: The monitoring laser was positioned vertical to the L1 segment of the spinal cord, the monitoring distance was 15 cm from the probe to the dorsal L1 segment, the resolution was 0.42 mm, the monitored region was a circle of 3.5 mm2 located in the center of the dorsal L1 segment, and the sampling frequency was 3 frames/second. Rats in the electroacupuncture group received electroacupuncture treatment for approximately 20 minutes, with dorsal blood flow being monitored simultaneously and for 10 minutes thereafter. Rats in the electroacupuncture group received one treatment per day for up to 14 days, and spinal cord blood flow was monitored for 10 minutes at the end of the treatment.
Assessment of motor function
Postoperatively, hindlimb motor function was assessed using the Basso, Beattie and Bresnahan locomotor rating scale (Basso et al., 1995) at 1, 4, 7 and 14 days after the establishment of the model (a full score of 21 points indicates that all motor functions are normal). As soon as the rats were completely conscious and the effect of the anesthesia completely wore off, scoring began, with that day being designated day 1.
Sample collection
All rats were anesthetized on day 14 after the surgery to establish the model of disc extrusion, and their thoracic cavity was exposed. A tube was inserted into the aortic arch, and then the right atrial appendage was cut. 200–250 mL of warm saline (37°C) was pumped into the body using the tube, followed by 400 mL of a 4% paraformaldehyde solution, whereupon clear liquid emerged from the right atrial appendage. The T13 segment of the spinal cord, which was compressed by the pad, was removed and soaked in a 4% paraformaldehyde solution overnight at 4°C. On the following day, the T13 segment was dehydrated using routine methods, embedded with paraffin, and serially sliced into 8-μm-thick sections.
Histopathological observation
Sections were used for conventional hematoxylin-eosin staining (Lim et al., 2007) or crystal violet staining (Courtine et al., 2008).
Immunofluorescence staining for microvessel density in the T13 segment of the rat spinal cord
Sections used for spinal microvascular immunofluorescence staining were dewaxed with xylene, rehydrated with a graded alcohol series, and subjected to antigen retrieval. These sections were then blocked to reduce non-specific labeling, and incubated with mouse anti-rat RECA-1 monoclonal antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1.5 hours at room temperature and FITC-goat anti-mouse IgG (1:10; Solarbio, Beijing, China) for 1.5 hours at room temperature. Sections were observed and photographed using an IX-71 microscope and CCD-DP72 image acquisition system (Olympus, Tokyo, Japan). The Beihang medical image analysis management system (Beihang University, Beijing, China) was used to evaluate microvessel density in the labeled spinal cord section. Density was calculated as follows: microvessel density = spinal microvascular area/total area of spinal cord section. Normal neurons, from the ventral horn, below the horizontal line of the central canal of the spinal cord, were counted using crystal violet stained sections.
Statistical analysis
Data were expressed as the mean ± SEM, and analyzed using Excel 2003 software (Microsoft Corporation, Redmond, WA, USA). Statistical significance between groups was evaluated using least significant range (when N is equal) or least significant difference (when N is unequal) test. A value of P < 0.05 was considered statistically significant.
Results
Motor assessment of a rat model of intervertebral disc extrusion
The Basso, Beattie and Bresnahan locomotor rating scale score of the left and right hind legs in the model group was significantly decreased compared to the control group at 1 day after surgery for generating the model (P < 0.01; Figure 2), which suggested a successfully established rat model of intervertebral disc extrusion. Compared with the control group, spinal cord blood flow was significantly lower in the model group (P < 0.01; Figure 3).
Figure 2.
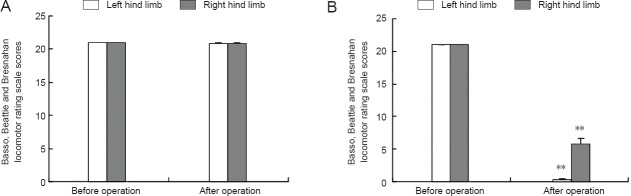
Motor function score of the hindlimbs in a rat model of intervertebral disc extrusion.
(A) The sham surgical procedure did not impact Basso, Beattie and Bresnahan locomotor rating scale scores in rats in the control group. (B) Basso, Beattie and Bresnahan locomotor rating scale scores were decreased in a rat model of intervertebral disc extrusion. The maximum possible Basso, Beattie and Bresnahan score was 21 points. A high score indicates good motor function. Data are expressed as the mean ± SEM. Statistical significance between groups was evaluated using the least significant range test. **P < 0.01, vs. before operation.
Figure 3.
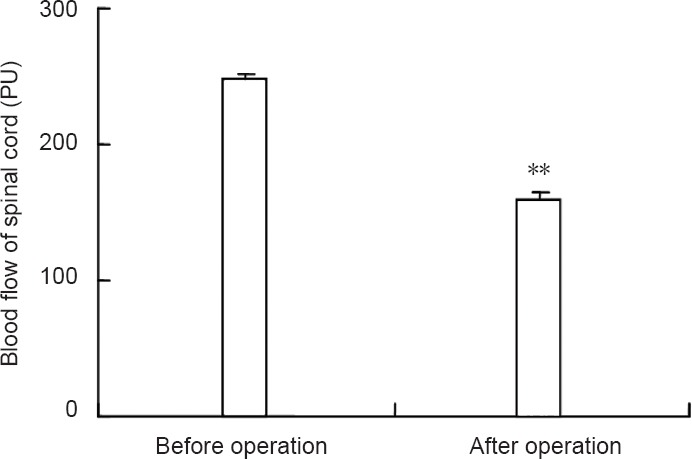
Spinal cord blood flow in the intervertebral disc extrusion model.
Data are expressed as the mean ± SEM. Statistical significance between groups was evaluated using least significant difference test (when N is unequal). **P < 0.01, vs. before operation.
Electroacupuncture elevates blood flow in the L1 spinal cord segment in a rat model of intervertebral disc extrusion
Spinal cord blood flow in the L1 segment in the electroacupuncture group was increased by approximately 22% after 20 minutes of electroacupuncture stimulation. Blood flow was significantly higher after electroacupuncture compared with before electroacupuncture or the model group (P < 0.01). Spinal cord blood flow gradually declined within 10 minutes following the 20-minute electroacupuncture treatment session. Although spinal cord blood flow was higher than in the model group, it was not significant (P > 0.05). After continuous electroacupuncture treatment for 14 days, spinal cord blood flow was significantly higher than in the model group (P < 0.01; Figure 4), indicating the effectiveness of electroacupuncture treatment.
Figure 4.
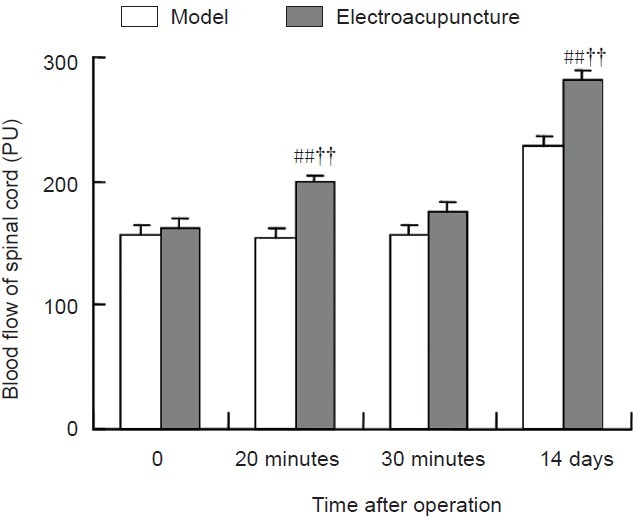
Influence of electroacupuncture on blood flow in the L1 spinal cord in the rat model of intervertebral disc extrusion.
Data are expressed as the mean ± SEM. Statistical significance between groups was evaluated with the least significant difference test. ##P < 0.01, vs. model group; ††P < 0.01, vs. immediately after operation (0).
Electroacupuncture increases spinal cord microvessel density in the T13 spinal cord segment in a rat model of intervertebral disc extrusion
Fluorescent staining showed that microvessel density in the T13 spinal cord segment in the model group was significantly lower than in the control group (P < 0.01). Microvessel density in the T13 spinal cord segment in the electroacupuncture group was significantly higher than in the model group (P < 0.01), and significantly lower than in the control group (P < 0.05; Figure 5).
Figure 5.
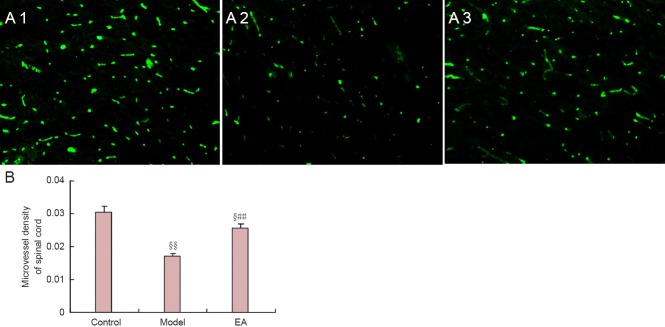
Effects of electroacupuncture (EA) on microvessel density in the T13 spinal cord segment in a rat model of intervertebral disc extrusion.
(A) Spinal cord microvessel in the rat model of intervertebral disc extrusion (immunofluorescence staining, × 100). Fluorescent dye is FITC. A1, A2 and A3: Control, model and electroacupuncture groups, respectively. (B) Spinal cord microvessel density values. Microvessel density was calculated as follows: spinal microvascular area/total area of spinal cord section. Data are expressed as the mean ± SEM. Statistical significance between groups was evaluated using the least significant range test. §P < 0.05, §§P < 0.01, vs. control group; ##P < 0.01, vs. model group.
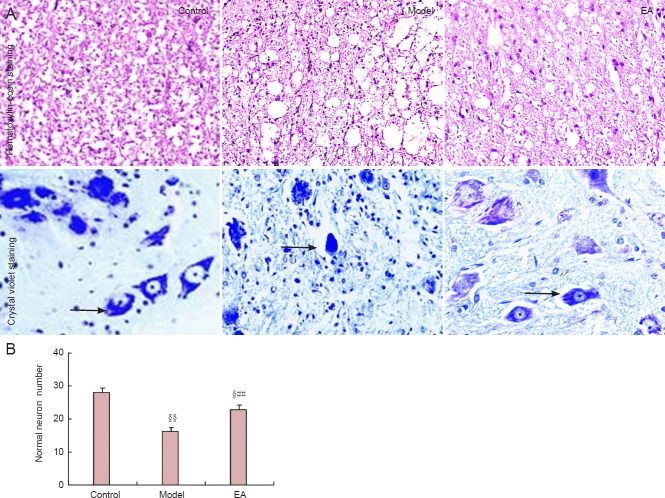
Electroacupuncture increases the number of normal neurons in the ventral spinal cord in a rat model of intervertebral disc extrusion
In the control group, the structure of the white matter in the ventral spinal cord was normal; neurons in the ventral horn were large and multipolar, deeply blue-stained, and big nuclei were visible. In contrast, the white matter in the ventral spinal cord in the model group showed marked vacuolization, a significant reduction in the number of normal neurons in the ventral horn, the disappearance of neurites, neuronal pyknosis, necrosis, fragmented nuclei, and glial cell hyperplasia. Moreover, the model group displayed a significant reduction in the quantity of normal neurons in the ventral spinal cord compared with the control group (P < 0.01). In comparison, white matter in the ventral spinal cord of rats in the electroacupuncture group showed mild vacuolization, and individual neurons demonstrated pyknosis and necrosis, but the neurites of most neurons were still present. Furthermore, nuclei were visible and glial hyperplasia was not observed. Overall, the quantity of normal neurons in the ventral spinal cord in the electroacupuncture group was higher than in the model group (P < 0.01), but lower than in the control group (P < 0.01; Figure 6).
Figure 6.
Effects of electroacupuncture (EA) on neurons in the white matter in the ventral spinal cord of a rat model of intervertebral disc extrusion.
(A) Effects on neuronal morphology (× 400). Arrows indicate neurons. (B) Effects on neuronal number in the ventral horn of the spinal cord. Data are expressed as the mean ± SEM. Statistical significance between groups was evaluated with the least significant range test. §P < 0.05, §§P < 0.01, vs. control group; ##P < 0.01, vs. model group.
Electroacupuncture enhances hindlimb motor function in a rat model of intervertebral disc extrusion
The Basso, Beattie and Bresnahan locomotor rating scale scores for the left and right hindlimbs increased with time after model establishment in the model and electroacupuncture groups. The scores for the left and right hindlimbs were higher at 7 and 14 days than at 1 day after model establishment in the electroacupuncture group (P < 0.01 or P < 0.05). The scores were higher in the electroacupuncture group than in the model group at 14 days after model establishment (P < 0.01; Figure 7).
Figure 7.
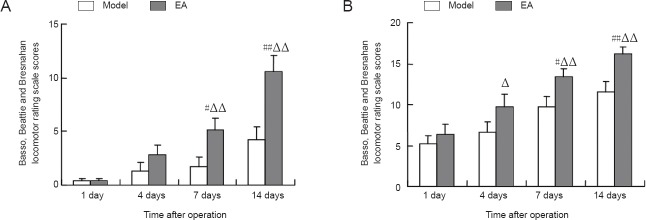
Effect of electroacupuncture (EA) on the recovery of hindlimb motor function in a rat model of intervertebral disc extrusion.
(A) Left hindlimb; (B) right hindlimb. Data are expressed as the mean ± SEM. #P < 0.05, ##P < 0.01, vs. model group. Statistical significance between groups was evaluated using the least significant range multiple group comparison.ΔP < 0.05, ΔΔP < 0.01, vs. 1 day after operation.
Discussion
In severe intervertebral disc extrusion, the nucleus pulposus prolapses. As such, a short-term injury like a hit or punch cannot accurately reflect or replicate the actual situation. Therefore, we attempted to replicate/simulate intervertebral disc extrusion in the dog by inserting a self-made silica gel pad into the T13 vertebral cavity of a rat to chronically compress the ventral horn of the spinal cord, and we assessed hindlimb motor function with the Basso, Beattie and Bresnahan locomotor rating scale. We found that all rats in the model group suffered from severe hindlimb movement dysfunction, indicating that we successfully established a model of intervertebral disc extrusion in rats.
Currently, methods used for spinal cord blood flow detection include the following: hydrogen clearance, the use of microspheres, the closed spinal cord window technique, and laser Doppler methods. The first three methods have limitations which can impact their application (Bartsch and Goadsby, 2002; Crystal and Salem, 2002); however, the laser Doppler method can detect spinal cord blood flow in high resolution, in a non-invasive and real-time manner. Therefore, we used this method to monitor spinal cord microcirculation in our study (Kato et al., 2008; Su et al., 2009). We observed a significant reduction in spinal cord blood flow, resulting from compression by the silica gel pad. In addition, the hindlimbs appeared to suffer from serious movement disorder, indicating that our rat model of intervertebral disc extrusion possessed symptoms of qi-stagnation, blood stasis and limb weakness, similar to canine intervertebral disc extrusion cases in the clinic. Our results demonstrate that electroacupuncture treatment, whether it was immediately applied after generating the model or applied continuously for up to 14 days, significantly increased spinal cord blood flow, which corroborates results of a previous study by Wu et al. (1995). These investigators used electroacupuncture to stimulate the Dazhui point (Du meridian 14, DU14) and Mingmen point (Du meridian 4, DU4) in a rat model of spinal cord impact injury, and observed an increase in spinal cord blood flow in the T13–L1 segments.
In recent years, the recovery of motor function after spinal cord injury has become a major research goal (Butcher, 2000; Sharp et al., 2012). It has been discovered that microvascular injury post spinal cord injury is a key contributor to secondary damage in the spinal cord (Benton et al., 2008). Therefore, many researchers have focused attention on the changes in microvascular function following injury (Benton et al., 2009; Hamamoto et al., 2009; Sharma, 2011). Whetstone et al. (2003) demonstrated that microvascular injury occurs instantly when the spinal cord is damaged; however, the revascularization and regeneration of damaged capillary vessels proceeds over time. In the present study, we found that the spinal cord microvessel density in the electroacupuncture group was significantly higher than in the model group after 2 weeks of treatment. In addition, hindlimb motor function improved significantly, suggesting that electroacupuncture promotes the regeneration of spinal cord capillary vessels and improves hindlimb motor function. Overall, the therapeutic effect in the rat model of intervertebral disc extrusion is consistent with clinical canine cases. The survival of neurons can be enhanced by the early recovery of microvascular endothelial cells (Li et al., 2009), and microvascular neogenesis can improve the regeneration of neurites of damaged neurons (Dray et al., 2009). Acupuncture is thought to mobilize and modulate the regeneration and reorganization of intrinsic short fibers in the spinal cord, and thereby accelerate the recovery of signal conduction function (Courtine et al., 2008). Our results demonstrate that spinal cord histology and hind limb motor function in the electroacupuncture group were significantly better than in the model group; there was a significant increase in the number of normal neurons, an abatement of white matter damage, and a significant improvement in hind limb motor function.
In summary, electroacupuncture increases spinal cord blood flow and microvessel density to improve microcirculation, reduce local secondary damage, and promote the rapid recovery of hindlimb motor functions.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 31372473, 30871886; the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of Beijing Municipality of China, No. PHR201107134; the 2012 Scientific Research Quality Raising Funds of Beijing University of Agriculture of China, No. PXM2012_014207_000010.
Conflicts of interest: None declared.
Copyedited by Patel B, Raye W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002;125:1496–1509. doi: 10.1093/brain/awf166. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Worth CA, Mahoney ET, Hagg T, Whittemore SR. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008;28:1771–1785. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Gruenthal MJ, Hagg T, Whittemore SR. Neutralizing endogenous VEGF following traumatic spinal cord injury modulates microvascular plasticity but not tissue sparing or functional recovery. Curr Neurovasc Res. 2009;6 doi: 10.2174/156720209788185678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher J. Spinal cord recovery moves one step closer. Lancet. 2000;356:405. doi: 10.1016/S0140-6736(05)73554-6. [DOI] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal GJ, Salem MR. Beta-adrenergic stimulation restores oxygen extraction reserve during acute normovolemic hemodilution. Anesth Analg. 2002;95:851–857. doi: 10.1097/00000539-200210000-00011. [DOI] [PubMed] [Google Scholar]
- Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A. 2009;106:9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto Y, Ogata T, Morino T, Hino M, Yamamoto H. Prostaglandin E1 analog increases spinal cord blood flow at the point of compression during and after experimental spinal cord injury. Spinal Cord. 2009;48:149–153. doi: 10.1038/sc.2009.99. [DOI] [PubMed] [Google Scholar]
- Han HJ, Yoon HY, Kim JY, Jang HY, Lee B, Choi SH, Jeong SW. Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am J Chin Med. 2010;38:1015–1025. doi: 10.1142/S0192415X10008433. [DOI] [PubMed] [Google Scholar]
- Hayashi AM, Matera JM, da Silva TS, Pinto AC, Cortopassi SR. Electro-acupuncture and Chinese herbs for treatment of cervical intervertebral disk disease in a dog. J Vet Sci. 2007;8:95–98. doi: 10.4142/jvs.2007.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JT. TCM viscera pathogenesis of lumbar intervertebral disc protrusion. Gansu Zhongyi Xueyuan Xuebao. 2005;22:16–18. [Google Scholar]
- Kato S, Kawahara N, Tomita K, Murakami H, Demura S, Fujimaki Y. Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine (Phila Pa 1976) 2008;33:1533–1541. doi: 10.1097/BRS.0b013e318178e5af. [DOI] [PubMed] [Google Scholar]
- Li W, Li P, Hua Q, Hou J, Wang J, Du H, Tang H, Xu Y. The impact of paracrine signaling in brain microvascular endothelial cells on the survival of neurons. Brain Res. 2009;1287:28–38. doi: 10.1016/j.brainres.2009.06.057. [DOI] [PubMed] [Google Scholar]
- Li ZR. Beijing: China Press of Traditional Chinese Medicine; 2007. Experimental Acupuncture Science. [Google Scholar]
- Lim JH, Jung CS, Byeon YE, Kim WH, Yoon JH, Kang KS, Kweon OK. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci. 2007;8:89–94. doi: 10.4142/jvs.2007.8.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm. 2011;118:155–176. doi: 10.1007/s00702-010-0514-4. [DOI] [PubMed] [Google Scholar]
- Sharp KG, Dickson AR, Marchenko SA, Yee KM, Emery PN, Laidmåe I, Uibo R, Sawyer ES, Steward O, Flanagan LA. Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol. 2012;235:345–356. doi: 10.1016/j.expneurol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Zhu ZH, Duan XL, Liu XJ, Fan J, Ju G, Wang BR. Effects of electroacupuncture at “Zusanli” (ST 36) on ERK1/2 phosphorylation in the dorsal horn of spinal cord of the rat. Zhongguo Zhen Jiu. 2006;26:362–366. [PubMed] [Google Scholar]
- Su H, Zheng QX, Luo QM. Monitoring of spinal cord hemodynamics by laser speckle imaging technique. Huazhong Keji Daxue Xuebao: Yixue Ban. 2009;38:106–109. [Google Scholar]
- Tsuchiya M, Sato EF, Inoue M, Asada A. Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth Analg. 2007;104:301–307. doi: 10.1213/01.ane.0000230622.16367.fb. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YG, Sun ZR, Li XY, Wang H. Studies on the influence of acupuncture on the changes of SCBF in rat’ injuried spinal. Zhongguo Zhongyiyao Keji. 1995;2:14–16. [Google Scholar]
- Yang JW, Jeong SM, Seo KM, Nam TC. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003;4:97–101. [PubMed] [Google Scholar]
- Zhu YD, Zhang JF. EMG change and numb lower limbs of protrusion of the lumbar intervertebral disc after ele-acupuncture therapy. Zhejiang Zhongyiyao Daxue Xuebao. 2012;36:565–567. [Google Scholar]